Abstract
Background
In glioblastoma (GBM), the gene for epidermal growth factor receptor (EGFR) is frequently amplified. EGFR mutations also are common, including a truncation mutation that yields a constitutively active variant called EGFR variant (v)III. EGFRvIII-positive GBM progresses rapidly; however, the reason for this is not clear because the activity of EGFRvIII is attenuated compared with EGF-ligated wild-type EGFR. We hypothesized that EGFRvIII-expressing GBM cells selectively express other oncogenic receptors that support tumor progression.
Methods
Mining of The Cancer Genome Atlas prompted us to test whether GBM cells in culture, which express EGFRvIII, selectively express vascular endothelial growth factor receptor (VEGFR)2. We also studied human GBM propagated as xenografts. We then applied multiple approaches to test the effects of VEGFR2 on GBM cell growth, apoptosis, and cellular senescence.
Results
In human GBM, EGFR overexpression and EGFRvIII positivity were associated with increased VEGFR2 expression. In GBM cells in culture, EGFRvIII-initiated cell signaling increased expression of VEGFR2, which prevented cellular senescence and promoted cell cycle progression. The VEGFR-selective tyrosine kinase inhibitor cediranib decreased tumor DNA synthesis, increased staining for senescence-associated β-galactosidase, reduced retinoblastoma phosphorylation, and increased p27Kip1, all markers of cellular senescence. Similar results were obtained when VEGFR2 was silenced.
Conclusions
VEGFR2 expression by GBM cells supports cell cycle progression and prevents cellular senescence. Coexpression of VEGFR2 by GBM cells in which EGFR signaling is activated may contribute to the aggressive nature of these cells.
Keywords: cellular senescence, EGF receptor, EGFRvIII, glioblastoma, VEGF receptor-2
Glioblastoma (GBM) is a highly malignant primary brain tumor in which the gene encoding the receptor tyrosine kinase (RTK), epidermal growth factor receptor (EGFR), is amplified or overexpressed in 50% or more of cases.1–3 In tumors with EGFR gene amplification, EGFR mutations are observed,3 including a truncation mutation in which exons 2–7 are absent.4,5 The resulting mutated form of EGFR, EGFR variant (v)III, is incapable of binding EGF but demonstrates constitutive tyrosine kinase activity in the absence of growth factor.5,6
GBM tumors in which EGFR is amplified and in which EGFRvIII is expressed progress rapidly and carry a poor prognosis.2,5,7 How EGFRvIII affects GBM progression remains incompletely understood. In EGFRvIII-positive GBM, the percentage of tumor cells that express the mutated EGFR is quite variable and can be small.8 Furthermore, the enzymatic activity of EGFRvIII is attenuated by as much as 90% compared with EGF-ligated wild-type (wt)EGFR.6 We hypothesized that the constitutive signaling activity of EGFRvIII induces expression of additional oncogenic receptors, which synergize with EGFRvIII to promote tumor progression. In support of this hypothesis, we recently demonstrated that EGFRvIII-positive GBM cells express increased levels of urokinase-type plasminogen activator receptor (uPAR).9 Even though uPAR is glycosylphosphatidylinositol anchored, it expresses potent cell-signaling activity and synergizes with EGFRvIII to activate the mitogenic transcription factor, signal transducer and activator of transcription 5b.10 The goal of the present study was to determine whether RTKs are selectively overexpressed in EGFRvIII-positive GBM cells.
We began our research by mining transcriptome profiling data from The Cancer Genome Atlas (TCGA). Our analysis demonstrated a significant correlation between the level of expression of EGFR and vascular endothelial growth factor receptor 2 (VEGFR2/kinase insert domain receptor [KDR]). We also determined that VEGFR2 is expressed at significantly higher levels in tumors that express EGFRvIII. Because TCGA data reflect total harvested RNA, we sought to confirm our findings using cell culture model systems and xenografts of human GBM in mice. VEGFR2 is expressed by endothelial cells and implicated in angiogenesis and vasculogenesis.11,12 Drugs targeting VEGF-VEGFR2 signaling have been used to treat a variety of cancers.13,14 GBM tumors are notable for intense microvascular proliferation, thought to reflect VEGF released by tumor cells acting through endothelial cell VEGFR2.15 However, previous studies have shown that in addition to endothelial cells, neoplastic GBM cells also express VEGFR2.16–18
The results presented in this paper demonstrate that VEGFR2 expression is increased in GBM cells in which EGFR signaling is activated. In these cells, VEGFR2 inhibits cellular senescence and promotes cell cycle progression. Cellular senescence is usually considered an irreversible process in which cells enter cell cycle arrest at the G1/G0 phase.19,20 Molecular markers of senescence include the cyclin-dependent kinase inhibitors (CDKIs) p16INK4a/CDKN2a, p21Cip1a, and p27Kip1 and hypophosphorylation of retinoblastoma protein (Rb).19,20 In premalignant conditions, senescence pathways may be activated to halt unrestricted cell growth.21,22 When senescence occurs within the context of cancer therapeutics, the response is termed therapy-induced senescence (TIS).20 Although replicative arrest, which is characteristic of TIS, may be favorable in cancer treatment, the concomitant resistance to apoptosis may make absolute cancer eradication more difficult. The activity of VEGFR2 in preventing cellular senescence in GBM cells in which EGFR is activated may contribute to the aggressive nature of these tumor cells. We further propose that anti-angiogenic drugs, which target VEGF signaling, may have a direct effect on GBM cells, inducing cellular senescence, in addition to the known effects on tumor vasculature.
Materials and Methods
Mining The Cancer Genome Atlas Data with cBioPortal
TCGA mRNA data were downloaded for a panel of GBM samples from the cBioPortal for Cancer Genomics including RNA-Seq Version 2 RSEM and Agilent microarray z-scores.23–25 EGFRvIII-positive or -negative status was derived from the Nanostring nCounter platform.26 Mean z-scores for EGFRvIII-negative and -positive tumors were compared by unpaired t-tests. Log2 RSEM values for EGFR were plotted against values for VEGFR2, stem cell growth factor receptor (SCGFR/c-Kit), platelet derived growth factor receptor (PDGFR)β, and hepatocyte growth factor receptor (HGFR/c-Met). Statistical analysis was performed to determine the Pearson correlation coefficient (r) and the corresponding P-value.
