Abstract
Neurofibromatosis type 1 (NF1), neurofibromatosis type 2 (NF2), and schwannomatosis (SWN) are tumor-suppressor syndromes. Each syndrome is an orphan disease; however, the tumors that arise within them represent the most common tumors of the nervous system worldwide. Systematic investigation of the pathways impacted by the loss of function of neurofibromin (encoded by NF1) and merlin (encoded by NF2) have led to therapeutic advances for patients with NF1 and NF2. In the syndrome of SWN, the genetic landscape is more complex, with 2 known causative genes (SMARCB1 and LZTR1) accounting for up to 50% of familial SWN patients. The understanding of the molecular underpinnings of these syndromes is developing rapidly and offers more therapeutic options for the patients. In addition, common sporadic cancers harbor somatic alterations in NF1 (ie, glioblastoma, breast cancer, melanoma), NF2 (ie, meningioma, mesothelioma) and SMARCB1 (ie, atypical teratoid/rhabdoid tumors) such that advances in management of syndromic tumors may benefit patients both with and without germline mutations. In this review, we discuss the clinical and genetic features of NF1, NF2 and SWN, the therapeutic advances for the tumors that arise within these syndromes and the interaction between these rare tumor syndromes and the common tumors that share these mutations.
Keywords: NF1, NF2, schwannomatosis, therapeutics, tumor suppressor syndrome
Tumor suppressor genes (TSGs) encode proteins that are responsible for regulating cell division. Tumor suppressor syndromes are due to mutations in a TSG, which results in dysregulation of pathways responsible for cell division and proliferation and ultimately makes cells vulnerable to additional genetic alterations that contribute to cancer formation. The classic example of a tumor suppressor syndrome is Li Fraumeni syndrome, in which a germline mutation in TP53 results in a roughly 100 times greater risk of breast cancer, soft tissue sarcomas, brain cancer, hematologic malignancies, and adrenocortical carcinoma.1 Neurofibromatosis type 1 (NF1), neurofibromatosis type 2 (NF2), and schwannomatosis (SWN) are also tumor suppressor syndromes caused by germline mutations in a TSG. The resting state of one normal and one mutant allele results in the clinical syndrome; however, inactivation of the second allele of the TSG leads to tumor formation (according to Knudson's “2-hit hypothesis”).2 Unlike Li Fraumeni syndrome, however, the tumors that develop most frequently in patients with NF1, NF2, and SWN are histologically benign with an overall low incidence of malignancy. That said, these benign tumors are relentless and predominantly involve the central and peripheral nervous system (CNS and PNS), thereby causing significant neurologic morbidity and in some cases mortality due to loss of nervous system function. Hence, patients with NF1, NF2, and SWN present unique therapeutic challenges for the neuro-oncology community. In addition, understanding NF1, NF2, and SWN provides an opportunity to gain insight and develop effective therapies for histologically benign CNS and PNS tumors including meningiomas and schwannomas, which together represent >50% of all CNS tumors in the United States, afflict millions of people across the world and for which there are no approved therapies.3 Identification of the NF1, NF2, LZTR1, and SMARCB1 genes underlying the tumors found in NF1, NF2, and SWN allows interrogation of drugs active at nodes along specific cellular pathways dysregulated by the loss of function of the respective TSG. The resultant discoveries may ultimately benefit people both with NF syndromes and nonsyndromic cancers that are related to somatic mutations in NF genes.
Neurofibromatosis Type 1
Clinical Presentation
NF1 is a rare disease, with an estimated birth incidence of 1 in every 2500–3500 individuals.4 However, it is the most common autosomal dominant disorder of the nervous system and one of the most common single-gene inherited conditions, rivaling cystic fibrosis or fragile X syndrome.4,5 The manifestations of NF1 can impact essentially every organ system, however, as a neurocutaneous syndrome, its hallmark lesions involve the skin, CNS, and PNS (Table 1). The skin lesions, including café au lait spots, intertriginous freckling, and cutaneous and subcutaneous neurofibromas, can cause significant deformity and discomfort but are not medically threatening (Fig. 1, A–C). Café au lait spots are often present at birth and grow in size and number across childhood but may fade in adulthood.6 Cutaneous and subcutaneous neurofibromas generally start to appear in late childhood and can grow in size and number throughout adulthood.4,6
Table 1.
Diagnostic criteria for neurofibromatosis type 1, neurofibromatosis type 2, and schwannomatosis
| NF1 | NF2 | Schwannomatosis |
|---|---|---|
| Clinical Criteria | ||
Presence of ≥2 of the following151:
|
Definite152: Bilateral VS or Family history of NF2 (first-degree family relative) plus
|
Definite119,153,154: Age > 30 y AND has ≥ 2 or more nonintradermal schwannomas, at least 1 with histological confirmation AND has no evidence of VS on high-quality MRI scan AND has no known constitutional NF2 mutation OR Has 1 pathologically confirmed nonvestibular schwannoma plus a first-degree relative who meets the above criteria Possible schwannomatosis: Age < 30 y AND has ≥2 nonintradermal schwannomas, at least 1 with histological confirmation AND no evidence of VS on high-quality MRI scan AND no known constitutional NF2 mutation OR Individual is age > 45 y AND has > 2 nonintradermal schwannomas, at least 1 with histological confirmation AND has no symptoms of 8th cranial nerve dysfunction AND has no known constitutional NF2 mutation OR Has radiographic evidence of a nonvestibular schwannoma and first-degree relative meeting criteria for definite schwannomatosis |
| Genetic Testing155 | ||
|
NF1 testing Comprehensive NF1 testing
|
Comprehensive NF2 testing
|
SMARCB1/INI1 LZTR1
|
Abbreviations: NF1, neurofibromatosis, type 1; NF2, neurofibromatosis, type 2; VS, vestibular schwannoma; y, year.
*First-degree relative: parent, sibling, or offspring.
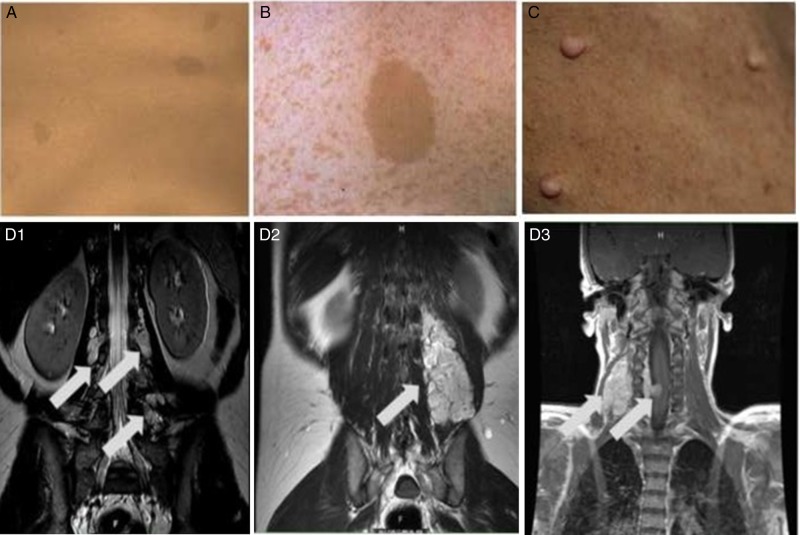
Fig. 1.
Common manifestations of neurofibromatosis type 1 including: (A) multiple café-au-lait macules, (B) skin fold freckling with a café-au-lait macule, (C) cutaneous neurofibromas and plexiform neurofibromas that are nodular (D1), diffuse (D2) and multifocal and infiltrating (D3).
The major peripheral nerve tumor impacting patients with NF1 is the plexiform neurofibroma (pNF). pNFs are multicellular tumors composed of a variety of cell types including neuronal axons, Schwann cells, fibroblasts, mast cells, macrophages, perineural cells, and extracellular matrix.7 They effect up to 50% of NF1 patients and can involve nerves anywhere from the spinal root to the distal periphery.4,8–10 pNFs range in their extent of involvement and configuration from confined and nodular to diffuse, crossing tissue planes or involving multiple body regions (Fig. 1, D1–D3). They occur most commonly in the trunk including the paraspinal region (31%), head and neck (31%), and the extremities (25%).9,10 Importantly, pNFs grow most rapidly in early childhood, often increasing by ≥20% volume per year in young children.8 Surgery is the current mainstay of therapy for pNF; however, the tumors can rarely be fully resected due to their involvement of critical structures, resulting frequently in regrowth after surgery. The limitations of surgery, combined with the known high growth rate in children, are the motivators for highly active efforts to discover drugs for pNF.
pNFs are a common source of neuropathic pain and neurologic dysfunction ranging from minor sensory alteration to complete myelopathy. Moreover, the lifetime risk of developing a malignant peripheral nerve-sheath tumor (MPNST) in people with NF1 is 8%–13%.11 MPNST is a rare form of sarcoma that occurs with disproportionate frequency in patients with NF1. Outside of complete resection (requiring wide margins of nerve and neighboring soft tissue), there are limited treatment options, and the 5-year survival is below 50%.11,12
In the CNS, patients with NF1 are at risk for low-grade gliomas more so than high-grade gliomas.13 The most common form of glioma seen in patients with NF1 is optic pathway gliomas (OPGs), which can involve any portion of the ophthalmologic pathway. An estimated 15%–20% of people with NF1 have OPGs, accounting for up to 70% of all patients diagnosed with OPG.14,15 Symptomatic OPGs are almost exclusively diagnosed in children <8 years of age, but there are rare patients who present later in life and may require treatment.15,16 Many OPGs remain clinically silent and in fact may regress over time as children enter adolescence and adulthood.15 Only roughly one-third of patients require intervention for OPG. Indeed, a major role of NF specialty clinics is to ensure that children with clinically silent OPG do not undergo treatment that can cause unnecessary complications. However, progressive OPGs can cause vision loss, proptosis, and hypothalamic dysfunction.16 Ongoing research is standardizing the metrics for assessing vision loss associated with OPG through both traditional visual acuity measures and novel approaches such as optic coherence tomography (OCT).17,18 OCT allows objective assessment of the retinal nerve fiber layer thickness (RNFL). Initial studies indicate that RNFL and vision are associated. If validated, OCT may provide a sensitive and specific way to assess vision in the youngest patients who are both at the highest risk for vision loss and the most challenging to assess with traditional measures.18,19
When patients have confirmed decline in vision or evidence of hypothalamic involvement, treatment with chemotherapies to address low-grade gliomas should be considered.20,21 Chemotherapy results in stabilization of the OPG with preserved or improved vision in roughly 72% of children.14 Notably, radiation therapy (RT) is not recommended for the majority of NF- associated OPG as it is associated with multiple adverse effects including moyamoya syndrome and secondary cancers.15,22 In the subset of patients that do not have stabilization or response to first-line chemotherapy, there are ongoing clinical trials evaluating inhibition of key nodes on molecular pathways regulated by neurofibromin such as mTOR and MEK (NCT01158651 and NCT01089101) as well as modulators of the tumor microenvironment such as PEG-interferon (NCT02343224). There is also a report of the antiangiogenesis inhibitor bevacizumab resulting in improvement in vision in some patients with progressive OPG19 and a provocative report of topical murine nerve growth factor improving visual-evoked potentials in children with optic nerve atrophy due to OPG,23 suggesting that there may be yet unexplored possibilities for vision restoration in children with vision loss due to OPG.
