Abstract
Background
Primary central nervous system lymphomas may present as diffuse, nonenhancing infiltrative lesions. This rare variant is termed lymphomatosis cerebri (LC). We did a systematic review and analysis of the literature, adding our own cases, to better characterize LC in order to improve early diagnosis and treatment.
Methods
PubMed, ISI Web of Knowledge, and hospital databases were reviewed. Information was extracted regarding demographic, clinical, histological, cerebrospinal fluid (CSF), neuroimaging, and treatment variables. The impact of single parameters on overall survival (OS) was determined by applying univariate and multivariate analyses.
Results
Forty-two patients were included (median age: 58 y; range: 28–80 y). At consultation, 52% of patients had a poor KPS. The most common presenting symptom was cognitive decline (59.5%). Imaging studies showed supratentorial and infratentorial infiltration in 55% of patients and bilateral hemispheric involvement in 95%. CSF pleocytosis was present in 51.5% of the patients. Median time to diagnosis was 4.5 (range: 1–30) months, and the diagnosis was not established until autopsy for 33% of patients. The median OS was 2.95 (range: 0.33–56) months; however, those patients who received methotrexate had a median OS of 13.8 (range: 0.7–56) months. Analysis identified KPS ≥ 70 (HR: 0.32; 95% CI: 0.114–0.894; P = .03) and treatment with methotrexate (HR: 0.19; 95% CI: 0.041–0.886; P = .034) as independent favorable prognostic factors, whereas T-cell lymphoma was independently related with a worse outcome (HR: 6.62; 95% CI: 1.317–33.316; P = .022).
Conclusions
LC is a misdiagnosed entity associated with considerable diagnostic delay. MRI evidence of bilateral hemispheric involvement and CSF pleocytosis should be alerts for this diagnosis. Treatment with methotrexate-based chemotherapy must be considered, especially for patients with good KPS.
Keywords: diffuse infiltrative lesion, leukoencephalopathy, lymphomatosis cerebri, primary central nervous system lymphoma
Primary central nervous system lymphoma (PCNSL) is an unusual form of non-Hodgkin lymphoma that represents 6.2% of all malignant primary brain tumors.1 Besides the brain, PCNSL can involve the meninges (7%–42%),2 eyes (15%–25%),3 and spinal cord (1%–2%).4 The majority of PCNSLs are diffuse large B-cell lymphomas, while the remaining cases are low-grade B-cell (8%), Burkitt (5%), or T-cell lymphomas (2%–3%).5,6 Patients with PCNSLs commonly present with focal neurological deficits (70%) and altered mental status (43%).7 Brain MRI usually reveals single (65%) or multiple (35%) lesions with variable mass effect4,8 and intense homogeneous or ring contrast enhancement.9 The most frequently affected areas are the cerebral hemispheres and basal ganglia in >50% of patients.4,9 The optimal therapy for PCNSL is not fully established. Resection is usually not considered because of the infiltrative nature of these tumors, and chemotherapy based on high-dose methotrexate (≥3 g/m2) is the cornerstone of treatment.5
PCNSLs may (rarely) present as diffuse, nonenhancing infiltrative lesions without mass effect. This very rare variant is termed lymphomatosis cerebri (LC).6 Due to the nonspecific clinical and neuroimaging features, LC frequently becomes a diagnostic challenge with a wide differential diagnosis to be considered. Typically, gliomatosis cerebri presents with similar neuroimaging findings in the brain MRI. Likewise, central nervous system infections and inflammatory, toxic, and metabolic disorders can also mimic the radiologic features of LC.10,11 Therefore, this diagnostic dilemma can lead to delay in the diagnosis of LC and its appropriate treatment. This study aimed to describe the clinical, radiological, pathological, and outcome characteristics as well as the prognostic factors of LC by adding 7 newly described patients (diagnosed in our centers) and conducting a systematic review of reported cases with the aim of improving knowledge of this entity.
Material and Methods
Search Strategy and Selection Criteria
References for this review were identified by searches of PubMed and ISI Web of Knowledge databases from January 1986 to December 2014 using the terms “lymphomatosis,” “lymphoma,” “primary,” “cerebri,” “cerebral,” “brain,” diffuse,” “central nervous system,” and “nervous system” joined in logical combinations. Articles were also identified through searches of the authors' own files. No language restrictions were applied.
According to information from abstracts, selection was based on the following criteria: (i) presence of diffuse white-matter disease in the brain MRI without contrast enhancement (although patchy contrast enhancement was also allowed) and (ii) histology revealing lymphoma. Exclusion criteria were: (i) presence of nodular enhancement at first MRI, (ii) concurrent systemic lymphoma, (iii) inconclusive histological data, or (iv) intravascular lymphoma. Four authors independently extracted data, and disagreements were resolved by consensus with other group members. When disagreement occurred, the opinion of a fifth investigator was requested. Initially, 836 articles were identified, but only 38 patients from 35 reports fulfilled all inclusion criteria. Three of these patients were excluded: 2 because the article was not available and one because of insufficient information. Eventually, 35 cases from the literature were included and herein described. Moreover, after reviewing the primary central nervous system tumor database at our hospitals, 7 additional patients were identified who fulfilled the previous diagnostic criteria for LC and added to the series. The detailed process is shown in Fig. S1 in the Supplementary material.