Immunohistochemistry
An EGFRvIII-expressing human GBM (GBM39) and an EGFRvIII-negative GBM in which wtEGFR was amplified (GBM8) were propagated as xenografts as previously described10,27 and kindly provided by C. David James (Department of Neurological Surgery, Feinberg School of Medicine, Northwestern University). Harvested tumor tissue was formalin fixed and paraffin embedded for sectioning and immunohistochemistry (IHC) using an antibody against VEGFR2. Detailed IHC methods are listed in the Supplementary material.
Cell Lines
U87MG cells that express EGFRvIII or overexpress wtEGFR have been previously described,5 as have U373MG cells that express EGFRvIII under the control of a doxycycline (Dox)-repressible promoter and U373MG cells that overexpress wtEGFR.28 Detailed cell culture maintenance methods are listed in the Supplementary material.
Immunoblot Analysis
Immunoblot analysis was performed as previously described.10 Detailed methods are listed in the Supplementary material.
Relative Real-time Quantitative PCR
Total RNA was isolated using the NucleoSpin RNA II Kit (Macherey-Nagel). Complementary DNA was synthesized with the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative (q)PCR was carried out using TaqManFast Universal PCR Mastermix 2x, and TaqMan primers and probes. Detailed methods and descriptions of primers/probes are provided in the Supplementary material.
Small Interfering RNA Transfection
VEGFR2-specific ON-TARGETplus small interfering (si)RNA or ON-TARGETplus nontargeting control (NTC) siRNA was introduced into cells by incubation with Lipofectamine 2000 (Invitrogen). The extent of gene silencing was determined by qPCR and immunoblot analysis. Detailed sequences and methods are provided in the Supplementary material.
Bromodeoxyuridine Incorporation
Cell proliferation was measured by bromodeoxyuridine (BrdU) incorporation using a fluorescently conjugated BrdU-specific antibody as previously described.10 Detailed methods are provided in the Supplementary material.
Cell Death Assay
Cell death was determined using the Cell Death Detection Enzyme-Linked Immunosorbent Assay (ELISA) Plus Kit (Roche Applied Science) according to the manufacturer's instructions. Cells were transfected with siRNA or treated with cediranib prior to performing the assay. Statistical significance was determined by an unpaired t-test.
Senescence-associated β-galactosidase Staining
Senescence was measured by immunostaining for senescence-associated β-galactosidase (SA-β-Gal) using the Senescence β-Galactosidase Staining Kit (Cell Signaling Technologies). Detailed methods for imaging and analysis are provided in the Supplementary material.
Results
EGFR and Receptor Tyrosine Kinase Coexpression in Human GBM
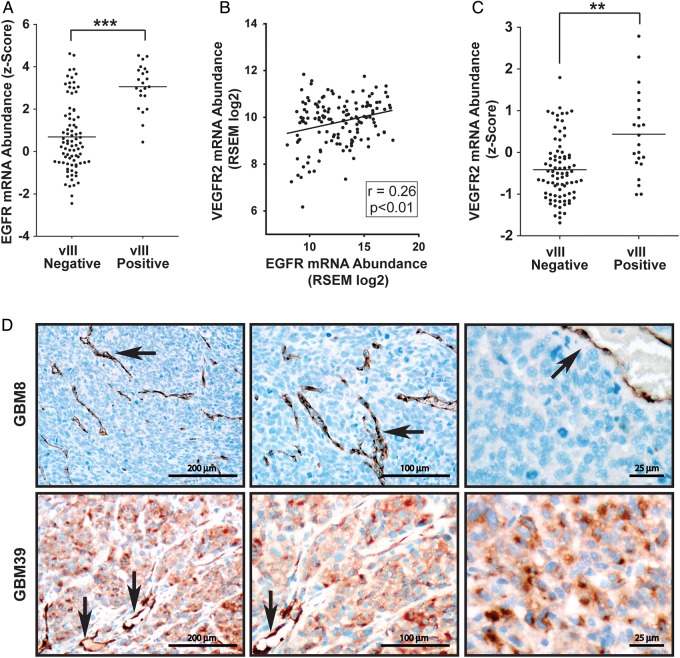
To compare gene expression in human GBM, we mined TCGA RNA-Seq and Agilent microarray transcriptome profiling data. Tumors were classified as EGFRvIII positive or negative based on Nanostring nCounter analyses.26 Figure 1A shows that EGFR expression was significantly increased in tumors that were EGFRvIII positive, as anticipated (P < .001). The z-score, shown in Fig. 1A, reports gene expression as the number of standard deviations displaced from a mean value, determined using a reference group of TCGA tumors. These results support the conclusion that EGFRvIII occurs in the context of EGFR gene amplification or overexpression.4,26
Fig. 1.
EGFR and VEGFR2 expression in human GBM. (A) EGFR mRNA expression z-scores in EGFRvIII-positive and -negative GBM from TCGA data are compared (mean ± SEM, n = 106, ***P < .001). (B) Scatter plot comparing VEGFR2 and EGFR mRNA abundance in GBM mined from TCGA data. Linear regression and Pearson’s correlation determination showed a statistically significant positive correlation. (C) VEGFR2 mRNA expression z-scores are compared in EGFRvIII-positive and -negative GBM from TCGA data (mean ± SEM, n = 106, **P < .01). (D) Immunohistochemical staining for VEGFR2 in human GBM tumors propagated as xenografts in mice. Harvested tumor tissue was immunostained for VEGFR2 (brown) using hematoxylin as a counterstain (blue). The top row shows GBM8, in which EGFR is amplified. GBM8 does not express EGFRvIII. Immunopositivity is evident only in blood vessels (black arrows). VEGFR2-positive tumor cells were not observed. The bottom row shows GBM39, which is EGFRvIII positive. Tumor cells and blood vessels (black arrows) are both immunopositive (100×, 200×, and 400× original magnifications).
Next, we mined RNA-Seq data to determine whether there is a correlation between expression of EGFR and other RTKs implicated in GBM progression.18,29,30 Of the RTKs examined, VEGFR2 showed the strongest positive correlation (Fig. 1B). Although the Pearson correlation coefficient was only 0.26, the correlation was statistically significant (P < .01). Additional RTKs examined included PDGFRβ, c-Kit, and c-Met. TCGA data revealed a weak (r = 0.17) but statistically significant (P < .05) correlation between PDGFRβ mRNA and EGFR mRNA (Supplementary Fig. S1A). Expression of c-Kit did not correlate with EGFR expression (Supplementary Fig. S1B). A significant negative correlation was demonstrated with c-Met (Supplementary Fig. S1C).
