Abstract
Background
Evaluating population-level patterns of heavy episodic drinking by age, period, and cohort is critical to understanding population-level influences on rates over time and to forecasting future trends for public health planning efforts. The present study examined trends in heavy episodic drinking in the US from 1985 through 2009 in a nationally representative sample that included adolescents and adults.
Methods
Data are drawn from repeated cross-sectional surveys of US households as part of the National Household Survey on Drug Use and Health conducted in 1985, 1988, and annually from 1990 though 2009, inclusive (N = 809,281). Heavy episodic drinking was defined as any instance of consuming five or more drinks in one sitting in the past month. Age–period–cohort models were identified using the Intrinsic Estimator algorithm.
Results
Heavy episodic drinking is decreasing in the US among adolescents and young adults, with the most recently born cohorts (born in the 1990s) at lower odds of heavy episodic drinking compared with cohorts born in the 1960s, 1970s, and 1980s. Results were consistent across sex and race/ethnicity, with the exception that the decrease is not apparent among Hispanics.
Conclusions
These data are promising in that young cohorts appear to be reducing heavy episodic drinking, however the lack of decrease among Hispanics suggests targeted intervention and prevention as well as increased surveillance are necessary.
Keywords: Heavy episodic drinking, Binge drinking, United States, Age–period–cohort effects, Intrinsic Estimator, National Household Survey on Drug Use and Health, NHSDUH
1. Introduction
Heavy episodic drinking, or consumption of a large amount of alcohol in a short period of time with the primary goal of intoxication, is implicated in over half of the alcohol-attributable deaths in the U.S. (Naimi et al., 2003a). Acute effects of heavy episodic drinking include intentional as well as unintentional injury (Hingson et al., 2002), intimate partner violence (Thompson and Kingree, 2006), unintended pregnancy (Naimi et al., 2003b), and fetal alcohol syndrome (May and Gossage, 2001), and chronic heavy episodic drinking is associated with several forms of cancer (Longnecker, 1994), disruption in liver function, and premature mortality (Holman et al., 1996). While surveillance estimates indicate overall reductions in heavy episodic drinking in the US over the last 10 years (Johnston et al., 2011), the prevalence remains high and reducing consequences of heavy drinking continues to be a significant public health priority (Casswell and Thamarangsi, 2009). Evaluating patterns of heavy episodic drinking by age, period, and cohort is critical to understanding population-level influences on rates over time and to forecasting future trends.
Existing literature on trends in overall alcohol consumption and alcohol use disorders generally indicate that strong cohort effects are operative in shaping patterns over time (Keyes et al., 2011). With regard to heavy episodic drinking specifically, however, available data are inconsistent. Kerr et al. (2009) documented in sequentially conducted cross-sectional samples over 26 years that two simultaneous processes are operative in alcohol consumption trends (Kerr et al., 2009). While cohorts born in the late 1970s and early 1980s consume alcohol less frequently and have a lower overall consumption mean compared with previously born cohorts, heavy episodic drinking is more prevalent. If true, this suggests that increases in risky patterns of alcohol consumption may portend greater drinking consequences for these cohorts later in life. However, other national studies have not found similar results, and instead documented that heavy episodic drinking is either decreasing in cohorts born in the 1980s (Bachman et al., 1999; Keyes et al., 2008), or that period effects explain trends over time in heavy episodic drinking rather than cohort effects (Karlamangla et al., 2006). These latter studies have not used age–period–cohort models to formally estimate cohort effects, which could be a source of divergence in results across study. Outside of the US, data has predominately shown increases in heavy episodic drinking among younger cohorts (Bjork et al., 2008; Kemm, 2003). No study to date has documented patterns among cohorts of the 1990s, who are now in the primary age of risk for heavy episodic drinking and the development of alcohol use disorders.
Of further interest is variation in heavy episodic drinking trends over time by sex and race/ethnicity. Accumulating evidence, both in the US and cross-nationally, indicates that the gender differences in heavy episodic drinking and alcohol disorders are converging in more recently born cohorts, primarily due to increases among women (Bjork et al., 2008; Grucza et al., 2008a,b; Holdcraft and Iacono, 2002; Kemm, 2003; Kerr et al., 2009; Keyes et al., 2008, 2011; Rice et al., 2003). Whether these trends will continue in the birth cohorts of the late 1980s and early 1990s, who are now passing through the primary age at risk for heavy episodic drinking and alcohol disorders, has not been investigated to date.
Information on trends by race and ethnicity is generally overlooked in national studies of alcohol consumption, and remains a critical epidemiologic gap. Large-scale surveys generally find that lifetime rates of heavy episodic drinking are lower among Blacks compared to non-Hispanic Whites, and that current rates are more comparable if slightly lower among Blacks (Hasin et al., 2007). Heavy episodic drinking patterns among Latinos are comparable if not slightly higher compared to non-Hispanic Whites (Caetano, 1984b), with Puerto Ricans evidencing the highest rate of heavy episodic drinking compared to Cuban, Mexican, and South American Latino subgroups. Further, while heavy episodic drinking and frequent heavy episodic drinking have decreased among non-Hispanic Whites, rates remained stable among Latinos and increased among Latino women across the 1980s and 1990s (Caetano and Clark, 1998). Additionally, several large-scale epidemiologic studies have documented a ‘cross-over’ effect (Caetano, 1984a; Robins, 1985; Watt, 2008), whereby non-Hispanic Whites have higher rates of alcohol use and problematic patterns of use compared to Latinos and Blacks only in adolescence; this difference not only converges in adulthood, it changes direction. Data on racial/ethnic differences in alcohol consumption in more recent decades is critical to understand more recent trends by race and ethnicity. If data indicate further increases in heavy episodic drinking among Latinos, target messages may be necessary. Further, age–period–cohort modeling across racial/ethnic subgroup is necessary to control for crossover patterns in the epidemiologic data when assessing period and cohort effects, which previous studies have not done.
The present study aims to provide updated information on age, period, and cohort effects in the prevalence of heavy episodic drinking in the US across the last 25 years using nationally representative surveillance data. Further, we focus on differences in these effects across sex and race/ethnicity due the paucity of available evidence on heavy episodic drinking trends in cohorts born in the late 1980s and early 1990s by these critical demographic axes.