Data Collection
Demographic, clinical, cerebrospinal fluid (CSF), brain and spinal MRI, pathological, and treatment data were extracted. Karnofsky Performance Status (KPS) at consultation was categorized according to the patient's ability to perform daily activities (with KPS ≥ 70 being considered as independent and KPS < 70 as dependent), and it was extrapolated from clinical information with the consensus of the 4 main reviewers when not directly reported. Symptoms at diagnosis were classified in 8 categories: behavior disturbance; cognitive, sensory, or motor impairment; gait disturbance; language disorders; seizures; and headache. Patients were allowed to be allocated to more than one category. Gadolinium enhancement in the MRI was defined as patchy when a minimally or moderately intense heterogeneous, not well-defined area of contrast enhancement was present, regardless of size.12 CSF parameters were categorized as follows: pleocytosis (>5 cells) yes/no, abnormal protein (>0.45 g/L), or glucose (<0.51 g/L) concentration. The diagnosis of lymphoma was based on 2007 WHO diagnostic criteria6 for PCNSL, and the cases in the literature were diagnosed according to the recognized anatomopathological criteria at the time of diagnosis. When T-cell lymphoma was suspected in our cases, a demonstration of monoclonal lymphoid proliferation was performed using T-cell receptor gene rearrangement analysis. Whenever possible, immunohistochemical classification according to the Hans algorithm as germinal center B cell-like (GCB) (CD10+ or CD10-, BCL6+, MUM1-) and the non-GCB (CD10-, BCL6- or CD10-, BCL6+, MUM1+) subtype was established.13 Four categories of therapy were considered: no treatment, corticosteroids only, chemotherapy-based methotrexate (including those patients also treated with radiotherapy), and other chemotherapy regimens different from methotrexate. In addition, the time from the first symptom to LC pathological diagnosis and overall survival (OS), considered from the pathological diagnosis to the date of the last follow-up or death, were recorded. In patients diagnosed by necropsy, the time between first consultation and death was also recorded. Detailed clinical presentation, neuroimaging, pathology data, treatment options, and survival information of each case are summarized in Supplementary material, Table S1.9–11,14,23–50
Statistical Analysis
Descriptive data analysis presented categorical variables as observed counts and weighted percentages and continuous variables as mean or median with the corresponding standard error or range, depending on the nature of the variable. Chi-square, Mann-Whitney U, and Student t tests were used to identify differences among groups. A 2-sided P value <.05 was considered significant. Univariate survival analysis was made by constructing probability curves according to the Kaplan-Meier method and comparing them using the log-rank test. Evaluated variables included: patient (age, sex), clinical characteristics (KPS, onset symptoms, and time from onset symptoms to consultation), radiological features (localization, contrast enhancement pattern), histology (lymphoma B or T), CSF parameters (glucose, protein, and cell count), and treatment. Variables that achieved a P value <.1 in the univariate analysis were subsequently introduced in a backward stepwise proportional-hazard analysis (Cox model) to identify independent survival predictors. The cutoff level chosen for continuous variables was their median value. All calculations were performed using SPSS software package version 18.0 (SPSS Inc.), and P values < .05 were considered significant.
Results
Demographic Characteristics
Forty-two patients: 7 from our centers and 35 from the literature were identified and analyzed. Median age was 58 years (range: 28–80 y) with neither sex being predominant (female-to-male ratio: 1:1.2) (Table 2, Supplementary material). Only 3 patients were immunosuppressed (2 with HIV infections and 1 due to kidney transplant (patients 9, 11, and 38, Supplementary material). Immunosuppressed patients were significantly younger (median age: 41 y; range: 28–45 y) compared with immunocompetent patients (median age: 58 y; range: 31–80 y, P = .012). Nineteen (45.2%), 14 (33.3%), and 9 (21.4%) patients corresponded to reports from European, Asiatic, and North-American countries, respectively.
Clinical Features
Twenty-two patients (52.4%) had a KPS < 70 at diagnosis. The most common presenting symptoms were cognitive decline and gait disturbances in 25 (59.5%) and 23 (54.8%) patients, respectively, followed by behavioral changes in 21 (50%) patients. Focal neurological motor deficits were present in about one-third of patients. The frequency of seizures was low, and they were reported in 2 (4.8%) cases at diagnosis and 4 (9.5%) cases during the disease course (Table 2, Supplementary material). Two patients (5%) also presented with uveitis (patients 18 and 20, Supplementary material). Median time from the onset of symptoms to first consultation was 60 days (range: 1–180 d), although one patient presented with a stroke-like onset (patient 36, Supplementary material) and proceeded to consultation on day one.