Next we examined VEGFR2 expression in EGFRvIII-positive vs -negative GBM and showed that VEGFR2 was significantly increased in EGFRvIII-positive tumors (P < .01) (Fig. 1C). Given the nature of TCGA transcriptome profiling data, the source of VEGFR2 (tumor cells vs nonmalignant cells, such as endothelium) could not be determined. To further examine the relationship between EGFR and VEGFR2 in human samples, we compared 2 previously characterized human GBM tumors that had been propagated as xenografts and shown to retain the original molecular characteristics of the parent tumors.27,31 IHC studies were performed to detect VEGFR2. As shown in Fig. 1D (top panels), VEGFR2 was not detected in tumor cells in EGFRvIII-negative GBM (GBM8), in which EGFR was amplified. Blood vessels provided an internal VEGFR2-positive control (arrows). By contrast, in EGFRvIII-positive GBM (GBM39), the tumor cells were robustly immunopositive for VEGFR2. Again, arrows in Fig. 1D point to blood vessels, which provided an internal positive control.
VEGFR2 Expression in EGFRvIII-positive GBM Cell Lines
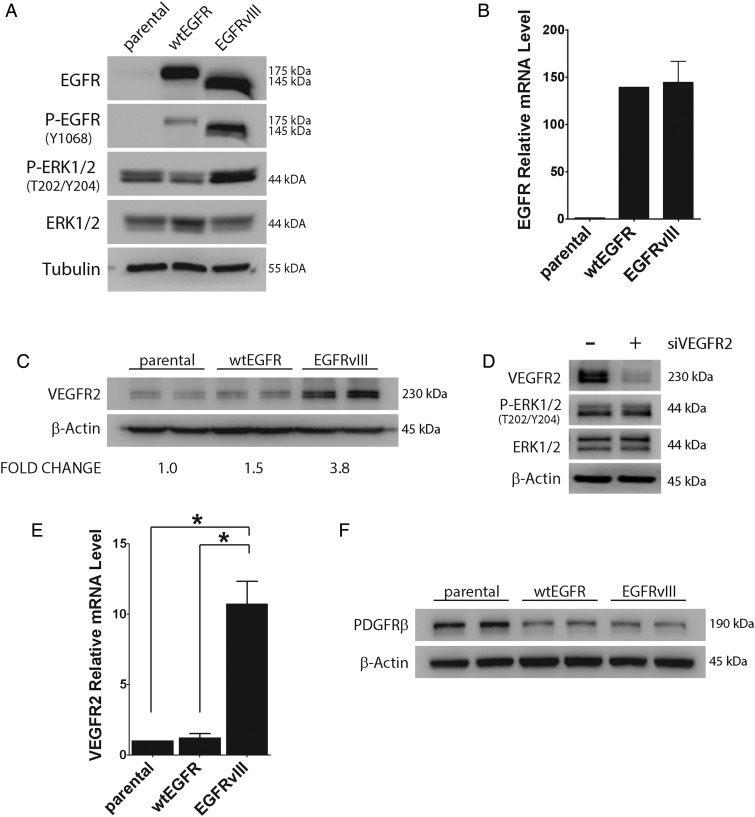
To test whether EGFR induces VEGFR2 expression in GBM cells, first we studied the U87MG GBM model system.5 Cells that express EGFRvIII or overexpress wtEGFR and parental U87MG cells were compared. Figure 2A shows that the total level of EGFR was similar in cells that express EGFRvIII or overexpress wtEGFR. The lower molecular mass of EGFRvIII is due to truncation of exons 2–7.4,5 EGFR was detected in parental cells only when immunoblots were exposed for longer periods of time (results not shown). Tyr-1068 in EGFR was phosphorylated in EGFRvIII-expressing cells, reflecting the constitutive activity of this mutant.4,5 Low levels of phospho-Tyr-1068 in wtEGFR-overexpressing cells may reflect endogenously produced ligands or ligand-independent signaling.32 Extracellular signal-regulated kinase (ERK)1/2, a well-defined downstream target of EGFR, was phosphorylated to a greater extent in EGFRvIII-expressing U87MG cells, as anticipated. EGFR mRNA was increased similarly in U87MG cells that expressed EGFRvIII or overexpressed wtEGFR, confirming the results of our immunoblotting studies (Fig. 2B).
Fig. 2.
VEGFR2 is increased in EGFRvIII-expressing U87MG GBM cells. (A) Cell extracts from parental, wtEGFR-overexpressing, and EGFRvIII-expressing U87MG cells were subjected to immunoblot analysis using the indicated antibodies. Blots were immunostained to detect tubulin as a loading control. (B) Total EGFR mRNA (wtEGFR + EGFRvIII) was determined by qPCR and standardized against the level present in parental cells (mean ± SEM, n = 3). (C) Immunoblot analysis to detect VEGFR2 was performed comparing parental, wtEGFR-overexpressing, and EGFRvIII-expressing U87MG cells. Each cell type is shown in duplicate. VEGFR2 signal intensity was standardized against actin and shown relative to that measured in parental cells (n = 3). (D) EGFRvIII-expressing U87MG cells were transfected with VEGFR2-specific (+) or NTC siRNA (−) for 24 h and subjected to immunoblot analysis to detect VEGFR2, phosphorylated ERK1/2 (P-ERK1/2), and total ERK1/2. (E) VEGFR2 mRNA was determined by qPCR and standardized against levels present in parental cells (mean ± SEM, n = 3, *P < .05). (F) Immunoblot analysis to detect PDGFRβ was performed comparing parental, wtEGFR-overexpressing, and EGFRvIII-expressing U87MG cells (in duplicate).
VEGFR2 protein was increased >3-fold (n = 3) in EGFRvIII-expressing U87MG cells, compared with wtEGFR-overexpressing U87MG cells or parental cells, as determined by immunoblot analysis and densitometry (Fig. 2C). To confirm the specificity of our antibody, we silenced VEGFR2 gene expression using siRNA in EGFRvIII-positive cells. Figure 2D shows that the 230-kDa band, corresponding to VEGFR2, was nearly completely eliminated by VEGFR2 gene silencing. Phospho-ERK1/2 was unchanged by VEGFR2 gene silencing, confirming the dominant activity of EGFRvIII in controlling this signaling factor.
VEGFR2 mRNA was increased more than 10-fold in EGFRvIII-expressing U87MG cells compared with parental and wtEGFR-overexpressing cells, as determined by qPCR (Fig. 2E). Because PDGFRβ expression correlated with EGFR expression in our TCGA analysis, we examined PDGFRβ by immunoblot analysis in the U87MG model system. Figure 2F shows that neither overexpression of wtEGFR nor EGFRvIII induced expression of PDGFRβ in U87MG cells. In fact, PDGFRβ was decreased in cells that expressed either form of EGFR.