Patients with NF1 also have up to a 5-fold increased risk of glioblastoma (GBM) compared to the general population.24 It is not clear if patients with NF1 who develop GBM fare better or worse than patients with sporadic GBM. There are some reports of enhanced toxicity with standard therapies such as radiation (RT) and alkylating chemotherapies such as temozolomide due to the underlying TSG.22,25 However, other reports indicate improved survival for children with NF1 and GBM versus sporadic pediatric GBM, and a recent case series reported long postrecurrence survival for adult patients with NF1 and GBM treated with bevacizumab.26,27 Finally, there is increasing evidence that newly diagnosed tumors histologically consistent with pilocytic astrocytoma behave far more aggressively than anticipated in adults with NF1.28 Based on these confounding clinical observations, there are active efforts to understand the molecular and genetic subtypes of NF1 extraoptic gliomas and how they relate to sporadic gliomas.29
Outside of the CNS and PNS tumors, there are several important nontumor manifestations. At least 50% of patients have cognitive deficits than can range from mild difficulty with spatial processing to autism-like features.30 Specific neurocognitive manifestations common in NF1 include attention deficit disorder/hyperactivity, visuospatial deficits, expressive and receptive language difficulties, and executive functioning difficulty.31 As with all other aspects of NF1, the breadth and severity of neurocognitive deficits are highly variable. Additionally, patients with NF1 are at risk for bone malformations including tibial pseudoarthrosis, sphenoid wing dysplasia, and severe scoliosis.32 There are also increasingly recognized risks of vasculopathy, including moyamoya leading to stoke and renal artery stenosis leading to secondary hypertension.33 Finally, there is an increased risk of nonnervous system malignancies such as gastrointestinal stromal tumors, breast cancer in women < 50 years of age, leukemia, and neuroendocrine tumors including pheochromocytomas.34,35
Clearly, the range of possible manifestations of NF1 is vast. Moreover, there is a great deal of variability in the expression of these manifestations, such that some patients appear to be minimally affected, while others have multiple disabling or life-threatening manifestations. In addition, the risk of the various manifestations changes across developmental stages. For example, the risk of symptomatic OPG is greatest in childhood, whereas cutaneous neurofibromas have their biggest impact in adulthood. With rare exceptions, there are no clear genotype-phenotype relationships, and there can be extensive variability in disease within a family and even between identical twins.36–38 This observation suggests that genetic, epigenetic, and environmental factors modify the clinical phenotype of individual patients, and this has important implications for translational research in NF1. Clinically, this inherent heterogeneity of disease severity within and across patients means that patients with NF1 need regular evaluation by experts familiar with the multiple possible manifestations across the lifespan in order to ensure that necessary treatments are provided and that unnecessary treatments are avoided.
Genetics and Molecular Pathophysiology
The NF1 syndrome results from a germline mutation in NF1 gene on chromosome 17q11.2.39,40 The mutation can be de novo or familial with an autosomal dominant inheritance pattern. Roughly 50% of patients with NF1 have a spontaneous mutation (ie, de novo) as the mutation rate for the NF1 gene is one of the highest known for any human gene.41 More than 500 mutations in the NF1 gene have been identified, with the majority resulting in a loss of function of neurofibromin, the protein encoded on the NF1 gene.42 Neurofibromin is widely expressed in almost all tissues, but it is most abundant in the brain, spinal cord, and PNS.43 A mutation in one germline allele is sufficient to result in the syndrome of NF1. However, tumor formation appears to require the loss of function in the second allele, at least in the tumor cell of origin, consistent with NF1 being a TSG.
The most deeply investigated domain of neurofibromin is the guanosine triphosphatase (GTPase) activating protein (GAP)- related domain. This is because GAP regulates Ras, perhaps the most prevalent proto-oncogene across all tumors, both malignant and nonmalignant.44,45 In healthy cells, Ras regulates proliferation, differentiation, transformation, and apoptosis and is most often in the inactive (GDP-bound) conformation. In contrast, Ras-GTP stimulates progrowth pathways including the RAF/MEK/ERK and PI3K/AKT/mTOR pathways.44
Neurofibromin functions, at least in part, by regulating Ras to maintain it in the Ras- GDP (inactive) confirmation.42 In the absence of neurofibromin, Ras-GTP is constitutively activated, which results in excessive stimulation of multiple progrowth pathways. This has implications not only for patients with NF1 but also for most cancers as mutations in, and overexpression of Ras genes (H-Ras, K-Ras, and N-Ras), are found in almost all known solid tumors including breast cancer, thyroid cancer, prostate cancer, lung cancer, colorectal cancer, and brain cancer.45 Moreover, mutations in NF1 have been linked to sporadic cancers including GBM, juvenile myelomonocytic leukemia, desmoplastic neurotrophic melanoma, gastrointestinal stromal tumors, pheochromocytoma, primary lung adenocarcinoma, breast cancer, and ovarian cancer.46–52 The shared genetic and molecular underpinnings between the relatively rare tumors in patients with NF1 and some of the worlds' most common and treatment-resistant cancers implies that the therapeutics developed to address neurofibromin dysfunction may benefit patients far beyond the syndrome of NF1, as was elegantly presented in a review of the role of neurofibromin and tumor pathophysiology.53
The involvement of Ras and the proteins in Ras-effector pathways may also provide opportunities for synergy across several of the manifestations of NF1. For example, MEK inhibitors have shown activity in pNF, low-grade gliomas, bone pathology, and brain formation preclinically.53–56 In the case of pNF and OPG, investigation of MEK inhibitors has now entered clinical trials (NCT02407405 and NCT01089101). Hence, there are new data to support optimism that rational targeting of key nodes affected by loss of neurofibromin will result in clinical benefit across a range of NF1 manifestations.53 Moreover, NF1 offers an opportunity to understand the interaction between the tumor-initiating cell and the microenvironment. Studies of tumorigenesis in MPNST, pNF, and OPG models have all suggested an interaction between the cell nullizygous for NF1 (the presumed tumor-initiating cell) and NF1 heterozygous cells in the microenvironment,57–59 suggesting that modulation of the microenvironment may be an independent therapeutic target for tumors. For example, imatinib, which targets the c-kit ligand secreted by Nf1−/− Schwann cells as a chemotactic agent for Nf1−/− mast cells, decreases the volume of pNF in genetically engineered mouse models (GEMMs), and a subset of patients.57,60 Similarly, pegylated interferon α-2b has shown some signs of efficacy in both pNF and gliomas61,62 and is in active clinical trials for both tumors (NCT00846430 and NCT02343224).
Finally, NF1 provides an interesting platform in which to investigate the stages of tumor pathogenesis. For example, OPGs are known to be of highest risk in early childhood but then often become clinically quiescent.16 Similarly, pNFs have a high growth period in early childhood but have little growth in adulthood.9,63 Preclinical models suggest that the heterozygous cell (ie, NF1+/− Schwann cell in pNF) has the capacity for proliferation but is not otherwise tumorigenic; in fact, additional TSGs can maintain senescence.64,65 Through yet undefined mechanism, when the second NF1 allele is lost, Ras- and cAMP-mediated pathways are permitted to be constitutively active, ultimately supporting tumor expansion. If this hypothesis is accurate, investigation of these feedback loops and the factors that support senescence may provide novel opportunities to prevent pathologic tumor progression in both the tumors seen within the NF1 syndrome and across common cancers driven in part by an NF1 mutation.
Therapeutic Development
Although all of the tumors that arise in the setting of NF1 are thought to be caused by biallelic mutations in NF1, there is a great deal of heterogeneity in the development and behavior of tumors both across and within patients. As a result, therapeutic development to date has been tumor specific and has focused on OPG, pNF, and MPNST. All 3 of these tumors have had dedicated preclinical and clinical efforts to develop therapeutics, but we will focus on pNF here as an illustration of the therapeutic development pipeline for NF1-associated tumors.
There have been 21 clinical trials for pNF, some of which are ongoing (Table 2). These studies have progressed from testing empirically selected drugs to drugs that are vetted through a well-established preclinical-to-clinical pipeline with the benefit of sophisticated GEMM for each tumor manifestation of NF1.66 The studies are relatively equally distributed across agents that target a node in the Ras-regulated pathway and those that target elements of the tumor microenvironment. Both strategies have shown initial signs of success (Table 2).
Table 2.