2. Methods
2.1. Sample
Data were drawn from the National Household Survey on Drug Use and Health (NHSDUH), a series of nationally representative cross-sectional surveys of the US civilian, non-institutionalized population. Data for the present study was collected in 1985, 1988, and annually from 1990 though 2009, inclusive. A primary purpose of the NHSDUH was to provide representative estimates of drug and alcohol use in the US population, thus the sampling scheme was a multistage probability sample, with over-representation of African Americans, Hispanics, and young adults adjusted to be representative of the nearest census using sampling weights. This analysis focuses on respondents aged 15–64 at each survey wave, with a total of 809,281 respondents.
Two methodological changes in the survey administration are worth noting. First, in 1999 the survey was administered using computer-assisted software rather than paper and pencil. Evidence indicates that reporting of substance use increased to some degree when the instrument was administered via computer (Barker et al., 1998; Penne et al., 1998; Wright et al., 1998). Second, in 2002 respondents were offered monetary incentives to participate. This change improved response rates, and evidence indicates a slight increase in the prevalence of substance use when these harder to reach respondents were included (Office of Applied Studies, 2002, 2003).
Interviews were conducted in the home by trained interviewers. Response rates were typically 80% or higher. All information is self-reported. More detailed information about the survey is available at the SAMHSA website at (http://www.oas.samhsa.gov/nhsda.htm).
2.2. Measures
2.2.1. Heavy episodic drinking
Heavy episodic drinking was defined as any instance of consuming five or more drinks in one sitting in the past month. While studies generally now define a lower threshold of drinks for women, these data were collected over the past twenty years and this was the only stably queried measure across the full study time. Further detail on the alcohol measures collected in the NHSDUH and changes over time in these questions can be found at (National Household Survey on Drug Use and Health, 2012).
2.2.2. Demographics
Birth cohort was defined by subtracting current age from year of the survey. Because of the large sample size, we were able to group birth cohorts into five-year intervals, which is standard in age–period–cohort modeling of surveillance data. Age and period groupings were also across five-year categories. We conducted sensitivity analyses by categorizing age, period, and birth cohort into four-year age groups in order to examine the robustness of our effects.
Sex and race/ethnicity were self-reported; racial categories were based on US census categories. Hispanic ethnicity was assessed separately. We divided the sample into those reporting non-Hispanic White race/ethnicity (heretofore referred to as “White”), non-Hispanic Black race/ethnicity (heretofore referred to as “Black”), and Hispanic ethnicity regardless of race. Other racial groups were not analyzed separately in the present study.
2.3. Statistical analysis
Prevalence estimates were generated from basic cross-tabulations. All analyses were weighted to take into account the complex survey design. In analyses of men, women, and racial/ethnic groups data from all survey years were included in one analysis pool and data from each survey were assigned unique strata numbers to adjust standard errors for design effects (Korn and Graubard, 1999). We first examined the prevalence of heavy episodic drinking by age and period in order to guide statistical model choice. This included assessment of potential age by period interactions, non-linearity, and/or the potential presence of cohort effects (Keyes and Li, 2010).
Age–period–cohort analysis was conducted using the Intrinsic Estimator approach developed by Yang and colleagues (Fu, 2000; Yang et al., 2004, 2008). Because age, period, and cohort are linear functions of one another (Cohort = Period − Age), attempts to simultaneously model the linear effects of age, period, and cohort in a traditional least squares regression will not be identified; i.e., they will produce an infinite number of equally-plausible estimates for which it is not possible to determine which are the best fit for the model. Therefore, researchers have either focused on estimating non-linear effects, or imposing some constraint on the model purely for identification purposes. Typically, this is done by equating one or more parameters to be equal (for example, setting the effects of first and second age groups to equal, or the first age group and the first time period, etc.). Unfortunately, parameter estimates are sensitive to the constraint chosen and the validity of the constraints is difficult to empirically assess. The IE is an approach that places a constraint on the model, but not a constraint that affects the estimation of regression parameters for age, period, and cohort in any way. That is, the regression parameter estimates are unbiased by the constraint placed, and a unique set of regression estimates can be estimated.
Technical descriptions of the IE can be found elsewhere (Yang et al., 2004, 2008). Briefly, the central premise of the IE is that a unique estimable function can be found by decomposing the parameter space of the age–period–cohort regression design matrix into two additive components that are geometrically perpendicular in the orthogonal subspace. It has been demonstrated that one of these additive components corresponds to the unique zero eigenvalue of the design matrix. As elegantly demonstrated by Yang and colleagues (Yang et al., 2004, 2008), this additive component has some unique properties that can be exploited for use in an age–period–cohort analysis. Specifically, it is independent of the actual age–period–cohort effects that gave rise to the data; it is a function of the number of age groups and period groups only. As such, it takes on a fixed vector form and does influence coefficient estimation. Further, using this decomposition, an Intrinsic Estimator can be estimated that is invariant to model constraints identified through the Moore–Penrose generalized inverse (Searle, 1971).
We use the publicly available add-on file for the “Intrinsic Estimator” algorithm (Yang et al., 2008) available in StataMP version 9.0. (StataCorp, 2005).
3. Results
Fig. 1 presents descriptive trends in the prevalence of past month heavy episodic drinking in the whole sample by age groups. We note that there is a notable decrease in the prevalence of heavy episodic drinking among individuals aged 15–19 in more recent years; specifically, the prevalence decreases 18% from 25.2% in 2004 to 20.8% in 2008. The prevalence increases across all age groups in 1999 and 2002, which could be an artifact of the changes in NHSUDH design. In summary, trends over time were different by age group, suggesting the possibility of a cohort effects; effects of methodological change on prevalence estimates were apparent, suggesting the possibility of a period effects. Because period effects in these data are hypothesized to capture methodological change, we operationalized age, period, and cohort effects as independent of each other in the statistical model (i.e., no interaction).
Fig. 1.
Prevalence of past-month heavy episodic drinking from 1985 to 2009 by age: results from the National Drug Use and Health surveys.