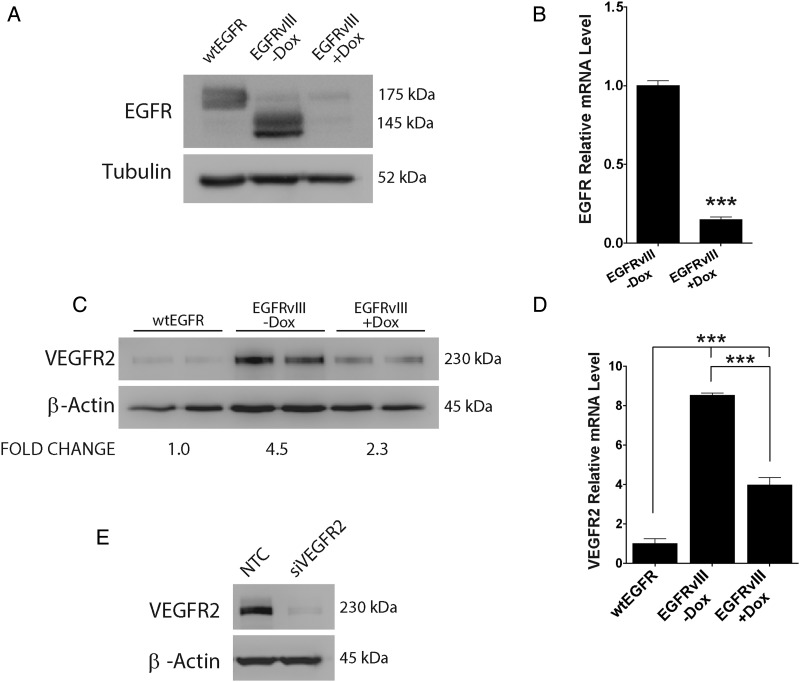
As a second GBM model system, we studied U373MG cells in which wtEGFR was overexpressed or EGFRvIII was expressed downstream of a Dox-repressible promoter.28 Figure 3A compares total EGFR protein expression. The U373MG cells that carry the regulated EGFRvIII promoter system were cultured in the presence or absence of Dox for 4 days to control EGFRvIII expression. Dox dramatically reduced EGFRvIII expression, as determined by immunoblot analysis, confirming the effectiveness of the promoter system. By qPCR, Dox treatment decreased EGFRvIII mRNA by about 80% (P < .001) (Fig. 3B).
Fig. 3.
EGFRvIII neutralization in U373MG GBM cells inhibits VEGFR2 expression. (A) U373MG cells expressing EGFRvIII under the control of a Dox-repressible promoter were treated with Dox (1 µg/mL) (+Dox) or vehicle (-Dox) for 4 days. Cell extracts were immunoblotted for EGFR. WtEGFR-overexpressing U373MG cells are shown for comparison. (B) Total EGFR mRNA (wtEGFR + EGFRvIII) was determined by qPCR in Dox treated (+Dox) EGFRvIII-expressing U373MG cells and standardized against the levels detected in cells that were not Dox treated (-Dox) (mean ± SEM, n = 3, ***P < .001). (C) Immunoblotting for VEGFR2 was performed to compare wtEGFR-overexpressing U373MG cells and EGFRvIII-expressing U373MG cells treated with Dox (+Dox) or vehicle (-Dox) for 4 days. Mean relative signal intensities for VEGFR2 are shown under the blot (n = 3). (D) VEGFR2 mRNA was determined by qPCR for EGFRvIII-expressing U373MG cells treated with Dox (+Dox) or vehicle (-Dox) for 4 days and standardized against the level detected in wtEGFR-overexpressing U373MG cells (mean ± SEM, n = 3, ***P < .001). (E) EGFRvIII-expressing U373MG cells were transfected with VEGFR2-specific or NTC siRNA for 24 h and subjected to immunoblot analysis to detect VEGFR2.
VEGFR2 protein was increased >4-fold in U373MG cells in which EGFRvIII was expressed compared with wtEGFR-overexpressing cells (Fig. 3C). When EGFRvIII-expressing cells were treated with Dox, VEGFR2 protein expression was decreased (Fig. 3C). Dox also significantly decreased expression of VEGFR2 mRNA (P < .001), as determined by qPCR (Fig. 3D). Figure 3E shows that VEGFR2 gene silencing nearly completely blocked detection of VEGFR2 in U373MG cells, once again confirming the specificity of our immunoblotting system. Although EGFRvIII significantly increased VEGFR2 expression in EGFRvIII-expressing U87MG cells and U373MG cells, the amount of VEGFR2 detected in these cells was still lower than that detected in extracts of human umbilical vein endothelial cells, which are frequently examined as a positive control for this RTK (Supplementary Fig. S2). The VEGFR2 detected in GBM cells did not reflect contamination by endothelial cells, since Tie2 was not observed in GBM cell extracts.
VEGFR2 Expression in GBM Cells Is Regulated by EGFR Signaling
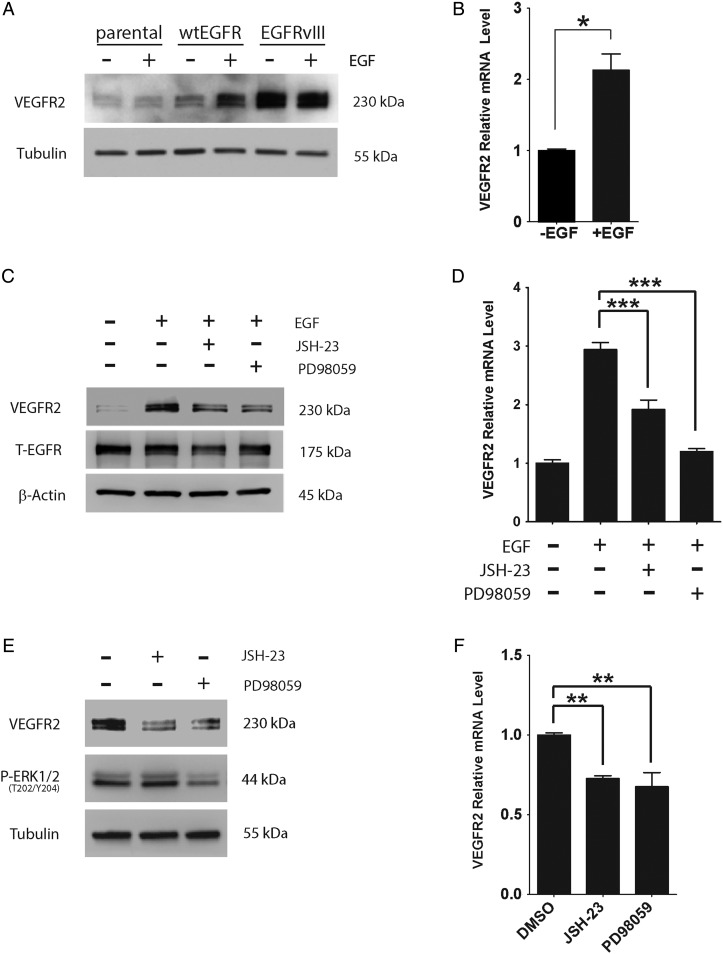
We hypothesized that VEGFR2 expression is increased in GBM cells as a consequence of activated EGFR signaling. To test this hypothesis, we treated wtEGFR-overexpressing U87MG cells with EGF (10 ng/mL). VEGFR2 protein levels were increased, as determined by immunoblot analysis (Fig. 4A). VEGFR2 mRNA was increased as well (P < .05) (Fig. 4B). EGF treatment also increased VEGFR2 protein (Fig. 4C) and mRNA (Fig. 4D) in wtEGFR-overexpressing U373MG cells. The EGF-induced increase in VEGFR2 was partially blocked by the mitogen/ERK (MEK) inhibitor PD98059 and by the nuclear factor–kappaB (NFκB) nuclear translocation inhibitor JSH-23.