Completed or ongoing clinical trials for neurofibromatosis type 1-associated plexiform neurofibroma
| Drug | Phase | Target | Age (y) | Endpoint | Results |
|---|---|---|---|---|---|
| Sorafenib156 | 1 | Raf, PDGFRβ, c-kit,VEGFR2 | 3–18 | Toxicity, PK, 3D ORR | Intolerable – pain |
| Tipifarnib67 | 1,2 | Farnesyl Transferase | 3–25 | WHO; TTP 3D ORR | Inactive |
| Pirfenidone157,158 | 1,2 | Fibroblast | 3–21 | 3D ORR | Inactive/unclear |
| Thalidomide159 | 1 | Angiogenesis | >5 | WHO ORR | Inactive/unclear |
| Ketotifen fumarate160 | 2 | Anti-histamine | 6–55 | Symptom improvement | Inactive/unclear |
| PEG-Interferon alpha 2b61 | 1, 2 | Immune; Angiogenesis | 1–34 | TTP, 3D ORR | 29% 3D ORR |
| Sirolimus68 | 2 | mTOR | >3 | TTP, 3D ORR | TTP – active No RR |
| Imatinib60 | 2 | c-kit, PDGFRβ | 3–65 | RECIST, 3D ORR | 17% 3D ORR |
| Selumetinib69 NCT01362803 |
1,2 | MEK | 3–18 | Toxicity, PK, 3D ORR | MTD defined, 3D ORR in 100% of first cohort reported69; phase 2 opening 2015 |
| Cediranib NCT00326872 |
2 | VEGFR-1,-2,-3 | ≥18 | 3D ORR | Ongoing |
| Vinblastine/Methotrexate NCT00030264 |
2 | cytotoxic | ≤25 | TTP | Ongoing |
| Everolimus NCT01412892 |
2 | mTOR | 18–60 | 3D ORR | Ongoing |
| Everolimus NCT01365468 |
2 | mTOR | >10 | 3D ORR, TTP | Ongoing |
| Nilotinib NCT01275586 |
2 | c-kit, BCR-ABL, MAPK11, PDGFRβ | ≥18 | REIST; 3D ORR | Ongoing |
| Celecoxib; PEG-Interferon alpha 2b NCT00846430 |
2 | Immune; Angiogenesis | 2–30 | Symptom improvement and RECIST | Ongoing |
| PLX3397 NCT02390752 |
1,2 | c-kit, CSF1R and FLT3 | 3–31 | Toxicity, PK, PD, ORR | Ongoing |
| Selumetinib NCT02407405 |
2 | MEK | ≥18 | Toxicity, PK, 3D ORR | Ongoing |
| PD-0325901 NCT02096471 |
2 | MEK | ≥16 | 3D, ORR | Ongoing |
| Trametinib NCT02124772 |
1 | MEK | 1 m–17 | Toxicity, PK, PD | Pending |
| Cabozantinib NCT02101736 |
2 | VEGFR, c-Met, RET | ≥16 | 3D, ORR | Ongoing |
| Sunitinib NCT01402817 |
2 | PDGFR, VEGFR, c-kit | 3–65 | 3D ORR | Suspended |
Abbreviations: 3D ORR, volumetric objective radiographic response; BCR-ABL, fusion gene of breakpoint cluster region and Abl1; c-kit, Kit ligand or stem cell factor; c-MET, MET proto-oncogene or hepatocyte growth factor receptor; CSF1R, colony stimulating factor 1 receptor; FLT3, Fms-like tyrosine kinase 3; MAPK11, mitogen-activated protein kinase 11; MEK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; PD, pharmacoodynamic; PDGF/R, platelet-derived growth factor/receptor; PK, pharmacokinetics; Raf, serine/threonine-protein kinase; RECIST, Response Evaluation Criteria In Solid Tumors; RET, rearranged during transfection proto-oncogene; TTP, time to progression; VEGF, vascular endothelial growth factor; WHO ORR, World Health Organization objective response rate.
Notable advances in the developing therapeutic landscape for pNF reflect both increasing experience with the natural history of the tumor and improved understanding of the pathophysiology. For example, a placebo-controlled crossover study of tipifarnib established the natural history of the growth rates of progressive pNF in children with NF1.67 Ultimately, tipifarnib did not reach the study goal of doubling the time to progression (TTP), but the study did reveal an average 12-month TTP for symptomatic pNF.67 A subsequent study of sirolimus in participants with symptomatic pNF showed no reduction in tumor mass but did achieve an improvement in TTP to 15.4 months compared with the TTP established by the tipifarnib study.68
The observed long intervals required for TTP, maturing natural history data indicating that spontaneous regression of pNF is vanishingly rare, and the dramatic tumor responses observed with a handful of drugs in GEMM have led to a greater focus on response endpoints. The first agent to show radiographic response (RR) was imatinib. A phase 2 clinical trial showed RR in 6/36 (17%) of participants with pNF (ages 3–65 y), with a suggestion that head-and-neck region tumors have the most robust results.60 Subsequently, in a phase 1 study of pegylated interferon-α-2b, there was a 29% (5/17) RR in participantts aged 2–35 years with NF1 associated pNF.61 The single agent phase 2 study is now nearing completion of enrollment (NCT00396019). Most recently, 2 small molecules targeting MEK have entered clinical trials. The phase 1 study of selumetinib defined twice daily 20 mg/m2 dosing as the recommended phase 2 dose and showed exciting signs of activity, with 100% of the 11 participants evaluable for response having a decrease in tumor volume.69 Based on the tolerability and the signs of clinical efficacy from phase 1, the phase 2 is in development and is enrolling (NCT02407405). There are several additional studies with pending results representing a relatively rapid expansion in clinical trial options for patients with NF1-associated pNF.66
Neurofibromatosis Type 2
Clinical Presentation
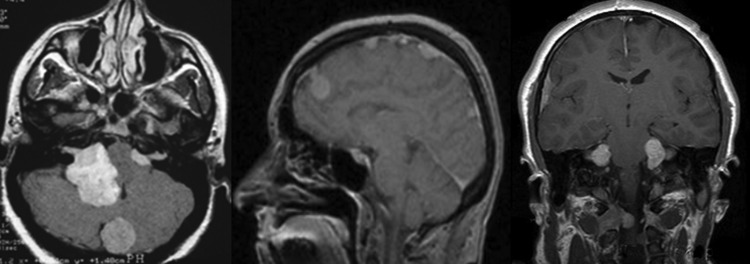
NF2 is far more rare than NF1, with an estimated incidence of1 in 25 000–33 000 births.70 Similar to NF1, NF2 is associated with multiple tumors throughout the CNS and PNS that progress to cause neurologic morbidity over time.5,70 The tumors that occur most commonly in the setting of NF2 are schwannomas and meningiomas (Table 1). Schwannomas can occur on any intracranial or extracranial peripheral nerve; however, the hallmark of NF2 is bilateral vestibular schwannomas (VSs) (Fig. 2).71 These tumors are the most common cause of morbidity for patients with NF2 and result in bilateral sensorineural hearing loss, tinnitus, balance difficulty, and ultimately deafness, facial nerve weakness, and possible brainstem compression.71,72
Fig. 2.
Cranial MRI scans of patients with neurofibromatosis type 2 demonstrating bilateral vestibular schwannomas and multiple meningiomas.
The primary treatment for nonsyndromic VS is surgical resection or increasingly, radiosurgery, especially for tumors <3 cm.73,74 These options are less attractive in the setting of NF2 as the neurologic disabilities that can occur with surgery (eg, hearing loss, facial nerve weakness, swallowing dysfunction) have significantly greater impact on morbidity and mortality when the tumors are multifocal and progressive over a lifetime.75 In addition, the standard treatments for VS (surgery and radiation therapy [RT]) appear to have lower rates of efficacy and higher rates of complications in the setting of NF2 than in nonsyndromic VS.72,76 For example, long-term follow-up of patients treated with RT for NF2-associated VS shows that there is a 50% chance of tumor control at 8 years with a low rate of long-term hearing preservation (40% at 3 years).77 Moreover, there is some concern about both early and late complications, including the potential of an increased rate of late malignant transformation in patients with NF2.78,79
There is substantial variability in disease severity across all patients with NF2. More severe disease, and poorer prognosis is associated with diagnoses made in childhood and less severe disease/better prognosis is observed in patients with mosaic NF2.80–82 In patients with mosaicism, only a subset of cells have the NF2 gene mutation, and these patients often have fewer tumors, milder symptoms, and a generally better prognosis.80–82 Independent of these factors, there is substantial variability in growth rate of any individual tumor, with the right and the left VS often growing at different rates and importantly with no clear association between the rate of growth and the rate of hearing loss.81,83,84
There have been major efforts to understand the natural history of patients with NF2 to help define the optimal timing for intervention and to establish benchmarks for testing new therapeutics. An early consortium-based study of 540 participants with NF2 showed the average age at diagnosis to be 27 years with a roughly 7-year delay from symptom onset to diagnosis.83,84 There was an average of 1 mm/year rate of tumor growth, and roughly 30% of participants had surgery within 2 years of diagnosis. A more recent analysis assessed endpoints commonly used in clinical trials for VS including tumor volume and word recognition score (WRS).85 In this study, the mean rate of hearing decline across 120 NF2 participants (200 VS) was 16% 3 years from diagnosis. Concurrently, the rate of VS radiographic progression (defined as ≥20% increase in tumor volume compared with baseline) was 31% at 1 year and 79% at 3 years, again highlighting the lack of correlation between tumor size or growth rate and hearing function in NF2 associated NF2-associated VS.85 In another study, across 46 participants with NF2 (92 VS) who were followed clinically for a mean of 6 years, the mean baseline VS size was 1.3 cm, and the mean rate of growth was 1.8 mm/year. Most tumors (88%) did not require surgery over 5 years of follow-up, and most participants (66%) maintained bilateral hearing for the study period.86 Hence, for select patients with NF2, careful observation alone is reasonable.
For a significant group of patients, there is continued progression of bilateral VS, ultimately leading to deafness, facial palsy, and other cranial nerve deficits. Historical actuarial survival in patients with NF2 from the time of diagnosis is 85% at 5 years, 67% at 10 years, and 38% at 20 years.82 More recent population studies report a median life expectancy for patients with NF2 of 69.0 years (95% CI: 58.9–79.0 y).87 Hence, although there is variability across patients, NF2-associated tumors (and their treatments) often contribute to earlier-than-expected death.