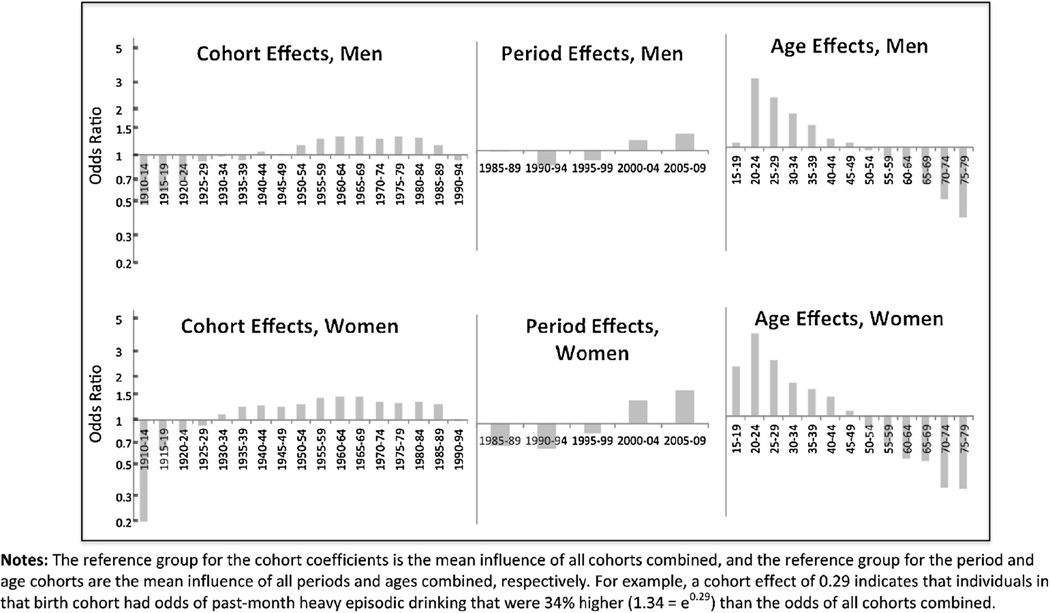
Figs. 2 and 3 present odds ratios from the full age–period–cohort results by gender (Fig. 2) and race/ethnicity (Fig. 3), and Table 1 contains the unexponentiated coefficients, standard errors, and p-values for the odds ratios shown in Figs. 2 and 3.
Fig. 2.
Age, period, and cohort effects for past-month heavy episodic drinking for men and women.
Fig. 3.
Age, period, and cohort effects for past-month heavy episodic drinking among Whites, Blacks, and Hispanics.
Table 1.
Results from age–period–cohort modeling showing the log odds of past-month heavy episodic drinking among men, women, Whites, Blacks, and Hispanics from 1985 to 2009.
| Men (N = 371,708) |
Women (N = 424,896) |
Whites (N = 503,745) |
Blacks (N = 113,330) |
Hispanics (N = 130,522) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log odds | SE | p-Value | Log odds | SE | p-Value | Log odds | SE | p-Value | Log odds | SE | p-Value | Log odds | SE | p-Value | |
| Age | |||||||||||||||
| 15–19 | 0.07 | 0.07 | 0.33 | 0.81 | 0.12 | <0.01 | 0.41 | 0.09 | <0.01 | −0.50 | 0.11 | <0.01 | 0.01 | 0.12 | 0.92 |
| 20–24 | 1.06 | 0.06 | <0.01 | 1.32 | 0.10 | <0.01 | 1.20 | 0.07 | <0.01 | 0.44 | 0.08 | <0.01 | 0.76 | 0.10 | <0.01 |
| 25–29 | 0.78 | 0.05 | <0.01 | 0.90 | 0.08 | <0.01 | 0.82 | 0.06 | <0.01 | 0.51 | 0.07 | <0.01 | 0.66 | 0.08 | <0.01 |
| 30–34 | 0.53 | 0.04 | <0.01 | 0.53 | 0.06 | <0.01 | 0.52 | 0.04 | <0.01 | 0.43 | 0.06 | <0.01 | 0.53 | 0.06 | <0.01 |
| 35–39 | 0.34 | 0.03 | <0.01 | 0.42 | 0.05 | <0.01 | 0.37 | 0.04 | <0.01 | 0.44 | 0.06 | <0.01 | 0.48 | 0.06 | <0.01 |
| 40–44 | 0.14 | 0.03 | <0.01 | 0.31 | 0.04 | <0.01 | 0.19 | 0.03 | <0.01 | 0.34 | 0.06 | <0.01 | 0.26 | 0.06 | <0.01 |
| 45–49 | 0.06 | 0.04 | 0.10 | 0.08 | 0.05 | 0.11 | 0.08 | 0.04 | 0.03 | 0.28 | 0.08 | <0.01 | 0.17 | 0.07 | 0.02 |
| 50–54 | −0.04 | 0.05 | 0.40 | −0.18 | 0.07 | 0.01 | −0.11 | 0.05 | 0.02 | 0.22 | 0.10 | 0.03 | −0.01 | 0.10 | 0.92 |
| 55–59 | −0.19 | 0.06 | <0.01 | −0.48 | 0.09 | <0.01 | −0.30 | 0.06 | <0.01 | 0.16 | 0.12 | 0.19 | −0.25 | 0.12 | 0.04 |
| 60–64 | −0.29 | 0.07 | <0.01 | −0.69 | 0.11 | <0.01 | −0.43 | 0.07 | <0.01 | 0.07 | 0.14 | 0.60 | −0.27 | 0.13 | 0.05 |
| 65–69 | −0.57 | 0.08 | <0.01 | −0.71 | 0.13 | <0.01 | −0.63 | 0.08 | <0.01 | −0.50 | 0.18 | 0.01 | −0.49 | 0.16 | <0.01 |
| 70–74 | −0.80 | 0.09 | <0.01 | −1.15 | 0.13 | <0.01 | −0.97 | 0.09 | <0.01 | −0.70 | 0.19 | <0.01 | −0.67 | 0.21 | <0.01 |
| 75–79 | −1.07 | 0.11 | <0.01 | −1.16 | 0.17 | <0.01 | −1.15 | 0.11 | <0.01 | −1.19 | 0.31 | <0.01 | −1.18 | 0.25 | <0.01 |
| Period | |||||||||||||||
| 1985–1989 | −0.02 | 0.04 | 0.56 | −0.36 | 0.06 | <0.01 | −0.12 | 0.04 | <0.01 | −0.23 | 0.07 | <0.01 | −0.14 | 0.06 | 0.03 |
| 1990–1994 | −0.25 | 0.02 | <0.01 | −0.40 | 0.03 | <0.01 | −0.30 | 0.02 | <0.01 | −0.26 | 0.04 | <0.01 | −0.13 | 0.04 | <0.01 |
| 1995–1999 | −0.16 | 0.02 | <0.01 | −0.15 | 0.02 | <0.01 | −0.15 | 0.02 | <0.01 | −0.17 | 0.03 | <0.01 | −0.16 | 0.03 | <0.01 |
| 2000–2004 | 0.17 | 0.02 | <0.01 | 0.38 | 0.03 | <0.01 | 0.23 | 0.02 | <0.01 | 0.29 | 0.03 | <0.01 | 0.19 | 0.03 | <0.01 |
| 2005–2009 | 0.27 | 0.03 | <0.01 | 0.53 | 0.05 | <0.01 | 0.35 | 0.03 | <0.01 | 0.37 | 0.05 | <0.01 | 0.24 | 0.05 | <0.01 |
| Cohort | |||||||||||||||
| 1910–1014 | −0.74 | 0.51 | 0.15 | −2.46 | 0.84 | <0.01 | −1.16 | 0.68 | 0.09 | 0.44 | 0.66 | 0.51 | −0.60 | 0.87 | 0.49 |
| 1915–1919 | −0.55 | 0.30 | 0.07 | −0.48 | 0.59 | 0.42 | −0.56 | 0.32 | 0.08 | −0.15 | 0.41 | 0.71 | −0.56 | 0.43 | 0.20 |