Fig. 4.
EGFR signaling to MEK and NFκB controls VEGFR2 expression in GBM cells. (A) Parental, wtEGFR-overexpressing, and EGFRvIII-expressing U87MG cells were serum starved for 18 h and then incubated with EGF (10 ng/mL) (+) or vehicle (−) for 6 h. Immunoblotting was performed to detect VEGFR2. (B) VEGFR2 mRNA levels were determined in wtEGFR-overexpressing U87MG cells treated with EGF (+) or vehicle (−) by qPCR and standardized against vehicle-treated cells (mean ± SEM, n = 3, *P < .05). (C) WtEGFR-overexpressing U373MG cells were serum starved for 18 h and then pretreated for 15 min with either JSH-23 (10 µM), PD98059 (50 µM), or vehicle (dimethyl sulfoxide [DMSO]). The cells were subsequently treated with EGF (10 ng/mL) (+) or vehicle (−) for 6 h. Immunoblot analysis was performed to detect EGFR and VEGFR2. T-EGFR = Total not truncated EGFR. (D) WtEGFR-overexpressing U373MG cells were serum starved for 18 h and pretreated for 15 min with either JSH-23 (10 µM), PD98059 (50 µM), or vehicle and then treated with EGF (10 ng/mL) (+) or vehicle (−) for 6 h. VEGFR2 mRNA was determined by qPCR (mean ± SEM, n = 4, ***P < .001). (E) EGFRvIII-expressing U373MG cells were serum starved for 18 h and treated with either JSH-23 (10 µM), PD98059 (50 µM), or vehicle for 6 h. Immunoblot analysis was performed to detect VEGFR2, phospho-ERK1/2 (P-ERK1/2), and tubulin as a control for load. (F) EGFRvIII-expressing U373MG cells were serum starved for 18 h and treated with either JSH-23 (10 µM), PD98059 (50 µM), or vehicle (DMSO) for 6 h. VEGFR2 mRNA was determined by qPCR and standardized against the level present in vehicle-treated cells (mean ± SEM, n = 5, **P < .01).
Similar results were obtained when we studied EGFRvIII-expressing cells in the absence of EGF supplementation. JSH-23 and PD98059 significantly decreased VEGFR2 protein levels (Fig. 4E) and VEGFR2 mRNA levels (Fig. 4F) in EGFRvIII-expressing U373MG cells. Both ERK1/2 and NFκB are reported to be activated downstream of EGFRvIII.10,33
VEGFR2 Prevents Cellular Senescence in EGFRvIII-expressing GBM Cells
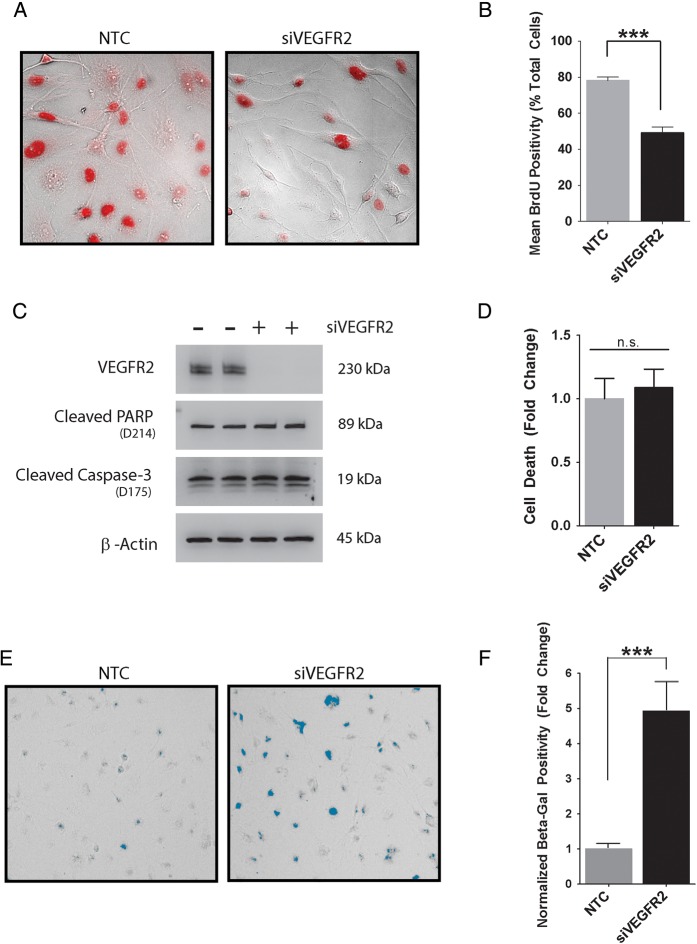
To determine whether VEGFR2 regulates growth or survival of GBM cells, we silenced the VEGFR2 gene in EGFRvIII-expressing U373MG cells. Control cells were transfected with NTC siRNA. BrdU incorporation was studied as an index of DNA synthesis and cell cycle progression. Figure 5A and B show that VEGFR2 gene silencing significantly decreases BrdU incorporation. VEGFR2 gene silencing did not increase apoptosis, as determined by measuring cleaved poly(ADP-ribose) polymerase (PARP) and activated/cleaved caspase-3 (Fig. 5C). We also probed for an increase in apoptosis using the Roche Cell Death ELISA Kit, which measures intracytoplasmic histone-associated DNA fragments. Figure 5D shows that silencing of VEGFR2 did not have an effect on cell death.