Meningioma is the most common primary brain tumor seen worldwide, and up to half of all patients with NF2 will develop intracranial meningiomas and in many cases will have multiple tumors or meningiomatosis (Fig. 2).88,89 Although many meningiomas in the setting of NF2 do not require intervention, one study has found that the presence of a meningioma is associated with 2.5-fold greater risk of mortality in patients with NF2 who have meningioma verus those who do not. This observation may be due to the fact that meningiomas are often the symptomatic tumor in children who are diagnosed with NF2 rather than VS, and NF2 diagnosed in childhood has a worse prognosis.90 If a NF2-associated meningioma is symptomatic or rapidly progressive, the primary treatment is surgery. RT is reserved for malignant or multiple recurrent meningiomas. However, if required, there are data to suggest that RT can be safely administered for NF2-associated meningiomas, although long-term safety and efficacy are unclear.91,92
The most clinically concerning NF2-associated meningiomas are the tumors involving the skull base, cavernous sinuses, and orbits or those in the parafalcine region, where they can occlude the sagittal sinus and contribute to venous hypertension. Surgery is often not feasible for these tumors, and the efficacy of RT is uncertain. Hence, like meningiomas in the general population, patients with NF2-associated meningiomas have a dire need for effective therapeutics that will slow progression or lead to tumor reduction.
Genetics and Molecular Pathology
The NF2 gene is on 22q11.2 and encodes the tumor suppressor protein merlin. Merlin is a member of the ERM protein family (MERlin: moesin-ezrin-radixin) responsible for membrane stabilization and regulation of several cellular growth pathways.93 Like neurofibromin in NF1, merlin is found predominantly in nervous system tissue. Activated merlin stabilizes cadherin-dependent cell-to-cell junctions, inhibiting the effects of receptor tyrosine kinases (RTKs) at the cell membrane.94 Merlin also facilitates endocytic trafficking of RTKs.95 In both sporadic and NF2-associated VS, the absence of merlin permits increased signaling of the ErbB/EGFR family RTKs.96–102 Importantly, merlin is active both at the cell membrane and in the nucleus, depending on its configuration,98,99 and loss of merlin allows activation of prosurvival and proliferation pathways via Ras modulation, sharing many of the same targets identified for NF1.97,103 Increased mTOR, RAC1, and FAK signaling have specifically been implicated as therapeutic targets in NF2-associated schwannomas.97,100–102 It has been hypothesized that the absence of merlin contributes to loss of normal semaphorin activity, resulting in enhanced angiogenesis possibly underlying the responses seen in NF2-associated VS to the antiangiogenic therapy bevacizumab.104 For each of the tumorgenic pathways influenced by the lack of merlin, there are therapeutic agents that can be repurposed from other disease indications (Table 3). This has resulted in relatively rapid translation of preclinical discoveries to clinical trials for patients with NF2-associated VS.
Table 3.
Neurofibromatosis type 2-associated vestibular schwannoma and meningioma trials
| Drug | Phase | Target | Age (y) | Endpoint | Results |
|---|---|---|---|---|---|
| Lapatinib113 | 2 | EGFR/ErBb2 | 4–80 | VS: 15% volume reduction | 4/17 (23.5%) with RR |
| RAD001161 | 2 | mTOR | ≥3 | VS: 15% volume reduction | No RR |
| RAD001112 | 2 | mTOR | >15 | VS: volume reduction | No RR; prolonged TTP |
| Lapatinib NCT00863122 |
0, translational | EGFR/ErBb2 | ≥18 | VS: tumor PK, molecular and gene mutation analysis | Completed: PK achieved in tissue, negative PD markers |
| Bevacizumab NCT01207687 |
2 | VEGF | ≥12 | VS: hearing response as measured by word recognition score | Completed: in review; 5/14 (36%) sustained hearing response |
| Bevacizumab NCT01767792 |
2 | VEGF | ≥12 | VS: hearing response as measured by word recognition score | Ongoing |
| RAD001 NCT01345136 |
2 | mTOR | 16–65 | VS: volume reduction | Ongoing |
| RAD001 NCT01880749 |
0, translational | mTOR | ≥18 | VS and MEN: tumor PK, molecular and gene mutation analysis | Ongoing |
| Sorafenib Not applicable |
0, translational | PDGF, VEGR, c-kit | ≥18 | Cutaneous SWN: PK, molecular studies | Ongoing |
| Axitinib NCT02129647 |
2 | VEGF, c-kit, PDGFR | ≥18 | VS: 20% volume reduction | Ongoing |
| Endostatin NCT02104323 |
2 | Anti-angiogenic | 16–30 | Tumor Volume | Ongoing |
| AR-42 NCT02282917 |
0, translational | HDAC | ≥18 | VS and MEN: tumor PK, molecular and gene mutation analysis | Recruiting |
| PTC 299 NCT00911248 |
2 | VEGF | ≥18 | VS : tumor volume or word recognition score | Suspended |
| Nilotinib NCT01201538 |
2 | PDGF, c-kit | ≥18 | VS: 20% volume reduction | Suspended |
Abbreviations: c-kit, Kit ligand or stem cell factor; EGFR/ErBb2, epidermal growth factor receptor; HDAC, histone deacetylases; MEN, meningiomas; mTOR, mammalian target of rapamycin; PD, pharmacoodynamic; PDGF/R, platelet-derived growth factor/receptor; PK, pharmacokinetics; RR, radiographic response; SWN, schwannomas; TTP, time to progression; VEGF, vascular endothelial growth factor; VS, vestibular schwannoma.
Interestingly, merlin is absent in all VSs studied to date, whether related to NF2 or de novo.105,106 This is significant because idiopathic VSs are common, with roughly 3000 new cases per year in the United States, and it may be that NF2-associated and sporadic VSs share a common therapeutic pathway.107,108 The majority of spontaneous meningiomas are also driven by NF2 regulated pathways,109 and there is evidence that merlin is a negative regulator of growth and progression of non-NF2-associated cancers.110,111 Here again, shared molecular genetic backgrounds between a rare tumor syndrome and some of the worlds' most common tumors support shared discovery efforts.
Therapeutic Development
The therapeutic trials developed for patients with NF2 thus far have been focused on VS because these tumors cause morbidity and mortality in the majority of patients with NF2. The majority of clinical trials have applied RTK inhibitors implicated in preclinical studies including lapatinib, everolimus, nilotinib, and sorafenib.112–114 Thus far, these agents have resulted in mixed rates of response, but there have been some signs of activity and evidence that agents such as everolimus and AR-42 may slow tumor growth.112,113,115 In contrast, the antiangiogenesis agent bevacizumab, initially given in a compassionate-use study for participants with progressive morbidity due to NF2-associated VS, had unprecedented efficacy.116,117 Specifically, the initial experience in 10 people with NF2 treated with bevacizumab at 5 mg/kg every 2 weeks resulted in 6 of 10 participants experiencing ≥20% reduction in tumor volume and 4 of 7 evaluable participants having significantly improved hearing.117 A subsequent series showed a hearing response in 13 of 23 (57%) participants with hearing loss due to VS.116 These results led to the development of 2 prospective clinical trials assessing bevacizumab in patients with progressive hearing loss due to VS (NCT01207687 and NCT01767792). The goal of these studies is to confirm the hearing response rate in people with NF2 and VS associated hearing loss, explore dosing regimens, determine short and long term tolerability and identify biomarkers that predict response.
Schwannomatosis
Clinical Presentation
Schwannomatosis is a third form of neurofibromatosis and is characterized by the predisposition for developing multiple schwannomas and, less commonly, meningiomas. Given the spectrum of tumors, this condition was initially felt to represent an attenuated form of NF2. While there is significant overlap between these conditions, subsequent research has confirmed that schwannomatosis is distinct from NF2 and has a different clinical phenotype and genetic etiology.
Patients with schwannomatosis most commonly develop symptoms in the second or third decade of life, but a formal diagnosis is usually delayed by ∼10 years.118 Patients typically present with complaints of pain (46%), a mass (27%), or both (11%).118 Indeed, pain is the most frequent symptom reported by patients, with 68% experiencing chronic pain in one study.118 Depression and anxiety are common complications of schwannomatosis, and managing these comorbidities is a cornerstone of treatment. Schwannomas commonly affect the spine (74%) and peripheral nerves (89%), whereas cranial nerve schwannomas (mostly trigeminal) are uncommon (8%) (Fig. 3).118 Neurologic dysfunction related to schwannomas is unusual and, when present, is often a complication of surgery. Anatomically limited disease, presumably due to genetic mosaicism, is seen in ∼30% of patients. In a series of 87 participants meeting clinical criteria for schwannomatosis, additional tumors identified included lipomas (11%) and angiolipomas (3%), but clinical findings consistent with NF1 such as café-au-lait macules, cutaneous lesions, learning disabilities and scoliosis were reported rarely (2–23% of participants) and no participants met clinical criteria for NF1 or NF2.118
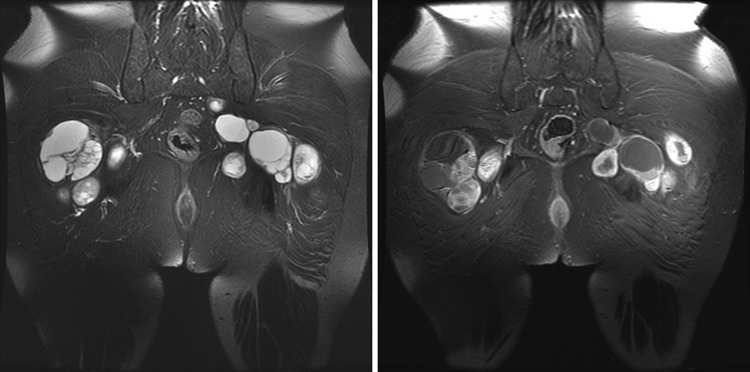
Fig. 3.
Representative MRI scan of the multiple schwannomas in a patient with schwannomatosis. Schwannomas appear hyperintense on T2-weighted images and enhance after administration of gadolinium contrast. The enhancement pattern can range from homogenous to heterogenous. Cysts may develop within some tumors.
In contrast to patients with NF2, VSs are rare in schwannomatosis, and the presence of bilateral VS in a patient with schwannomatosis should raise concern for an alternative diagnosis.119 However, unilateral VS has been described in patients with germline mutations in SMARCB1 and LZTR1.120,121 Patients with multiple nonvestibular schwannomas without VS may in fact have mosaic NF2 rather than schwannomatosis. This important finding was demonstrated by the identification of identical NF2 alteration in multiple tumors in 4 of 9 participants having sporadic schwannomatosis and multiple tumors available for analysis.122 Together these results emphasize the considerable phenotypic overlap between schwannomatosis and NF2.