Neuroimaging
A summary of MRI features is presented in Supplementary material, Table S2. The baseline brain MRI showed patchy enhancement in nearly one-third of cases (28.6%), and 23 (54.8%) had concurrent infratentorial and supratentorial infiltration (Fig. 1). The remaining patients (45.2%) presented with isolated supratentorial infiltration. It is noteworthy that all but 2 patients (95.2%) had bilateral hemispheric disease in the first MRI. Basal ganglia were involved in 21 cases (50%). Spinal cord MRI showed infiltration in 4 of the 6 patients. A follow-up brain MRI was performed in 28 patients, revealing changes in the contrast enhancement pattern in some patients. Eight of the 30 (26.6%) patients without contrast enhancement in the first MRI and 2 of the 12 patients (16.6%) who presented patchy gadolinium enhancement lesions developed nodular contrast enhancement. In 11 of the 28 patients for whom information was available, new contrast enhancement or changes in the pattern of contrast appeared before onset of treatment. MRI spectroscopy was performed in 5 patients, showing a peak of choline (n = 4), lactate (n = 2), and lipids (n = 1). Diffusion-weighted imaging (DWI) brain MRI showed a diffusion restriction pattern in 6 out of 9 (66.7%) patients. Brain positron emission tomography-fluorodeoxyglucose imaging (PET-FDG) was performed in 4 patients, showing increased metabolism in the deep white matter and thalamus in only one case (patient 16), and it was correlated with the hemispheric white and grey deep matter changes revealed by MRI. In univariate analysis, when comparing patients presenting patchy contrast enhancement in the first brain MRI with those patients without enhancement, a greater CSF protein concentration (≥0.65 g/L) was the only significant factor associated with the presence of contrast enhancement (P = .017). No other significant differences were found in terms of demographic, clinical, histopathological, or OS between both groups.
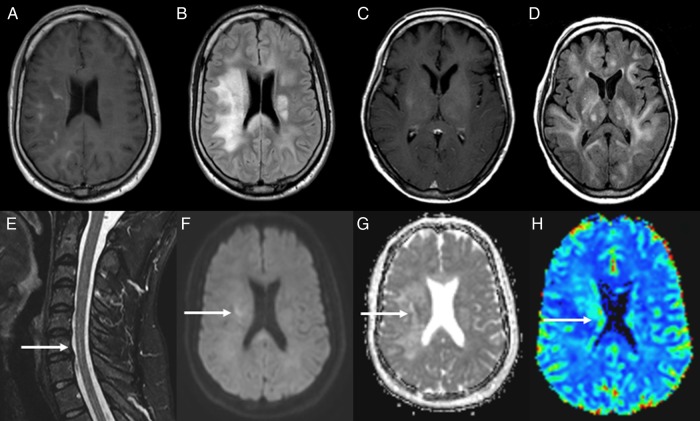
Fig. 1.
(A and B) MRI of a young male (patient 40) showing patchy contrast enhancement in the right hemisphere in axial T1 sequence (A) and diffuse hyperintense lesions in bilateral white matter on FSE-FLAIR sequence (B). (C and D) MRI of an adult female (patient 36) showing contrast in right subinsula and left thalamus (C) and diffuse bilateral abnormal hypersignal within the deep white matter in FLAIR sequence (D). (E–H) Short-TI inversion-recovery (STIR) spinal cord MRI from patient 40 showing an intramedullary hyperintense lesion (arrow) (E). Diffusion-weighted image shows elevated signal in right hemisphere (arrow) (F), and ADC map (G) shows (arrow) low signal in the lesion confirming that it is a true restricted diffusion. Perfusion-weighted image shows an elevated relative regional cerebral blood volume ratio (H).
Cerebrospinal Fluid Studies
CSF information was available from 36 patients, although not all parameters were registered in every case. CSF pleocytosis was present in 17 (n = 33, 51.5%) patients with a median cell count of 18 (range: 6–140). Protein levels were elevated in 26 (n = 34, 76.5%) cases (median: 0.65 g/L, range: 0.3–9.31), and low glucose was detected in 2 (n = 24, 8.3%) cases (Table 2, Supplementary material). CSF was normal in 7 cases (n = 36, 16.7%). CSF oligoclonal bands were detected in one case (n = 7, 14.3%). Cytology was negative for malignant cells in 72% (n = 32) of the cases. Cell flow cytometry was performed in 3 patients and diagnosed a lymphoma in 2 of them.
Pathology
Diagnosis was established by brain biopsy in 28 cases and at autopsy in 14. In 5 of 32 patients, the first biopsy was inconclusive; in one case, diagnosis was not established even after 2 biopsies. In this patient, only autopsy revealed the final diagnosis. The patient's median time from first symptoms to biopsy (n = 28) and to definitive diagnosis (n = 25) was 4.2 and 4.5 months (range: 1–30 m), respectively.
Most patients (n = 35/41; 85.4%) had diffuse large B-cell lymphoma, 5 (12.1%) patients had T-cell lymphoma, and one patient was diagnosed with a small B-cell lymphocytic lymphoma with lymphoplasmatic differentiation. In one case, the lymphoma subtype details were missing (Table 2, Supplementary material). Only one T-cell lymphoma was treated with corticosteroids before biopsy. According the Hans immunohistochemical algorithm,13 it was possible to determine the origin of lymphoma cells in 8 cases. Seven patients (87.5%) showed non-GBC, and one (12.5%) showed GBC cell immunophenotype.