Fig. 5.
VEGFR2 gene silencing induces cellular senescence in EGFRvIII-expressing GBM cells. (A) EGFRvIII-expressing U373MG cells were transfected with VEGFR2-specific or NTC siRNA for 18 h prior to adding BrdU (100 μM) for 12 h. BrdU-positive cells (red) were detected by immunofluorescence microscopy (representative fields shown). (B) The percentage of BrdU-positive cells is shown for cells transfected with VEGFR2-specific or NTC siRNA (mean ± SEM, n = 4, ***P < .01). (C) EGFRvIII-expressing U373MG cells were transfected with VEGFR2-specific (+) or NTC siRNA for 24 h. Immunoblot analysis was performed using the indicated antibodies. Each condition is shown in duplicate. (D) EGFRvIII-expressing U373MG cells were transfected with VEGFR2-specific or NTC siRNA. Cell death was determined using the Cell Death Detection ELISA Plus Kit (mean ± SEM, n = 6, n.s., not statistically significant). (E) EGFRvIII-expressing U373MG cells were transfected with VEGFR2-specific or NTC siRNA. Senescence was determined by SA-β-Gal staining (blue). Cells were imaged by brightfield microscopy. Representative fields are shown. (F) The number of SA-β-Gal-positive cells after transfection with VEGFR2-specific or NTC siRNA was determined using ImageJ and normalized against that observed in cultures of NTC siRNA-transfected cells (mean ± SEM, n = 4, ***P < .001).
Next, we stained cells to detect SA-β-Gal, a well-accepted biomarker of cellular senescence. Figure 5E shows representative images of EGFRvIII-expressing U373MG cells transfected with NTC or VEGFR2-specific siRNA. As shown in Fig. 5F, VEGFR2 gene silencing induced a 4- to 5-fold increase in cells positive for SA-β-Gal (P < .001). Induction of senescence provides a plausible explanation for the decrease in DNA synthesis in VEGFR2 gene-silenced EGFRvIII-expressing U373MG cells.
VEGFR-specific Tyrosine Kinase Inhibition Induces Senescence in EGFRvIII-positive GBM Cells
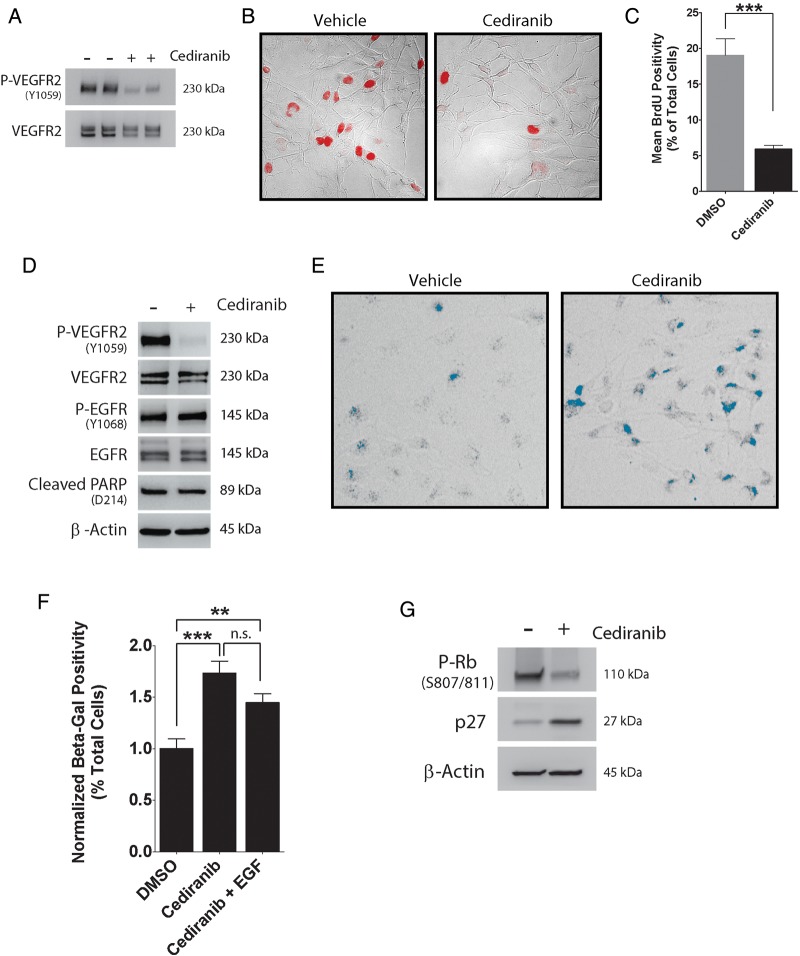
To further explore the role of VEGFR2 in preventing cellular senescence in GBM cells, we studied the VEGFR-specific tyrosine kinase inhibitor cediranib (AZD2171), which has been used to treat GBM in patients.34 Under basal cell culture conditions, VEGFR2 was at least partially activated in EGFRvIII-expressing U373MG cells, as determined by immunoblotting for VEGFR2 phospho-Tyr-1059 (Fig. 6A). Treating the cells with 5 µM cediranib for 24 h substantially decreased phospho-Tyr-1059.
Fig. 6.
Cediranib promotes cellular senescence in EGFRvIII-expressing GBM cells. (A) EGFRvIII-expressing U373MG cells were treated with cediranib (5 µM) (+) or vehicle (DMSO) (−) for 24 h and immunoblotted to detect phospho-VEGFR2 (P-VEGFR2 Y1059) and total VEGFR2. (B) EGFRvIII-expressing U373MG cells were treated with cediranib (5 µM) or vehicle for 24 h prior to adding BrdU (100 µM). BrdU-positive cells (red) were detected by immunofluorescence microscopy (representative fields are shown). (C) The percentage of BrdU-positive cells was determined for cediranib- or vehicle-treated cultures (mean ± SEM, n = 4, ***P < .01). (D) EGFRvIII-expressing U373MG cells were treated with cediranib (5 µM) or vehicle for 24 h. Immunoblot analysis was performed using the indicated antibodies. (E) EGFRvIII-expressing U373MG cells were treated with cediranib (5 µM) or vehicle. Senescence was determined by SA-β-Gal staining (blue). Representative fields are shown. (F) The percentage of SA-β-Gal-positive cells was determined, using ImageJ software, for cells treated with cediranib, cediranib + EGF (10 ng/mL), or vehicle (mean ± SEM, n = 4, **P < .01, ***P < .001, n.s., not statistically significant). (G) EGFRvIII-expressing U373MG cells were treated with cediranib (5 µM) or vehicle. Immunoblot analysis was performed using the indicated antibodies.
