Abstract
Background
Patients with WHO grade II glioma may respond to chemotherapy that is currently not standardized regarding timing and treatment duration. Metabolic changes during chemotherapy may precede structural tumor volume reductions. We therefore compared time courses of amino acid PET and MRI responses to temozolomide (TMZ) and assessed whether responses correlated with seizure control and progression-free survival (PFS).
Methods
PET and MRI were performed before and during TMZ chemotherapy. Tumor volumes were calculated using regions-of-interest analysis. Amino acid uptake was also quantified as metabolically active tumor volume and tumor-to-cerebellum uptake ratio.
Results
One hundred twenty-five PET and 125 MRI scans from 33 patients were analyzed. Twenty-five patients showed metabolic responses that exhibited an exponential time course with a 25% reduction of the active volume on average after 2.3 months. MRI responses followed a linear course with a 25% reduction after 16.8 months. Reduction of metabolically active tumor volumes, but not reduction of PET uptake ratios or MRI tumor volumes, correlated with improved seizure control following chemotherapy (P = .012). Receiver-operating-characteristic curve analysis showed that a decrease of the active tumor volume of ≥80.5% predicts a PFS of ≥60 months (P = .018) and a decrease of ≥64.5% a PFS of ≥48 months (P = .037).
Conclusions
Amino acid PET is superior to MRI for evaluating TMZ responses in WHO grade II glioma patients. The response delay between both imaging modalities favors amino acid PET for individually tailoring the duration of chemotherapy. Additional studies should investigate whether this personalized approach is appropriate with regard to outcome.
Keywords: chemotherapy, epilepsy, low-grade glioma, MRI, PET
Diffuse cerebral WHO grade II gliomas often present with epileptic seizures and show insidious tumor growth.1 The natural history and response to treatment of these tumors are predominantly determined by genetic alterations such as co-deletion on chromosomal arms 1p and 19q, isocitrate dehydrogenase 1 (IDH1/2) mutation, and methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter.2,3
Alkylating agent chemotherapy is effective in patients with WHO grade II glioma. Once given at tumor progression or recurrence, it yields objective response rates up to 60% and response durations of several years.4 In addition, chemotherapy can reduce the frequency of seizures. However, the evaluation of chemotherapy response based on MRI with T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI sequences is difficult.5 Accordingly, a reduction in seizure frequency has been proposed as a surrogate marker for a clinical benefit from chemotherapy.6,7 There is no current standard for the optimal drug choice, timing, or duration of chemotherapy. The European Organization for Research and Treatment of Cancer trial 22033-26033 prescribed 12 cycles of temozolomide (TMZ) chemotherapy, while other groups treated patients as long as MRI showed at least evidence of stable disease.4 Interestingly, tumors may continue to shrink on MRI even after termination of chemotherapy.8,9 We have previously reported WHO grade II glioma patients who experienced rapid metabolic deactivation in response to TMZ, which was confirmed by using PET with the amino acid O-(2-[18F]fluoroethyl)-L-tyrosine (FET).10 Our current study aimed to investigate metabolic responses in a large multicenter participant cohort treated with TMZ. We also assessed whether metabolic responses on PET correlate with seizure control and progression-free survival (PFS).
Materials and Methods
Clinical Data
Participants were retrospectively identified according to the following criteria: (i) supratentorial cerebral WHO grade II glioma at progression after surgery; (ii) no contrast enhancement on T1-weighted MRI; (iii) no previous radio- or chemotherapy; (iv) measurable disease on T2-weighted MRI before chemotherapy with 2 perpendicular diameters ≥10 mm; and (v) MRI and amino acid PET schedule including baseline evaluation and at least 2 examinations during and at the end of chemotherapy. Participant data were extracted from the hospital medical records and included demographic as well as tumor and treatment characteristics, PFS, and overall survival (OS). Seizure frequencies before chemotherapy were documented during postsurgical surveillance in every 3 months. Seizure frequencies were recorded monthly during chemotherapy. We defined seizure control groups during chemotherapy as: group I = 0–50% seizure frequency reduction and group II = >50% reduction or seizure free.
Chemotherapy
TMZ chemotherapy was administered according to local policies (Table 1). Adverse effects were scored according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4.0).
Table 1.