Focusing on the patients in our centers (and for whom autopsy results were available), we observed blastic lymphocytic cells with large pleomorphic nuclei and distinct nucleoli diffusely infiltrating the cortical and subcortical structures, white matter, basal ganglia, and thalamus as well as the brain stem and cerebellum. Moreover, one patient with diffuse B-cell LC also presented hippocampus, amygdala, Meynert nuclei, hypothalamus, and leptomeningeal infiltration. These lymphocytes showed the typical angiocentric infiltration pattern, and tumor cells invaded the neural parenchyma with a diffuse growth pattern from these perivascular cuffs (Fig. 2). No large geographic necrotic areas were identified, while the brain distribution and the pattern infiltration of cells were indistinguishable between patients with B- or T-cell lymphoma. The comparison between T- and B-cell lymphoma cases only demonstrated that patients having T-cell lymphoma presented a better clinical performance status (KPS ≥ 70) (P = .010) and more frequently isolated supratentorial disease (P = .010) at diagnosis.
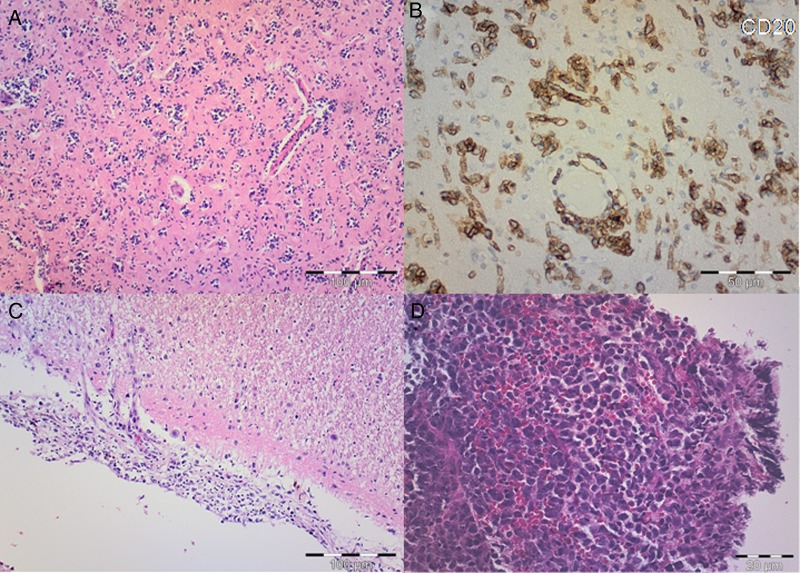
Fig. 2.
(A) Hematoxylin and eosin (H&E) staining shows scattered atypical lymphocytes with diffuse infiltration of the parenchyma and perivascular distribution. Scale bar, 100 µm. (B) On immunohistochemical staining, atypical lymphoid cells are strongly stained with CD20. Scale bar, 50 µm. (C) H&E shows atypical lymphocytes infiltrating meninges. Scale bar, 100 µm. (D) H&E microphotograph of typical nodular primary central nervous system lymphoma with atypical lymphocytes invading brain parenchyma in compact cellular aggregates. Scale bar, 20 µm.
Therapy
The great majority of patients (n = 39, 89.7%) received some form of therapy. Treatment was corticosteroids alone in 14 cases (35.9%), high-dose methotrexate, alone or in combination with other drugs or radiotherapy (RT) in 11 (28.2%) cases, and RT (total dose range: 30–50 Gy) or other chemotherapies without methotrexate in 10 (25.6%) cases. The chemotherapy schedule was not reported in 2 cases. Because treatment decisions were made arbitrarily, 2 groups of patients (patients receiving any type of treatment [chemotherapy and/or RT] vs others) were compared regarding baseline characteristics to identify any potential bias. Indeed, only differences regarding age at presentation were observed. Those patients receiving treatment (chemotherapy and/or radiotherapy) were significantly younger (median age: 55 y; range: 31–75 y) versus 62 years (range: 41–80), P = .021).
Survival
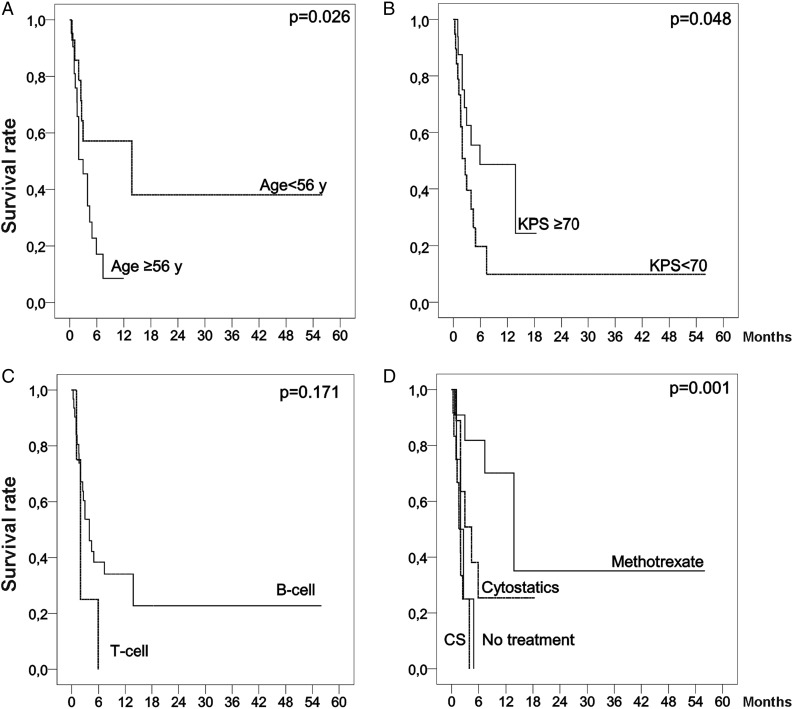
Median OS was 2.95 months (range: 0.33–56 m). The majority (64.3%) of patients were already dead at the time of reporting. According to treatment modality, median OS was 1.97 months (range: 1.08–4.91 m) and 1.63 months (range: 0.33–3.93 m) in the untreated patients and those receiving only corticosteroids, respectively. In patients receiving methotrexate-based treatments the OS was 13.8 months (range: 0.7–56 m), and the OS of patients treated with other cytoreductive treatments different from methotrexate (RT and/or chemotherapy) was 4.42 months (range: 1.0–18.4 m). Univariate analysis revealed that age <56 years (P = .026), KPS ≥ 70 at consultation (P = .048), and treatment with methotrexate (P = .001) were associated with a better OS. Other explored clinical, radiological, and CSF variables did not show any impact on survival; only the lymphoma subtype presented differentiated survival behavior, although it was not significant (Fig. 3). The multivariate analysis demonstrated that KPS ≥ 70 (HR: 0.32; 95% CI: 0.114–0.894; P = .03) and treatment with methotrexate (HR: 0.19; 95% CI: 0.041–0.886; P = .034) were independent favorable survival prognostic factors, while T-cell lymphoma was an independent prognostic factor of worse outcome (HR: 6.62; 95% CI: 1.317–33.316; P = .022). Other explored variables included in this analysis were age*treatment (HR: 1.029; 95% CI: 0.942–1.125; P = .527) to avoid the bias identified previously, and age (HR: 0.982; 95% CI: 0.877–1.099; P = .75), but were not identified as independent prognostic factors.
Fig. 3.
Kaplan-Meier curves showing overall survival of the patients with lymphomatosis cerebri stratified by age, KPS, histological subtype, and treatment options (any cytostatic treatment with methotrexate, cytostatic treatment without methotrexate, corticosteroids treatment, and no treatment).