Meningiomas occur in ∼5% of schwannomatosis patients and have a predilection for the cerebral falx.123 Families have been reported with multigenerational meningiomas and SMARCB1 germline mutations.123,124 However, germline SMARCB1 mutations are uncommon in individuals with multiple meningiomas and no other signs of schwannomatosis.125 The occurrence of malignancy in schwannomatosis has been debated. In a series of 87 participants with schwannomatosis from the United States,118 tumor specimens from 3 cases of MPNST were reviewed by an expert neuropathologist and reclassified as cellular schwannomas or melanoma. Similarly, in an English cohort of 104 patients, one case of MPNST was reported (without central pathology review).122 These reports suggest that the risk of MPNST in schwannomatosis is low, but not zero, and hence it is important to be aware of this possibility.
Genetics and Molecular Pathology
Germline mutations or deletions in SMARCB1 have been identified in 40%–50% of kindreds affected by familial schwannomatosis and 10% of sporadic schwannomatosis patients.126,127 Inherited mutations found in familial schwannomatosis are more likely to be nontruncating (ie, missense or splice-site).122,128 In contrast, sporadic schwannomatosis patients are more likely to harbor truncating mutations (ie, frameshift or nonsense), which are predicted to lead to loss of functional SMARCB1.122,127
Genetic analysis of blood and tumors from patients with schwannomatosis has revealed the presence of constitutional mutations in SMARCB1 with different somatic mutations in NF2. These findings support a 3- or 4-hit mechanism of tumorigenesis involving 2 distinct linked tumor suppressor genes: SMARCB1 and NF2.129 It is possible that the specific combination of resulting somatic mutations may regulate the severity of the resulting phenotype.122,128
SMARCB1 (also called hSNF5, INI1, and BAF47) is a subunit of the SWI/SNF complex, an ATP-dependent chromatin-remodeling complex.130,131 SMARCB1 exerts its tumor suppressor function by regulating the cell cycle and inducing senescence. In addition, SMARCB1 and the SWI/SNF components regulate lineage-specific gene expression and embryonic stem cell programming.132–136 Mutations in subunits of the SWI/SNF complex are common across human cancer. In an analysis of 44 exome-sequencing studies, SWI/SNF subunits were mutated in 19.6% of all human tumors.137
In 2014, germline mutations in LZTR1 were identified in about 80% of schwannomatosis cases lacking mutations in SMARCB1 but with loss of chromosome 22q in tumors.138 LZTR1 mutations have been identified in several cancers, and the gene functions as a tumor suppressor in glioblastoma where biallelic mutations have been reported. LZTR1 is located at22q11.21 in a region near SMARCB1 and NF2.138 The LZTR1 protein belongs to the BTB/POZ superfamily and is involved in multiple cellular processes including regulation of chromatin conformation and the cell cycle.139 As with SMARCB1-related schwannomatosis, different somatic mutations in NF2 were identified in schwannomas from people with LZTR1 mutation, supporting the 3- or 4-hit mechanism of tumorigenesis.138 Subsequent studies of LZTR1 in participants without proven involvement of the chromosome 22q locus revealed mutations in ∼ 40% of those with familial schwannomatosis, ∼25% with sporadic patients, and 5% of patients with unilateral VS and an additional nonvestibular schwannoma.121,140,141 Clinical testing for SMARCB1 and LZTR1 mutations is now available as part of the standard of care for patients with suspected schwannomatosis.
Therapeutic Development
Management of patients with schwannomatosis is primarily symptom oriented. Surgery is currently the treatment of choice for symptomatic schwannomas and can relieve local pain or symptoms arising from compression of neighboring tissues in many patients. However, recurrence of pain is common and may not be related to tumor size.118 The major risk of surgery is iatrogenic nerve injury; hence, surgeons with experience in nerve- sparing surgery should be involved when considering a schwannoma resection.
Experience with RT for management of schwannomatosis-related schwannomas is limited. As with any tumor suppressor syndrome, there is concern that RT treatment could increase the risk for malignant transformation of radiated tumors, as has been reported for NF1 and NF2.142 There are no available data on the risk of secondary malignant transformation of tumors in schwannomatosis patients. At this time, most experts reserve RT for patients who require treatment for life-threatening, enlarging schwannomas that cannot be treated with surgery or for the very rare patients with malignant schwannomas. There are individual reports about the clinical efficacy of bevacizumab in patients with progressive tumors and refractory pain; however, this has not yet been tested formally.143 There have been no clinical trials to date that have focused specifically on treating schwannomas or meningiomas in the setting of schwannomatosis.
Endpoints for Therapeutic Trials in the Neurofibromatoses
Most early NF trials adopted trial designs similar to those used in oncology trials with a variety of imaging-based response criteria (Table 2). However, due to a preponderance of histologically benign tumors, the prolonged survival of NF patients compared with cancer patients, and the irregular boundaries of tumors associated with NF, the standard endpoints used in oncology have limited application for NF trials. The use of a variety of nonuniform endpoints for clinical trials in NF has hindered the ability to assess efficacy across studies, and there was a clear need to establish uniform endpoints for the various tumors across NF1, NF2, and schwannomatosis. Using the Response Assessment in Neuro-Oncology (RANO) initiative as a model, the Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) International Collaboration was established in 2011 to achieve consensus within the NF community for clinical trial endpoints. The goal of the group is to recommend outcome measures and methodologies to standardize endpoints for NF clinical trials. To accomplish this, the REiNS collaboration is organized around 7 working groups: (i) imaging of tumor response, (ii) functional outcomes, (iii) visual outcomes, (iv) patient-reported outcomes, (v) neurocognitive outcomes, (vi) whole-body MRI, and (vii) biomarkers.
In 2013, the REiNS International Collaboration published its initial recommendations for clinical trial endpoints in NF.144 Regarding imaging endpoints, volumetric MRI assessment of pNF and VS was recommended as the standard imaging metric for clinical investigations in NF1 and NF2.63 This conclusion was based on the comparison of linear versus bidimensional versus volumetric measurements for pNF and VS that demonstrated that volumetric assessments are far more sensitive to change (growth or regression) than 1- or 2-dimensional measures.63,145 The goal is for all future studies to use these imaging assessment metrics to allow more uniform assessment across studies of the same tumor type. Similarly, whole body MRI (WBMRI) has been administered to patients with NF1, NF2, and schwannomatosis because it is well suited for evaluating large tumors that cross traditional body regions as well as providing more accurate longitudinal assessment of complex tumors.146–148 The REiNS group is now working to develop standardized WBMRI approaches for use in NF clinical trials.
Regarding clinical outcome assessment (COA) endpoints, given the frequency of pain in NF1, NF2, and schwannomatosis, the group reviewed a variety of pain intensity measures and ultimately recommended the numeric rating scale-11 (NRS-11) for use in NF clinical trials based on strong psychometric data that include sensitivity to change and excellent feasibility in people ≥aged 8 years.149 For NF1-OPG therapeutic trials, the group recommended functional assessment of vision via visual acuity (VA) using consistent quantitative testing methods as the main functional outcome measure.17 They also recommended assessment of the optic disc for pallor because this appears to be a contributory variable that may affect the interpretation of VA change over time. For NF2 COA endpoints, the group endorsed the use of maximum word recognition score as a primary endpoint for hearing and the scaled measurement of improvement in lip excursion (SMILE) system for studies of facial function.150
The REiNS International Collaboration continues to develop consensus endpoints for NF trials, with ongoing efforts to validate the endpoints across a range of patients and tumors. Future efforts will be directed toward evaluation of neurocognitive outcomes for attention and executive function, patient-reported scales for pain interference, pulmonary outcomes for pNF affecting the airway, and imaging outcomes for OPG. Through these efforts and ongoing discussions with the US FDA, the REiNS group is working to establish a clear pathway for developing measurement tools that will ultimately support drug approvals for NF-associated tumors.
Future Directions
Therapeutic trials for the neurofibromatoses have progressed rapidly over the past 2 decades. During that time, reliable preclinical models have been developed and refined, enabling translational science including high throughput drug screening of tumor cell lines (across the syndromes and tumor types), xenograft mouse models, and GEMM with imaging and functional measures that parallel human clinical endpoints. Such tools have been used to identify and fully investigate candidate compounds before advancing to clinical trials. Single institution trials of drugs against many tumors of the neurofibromatoses are underway, and multi-institutional trials have been launched since establishment of the Department of Defense NF Clinical Trials Consortium in 2007, providing infrastructure, shared resources, and a community of thought leaders to generate high-impact and resource-efficient clinical trials.66 The academic, federal regulatory, and foundation communities are collaborating to develop consensus recommendations for clinical trial endpoints in NF, targeting sensitive, reproducible, and clinically meaningful endpoints in an effort to identify drugs with the best safety profiles as well as the greatest clinical impact against the manifestations of disease. These efforts have resulted in a number of small, early successes for tumors afflicting patients with NF1 and NF2. Although new and better compounds with activity against NF-associated tumors are needed, the collaboration and tools required for a successful therapeutic development pipeline are strong and growing.
Funding
Jaishri Blakeley receives salary support from the Neurofibromatosis Therapeutic Acceleration Program.
Acknowledgments
The authors gratefully acknowledge the administrative support provided by Ms. Rhonda Jackson.
Conflict of interests statement. Jaishri Blakeley received research support (non-salary) for a clinical trial in participants with NF2 from GlaxoSmithKline. Jaishri Blakeley served on the advisory committee for Abbvie for patient-reported outcomes in glioblastoma and will be paid a consultancy fee for this one-time service. Scott Plotkin has equity in NFlection.