| 1920–1924 | −0.40 | 0.20 | 0.04 | −0.16 | 0.35 | 0.64 | −0.37 | 0.21 | 0.08 | −0.38 | 0.36 | 0.30 | −0.24 | 0.36 | 0.50 |
| 1925–1929 | −0.10 | 0.14 | 0.47 | −0.08 | 0.22 | 0.72 | −0.11 | 0.15 | 0.48 | −0.28 | 0.28 | 0.32 | −0.25 | 0.26 | 0.34 |
| 1930–1934 | −0.01 | 0.11 | 0.93 | 0.08 | 0.18 | 0.65 | −0.01 | 0.13 | 0.92 | −0.39 | 0.21 | 0.07 | 0.02 | 0.20 | 0.91 |
| 1935–1939 | −0.07 | 0.10 | 0.46 | 0.21 | 0.17 | 0.21 | −0.05 | 0.11 | 0.67 | −0.06 | 0.18 | 0.75 | −0.13 | 0.19 | 0.49 |
| 1940–1944 | 0.05 | 0.09 | 0.55 | 0.24 | 0.15 | 0.12 | 0.04 | 0.10 | 0.69 | −0.19 | 0.17 | 0.25 | 0.24 | 0.16 | 0.14 |
| 1945–1949 | 0.02 | 0.08 | 0.81 | 0.22 | 0.13 | 0.09 | 0.02 | 0.09 | 0.85 | −0.24 | 0.14 | 0.08 | −0.05 | 0.14 | 0.73 |
| 1950–1954 | 0.14 | 0.06 | 0.03 | 0.25 | 0.11 | 0.02 | 0.12 | 0.07 | 0.10 | 0.01 | 0.11 | 0.92 | 0.25 | 0.12 | 0.03 |
| 1955–1959 | 0.25 | 0.05 | <0.01 | 0.34 | 0.08 | <0.01 | 0.25 | 0.06 | <0.01 | 0.04 | 0.09 | 0.63 | 0.11 | 0.09 | 0.22 |
| 1960–1964 | 0.29 | 0.04 | <0.01 | 0.38 | 0.06 | <0.01 | 0.28 | 0.04 | <0.01 | 0.18 | 0.07 | 0.01 | 0.09 | 0.07 | 0.17 |
| 1965–1969 | 0.27 | 0.03 | <0.01 | 0.36 | 0.04 | <0.01 | 0.29 | 0.03 | <0.01 | 0.09 | 0.05 | 0.06 | 0.13 | 0.05 | 0.01 |
| 1970–1974 | 0.24 | 0.02 | <0.01 | 0.29 | 0.03 | <0.01 | 0.28 | 0.02 | <0.01 | 0.11 | 0.04 | 0.01 | 0.08 | 0.03 | 0.02 |
| 1975–1979 | 0.27 | 0.02 | <0.01 | 0.27 | 0.03 | <0.01 | 0.31 | 0.02 | <0.01 | 0.25 | 0.04 | <0.01 | 0.13 | 0.04 | <0.01 |
| 1980–1984 | 0.26 | 0.03 | <0.01 | 0.30 | 0.04 | <0.01 | 0.34 | 0.03 | <0.01 | 0.27 | 0.05 | <0.01 | 0.27 | 0.06 | <0.01 |
| 1985–1989 | 0.15 | 0.04 | <0.01 | 0.25 | 0.07 | <0.01 | 0.28 | 0.05 | <0.01 | 0.29 | 0.08 | <0.01 | 0.30 | 0.08 | <0.01 |
| 1990–1994 | −0.08 | 0.06 | 0.18 | −0.01 | 0.09 | 0.87 | 0.06 | 0.06 | 0.38 | <0.01 | 0.11 | 0.97 | 0.21 | 0.11 | 0.06 |
Note: The reference group for the cohort coefficients is the mean influence of all cohorts combined, and the reference group for the period and age cohorts are the mean influence of all periods and ages combined, respectively. For example, a cohort effect of 0.29 indicates that individuals in that birth cohort had odds of past-month heavy episodic drinking that were 34% higher (1.34 = e0.29) than the odds of all cohorts combined.
Results are relatively consistent across sex (Fig. 2). Cohort effects. Both men and women in the youngest born cohort (1990–1994) have odds of heavy episodic drinking that are lower than cohorts born in the previous decades. Among men, odds ratios for cohorts born 1955 through 1985 ranged from 1.16 [1985–1989] to 1.33 [1960–1964], whereas the 1990–1994 cohort had 0.93 times the odds of past-month heavy episodic drinking compared to the mean. A similar pattern was observed for women; those born in 1960–1964 has the highest odds of heavy episodic drinking compared to the mean (OR = 1.44), and those in the most recently born cohort had 0.99 times the odds of drinking, approximately equal to the mean. Period effects. Both men and women evidence an increasing period effect across the time period of the surveys. Age effects. Those aged 20–24 have the highest odds of heavy episodic drinking among both men (OR = 2.89) and women (OR = 3.75). Women aged 15–19 have odds of heavy episodic drinking higher than the mean (OR = 2.24, p < 0.01), whereas men aged 15–19 have odds of heavy episodic drinking similar to the mean odds (OR = 1.07, p = 0.33).