Clinical data, histology and molecular genetic status, imaging response, and survival
| ID | Age (y) | Histologya WHO II | Extent of surgery | IDH1a | LOH 1p19qa | MGMTa | TMZ dose (mg) | PETb | MRIb | PFS (mo) | OS (mo) | Histologyc (WHO grade) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | A | b | mut | no | – | 20'580 | R (−70) | MR (−31) | 27 | 31 | n.d. |
| 2 | 49 | A | p | – | – | – | 67'620 | R (−56) | SD (−4) | 24 | 31 | n.d. |
| 3 | 35 | A | p | mut | no | m | 29'400 | R (−21) | MR (−25) | 38 | 75 | n.d. |
| 4 | 23 | A | b | wt | no | m | 70'560 | R (−94) | SD (−9) | 19 | 21 | GBM (IV) |
| 5 | 38 | A | b | wt | no | u | 26'880 | n.a.v. | SD (−1) | 40 | 61 | GBM (IV) |
| 6 | 32 | A | p | mut | no | u | 20'160 | NR (+354) | SD (+18) | 13 | 99+ | A (II) |
| 7 | 33 | OA | p | mut | no | – | 45'360 | n.a.v. | PD (+29) | 16 | 27+ | n.d. |
| 8 | 38 | OA | p | mut | yes | m | 61'740 | R (−73) | MR (−40) | 34 | 51+ | n.d. |
| 9 | 28 | OA | p | mut | no | m | 30'500 | R (−94) | MR (−39) | 66 | 119+ | OA (II) |
| 10 | 27 | OA | p | mut | yes | m | 81'600 | R (−69) | SD (−10) | 20 | 44 | AA (III) |
| 11 | 20 | OA | b | mut | no | m | 23'100 | NR (−8) | SD (+0) | 6 | 61+ | OA (II) |
| 12 | 31 | OA | p | mut | no | m | 58'100 | R (−99) | SD (−21) | 73 | 125+ | n.d. |
| 13 | 40 | OA | b | wt | no | u | 53'200 | NR (−6) | SD (−20) | 66 | 139+ | n.d. |
| 14 | 45 | O | b | – | – | – | 37'800 | R (−12) | PR (−67) | 45 | LFU | n.d. |
| 15 | 29 | O | gt | – | – | – | 24'500 | R (−91) | MR (−48) | 17+ | 17+ | n.d. |
| 16 | 41 | O | gt | – | – | – | 10'780 | R (−62) | MR (−34) | 28 | 63+ | O (III) |
| 17 | 43 | O | gt | – | – | – | 8'250 | R (−98) | SD (−4) | 61+ | 61+ | n.d. |
| 18 | 36 | O | b | – | – | – | 13'000 | R (−29) | SD (+2) | 6 | 28+ | n.d. |
| 19 | 41 | O | b | – | – | – | 37'080 | NR (+116) | SD (+3) | 24 | 44+ | n.d. |
| 20 | 40 | O | gt | mut | yes | m | 43'900 | R (−82) | PR (−76) | 61 | 67+ | n.d. |
| 21 | 34 | O | b | mut | yes | m | 36'960 | R (−65) | PR (−61) | 84 | 118+ | n.d. |
| 22 | 44 | O | p | mut | yes | m | 43'680 | R (−91) | PR (−57) | 81+ | 81+ | n.d. |
| 23 | 34 | O | gt | mut | yes | m | 29'820 | R (−98) | PR (−53) | 66+ | 66+ | n.d. |
| 24 | 51 | O | p | wt | no | m | 17'640 | R (−64) | MR (−42) | 23 | 53 | n.d. |
| 25 | 38 | O | p | mut | yes | m | 17'640 | R (−72) | MR (−40) | 36 | 61+ | n.d. |
| 26 | 45 | O | p | mut | yes | m | 26'420 | R (−67) | SD (−8) | 10 | 68+ | n.d. |
| 27 | 37 | O | p | mut | no | m | 24'000 | R (−37) | SD (+1) | 7 | 74+ | O (III) |
| 28 | 51 | O | p | mut | yes | m | 108'000 | n.a.v. | SD (+2) | 47+ | 47+ | n.d. |
| 29 | 43 | O | gt | mut | yes | m | 85'600 | NR (−6) | SD (+5) | 6 | 8+ | n.d. |
| 30 | 53 | O | p | mut | yes | m | 42'300 | R (−56) | SD (+6) | 52+ | 52+ | n.d. |
| 31 | 29 | O | p | mut | yes | m | 37'800 | R (−59) | MR (−42) | 72+ | 72+ | n.d. |
| 32 | 39 | O | p | mut | no | m | 12'750 | R (−27) | SD (−19) | 47 | 73+ | O (II) |
| 33 | 34 | O | p | mut | yes | u | 33'500 | R (−79) | SD (+9) | 16 | 57+ | n.d. |
Abbreviations:
Histologya WHO grade II: A, astrocytoma; O, oligodendroglioma; OA, oligoastrocytoma; AA, anaplastic astrocytoma; n.d., not done.