References
- 1.McBride KA, Ballinger ML, Killick E et al. Li-Fraumeni syndrome: cancer risk assessment and clinical management. Nat Rev Clin Oncol. 2014;11(5):260–271. [DOI] [PubMed] [Google Scholar]
- 2.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68(4):820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Liao P et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–i63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huson SM, Harper PS, Compston DA. Von Recklinghausen neurofibromatosis. A clinical and population study in south-east Wales. Brain. 1988;111(Pt 6):1355–1381. [DOI] [PubMed] [Google Scholar]
- 5.Evans DG, Howard E, Giblin C et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327–332. [DOI] [PubMed] [Google Scholar]
- 6.Shah KN. The diagnostic and clinical significance of cafe-au-lait macules. Pediatr Clin North Am. 2010;57(5):1131–1153. [DOI] [PubMed] [Google Scholar]
- 7.Le LQ, Liu C, Shipman T, Chen Z, Suter U, Parada LF. Susceptible stages in Schwann cells for NF1-associated plexiform neurofibroma development. Cancer Res. 2011;71(13):4686–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dombi E, Solomon J, Gillespie AJ et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology. 2007;68(9):643–647. [DOI] [PubMed] [Google Scholar]
- 9.Tucker T, Friedman JM, Friedrich RE, Wenzel R, Funsterer C, Mautner VF. Longitudinal study of neurofibromatosis 1 associated plexiform neurofibromas. J Med Genet. 2009;46(2):81–85. [DOI] [PubMed] [Google Scholar]
- 10.Prada CE, Rangwala FA, Martin LJ et al. Pediatric plexiform neurofibromas: impact on morbidity and mortality in neurofibromatosis type 1. J Pediatr. 2012;160(3):461–467. [DOI] [PubMed] [Google Scholar]
- 11.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39(5):311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pourtsidis A, Doganis D, Baka M et al. Malignant peripheral nerve sheath tumors in children with neurofibromatosis type 1. Case Rep Oncol Med. 2014;2014:843749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albers AC, Gutmann DH. Gliomas in patients with neurofibromatosis type 1. Expert Rev Neurother. 2009;9(4):535–539. [DOI] [PubMed] [Google Scholar]
- 14.Fisher MJ, Loguidice M, Gutmann DH et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63–66. [DOI] [PubMed] [Google Scholar]
- 16.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61(3):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher MJ, Avery RA, Allen JC et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;81(21 Suppl 1):S15–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avery RA, Hwang EI, Ishikawa H et al. Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthalmol. 2014;132(3):265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avery RA, Hwang EI, Jakacki RI, Packer RJ. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014;132(1):111–114. [DOI] [PubMed] [Google Scholar]
- 20.Packer RJ, Ater J, Allen J et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. [DOI] [PubMed] [Google Scholar]
- 21.Gururangan S, Cavazos CM, Ashley D et al. Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol. 2002;20(13):2951–2958. [DOI] [PubMed] [Google Scholar]
- 22.Sharif S, Ferner R, Birch JM et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24(16):2570–2575. [DOI] [PubMed] [Google Scholar]
- 23.Falsini B, Chiaretti A, Barone G et al. Topical nerve growth factor as a visual rescue strategy in pediatric optic gliomas: a pilot study including electrophysiology. Neurorehabil Neural Repair. 2011;25(6):512–520. [DOI] [PubMed] [Google Scholar]
- 24.Korf BR. Malignancy in neurofibromatosis type 1. Oncologist. 2000;5(6):477–485. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura JL, Phong C, Pinarbasi E et al. Dose-dependent effects of focal fractionated irradiation on secondary malignant neoplasms in Nf1 mutant mice. Cancer Res. 2011;71(1):106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huttner AJ, Kieran MW, Yao X et al. Clinicopathologic study of glioblastoma in children with neurofibromatosis type 1. Pediatr Blood Cancer. 2010;54(7):890–896. [DOI] [PubMed] [Google Scholar]
- 27.Theeler BJ, Ellezam B, Yust-Katz S, Slopis JM, Loghin ME, de Groot JF. Prolonged survival in adult neurofibromatosis type I patients with recurrent high-grade gliomas treated with bevacizumab. J Neurol. 2014;261(8):1559–1564. [DOI] [PubMed] [Google Scholar]
- 28.Guillamo JS, Creange A, Kalifa C et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126(Pt 1):152–160. [DOI] [PubMed] [Google Scholar]
- 29.Jentoft M, Giannini C, Cen L et al. Phenotypic variations in NF1-associated low grade astrocytomas: possible role for increased mTOR activation in a subset. Int J Clin Exp Pathol. 2010;4(1):43–57. [PMC free article] [PubMed] [Google Scholar]
- 30.Acosta MT, Gioia GA, Silva AJ. Neurofibromatosis type 1: new insights into neurocognitive issues. Curr Neurol Neurosci Rep. 2006;6(2):136–143. [DOI] [PubMed] [Google Scholar]
- 31.Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65(7):1037–1044. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson DA, Little D, Armstrong L et al. Approaches to treating NF1 tibial pseudarthrosis: consensus from the Children's Tumor Foundation NF1 Bone Abnormalities Consortium. J Pediatr Orthop. 2013;33(3):269–275. [DOI] [PubMed] [Google Scholar]
- 33.Kaas B, Huisman TA, Tekes A, Bergner A, Blakeley JO, Jordan LC. Spectrum and prevalence of vasculopathy in pediatric neurofibromatosis type 1. J Child Neurol. 2013;28(5):561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montani D, Coulet F, Girerd B et al. Pulmonary hypertension in patients with neurofibromatosis type I. Medicine (Baltimore). 2011;90(3):201–211. [DOI] [PubMed] [Google Scholar]
- 35.Walker L, Thompson D, Easton D et al. A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br J Cancer. 2006;95(2):233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauer M, Lubs H, Lubs ML. Variable expressivity of neurofibromatosis-1 in identical twins. Neurofibromatosis. 1988;1(5–6):323–329. [PubMed] [Google Scholar]
- 37.Carey JC, Viskochil DH. Neurofibromatosis type 1: A model condition for the study of the molecular basis of variable expressivity in human disorders. Am J Med Genet. 1999;89(1):7–13. [PubMed] [Google Scholar]
- 38.Alkindy A, Chuzhanova N, Kini U, Cooper DN, Upadhyaya M. Genotype-phenotype associations in neurofibromatosis type 1 (NF1): an increased risk of tumor complications in patients with NF1 splice-site mutations? Hum Genomics. 2012;6:12 -7364-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballester R, Marchuk D, Boguski M et al. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990;63(4):851–859. [DOI] [PubMed] [Google Scholar]
- 40.Martin GA, Viskochil D, Bollag G et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63(4):843–849. [DOI] [PubMed] [Google Scholar]
- 41.Ars E, Kruyer H, Morell M et al. Recurrent mutations in the NF1 gene are common among neurofibromatosis type 1 patients. J Med Genet. 2003;40(6):e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Upadhyaya M, Osborn MJ, Maynard J, Kim MR, Tamanoi F, Cooper DN. Mutational and functional analysis of the neurofibromatosis type 1 (NF1) gene. Hum Genet. 1997;99(1):88–92. [DOI] [PubMed] [Google Scholar]
- 43.Daston MM, Scrable H, Nordlund M, Sturbaum AK, Nissen LM, Ratner N. The protein product of the neurofibromatosis type 1 gene is expressed at highest abundance in neurons, Schwann cells, and oligodendrocytes. Neuron. 1992;8(3):415–428. [DOI] [PubMed] [Google Scholar]
- 44.Weiss B, Bollag G, Shannon K. Hyperactive Ras as a therapeutic target in neurofibromatosis type 1. Am J Med Genet. 1999;89(1):14–22. [PubMed] [Google Scholar]
- 45.Harris TJ, McCormick F. The molecular pathology of cancer. Nat Rev Clin Oncol. 2010;7(5):251–265. [DOI] [PubMed] [Google Scholar]
- 46.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Side LE, Emanuel PD, Taylor B et al. Mutations of the NF1 gene in children with juvenile myelomonocytic leukemia without clinical evidence of neurofibromatosis, type 1. Blood. 1998;92(1):267–272. [PubMed] [Google Scholar]
- 48.Gutzmer R, Herbst RA, Mommert S et al. Allelic loss at the neurofibromatosis type 1 (NF1) gene locus is frequent in desmoplastic neurotropic melanoma. Hum Genet. 2000;107(4):357–361. [DOI] [PubMed] [Google Scholar]
- 49.Gutmann DH, Geist RT, Rose K, Wallin G, Moley JF. Loss of neurofibromatosis type I (NF1) gene expression in pheochromocytomas from patients without NF1. Genes Chromosomes Cancer. 1995;13(2):104–109. [DOI] [PubMed] [Google Scholar]
- 50.Furukawa K, Yanai N, Fujita M, Harada Y. Novel mutations of neurofibromatosis type 1 gene in small cell lung cancers. Surg Today. 2003;33(5):323–327. [DOI] [PubMed] [Google Scholar]