Results are also relatively consistent across race/ethnicity (Fig. 3), although with several notable exceptions. Age effects. When examining age effects, the age group with the highest odds of use is those aged 20–24 for both Whites and Hispanics (for age 20–24, OR = 3.13 among Whites and 2.14 among Hispanics); among Blacks, the 25–29 age group has the highest odds (OR = 1.67). Further, those age 15–19 have higher odds than the mean odds in Whites (OR = 1.51, p <0.01), lower odds than the mean odds in Blacks (OR = 0.61, p < 0.01), and approximately equal to the mean odds in Hispanics (OR = 1.01, p = 0.92). Period effects. All race/ethnic groups evidence an increasing period effect across the time period of the surveys. Cohort effects. Among Blacks and Hispanics, the youngest cohorts have lower odds of heavy episodic drinking than cohorts born within the previous decade. Among Hispanics, however, the youngest cohort remains at slightly elevated odds compared to the mean odds (OR = 1.23, p = 0.06).
Because we see a decrease in the cohort effect for the youngest cohort, we examined descriptive trends in the youngest age group by sex and race/ethnicity to verify the results given by the model (Supplementary Fig. 11). Descriptive trends show decreases in heavy episodic drinking among the youngest age group (who would comprise the most recently-born cohorts) across men, women, and Whites. Among Blacks, there is an increase in heavy episodic drinking from 8.6% to 11.4% from 2006 to 2008, and then a decrease in 2009 to 10.4%. Among Hispanics, the prevalence consistently decreased from 2004 to 2008, and then increased in 2009 to 19.2%.
In Supplementary Fig. 2, we present the results of a sensitivity analysis in which age, period, and cohort were categorized into four-year age groupings rather than five-year age groupings in the total sample. Similar to the findings in the analyses using five-year groups, cohort effects were largely unchanged across all cohorts from 1954–57 to 1982–85, and then declined among the most recent cohorts. For men the cohort effects declined from 0.26 (C.I. 0.19–0.33) in the 1982–85 cohort to −0.07 (C.I. −0.19 to 0.05) in the 1990–93 cohort, and for women the decline over the same period was from 0.33 (C.I. 0.23–0.43) to 0.02 (C.I. −0.15 to 0.19). Age and period effects in the four-year groups were similar to the five-year groups: there is an increasing period effect beginning in the early 2000s compared to the mean across periods, and those in young adulthood have increased odds of binge drinking compared to the mean across age groups (see Online Fig. 2).
4. Discussion
The birth cohort into which individuals are born can increase or decrease their odds of substance use over the entire life course. This cohort effect can result, in part, from cohort-specific norms and attitudes, which have an effect on substance abuse above and beyond an individual’s attitudes and beliefs (Keyes et al., 2012a,b). In this study we focus on today’s most recent cohorts and examine whether the sum effects of cohort-specific factors suggest an increase or decrease in binge drinking in comparison to cohort effects of the past.
The results indicate that membership in the most recent birth cohort born in 1990–94 confers a lower risk for binge drinking than membership in any cohort dating back to at least 1950–54. These results suggest that at any given age the members of the 1990–94 birth cohort will be less likely to binge drink than members of other cohorts when they were the same age, independent of any other influence that may affect binge drinking. Further, while the binge drinking patterns of all cohorts will ebb and flow over historical time, within this pattern the odds of binge drinking will remain lower for members of the 1990–94 cohort in comparison to birth cohorts dating back to 1950–54. However, we note that the 1990–94 cohort has limited data on episodic drinking patterns; in the 2005–2009 survey, individuals in this cohort range from age 11 to age 20. Thus, additional data need to be collected on this cohort as they progress through early adulthood to determine whether the pattern of decrease cohort effects persists in the long term, or is an artifact of their younger age compared with older cohorts.
These findings correspond to other surveillance information such as per capita alcohol consumption, which peaked in the early 1980s (when then cohorts born in the 1960s would be in the primarily risk period for heavy alcohol use). They are also consistent with national surveillance of adolescent heavy episodic drinking, in which prevalence rates of past two-week heavy episodic drinking among high school seniors have decreased more than seven percentage points since 2000 (Johnston et al, 2011). Further, the present study coheres with other age–period–cohort analyses of heavy episodic drinking in showing that cohorts born in the 1970s and 1980s had increased odds of heavy episodic drinking compared to earlier born cohorts (Bjork et al., 2008; Kemm, 2003; Kerr et al., 2009; Lakins et al., 2008); however, we add to this literature by demonstrating that this increase in risk may not apply to the cohorts of the 1990s. Finally, the study results are also consistent with age–period–cohort analyses of marijuana use in these data, which also indicated that cohort effects were declining for more recently born cohorts (Miech and Koester, 2012).
We note that a positive period effect was found, consistently across gender and race. Changes in NHSDUH methodology over the last 10 years limit inferential ability about these period effects. Methodology changes should manifest as period effects as they likely apply in effect equally across participants, and thus should be filtered out of our estimates of age and cohort effects. Indeed, the patterns of period effects observed here, with increases across time, are consistent with an increase in prevalence when the survey moved from paper and pencil to computer-assisted 1999, and when monetary incentives were introduced in 2002. Both of these changes should increase the observed prevalence of use, and as such we see increases in the prevalence across age groups at these two times points and believe this effect is primarily reflected in our increasing period effect.
These trends were remarkably consistent across gender, with cohorts born primarily in the 1960s, 1970s and 1980s at highest risk, and those born in the 1990s at lower risk. One gender difference in these effects was the positive age effect for those aged 15–19 among women, and no positive effect for men of the same age. This indicates that adolescent women have higher odds of heavy episodic drinking compared to the mean of all women, whereas adolescent men have no higher odds of heavy episodic drinking compared to the mean of all men. These results could indicate that men tend to drink more across the entire life course than women, such that the mean for 15–19 year olds is not substantially higher than the overall mean. Such an interpretation would be consistent with evidence from other studies indicating that men drink more than women at every point in the life course (Keyes et al, 2008). That is, there could be more desistence from heavy drinking patterns in women compared to men, such that early onset drinking is more pronounced when compared to the mean.
Studies of age–period–cohort effects in alcohol use disorders, which are more rare than heavy episodic drinking, have generally found that each successively younger cohort post World War II has a higher risk for alcohol disorders, and this risk is more pronounced for women compared with men (Grucza et al., 2008a,b; Holdcraft and Iacono, 2002; Keyes et al., 2008; Seedat et al., 2009). These results suggest that the same trends may not be operative in heavy episodic drinking, which is more prevalent than alcohol use disorders and may reflect different factors affecting prevalence. Instead, age, period, and cohort factors appear relatively similar for women and men, and suggest that risk is generally decreasing in the youngest cohorts.