Discussion
This study reports the largest series of LC obtained by systematic review of the main databases and presents 7 additional patients of our own. Our study shows that bilateral hemispheric involvement in the MRI and CSF pleocytosis should alert for this diagnosis and that treatment with methotrexate-based chemotherapy significantly prolongs survival.
The demographic characteristics of patients with LC are quite similar to PCNSL patients, presenting a peak age distribution between 50 and 70 years and similar distribution ratios between sexes (male-to-female ratios 1.5:1 and 1.2:1) in PCNSL and LC, respectively.6 LC is a rare variant of PCNSL. See Table 1 to compare the main differences between PCNSL and LC. The term is relatively new (introduced in 199914), so it was not unexpected to find that LC represents a challenging diagnosis and that correct diagnosis in life is obtained in only about one-third of the patients. Similarly, the confirmatory pathological diagnosis is associated with considerable delay (138 days) compared with other primary malignant brain tumors.15 Although this issue has also been identified in classical PCNSL patients, it is usually much less pronounced, with reports of 70 and 75 days in non-HIV and HIV patients, respectively.16 Several reasons may contribute to this diagnostic delay. First, clinical presentation is overlapped by cognitive impairment and/or personality changes that may be easily misinterpreted as dementia or depression—entities in which the requirement for urgent neuroimaging is not usually considered in routine practice.11 Second, the MRI findings in a typically nonspecific clinical syndrome can be easily misdiagnosed as being related to conditions such as infectious processes, toxic-metabolic disorders, small vessel vasculopathy, or inflammatory or neurodegenerative etiologies, which sometimes require further testing or even an observation period to confirm diagnosis and thus complicate an LC diagnosis.11 Another reason to explain the diagnostic delay (uncontrolled in this study by the lack of information in reported cases) is the use of corticosteroids before the biopsy. The role of this confounding variable constitutes an inherent bias. Notwithstanding, a large retrospective study on PCNSL did not reveal significant differences in diagnostic ratios between patients pretreated or not with corticosteroids.17 However, according to the present study, brain MRI features combined with CSF examination usually provide important clues that are useful for facilitating differential diagnosis in LC (Fig. 4). Diffuse involvement of both hemispheres is the rule in LC, and this finding is uncommon in other entities considered in the differential diagnosis. Extension of the MRI to the spinal cord and use of MR spectroscopy or DWI sequences may increase the suspicion for LC, whereas CSF flow cytometry (ie, a technique increasing the sensitivity and specificity of leptomeningeal disease detection in PCNSL18) seems potentially useful in LC diagnosis. These suggestions about complementary diagnostic tools have to be considered with caution, however, due to the limited number of patients available.
Table 1.
Main clinical, radiological, and histopathological features of primary central nervous system lymphoma6–8 versus lymphomatosis cerebri
| PCNSL | LC | |
|---|---|---|
| Clinical | Focal deficits (70%) | Focal deficits (38.1%) |
| Cognitive/behavioral (43%) | Cognitive (59.5%)/behavioral (50%) | |
| Headache (33%) | Headache (9.5%) | |
| Seizures (14%) | Seizures (4.8%) | |
| Gait disturbances (34%) | Gait disturbances (54.8%) | |
| Radiological | Single or multiple well-defined lesions | Diffuse poorly circumscribed lesions |
| Intense homogeneous CE | Absence or patchy CE | |
| Marked restricted diffusion | Variable restricted diffusion | |
| Rare spinal cord involvement (1%) | Not uncommon spinal cord involvement (67%) | |
| Histopathological | Tumor cells forming compact aggregates | No cohesive mass lesion |
| Common necrosis | Uncommon necrosis | |
| Perivascular cell tumor | Perivascular cell tumor |
Abbreviations: CE, contrast enhancement; LC, lymphomatosis cerebri; PCNSL, primary central nervous system lymphoma.