- 51.Sangha N, Wu R, Kuick R et al. Neurofibromin 1 (NF1) defects are common in human ovarian serous carcinomas and co-occur with TP53 mutations. Neoplasia. 2008;10(12):1362–1372, following 1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallace MD, Pfefferle AD, Shen L et al. Comparative oncogenomics implicates the neurofibromin 1 gene (NF1) as a breast cancer driver. Genetics. 2012;192(2):385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ratner N, Miller SJ. A RASopathy gene commonly mutated in cancer: the neurofibromatosis type 1 tumour suppressor. Nat Rev Cancer. 2015;15(5):290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaul A, Toonen JA, Cimino PJ, Gianino SM, Gutmann DH. Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth. Neuro Oncol. 2015;17(6):843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de la Croix Ndong J, Stevens DM, Vignaux G et al. Combined MEK inhibition and BMP2 treatment promotes osteoblast differentiation and bone healing in Nf1Osx -/- mice. J Bone Miner Res. 2015;30(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim E, Wang Y, Kim SJ et al. Transient inhibition of the ERK pathway prevents cerebellar developmental defects and improves long-term motor functions in murine models of neurofibromatosis type 1. Elife. 2014;3:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Staser K, Yang FC, Clapp DW. Pathogenesis of plexiform neurofibroma: tumor-stromal/hematopoietic interactions in tumor progression. Annu Rev Pathol. 2012;7:469–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun T, Gianino SM, Jackson E, Piwnica-Worms D, Gutmann DH, Rubin JB. CXCL12 alone is insufficient for gliomagenesis in Nf1 mutant mice. J Neuroimmunol. 2010;224(1–2):108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malone CF, Fromm JA, Maertens O, DeRaedt T, Ingraham R, Cichowski K. Defining key signaling nodes and therapeutic biomarkers in NF1-mutant cancers. Cancer Discov. 2014;4(9):1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robertson KA, Nalepa G, Yang FC et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 2012;13(12):1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jakacki RI, Dombi E, Potter DM et al. Phase I trial of pegylated interferon-alpha-2b in young patients with plexiform neurofibromas. Neurology. 2011;76(3):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warren K, Bent R, Wolters PL et al. A phase 2 study of pegylated interferon alpha-2b (PEG-Intron-R) in children with diffuse intrinsic pontine glioma. Cancer. 2012;118(14):3607–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21 Suppl 1):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Courtois-Cox S, Genther Williams SM, Reczek EE et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10(6):459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet. 1994;7(3):353–361. [DOI] [PubMed] [Google Scholar]
- 66.Gutmann DH, Blakeley JO, Korf BR, Packer RJ. Optimizing biologically targeted clinical trials for neurofibromatosis. Expert Opin Investig Drugs. 2013;22(4):443–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Widemann BC, Dombi E, Gillespie A et al. Phase 2 randomized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipifarnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro Oncol. 2014;16(5):707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiss B, Widemann BC, Wolters P et al. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: a neurofibromatosis Clinical Trials Consortium phase II study. Neuro Oncol. 2015;17(4):596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Widemann BC, Marcus LJ, Fisher MJ et al. Phase I study of the MEK1/2 inhibitor selumetinib (AZD6244) hydrogen sulfate in children and young adults with neurofibromatosis type 1 (NF1) and inoperable plexiform neurofibromas (PNs). J Clin Oncol. 2014;32(5s (suppl; abstr 10018). [Google Scholar]
- 70.Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26(1):93–97. [DOI] [PubMed] [Google Scholar]
- 71.Evans DG, Kalamarides M, Hunter-Schaedle K et al. Consensus recommendations to accelerate clinical trials for neurofibromatosis type 2. Clin Cancer Res. 2009;15(16):5032–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi JW, Lee JY, Phi JH et al. Clinical course of vestibular schwannoma in pediatric neurofibromatosis Type 2. J Neurosurg Pediatr. 2014;13(6):650–657. [DOI] [PubMed] [Google Scholar]
- 73.Rowe JG, Radatz MW, Walton L, Hampshire A, Seaman S, Kemeny AA. Gamma knife stereotactic radiosurgery for unilateral acoustic neuromas. J Neurol Neurosurg Psychiatry. 2003;74(11):1536–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.International RadioSurgery Association. Acoustic Neuroma & Patient Choice. An Education Publication. brain talk;2004;9(2) Stereotactic Radiosurgery for Patients with Vestibular Schwannomas. Radiosurgery Practice Guideline Report #4-06 Brain Talk® 9(2); Another Perspective® 2(1), 2006. www.IRSA.org/publications.html/ [Google Scholar]
- 75.Ferner RE, Shaw A, Evans DG et al. Longitudinal evaluation of quality of life in 288 patients with neurofibromatosis 2. J Neurol. 2014;261(5):963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Evans DG, Baser ME, O'Reilly B et al. Management of the patient and family with neurofibromatosis 2: a consensus conference statement. Br J Neurosurg. 2005;19(1):5–12. [DOI] [PubMed] [Google Scholar]
- 77.Rowe J, Radatz M, Kemeny A. Radiosurgery for type II neurofibromatosis. Prog Neurol Surg. 2008;21:176–182. [DOI] [PubMed] [Google Scholar]
- 78.Baser ME, Evans DG, Jackler RK, Sujansky E, Rubenstein A. Neurofibromatosis 2, radiosurgery and malignant nervous system tumours. Br J Cancer. 2000;82(4):998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balasubramaniam A, Shannon P, Hodaie M, Laperriere N, Michaels H, Guha A. Glioblastoma multiforme after stereotactic radiotherapy for acoustic neuroma: case report and review of the literature. Neuro Oncol. 2007;9(4):447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evans DG, Raymond FL, Barwell JG, Halliday D. Genetic testing and screening of individuals at risk of NF2. Clin Genet. 2012;82(5):416–424. [DOI] [PubMed] [Google Scholar]
- 81.Dirks MS, Butman JA, Kim HJ et al. Long-term natural history of neurofibromatosis Type 2-associated intracranial tumors. J Neurosurg. 2012;117(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Otsuka G, Saito K, Nagatani T, Yoshida J. Age at symptom onset and long-term survival in patients with neurofibromatosis Type 2. J Neurosurg. 2003;99(3):480–483. [DOI] [PubMed] [Google Scholar]
- 83.Slattery WH III, Fisher LM, Iqbal Z, Oppenhiemer M. Vestibular schwannoma growth rates in neurofibromatosis type 2 natural history consortium subjects. Otol Neurotol. 2004;25(5):811–817. [DOI] [PubMed] [Google Scholar]
- 84.Fisher LM, Doherty JK, Lev MH, Slattery WH. Concordance of bilateral vestibular schwannoma growth and hearing changes in neurofibromatosis 2: neurofibromatosis 2 natural history consortium. Otol Neurotol. 2009;30(6):835–841. [DOI] [PubMed] [Google Scholar]
- 85.Plotkin SR, Merker VL, Muzikansky A, Barker FG II, Slattery W III. Natural history of vestibular schwannoma growth and hearing decline in newly diagnosed neurofibromatosis type 2 patients. Otol Neurotol. 2014;35(1):e50–e56. [DOI] [PubMed] [Google Scholar]
- 86.Peyre M, Goutagny S, Bah A et al. Conservative management of bilateral vestibular schwannomas in neurofibromatosis type 2 patients: hearing and tumor growth results. Neurosurgery. 2013;72(6):907–913; discussion 914; quiz 914. [DOI] [PubMed] [Google Scholar]
- 87.Wilding A, Ingham SL, Lalloo F et al. Life expectancy in hereditary cancer predisposing diseases: an observational study. J Med Genet. 2012;49(4):264–269. [DOI] [PubMed] [Google Scholar]
- 88.Mautner VF, Lindenau M, Baser ME et al. The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery. 1996;38(5):880–885; discussion 885–6. [DOI] [PubMed] [Google Scholar]
- 89.Goutagny S, Kalamarides M. Meningiomas and neurofibromatosis. J Neurooncol. 2010;99(3):341–347. [DOI] [PubMed] [Google Scholar]
- 90.Ruggieri M, Iannetti P, Polizzi A et al. Earliest clinical manifestations and natural history of neurofibromatosis type 2 (NF2) in childhood: a study of 24 patients. Neuropediatrics. 2005;36(1):21–34. [DOI] [PubMed] [Google Scholar]
- 91.Wentworth S, Pinn M, Bourland JD et al. Clinical experience with radiation therapy in the management of neurofibromatosis-associated central nervous system tumors. Int J Radiat Oncol Biol Phys. 2009;73(1):208–213. [DOI] [PubMed] [Google Scholar]
- 92.Liu A, Kuhn EN, Lucas JT Jr, Laxton AW, Tatter SB, Chan MD. Gamma Knife radiosurgery for meningiomas in patients with neurofibromatosis Type 2. J Neurosurg. 2015;122(3):536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trofatter JA, MacCollin MM, Rutter JL et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75(4):826. [DOI] [PubMed] [Google Scholar]
- 94.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23(8):1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol. 2006;16(7):702–709. [DOI] [PubMed] [Google Scholar]
- 96.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177(5):893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67(2):520–527. [DOI] [PubMed] [Google Scholar]
- 98.Cooper J, Li W, You L et al. Merlin/NF2 functions upstream of the nuclear E3 ubiquitin ligase CRL4DCAF1 to suppress oncogenic gene expression. Sci Signal. 2011;4(188):pt6. [DOI] [PubMed] [Google Scholar]
- 99.Li W, Cooper J, Zhou L et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell. 2014;26(1):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shapiro IM, Kolev VN, Vidal CM et al. Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci Transl Med. 2014;6(237):237ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hennigan RF, Moon CA, Parysek LM et al. The NF2 tumor suppressor regulates microtubule-based vesicle trafficking via a novel Rac, MLK and p38(SAPK) pathway. Oncogene. 2013;32(9):1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.James MF, Stivison E, Beauchamp R et al. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol Cancer Res. 2012;10(5):649–659. [DOI] [PubMed] [Google Scholar]
- 103.Lim JY, Kim H, Kim YH et al. Merlin suppresses the SRE-dependent transcription by inhibiting the activation of Ras-ERK pathway. Biochem Biophys Res Commun. 2003;302(2):238–245. [DOI] [PubMed] [Google Scholar]
- 104.Wong HK, Lahdenranta J, Kamoun WS et al. Anti-vascular endothelial growth factor therapies as a novel therapeutic approach to treating neurofibromatosis-related tumors. Cancer Res. 2010;70(9):3483–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Neff BA, Welling DB, Akhmametyeva E, Chang LS. The molecular biology of vestibular schwannomas: dissecting the pathogenic process at the molecular level. Otol Neurotol. 2006;27(2):197–208. [DOI] [PubMed] [Google Scholar]
- 106.Hanemann CO. Magic but treatable? Tumours due to loss of merlin. Brain. 2008;131(Pt 3):606–615. [DOI] [PubMed] [Google Scholar]
- 107.Tos M, Stangerup SE, Caye-Thomasen P, Tos T, Thomsen J. What is the real incidence of vestibular schwannoma? Arch Otolaryngol Head Neck Surg. 2004;130(2):216–220. [DOI] [PubMed] [Google Scholar]
- 108.Stangerup SE, Tos M, Caye-Thomasen P, Tos T, Klokker M, Thomsen J. Increasing annual incidence of vestibular schwannoma and age at diagnosis. J Laryngol Otol. 2004;118(8):622–627. [DOI] [PubMed] [Google Scholar]