By race and ethnicity, we do not find strong evidence of a ‘cross-over effect’ whereby Whites drink more than Black and Hispanic individuals only at young ages (Caetano, 1984a; Robins, 1985; Watt, 2008). However, we do find that the distribution of alcohol consumption differs across race, such that Blacks have an older age at which heavy episodic drinking is mostly likely to occur. This is consistent with prior literature suggesting that Blacks have an older age of alcohol use onset (Grant et al., 2012; Johnson et al., 2005; Wagner et al., 2002), and also suggests that risky drinking practices are delayed in onset and persistence for Blacks as well. Hispanics were the only ethnic group that did not show a decrease in the cohort effect for more recently born cohorts. Descriptive trends from national samples in the 1980s and 1990s demonstrated that Whites were decreasing heavy episodic drinking but heavy episodic drinking was increasing in Hispanics Women (Caetano and Clark, 1998). More recent data indicated that the prevalence of alcohol abuse and dependence symptoms is increasing in Hispanics and Whites but not to the same extent among Blacks (Caetano et al., 2011). Overall, these results indicate that young Hispanics in the US may be increasingly in need of targeted prevention and intervention strategies, and these young cohorts should be monitored for potential service needs if higher rates of alcohol use disorders manifest in this group.
Age–period–cohort analyses are undertaken to decompose variance to determine the broad trends by age, period, and cohort. Age–period–cohort models do not typically test mechanisms to understand what factors drive trends, although methods are in development in order to construct models in which mechanisms can be tested (Keyes et al., 2012a,b). Given that converging evidence from these and other studies indicates that patterns of alcohol consumption including heavy episodic drinking aggregate by birth cohort (Kerr et al., 2009), an important next step in understanding cohort-specific trends over time includes developing theory and collecting data in order to test mechanisms. Factors that would explain the decreasing cohort effect for birth cohorts of the 1990s, for example, must be: (a) changing by birth cohort and (b) changing in a similar pattern that would be predicted by our models. Potential mechanisms that may be operative in this way could include changes in the population distribution of educational attainment, family structure, urban density and characteristics of urban populations, and social norms. Candidate factors must also be specific to the outcome of heavy drinking, given that a recent age–period–cohort analysis indicates that cohort effects are increasing the odds of nonmedical prescription pain medication use among today’s most recent cohorts (Miech et al., 2013). Unfortunately, it is possible that the decrease in heavy drinking among today’s youth is simply the result of their switch to prescription drug misuse as their drug of choice. Future studies examining switching and substitution across substances, time-varying population demographics, and other potential explanations for trends over time and across substance, are key to further developing our understanding of the population dynamics central to predicting future trends in substance use.
The study should be considered in light of several important limitations. Changes in NHSDUH methodology over the last 10 years limit inferential ability about trends over time. The first is the general limitations of self-reported data being potentially inaccurate, which is not unique to the present study but nonetheless important. Further, questions on alcohol consumption in the NHSUDH are limited; past-month is the only time frame assessed for binge drinking. While the past-month time frame may miss some frequent or problem episodic drinkers who may have not engaged in this level of drinking in the past month, we will likely capture those who are the most frequent heavy drinkers. Further, there was no information in the survey on the frequency of binge drinking; some individuals may have had a single episode of binge drinking in the past month whereas others engaged in weekly or even daily binge drinking. Age, period, and cohort effects for frequency of heavy episodic drinking may differ from overall prevalence of heavy episodic drinking. We note, however, the consistency in our results compared to other age–period–cohort analyses of heavy episodic drinking which operationalized episodic drinking in various ways (Bjork et al., 2008; Kemm, 2003; Kerr et al., 2009; Lakins et al., 2008), suggesting that any misclassification does not likely change the conclusions drawn from these data.
Specific to age–period–cohort studies, there may be secular trends in the acceptability of heavy episodic drinking which would affect the accuracy of reporting. Thus, there could be no trend in actual heavy episodic drinking but a trend in reporting. This is not testable in the present study but should be acknowledged as an alternative explanation. Further, as is true of all age–period–cohort studies, we are limited by the lack of information for the youngest and oldest birth cohorts. That is, age effects cannot be fully separated from cohort effects when there is no variation by age within birth cohort. Continued surveillance is necessary to confirm and extend these results as the youngest cohorts continue to pass through the age of high binge drinking risk. Additionally, assessing and recording race and ethnicity is generally problematic for a number of reasons. These are categories in constant flux, and no consensus regarding the concept of race has been formed in scientific research. Some researchers question the value of assessing race at all in research, given the ambiguities and lack of clear boundaries among groups, and the problematic history of scientific research on racial/ethnic minorities (Fullilove, 1998). However, assessment of racial/ethnic variation in heavy episodic drinking is necessary to document potential health disparities in the population, health status, and to allow for access to health resources.
Another limitation is a potential bias in self-reporting of heavy episodic drinking over time. It is theoretically possible that youth today are more reluctant to disclose heavy episodic drinking in population surveys than they were in the past, and the results of this study could reflect self-reporting trends more so than real drinking behavior. In order to explain the results of this study, such a bias would need to be specific to youth, given the historical period effect in the results indicating that reports of heavy episodic drinking have actually increased in the most recent historical period among the general population. Available evidence does not support self-reporting bias as a plausible explanation for findings of this study. Levels of youth attitude toward heavy episodic drinking, such as disapproval and perceived risk, have changed very little in the past ten years (Johnston et al., 2011), and level of these attitudes would be expected to have increased if binge drinking has become increasingly stigmatized in recent years and less likely to be self-reported. The same national survey of youth that tracks attitudes toward heavy episodic drinking also tracks its prevalence and the results indicate a substantial downturn in recent years (Johnston et al., 2011), which provides additional support for unique, lower odds of heavy episodic drinking among today’s youth.
Finally, we note that the subject of appropriate age–period–cohort modeling is one that remains an active area of research. The IE is a variation on the use of principal components regression and principal least squares regression to identify age–period–cohort models, which have a long history in trend research. While the IE does not solve the identification problem in age–period–cohort analysis, it provides a useful way to express the relations among age, period, and cohort in a statistically robust model. However, like all models, the research makes assumptions about the relation among the variables when applying the model (e.g., no interaction across age, period, or cohort), which may not correspond to theory (Keyes et al., 2010). We used basic graphical techniques to guide and confirm the results of our modeling strategy. Thus, the estimates reported confirm observed graphical trends are thus are unlikely to be an artifact of model assumptions. Overall, we believe the strategy used here provides and robust depiction of trends in heavy episodic drinking, but note that the IE may not be the appropriate model for all research questions.