Cediranib inhibited DNA synthesis and cell cycle progression in EGFRvIII-expressing U373MG cells, as determined by BrdU incorporation (Fig. 6B and C) (P < .001). Cleaved PARP was not increased in cediranib-treated cells, once again providing evidence that neutralizing VEGFR2 does not promote GBM cell apoptosis (Fig. 6D). Cediranib did not effect EGFRvIII phosphorylation at Tyr-1068. By contrast, cediranib increased the percentage of SA-β-Gal-staining cells, as shown in representative images (Fig. 6E) and in summary form in Fig. 6F (P < .001). Addition of EGF did not significantly offset the effects of cediranib on induction of senescence as determined by SA-β-Gal staining.
Our cediranib and VEGFR2 gene-silencing studies suggested that neutralizing VEGFR2 activity in EGFRvIII-expressing GBM cells may induce cellular senescence. To further test this hypothesis, we examined phosphorylation of Rb protein at Ser-807/811, which is known to promote transition into S phase.35 Phospho-Ser-807/811 was decreased in cediranib-treated cells (Fig. 6G), supporting the hypothesis that this drug induces cellular senescence. We also observed an increase in p27Kip1, a CDKI expressed at increased levels in senescent cells.19,20
Discussion
EGFR gene amplification and EGFRvIII are known drivers of an aggressive phenotype in GBM.2,5,7 We have demonstrated that tumor cells with activated EGFR signaling express increased levels of VEGFR2. The increase in VEGFR2 expression potentiates this RTK as a regulator of GBM cell physiology. Our results suggest that VEGFR2 synergizes with activated EGFR to promote cell cycle progression and avoid cellular senescence in GBM cells. When VEGFR2 is silenced, a significant fraction of the GBM cells become senescent. Similarly, targeting VEGFR2 with the specific tyrosine kinase inhibitor cediranib induces cellular senescence that cannot be rescued with exogenous EGF. The function of VEGFR2 in EGFRvIII-expressing GBM cells may help explain the aggressive nature of these cells despite the fact that the tyrosine kinase activity of EGFRvIII is attenuated compared with EGF-ligated wtEGFR.6
In clinical trials, cediranib has demonstrated efficacy in patients with recurrent GBM by decreasing overall tumor burden, normalizing tumor vasculature, decreasing vasogenic edema, and improving cerebral perfusion.36,37 An increase in overall survival also has been observed. Serial MR spectroscopy studies demonstrated that cediranib has a direct effect on GBM cells by increasing the N-acetylaspertase to choline ratio, a biomarker of decreased cellular metabolism.38 These changes are consistent with cediranib causing TIS in GBM cells, as described here, in addition to its effects on tumor vasculature.
Cellular senescence occurs secondary to different stimuli, including dysfunctional telomeres, DNA damage, chromatin alterations, and generalized cell stress.19 Cell-signaling proteins implicated in cellular senescence include p53, Rb, and the CDKIs p16Ink4a, p21Cip1a, and p27Kip1. Cellular pathways that induce senescence are thought to be activated in premalignant conditions, such as colonic adenoma.21,22 In these lesions, overriding senescence may be essential for malignant transformation.21,22 TIS is a form of cellular senescence induced in cancer cells by cytotoxic and targeted drug therapies.20,21 Previous studies have suggested that targeting VEGF signaling may induce TIS in colon cancer cells39 and renal cell carcinoma cells.40 In our GBM model system, evidence that cediranib induced TIS included increased SA-β-Gal staining, increased p27Kip protein expression, and decreased phosphorylation of Rb at Ser-807 and 811. We also observed a significant decrease in DNA synthesis, as determined by BrdU labeling, in the absence of apoptosis. Because we conducted experiments with homogeneous cell culture model systems, we can be certain that the effects of cediranib were due to direct interactions with the GBM cells and not an indirect consequence of targeting endothelial cells. Evidence for decreased proliferation and cellular senescence was also obtained when the VEGFR2 gene was silenced. These results suggest that in GBM receiving VEGFR2-directed chemotherapy, the cancer cells may be targets, in addition to endothelial cells.
The assumption that cellular senescence is associated with irreversible replicative arrest and resistance to apoptosis is important in the context of this study and TIS in general. The enhanced ability of senescent cancer cells to survive is evident when these cells are subjected to radiation or chemotherapy drugs in the alkylating agent family, which target dividing cells.20,22 Such therapies are commonly utilized in treating GBM and may be used in conjunction with VEGFR-targeting drugs.41 Our studies suggest that by inducing senescence, VEGFR-targeting drugs may inhibit the response to cytotoxic therapies. Testing this hypothesis is an important future goal. If TIS secondary to VEGFR-targeting drugs permits survival of cancer cells that would have otherwise been killed by cytotoxic therapies, then the irreversible nature of replicative arrest becomes increasingly critical. Gene products such as urokinase-type plasminogen activator and its receptor, uPAR, have been implicated in the release of tumor cells from states of replicative dormancy.42 Their function in TIS induced by VEGFR-targeting drugs remains to be determined.
Our decision to focus on the activity of VEGFR2 in EGFRvIII-expressing GBM cells was based on an analysis of TCGA data. We confirmed that VEGFR2 is upregulated downstream of EGFRvIII in multiple model systems, including a human GBM xenograft model.27 Our hypothesis that VEGFR2 is upregulated in EGFRvIII-expressing cells due to activation of EGFR-dependent cell signaling was supported by studies with pharmacologic inhibitors of the MEK-ERK1/2 pathway and NFκB signaling. In addition, we showed that treating wtEGFR-overexpressing GBM cells with EGF upregulates VEGFR2 expression. These results suggest that VEGFR2 expression in vivo may be controlled not only by EGFRvIII but also by wtEGFR, when EGF is abundant in the tumor microenvironment.
The cytoplasmic tail of VEGFR2 includes multiple Tyr residues that are phosphorylated upon receptor activation and serve as docking sites for cell-signaling proteins implicated in cell cycle progression, including phosphatidylinositol-3 kinase,43 protein tyrosine kinase 2 /focal adhesion kinase 1,44 mesenchymal epithelial transition factor,45 Src family kinases,46 phospholipase-C1γ,47 and Cdc42.48 Some of these cell-signaling proteins are activated downstream of EGFR as well. The cell-signaling factors activated selectively downstream of VEGFR2 to inhibit induction of cellular senescence in GBM cells remain to be determined. These signaling factors may also be candidate targets for inducing senescence as an approach to treating cancer.