- 109.Brastianos PK, Horowitz PM, Santagata S et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stamenkovic I, Yu Q. Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci. 2010;11(6):471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Petrilli AM, Fernández-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene. 2015:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goutagny S, Raymond E, Esposito-Farese M et al. Phase II study of mTORC1 inhibition by everolimus in neurofibromatosis type 2 patients with growing vestibular schwannomas. J Neurooncol. 2015;122(2):313–320. [DOI] [PubMed] [Google Scholar]
- 113.Karajannis MA, Legault G, Hagiwara M et al. Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2012;14(9):1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ammoun S, Schmid MC, Triner J, Manley P, Hanemann CO. Nilotinib alone or in combination with selumetinib is a drug candidate for neurofibromatosis type 2. Neuro Oncol. 2011;13(7):759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bush ML, Oblinger J, Brendel V et al. AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro Oncol. 2011;13(9):983–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Plotkin SR, Merker VL, Halpin C et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol. 2012;33(6):1046–1052. [DOI] [PubMed] [Google Scholar]
- 117.Plotkin SR, Stemmer-Rachamimov AO, Barker FG II et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361(4):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Merker VL, Esparza S, Smith MJ, Stemmer-Rachamimov A, Plotkin SR. Clinical features of schwannomatosis: a retrospective analysis of 87 patients. Oncologist. 2012;17(10):1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Plotkin SR, Blakeley JO, Evans DG et al. Update from the 2011 International Schwannomatosis Workshop: From genetics to diagnostic criteria. Am J Med Genet A. 2013;161A(3):405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith MJ, Kulkarni A, Rustad C et al. Vestibular schwannomas occur in schwannomatosis and should not be considered an exclusion criterion for clinical diagnosis. Am J Med Genet A. 2012;158A(1):215–219. [DOI] [PubMed] [Google Scholar]
- 121.Smith MJ, Isidor B, Beetz C et al. Mutations in LZTR1 add to the complex heterogeneity of schwannomatosis. Neurology. 2015;84(2):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smith MJ, Wallace AJ, Bowers NL et al. Frequency of SMARCB1 mutations in familial and sporadic schwannomatosis. Neurogenetics. 2012;13(2):141–145. [DOI] [PubMed] [Google Scholar]
- 123.van den Munckhof P, Christiaans I, Kenter SB, Baas F, Hulsebos TJ. Germline SMARCB1 mutation predisposes to multiple meningiomas and schwannomas with preferential location of cranial meningiomas at the falx cerebri. Neurogenetics. 2012;13(1):1–7. [DOI] [PubMed] [Google Scholar]
- 124.Bacci C, Sestini R, Provenzano A et al. Schwannomatosis associated with multiple meningiomas due to a familial SMARCB1 mutation. Neurogenetics. 2010;11(1):73–80. [DOI] [PubMed] [Google Scholar]
- 125.Hadfield KD, Smith MJ, Trump D, Newman WG, Evans DG. SMARCB1 mutations are not a common cause of multiple meningiomas. J Med Genet. 2010;47(8):567–568. [DOI] [PubMed] [Google Scholar]
- 126.Smith MJ, Wallace AJ, Bowers NL, Eaton H, Evans DG. SMARCB1 mutations in schwannomatosis and genotype correlations with rhabdoid tumors. Cancer Genet. 2014;207(9):373–378. [DOI] [PubMed] [Google Scholar]
- 127.Rousseau G, Noguchi T, Bourdon V, Sobol H, Olschwang S. SMARCB1/INI1 germline mutations contribute to 10% of sporadic schwannomatosis. BMC Neurol. 2011;11:9 –2377–11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boyd C, Smith MJ, Kluwe L, Balogh A, Maccollin M, Plotkin SR. Alterations in the SMARCB1 (INI1) tumor suppressor gene in familial schwannomatosis. Clin Genet. 2008;74(4):358–366. [DOI] [PubMed] [Google Scholar]
- 129.Sestini R, Bacci C, Provenzano A, Genuardi M, Papi L. Evidence of a four-hit mechanism involving SMARCB1 and NF2 in schwannomatosis-associated schwannomas. Hum Mutat. 2008;29(2):227–231. [DOI] [PubMed] [Google Scholar]
- 130.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108(4):475–487. [DOI] [PubMed] [Google Scholar]
- 131.Wang W, Xue Y, Zhou S, Kuo A, Cairns BR, Crabtree GR. Diversity and specialization of mammalian SWI/SNF complexes. Genes Dev. 1996;10(17):2117–2130. [DOI] [PubMed] [Google Scholar]
- 132.Krosl J, Mamo A, Chagraoui J et al. A mutant allele of the Swi/Snf member BAF250a determines the pool size of fetal liver hemopoietic stem cell populations. Blood. 2010;116(10):1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yoo AS, Crabtree GR. ATP-dependent chromatin remodeling in neural development. Curr Opin Neurobiol. 2009;19(2):120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Albini S, Puri PL. SWI/SNF complexes, chromatin remodeling and skeletal myogenesis: it's time to exchange! Exp Cell Res. 2010;316(18):3073–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Caramel J, Medjkane S, Quignon F, Delattre O. The requirement for SNF5/INI1 in adipocyte differentiation highlights new features of malignant rhabdoid tumors. Oncogene. 2008;27(14):2035–2044. [DOI] [PubMed] [Google Scholar]
- 136.Young DW, Pratap J, Javed A et al. SWI/SNF chromatin remodeling complex is obligatory for BMP2-induced, Runx2-dependent skeletal gene expression that controls osteoblast differentiation. J Cell Biochem. 2005;94(4):720–730. [DOI] [PubMed] [Google Scholar]
- 137.Kadoch C, Hargreaves DC, Hodges C et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45(6):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Piotrowski A, Xie J, Liu YF et al. Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat Genet. 2014;46(2):182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nacak TG, Leptien K, Fellner D, Augustin HG, Kroll J. The BTB-kelch protein LZTR-1 is a novel Golgi protein that is degraded upon induction of apoptosis. J Biol Chem. 2006;281(8):5065–5071. [DOI] [PubMed] [Google Scholar]
- 140.Kamada K, Houkin K, Abe H, Sawamura Y, Kashiwaba T. Differentiation of cerebral radiation necrosis from tumor recurrence by proton magnetic resonance spectroscopy. Neurol Med Chir (Tokyo). 1997;37(3):250–256. [DOI] [PubMed] [Google Scholar]
- 141.Paganini I, Chang VY, Capone GL et al. Expanding the mutational spectrum of LZTR1 in schwannomatosis. Eur J Hum Genet. 2014;23(7):963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Evans DG, Birch JM, Ramsden RT, Sharif S, Baser ME. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet. 2006;43(4):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blakeley J, Schreck KC, Evans DG et al. Clinical response to bevacizumab in schwannomatosis. Neurology. 2014;83(21):1986–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Widemann BC, Blakeley JO, Dombi E et al. Conclusions and future directions for the REiNS International Collaboration. Neurology. 2013;81(21 Suppl 1):S41–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harris GJ, Plotkin SR, Maccollin M et al. Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery. 2008;62(6):1314–1319; discussion 1319–20. [DOI] [PubMed] [Google Scholar]
- 146.Kluwe L, Nguyen R, Vogt J et al. Internal tumor burden in neurofibromatosis Type I patients with large NF1 deletions. Genes Chromosomes Cancer. 2012;51(5):447–451. [DOI] [PubMed] [Google Scholar]
- 147.Fayad LM, Blakeley J, Plotkin S, Widemann B, Jacobs MA. Whole Body MRI at 3 T with Quantitative Diffusion Weighted Imaging and Contrast-Enhanced Sequences for the Characterization of Peripheral Lesions in Patients with Neurofibromatosis Type 2 and Schwannomatosis. ISRN Radiol. 2013;2013:627932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Merker VL, Bredella MA, Cai W et al. Relationship between whole-body tumor burden, clinical phenotype, and quality of life in patients with neurofibromatosis. Am J Med Genet A. 2014;164A(6):1431–1437. [DOI] [PubMed] [Google Scholar]
- 149.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children's self-reports of pain intensity. Pain. 2009;143(3):223–227. [DOI] [PubMed] [Google Scholar]
- 150.Plotkin SR, Ardern-Holmes SL, Barker FG II et al. Hearing and facial function outcomes for neurofibromatosis 2 clinical trials. Neurology. 2013;81(21 Suppl 1):S25–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, Md., USA, July 13–15, 1987. Neurofibromatosis. 1988;1(3):172–178. [PubMed] [Google Scholar]
- 152.Gutmann D, Aylsworth A, Carey J et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA 1997;278:51–57. [PubMed] [Google Scholar]
- 153.MacCollin M, Chiocca EA, Evans DG et al. Diagnostic criteria for schwannomatosis. Neurology. 2005;64(11):1838–1845. [DOI] [PubMed] [Google Scholar]
- 154.Baser ME, Friedman JM, Evans DG. Increasing the specificity of diagnostic criteria for schwannomatosis. Neurology. 2006;66(5):730–732. [DOI] [PubMed] [Google Scholar]
- 155.University of Alabama School of Medicine Medical Genomics Laboratory Testing Services. Available at: http://www.uab.edu/medicine/genetics/medical-genomics-laboratory/testing-service. Accessed February 4, 2016.
- 156.Kim A, Dombi E, Tepas K et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas . Pediatr Blood Cancer. 2013;60(3):396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Babovic-Vuksanovic D, Widemann BC, Dombi E et al. Phase I trial of pirfenidone in children with neurofibromatosis 1 and plexiform neurofibromas. Pediatr Neurol. 2007;36(5):293–300. [DOI] [PubMed] [Google Scholar]
- 158.Widemann BC, Babovic-Vuksanovic D, Dombi E et al. Phase II trial of pirfenidone in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Pediatr Blood Cancer. 2014;61(9):1598–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gupta A, Cohen BH, Ruggieri P, Packer RJ, Phillips PC. Phase I study of thalidomide for the treatment of plexiform neurofibroma in neurofibromatosis 1. Neurology. 2003;60(1):130–132. [DOI] [PubMed] [Google Scholar]
- 160.Riccardi VM. Ketotifen suppression of NF1 neurofibroma growth over 30 years. Am J Med Genet A. 2015;167(7):1570–1577. [DOI] [PubMed] [Google Scholar]
- 161.Karajannis MA, Legault G, Hagiwara M et al. Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2014;16(2):292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]