In conclusion, the present study provides the largest population-based assessment of age, period, and cohort effects in US heavy episodic drinking ever conducted to date, and presents robust information on cohorts born in the 1990s who are now in early adulthood. Our results are promising in that young cohorts appear to be reducing heavy episodic drinking, however the lack of decrease among Hispanics is troubling and suggests targeted intervention and prevention as well as increased surveillance are necessary. Further, the downward shift in the age distribution of heavy episodic drinking to younger ages in women compared to men should be subject to further research, given the well-documented associations between early onset heavy alcohol use and later developmental health issues (McGue et al., 2001; Squeglia et al., 2009; White et al., 2002). Future research is necessary to understand the mechanisms of these changes over time, examining changes in educational attainment, family structures, urbanicity, and health status across gender and race/ethnicity to fully elucidate the mechanisms through which population-level changes manifest as trends over time.
Supplementary Material
Acknowledgments
Role of funding source
This research was supported in part by support from New York State Psychiatric Institute and the Department of Epidemiology at the Mailman School of Public Health (Keyes).The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2013.01.019.
Footnotes
Supplementary material can be found by accessing the online version of this paper. Please see Appendix A for more information.
Contributors
K. Keyes drafted the manuscript and conducted supplementary data analysis. R. Miech conducted data analysis and provided critical revisions to the text.
Conflict of interest
The authors report no conflicts of interest.
References
- Bachman JG, Freedman-Doan P, O’Malley PM, Johnston LD, Segal DR. Changing patterns of drug use among US military recruits before and after enlistment. Am. J. Public Health. 1999;89:672–677. doi: 10.2105/ajph.89.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker P, Gfroerer J, Caspar R, Lessler J. Proceedings of the 1998 Joint Statistical Meetings, American Statistical Association, Survey Research Methods Section, Dallas, TX. Alexandria, VA: American Statistical Association; 1998. Major Design Changes in the National Household Survey on Drug Abuse; pp. 732–737. [Google Scholar]
- Bjork C, Thygesen LC, Vinther-Larsen M, Gronbaek MN. Time trends in heavy drinking among middle-aged and older adults in Denmark. Alcohol. Clin. Exp. Res. 2008;32:120–127. doi: 10.1111/j.1530-0277.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Caetano R. Ethnicity and drinking in northern California: a comparison among whites, blacks and Hispanics. Alcohol Alcohol. 1984a;19:31–44. [PubMed] [Google Scholar]
- Caetano R. Self-reported intoxication among Hispanics in northern California. J. Stud. Alcohol. 1984b;45:349–354. doi: 10.15288/jsa.1984.45.349. [DOI] [PubMed] [Google Scholar]
- Caetano R, Baruah J, Chartier KG. Ten-year trends(1992 to 2002) in sociodemographic predictors and indicators of alcohol abuse and dependence among whites, blacks, and Hispanics in the United States. Alcohol. Clin. Exp. Res. 2011;35:1458–1466. doi: 10.1111/j.1530-0277.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano R, Clark CL. Trends in alcohol consumption patterns among whites, blacks and Hispanics: 1984 and 1995. J. Stud. Alcohol. 1998;59:659–668. doi: 10.15288/jsa.1998.59.659. [DOI] [PubMed] [Google Scholar]
- Casswell S, Thamarangsi T. Reducing harm from alcohol: call to action. Lancet. 2009;373:2247–2257. doi: 10.1016/S0140-6736(09)60745-5. [DOI] [PubMed] [Google Scholar]
- Fu WJ. Ridge estimator in singular design with application to age–period–cohort analysis of disease rates. Commun. Stat. Theory Methods. 2000;29:263–278. [Google Scholar]
- Fullilove MT. Abandoning “race” as a variable in public health research: an idea whose time has come. Am. J. Public Health. 1998;88:1297–1298. doi: 10.2105/ajph.88.9.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JD, Verges A, Jackson KM, Trull TJ, Sher KJ, Bucholz KK. Age and ethnic differences in the onset, persistence and recurrence of alcohol use disorder. Addiction. 2012;107:756–765. doi: 10.1111/j.1360-0443.2011.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Bucholz KK, Rice JP, Bierut LJ. Secular trends in the lifetime prevalence of alcohol dependence in the United States: a re-evaluation. Alcohol. Clin. Exp. Res. 2008a;32:763–770. doi: 10.1111/j.1530-0277.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Norberg K, Bucholz KK, Bierut LJ. Correspondence between secular changes in alcohol dependence and age of drinking onset among women in the United States. Alcohol. Clin. Exp. Res. 2008b;32:1493–1501. doi: 10.1111/j.1530-0277.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Zakocs RC, Kopstein A, Wechsler H. Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18–24. J. Stud. Alcohol. 2002;63:136–144. doi: 10.15288/jsa.2002.63.136. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cohort effects on gender differences in alcohol dependence. Addiction. 2002;97:1025–1036. doi: 10.1046/j.1360-0443.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- Holman CD, English DR, Milne E, Winter MG. Meta-analysis of alcohol and all-cause mortality: a validation of NHMRC recommendations. Med. J. Aust. 1996;164:141–145. doi: 10.5694/j.1326-5377.1996.tb122011.x. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Richter L, Kleber HD, McLellan AT, Carise D. Telescoping of drinking-related behaviors: gender, racial/ethnic, and age comparisons. Subst. Use Misuse. 2005;40:1139–1151. doi: 10.1081/JA-200042281. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg J. Volume I: Secondary School Students. Ann Arbor: Institute for Social Research, The University of Michigan; 2011. Monitoring the future national survey results on drug use, 1975–2010. [Google Scholar]
- Karlamangla A, Zhou K, Reuben D, Greendale G, Moore A. Longitudinal trajectories of heavy drinking in adults in the United States of America. Addiction. 2006;101:91–99. doi: 10.1111/j.1360-0443.2005.01299.x. [DOI] [PubMed] [Google Scholar]
- Kemm J. An analysis by birth cohort of alcohol consumption by adults in Great Britain 1978–1998. Alcohol Alcohol. 2003;38:142–147. doi: 10.1093/alcalc/agg039. [DOI] [PubMed] [Google Scholar]