Supplementary Material
Funding
This work was supported by NIH grant R01 CA169096 (S.L.G.).
Supplementary Material
Acknowledgments
The authors thank Drs Webster K. Cavenee and Frank B. Furnari for kindly providing glioma cell lines. We thank Dr Jingjing Hu for assistance with experiments.
Conflict of interest statement. None declared.
References
- 1.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353(8):811–822. [DOI] [PubMed] [Google Scholar]
- 2.Heimberger AB, Hlatky R, Suki D et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11(4):1462–1466. [DOI] [PubMed] [Google Scholar]
- 3.Brennan CW, Verhaak RGW, McKenna A et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugawa N, Ekstrand AJ, James CD et al. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci U S A. 1990;87(21):8602–8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishikawa R, Ji XD, Harmon RC et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91(16):7727–7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su Huang HJ, Nagane M, Klingbeil CK et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272(5):2927–2935. [DOI] [PubMed] [Google Scholar]
- 7.Shinojima N, Tada K, Shiraishi S et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–6970. [PubMed] [Google Scholar]
- 8.Nishikawa R, Sugiyama T, Narita Y et al. Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumor Pathol. 2004;21(2):53–56. [DOI] [PubMed] [Google Scholar]
- 9.Gilder AS, Jones KA, Hu J et al. Soluble uPAR is released selectively by glioblastoma cells that express EGF receptor variant III and promotes tumor cell migration and invasion. J Biol Chem. 2015;290:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, Jo M, Cavenee WK et al. Crosstalk between the urokinase-type plasminogen activator receptor and EGF receptor variant III supports survival and growth of glioblastoma cells. Proc Natl Acad Sci U S A. 2011;108(38):15984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front Biosci. 2006;11:818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. [DOI] [PubMed] [Google Scholar]
- 13.Goodman VL, Rock EP, Dagher R et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13(5):1367–1373. [DOI] [PubMed] [Google Scholar]
- 14.Kaley TJ, Wen P, Schiff D et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17(1):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman CK, Kim J, Wong WL et al. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4(1):121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll RS, Zhang J, Bello L et al. KDR activation in astrocytic neoplasms. Cancer. 1999;86(7):1335–1341. [DOI] [PubMed] [Google Scholar]
- 17.Knizetova P, Ehrmann J, Hlobilkova A et al. Autocrine regulation of glioblastoma cell cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle. 2008;7(16):2553–2561. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimoto K, Ma X, Guan Y et al. Expression of stem cell marker and receptor kinase genes in glioblastoma tissue quantified by real-time RT-PCR. Brain Tumor Pathol. 2011;28(4):291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. [DOI] [PubMed] [Google Scholar]
- 20.Ewald JA, Desotelle JA, Wilding G et al. Therapy-induced senescence in cancer. J Natl Cancer Inst. 2010;102(20):1536–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer 2010;10(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon RR, Nelson PS. Cellular senescence and cancer chemotherapy resistance. Drug Resist Updat. 2012;15:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerami E, Gao J, Dogrusoz U et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J, Aksoy BA, Dogrusoz U et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kastenhuber ER, Huse JT, Berman SH et al. Quantitative assessment of intragenic receptor tyrosine kinase deletions in primary glioblastomas: their prevalence and molecular correlates. Acta Neuropathol. 2014;127(5):747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkaria JN, Yang L, Grogan PT et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6(3):1167–1174. [DOI] [PubMed] [Google Scholar]
- 28.Mukasa A, Wykosky J, Ligon KL et al. Mutant EGFR is required for maintenance of glioma growth in vivo, and its ablation leads to escape from receptor dependence. Proc Natl Acad Sci U S A. 2010;107(6):2616–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joensuu H, Puputti M, Sihto H et al. Amplification of genes encoding KIT, PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J Pathol. 2005;207(2):224–231. [DOI] [PubMed] [Google Scholar]
- 30.Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro Oncol. 2005;7(4):436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkaria JN, Carlson BL, Schroeder MA et al. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin Cancer Res. 2006;12(7):2264–2271. [DOI] [PubMed] [Google Scholar]
- 32.Andreev J, Galisteo ML, Kranenburg O et al. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem. 2001;276(23):20130–5. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Babic I, Nathanson D et al. Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011;1(6):524–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batchelor TT, Mulholland P, Neyns B et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dick FA, Rubin SM. Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol. 2013;14(5):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wedge SR, Kendrew J, Hennequin LF et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65(10):4389–4400. [DOI] [PubMed] [Google Scholar]
- 37.Batchelor TT, Gerstner ER, Emblem KE et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A. 2013;110(47):19059–19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Catana C, Ratai E-M et al. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 2011;71(11):3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasan MR, Ho SHY, Owen DA et al. Inhibition of VEGF induces cellular senescence in colorectal cancer cells. Int J Cancer. 2011;129(9):2115–2123. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Xu L, Zhang J et al. Sunitinib induces cellular senescence via p53/Dec1 activation in renal cell carcinoma cells. Cancer Sci. 2013;104(8):1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wachsberger PR, Lawrence RY, Liu Y et al. Cediranib enhances control of wild type EGFR and EGFRvIII-expressing gliomas through potentiating temozolomide, but not through radiosensitization: implications for the clinic. J Neurooncol. 2011;105(2):181–190. [DOI] [PubMed] [Google Scholar]
- 42.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147(1):89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanes MG, Oubaha M, Rautureau Y et al. Phosphorylation of tyrosine 801 of vascular endothelial growth factor receptor-2 is necessary for Akt-dependent endothelial nitric-oxide synthase activation and nitric oxide release from endothelial cells. J Biol Chem. 2007;282(14):10660–9. [DOI] [PubMed] [Google Scholar]
- 44.Dougher M, Terman BI. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene. 1999;18(8):1619–1627. [DOI] [PubMed] [Google Scholar]
- 45.Lu KV, Chang JP, Parachoniak CA et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer RD, Sacks DB, Rahimi N. IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PLoS One. 2008;3(12):e3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi T, Shibuya M. The 230kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 1997;14(17):2079–2089. [DOI] [PubMed] [Google Scholar]
- 48.Lamalice L, Houle F, Jourdan G et al. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene. 2004;23(2):434–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.