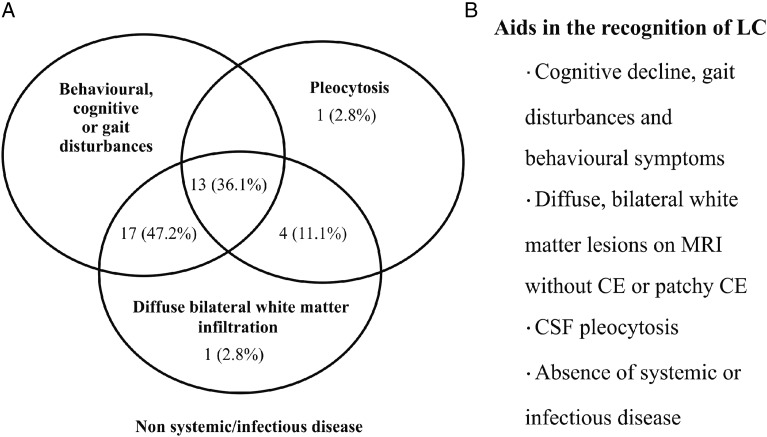
Fig. 4.
Lymphomatosis cerebri features that would help when considering the diagnosis and a diagram performed with all patients (n = 36) whose radiological, cerebrospinal fluid, and clinical data were available.
The outcome of LC patients is poor. In terms of survival, nearly half of the patients (47.2%) in this study were dead within 3 months after the onset of symptoms. However, we have shown that most patients either presented with a low KPS or were misdiagnosed, which may have a negative influence on the time of final treatment decision. Although not significant (P = .14), patients with a KPS ≥ 70 were diagnosed earlier (median of 138 days) than patients with lower KPS < 70 (median of 240 days). In addition, OS of patients with LC treated with chemotherapy schedules (including methotrexate and RT) were lower than that reported in main trials or retrospective studies of PCNSL (13.8 vs 33–60 m).19,20 These differences could be related to low KPS, delay in diagnosis, or sample size of the present series.
Our study identifies KPS ≥ 70 as an independent survival prognostic factor and demonstrates that treatment with methotrexate has a favorable influence on OS prognosis despite differences in the impact of treatment compared with the PCNSL. Our observation concurs with that found in series of PCNSL and emphasizes the need to start conventional treatment for PCNSL early, when the chances of a good KPS are probably higher. Lastly, the prognostic effect of the histological subtype is controversial. Some reports suggest that a T-cell phenotype possibly confers a worse prognosis than B-cell PCNSL.21 In contrast, other series have suggested a possible better outcome or found no differences.22 However, these results should be treated with caution because of the retrospective data and the small number of T-cell LC cases reported. Nonetheless, in our study, the multivariate analysis showed that T-cell LC is an independent prognostic factor related to a worse outcome.
On clinical grounds, our study provides a general picture of clinical, radiological, and cytological features to be considered for LC diagnosis. Physicians should be aware that MRI findings of diffuse bilateral hemispheric involvement and CSF pleocytosis, when seen in patients without evidence of systemic and infectious diseases, are useful clues for diagnosing this rare syndrome and that prompt biopsy should be performed to avoid diagnostic delay. Furthermore, despite the poor outcome, intensive treatment should be considered, especially in those LC patients' with a better KPS.
Supplementary Material
Funding
No funding supported the research.
Supplementary Material
Acknowledgments
Robert Nakayama from the Department of Surgery, School of Medicine, Keio University, Tokyo, Japan for helping with the Japanese manuscripts.
Conflict of interest statement. None declared.