- Kerr WC, Greenfield TK, Bond J, Ye Y, Rehm J. Age–period–cohort modelling of alcohol volume and heavy drinking days in the US National Alcohol Surveys: divergence in younger and older adult trends. Addiction. 2009;104:27–37. doi: 10.1111/j.1360-0443.2008.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 2008;93:21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Li G. A multiphase method for estimating cohort effects in age–period contingency table data. Ann. Epidemiol. 2010;20:779–785. doi: 10.1016/j.annepidem.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Li G, Hasin DS. Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis. Alcohol. Clin. Exp. Res. 2011;35:2101–2112. doi: 10.1111/j.1530-0277.2011.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Liu XC, Cerda M. The role of race/ethnicity in alcohol-attributable injury in the United States. Epidemiol. Rev. 2012a;34:89–102. doi: 10.1093/epirev/mxr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Schulenberg JE, O’Malley PM, Johnston LD, Bachman JG, Li G, Hasin D. Birth cohort effects on adolescent alcohol use: the influence of social norms from 1976 to 2007. Arch. Gen. Psychiatry. 2012b:1–10. doi: 10.1001/archgenpsychiatry.2012.787. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Utz RL, Robinson W, Li G. What is a cohort effect? Comparison of three statistical methods for modeling cohort effects in obesity prevalence in the United States, 1971–2006. Soc. Sci. Med. 2010;70:1100–1108. doi: 10.1016/j.socscimed.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn EL, Graubard BI. Analysis of Health Surveys. New York: John Wiley & Sons; 1999. [Google Scholar]
- Lakins NE, LaVallee RA, Williams GD, Yi H. Surveillance Report #85: Apparent Per Capita Alcohol Consumption: National, State, and Regional Trends, 1977–2006. Bethesda, MD: NIAAA, Division of Epidemiology and Prevention Research, Alcohol Epidemiologic Data System; 2008. (November 2008), Epub: http://pubsniaaanihgov/publications/surveillance85/CONS06htm. [Google Scholar]
- Longnecker MP. Alcoholic beverage consumption in relation to risk of breast cancer: meta-analysis and review. Cancer Causes Control. 1994;5:73–82. doi: 10.1007/BF01830729. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res. Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol. Clin. Exp. Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Miech R, Koester S. Trends in U.S., past-year marijuana use from 1985 to 2009: an age–period–cohort analysis. Drug Alcohol Depend. 2012;124:259–267. doi: 10.1016/j.drugalcdep.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech R, Bohnert A, Heard K, Boardman J. Increasing use of nonmedical analgesics among younger cohorts in the United States: a birth cohort effect. J. Adolesc. Health. 2013;52(1):35–41. doi: 10.1016/j.jadohealth.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. 2003a;289:70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- Naimi TS, Lipscomb LE, Brewer RD, Gilbert BC. Binge drinking in the preconception period and the risk of unintended pregnancy: implications for women and their children. Pediatrics. 2003b;111:1136–1141. [PubMed] [Google Scholar]
- National Household Survey on Drug Use and Health. 2012 http://www.samhsa.gov/data/NSDUH.aspx.
- Office of Applied Studies. National Household Survey on Drug Abuse: Incentive Experiment Combined Quarter 1 and Quarter 2 Analysis (RTI/07190388100) Rockville, MD: Substance Abuse and Mental Health Services Administration; 2002. [Google Scholar]
- Office of Applied Studies. Appendix C: NSDUH Changes and Their Impact on Trend Measurement. Results from the 2002 National Survey on Drug Use and Health: National findings (DHHS Publication No SMA 03-3836, NSDUH Series H-22) Rockville, MD: Substance Abuse and Mental Health Services Administration; 2003. pp. 107–137. [Google Scholar]
- Penne MA, Lessler J, Bieler G, Caspar R. Proceedings of the 1998 Joint Statistical Meetings, American Statistical Association, Social Statistics Section, Dallas, TX. Alexandria, VA: American Statistical Association; 1998. Effects of experimental audio computer-assisted self-interviewing (ACASI) procedures on reported drug use in the NHSDA: results from the 1997 CAI field experiment; pp. 744–749. [Google Scholar]
- Rice JP, Neuman RJ, Saccone NL, Corbett J, Rochberg N, Hesselbrock V, Bucholz KK, McGuffin P, Reich T. Age and birth cohort effects on rates of alcohol dependence. Alcohol. Clin. Exp. Res. 2003;27:93–99. doi: 10.1097/01.ALC.0000047303.89421.AA. [DOI] [PubMed] [Google Scholar]
- Robins L. Alcohol abuse in blacks and whites as indicated in the epidemiological catchment area program. In: Spiegler D, editor. Alcohol Use Among US Ethnic Minorities. Bethesda: U.S. Department of Health and Human Services; 1985. [Google Scholar]
- Searle SR. Linear Models. New York: Wiley & Sons; 1971. [Google Scholar]
- Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, Demyttenaere K, de Girolamo G, Haro JM, Jin R, Karam EG, Kovess-Masfety V, Levinson D, Medina Mora ME, Ono Y, Ormel J, Pennell BE, Posada-Villa J, Sampson NA, Williams D, Kessler RC. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch. Gen. Psychiatry. 2009;66:785–795. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin. EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software, Release 9. College Station, TX: StataCorp LP; 2005. [Google Scholar]
- Thompson MP, Kingree JB. The roles of victim and perpetrator alcohol use in intimate partner violence outcomes. J. Interpers. Violence. 2006;21:163–177. doi: 10.1177/0886260505282283. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Lloyd DA, Gil AG. Racial/ethnic and gender differences in the incidence and onset age of DSM-IV alcohol use disorder symptoms among adolescents. J. Stud. Alcohol. 2002;63:609–619. doi: 10.15288/jsa.2002.63.609. [DOI] [PubMed] [Google Scholar]
- Watt TT. The race/ethnic age crossover effect in drug use and heavy drinking. J. Ethn. Subst. Abuse. 2008;7:93–114. doi: 10.1080/15332640802083303. [DOI] [PubMed] [Google Scholar]
- White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcohol. Clin. Exp. Res. 2002;26:960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- Wright DL, Aquilino WS, Supple AJ. A comparison of computer-assisted and paper-and-pencil self-administered questionnaires in a survey on smoking, alcohol, and drug use. Public Opin. Q. 1998;62:331–353. [Google Scholar]
- Yang Y, Fu WJ, Land KC. A methodological comparison of age–period–cohort models: the intrinsic estimator and conventional generalized linear models. Sociol. Methodol. 2004;34:75–110. [Google Scholar]
- Yang Y, Schulhofer-Wohl S, Fu WJ, Land KC. The intrinsic estimator for age–period–cohort analysis: what it is and how to use it. Am. J. Sociol. 2008;113:1697–1736. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.