References
- 1.Ostrom QT, Gittleman H, Farah P et al. . CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiewe P, Fischer L, Martus P, Thiel E, Korfel A. Meningeal dissemination in primary CNS lymphoma: diagnosis, treatment, and survival in a large monocenter cohort. Neuro Oncol. 2010;12(4):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong JT, Chae JB, Lee JY, Kim JG, Yoon YH. Ocular involvement in patients with primary CNS lymphoma. J Neurooncol. 2011;102(1):139–145. [DOI] [PubMed] [Google Scholar]
- 4.Kuker W, Nagele T, Thiel E, Weller M, Herrlinger U. Primary central nervous system lymphomas (PCNSL): MRI response criteria revised. Neurology. 2005;65(7):1129–1131. [DOI] [PubMed] [Google Scholar]
- 5.Ferreri AJ, Marturano E. Primary CNS lymphoma. Best Pract Res Clin Haematol. 2012;25(1):119–130. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. Tumours of the haematopoietic system, malignant lymphomas. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO Classification of Tumours of the Central Nervous System. Vol 1 4th ed Lyon: IARC; 2007:188–196. [Google Scholar]
- 7.Gallop-Evans E. Primary central nervous system lymphoma. Clin Oncol (R Coll Radiol). 2012;24(5):329–338. [DOI] [PubMed] [Google Scholar]
- 8.Yap KK, Sutherland T, Liew E, Tartaglia CJ, Pang M, Trost N. Magnetic resonance features of primary central nervous system lymphoma in the immunocompetent patient: a pictorial essay. J Med Imaging Radiat Oncol. 2012;56(2):179–186. [DOI] [PubMed] [Google Scholar]
- 9.Thurnher MM, Rieger A, Kleibl-Popov C et al. . Primary central nervous system lymphoma in AIDS: a wider spectrum of CT and MRI findings. Neuroradiology. 2001;43(1):29–35. [DOI] [PubMed] [Google Scholar]
- 10.Kitai R, Hashimoto N, Yamate K et al. . Lymphomatosis cerebri: clinical characteristics, neuroimaging, and pathological findings. Brain Tumor Pathol. 2012;29(1):47–53. [DOI] [PubMed] [Google Scholar]
- 11.Lewerenz J, Ding XQ, Matschke J et al. . Dementia and leukoencephalopathy due to lymphomatosis cerebri. BMJ Case Rep. 2009;2009 pii: bcr08.2008.0752. doi: 10.1136/bcr.08.2008.0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallud J, Capelle L, Taillandier L et al. . Prognostic significance of imaging contrast enhancement for WHO grade II gliomas. Neuro Oncol. 2009;11(2):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hans CP, Weisenburger DD, Greiner TC et al. . Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. [DOI] [PubMed] [Google Scholar]
- 14.Bakshi R, Mazziotta JC, Mischel PS, Jahan R, Seligson DB, Vinters HV. Lymphomatosis cerebri presenting as a rapidly progressive dementia: clinical, neuroimaging and pathologic findings. Dement Geriatr Cogn Disord. 1999;10(2):152–157. [DOI] [PubMed] [Google Scholar]
- 15.Graus F, Bruna J, Pardo J et al. . Patterns of care and outcome for patients with glioblastoma diagnosed during 2008–2010 in Spain. Neuro Oncol. 2013;15(6):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haldorsen IS, Espeland A, Larsen JL, Mella O. Diagnostic delay in primary central nervous system lymphoma. Acta Oncol. 2005;44(7):728–734. [DOI] [PubMed] [Google Scholar]
- 17.Porter AB, Giannini C, Kaufmann T et al. . Primary central nervous system lymphoma can be histologically diagnosed after previous corticosteroid use: a pilot study to determine whether corticosteroids prevent the diagnosis of primary central nervous system lymphoma. Ann Neurol. 2008;63(5):662–667. [DOI] [PubMed] [Google Scholar]
- 18.Schroers R, Baraniskin A, Heute C et al. . Diagnosis of leptomeningeal disease in diffuse large B-cell lymphomas of the central nervous system by flow cytometry and cytopathology. Eur J Haematol. 2010;85(6):520–528. [DOI] [PubMed] [Google Scholar]
- 19.Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18(17):3144–3150. [DOI] [PubMed] [Google Scholar]
- 20.Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H et al. . High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21(24):4483–4488. [DOI] [PubMed] [Google Scholar]
- 21.Gijtenbeek JM, Rosenblum MK, DeAngelis LM. Primary central nervous system T-cell lymphoma. Neurology. 2001;57(4):716–718. [DOI] [PubMed] [Google Scholar]
- 22.Lim T, Kim SJ, Kim K et al. . Primary CNS lymphoma other than DLBCL: a descriptive analysis of clinical features and treatment outcomes. Ann Hematol. 2011;90(12):1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provinciali L, Signorino M, Ceravolo G, Pasquini U. Onset of primary brain T-lymphoma simulating a progressive leukoencephalopathy. Ital J Neurol Sci. 1988;9(4):377–381. [DOI] [PubMed] [Google Scholar]
- 24.Kazahaya Y, Doi A, Hamaya K. An autopsy case of primary central nervous system lymphoma with diffuse intracerebral lesion [in Japanese]. No To Shinkei. 1992;44(1):65–70. [PubMed] [Google Scholar]
- 25.Carlson BA. Rapidly progressive dementia caused by nonenhancing primary lymphoma of the central nervous system. AJNR Am J Neuroradiol. 1996;17(9):1695–1697. [PMC free article] [PubMed] [Google Scholar]
- 26.Terae S, Ogata A. Nonenhancing primary central nervous system lymphoma. Neuroradiology. 1996;38(1):34–37. [DOI] [PubMed] [Google Scholar]
- 27.Furusawa T, Okamoto K, Ito J et al. . Primary central nervous system lymphoma presenting as diffuse cerebral infiltration. Radiat Med. 1998;16(2):137–140. [PubMed] [Google Scholar]
- 28.Brecher K, Hochberg FH, Louis DN, de la Monte S, Riskind P. Case report of unusual leukoencephalopathy preceding primary CNS lymphoma. J Neurol Neurosurg Psychiatry. 1998;65(6):917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayuso-Peralta L, Orti-Pareja M, Zurdo-Hernandez M et al. . Cerebral lymphoma presenting as a leukoencephalopathy. J Neurol Neurosurg Psychiatry. 2001;71(2):243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moulignier A, Galicier L, Mikol J, Masson H, Molho M, Thiebaut JB. Primary cerebral lymphoma presenting as diffuse leukoencephalopathy. AIDS. 2003;17(7):1111–1113. [DOI] [PubMed] [Google Scholar]
- 31.Rendel Mathew PA, Sarah T, Newel K. A unique presentation of lymphomatosis cerebri. Neuro Oncol 2007;9(4):542. [Google Scholar]
- 32.Rollins KE, Kleinschmidt-DeMasters BK, Corboy JR, Damek DM, Filley CM. Lymphomatosis cerebri as a cause of white matter dementia. Hum Pathol. 2005;36(3):282–290. [DOI] [PubMed] [Google Scholar]
- 33.Vital A, Sibon I. A 64-year-old woman with progressive dementia and leukoencephalopathy. Brain Pathol. 2007;17(1):117–118, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Toledo M, Lopez-Valdes E, Ferreiro M et al. . Lymphomatosis cerebri as the cause of leukoencephalopathy. Rev Neurol. 2008;46(11):667–670. [PubMed] [Google Scholar]
- 35.Kanai R, Shibuya M, Hata T et al. . A case of ‘lymphomatosis cerebri’ diagnosed in an early phase and treated by whole brain radiation: case report and literature review. J Neurooncol. 2008;86(1):83–88. [DOI] [PubMed] [Google Scholar]
- 36.Sugie M, Ishihara K, Kato H, Nakano I, Kawamura M. Primary central nervous system lymphoma initially mimicking lymphomatosis cerebri: an autopsy case report. Neuropathology. 2009;29(6):704–707. [DOI] [PubMed] [Google Scholar]
- 37.Raz E, Tinelli E, Antonelli M et al. . MRI findings in lymphomatosis cerebri: description of a case and revision of the literature. J Neuroimaging. 2011;21(2):e183–e186. [DOI] [PubMed] [Google Scholar]
- 38.Pandit L, Chickabasaviah Y, Raghothaman A, Mustafa S, Vasudevan A. Lymhomatosis cerebri–a rare cause of leukoencephalopathy. J Neurol Sci. 2010;293(1–2):122–124. [DOI] [PubMed] [Google Scholar]
- 39.Leschziner G, Rudge P, Lucas S, Andrews T. Lymphomatosis cerebri presenting as a rapidly progressive dementia with a high methylmalonic acid. J Neurol. 2011;258(8):1489–1493. [DOI] [PubMed] [Google Scholar]
- 40.Liao MF, Toh CH, Kuo HC, Chu CC, Jung SM, Huang CC. Diffusion tensor images and magnetic resonance spectroscopy in primary central nervous system T-cell lymphoma: a case report. Acta Neurol Taiwan. 2011;20(1):59–64. [PubMed] [Google Scholar]
- 41.Keswani A, Bigio E, Grimm S. Lymphomatosis cerebri presenting with orthostatic hypotension, anorexia, and paraparesis. J Neurooncol. 2012;109(3):581–586. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto T, Kojima K, Koibuchi K et al. . A case of primary central nervous system lymphoma presenting diffuse infiltrative leukoencephalopathy. Intern Med. 2012;51(9):1103–1106. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe M, Satoi H, Takahashi Y, Nishida N, Toda H, Matsumoto S. Remission of lymphomatosis cerebri induced by corticosteroid and high-doses intravenous methotrexate [in Japanese]. Rinsho Shinkeigaku. 2012;52(7):486–490. [DOI] [PubMed] [Google Scholar]
- 44.Choi CY, Lee CH, Joo M. Lymphomatosis cerebri. J Korean Neurosurg Soc. 2013;54(5):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugino T, Mikami T, Akiyama Y, Wanibuchi M, Hasegawa T, Mikuni N. Primary central nervous system anaplastic large-cell lymphoma mimicking lymphomatosis cerebri. Brain Tumor Pathol. 2013;30(1):61–65. [DOI] [PubMed] [Google Scholar]
- 46.Sato H, Takahashi Y, Wada M et al. . Lymphomatosis cerebri with intramedullary spinal cord involvement. Intern Med. 2013;52(22):2561–2565. [DOI] [PubMed] [Google Scholar]
- 47.Samani A, Davagnanam I, Cockerell OC, Ramsay A, Patani R, Chataway J. Lymphomatosis cerebri: a treatable cause of rapidly progressive dementia. J Neurol Neurosurg Psychiatry. 2014;86(2):238–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miki Y, Tomiyama M, Kurotaki H, Wakabayashi K, Baba M. Primary central nervous system lymphoma mimicking Bickerstaff's encephalitis. Neurol Sci. 2014;35(1):139–141. [DOI] [PubMed] [Google Scholar]
- 49.Rivero Sanz E, Torralba Cabeza MA, Sanjuan Portugal F, Garcia-Bragado F. Lymphomatosis cerebri mimicking iatrogenic Creutzfeldt-Jakob disease. BMJ Case Rep. 2014;2014 doi:10.1136/bcr-2013-201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imperiale D, Taraglio S, Atzori C, Testi R. Diffuse leukoencephalopathy due to lymphomatosis cerebri: a clinicopathological report. Neurol Sci. 2015;36(6):1071–1073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.