Extent of surgery: b, biopsy; gt, gross total resection; p, partial resection.
IDH1, isocitrate dehydrogenase 1; mut, mutated; wt, wild type; –, no tissue available.
LOH, co-deletion on chromosomal arms 1p and 19q; no, absent; yes, present; –, no tissue available.
MGMT, O6-methylguanine-DNA methyltransferase; m, methylated; u, unmethylated; –, no tissue available.
PET: n.a.v., no active tumor volume; NR, nonresponder; R, responder.
MRI (RANO): MR, minor response; PD, progressive disease; PR, partial response; SD, stable disease.
PFS, progression-free survival; +, progression-free at end of follow-up.
OS, overall survival; LFU, lost during follow-up; +, alive at end of follow-up.
Histologyc (WHO grade): AA, anaplastic astrocytoma; A, astrocytoma; GBM, glioblastoma; n.d., not done; O, oligodendroglioma; OA, oligoastrocytoma.
alast operation before start of chemotherapy.
bnumber in bracket = maximal volume decrease (−) or increase (+) in percent from baseline.
cafter progression following chemotherapy.
Imaging Data Acquisition
Baseline imaging was performed within one month before chemotherapy initiation, and follow-up imaging started between 2 and 6 months thereafter. MRI examinations included T2-weighted and gadolinium-enhanced T1-weighted sequences according to clinical routine protocols on 1.5 or 3.0 Tesla MRI scanners. PET data were acquired after intravenous administration of 150–240 MBq FET (Switzerland, Austria, Germany) or ∼650 MBq L-[methyl-11C]methionine (MET, Italy). Grosu et al reported that both tracers yield virtually identical amino acid uptake values in gliomas.11 Their whole cohort of patients with WHO grade II-IV gliomas showed mean tumor-to-brain uptake ratios of 2.5 for FET and of 2.6 for MET, which represent a difference of 4%. Uptake ratios differed by 1% for the subgroup of WHO II gliomas.11 We therefore consider both MET and FET suitable to comparably characterize low-grade gliomas on amino acid PET. Static emission scans were acquired at the following time periods after tracer administration: 40–60 minutes (Switzerland; Discovery LS, GE Medical Systems), 30–45 minutes (Austria; Advance, GE Healthcare), 20–40 minutes (Germany; ECAT Exact HR+, Siemens), and 30–45 minutes (Italy; Discovery LS, GE Healthcare). After correction for random and scattered coincidences and dead time, images were reconstructed with the specific scanner software. The reconstructed image resolution was ∼5.5 mm.
Image Data Analysis, Determination of Response, and Progression
Response assessment on PET and MRI was centrally performed by M.Wy. (nuclear medicine) and U.R. and N.G. (neuro-oncologists) with long-standing experience in PET and MRI analysis. Each participant served as his or her own control, and values during chemotherapy were expressed as percent from baseline (ie, before the start of chemotherapy). Quantification of PET and MRI data was performed on co-registered images using PMOD (PMOD Technologies Ltd).12 MRI tumor volumes were measured on T2-weighted axial sequences by manually outlining regions-of-interest (ROIs) around tissue exhibiting T2-hyperintensity. Finally, all tumor-containing slices were summed. Values during chemotherapy were expressed as percent change from baseline MRI before the initiation of chemotherapy. Response was also determined according to the Response Assessment in Neuro-Oncology (RANO) criteria, which quantify the product of the perpendicular tumor diameters on the slice showing the largest tumor area (mm2).5 The RANO criteria categorize size reductions between 25% and 50% as minor response, ≥50% as partial response, and disappearance of the lesion on T2–weighted or FLAIR MR imaging as complete response. Objective response is defined as the sum of minor response, partial response, and complete response. Size increases ≥25% were scored as progressive disease. Stable disease was defined when MRI changes did not qualify for progression or for complete, partial, or minor response. We also diagnosed progression if new contrast enhancement on T1-weighted sequences or clinical signs (eg, recurrence or new onset of seizures) were accompanied by tumor size increase <25%.
Amino acid uptake was also quantified using ROI analysis. The cerebellum served as reference region. ROIs covering the whole cerebellum were drawn for each slice. Counts of all cerebellar ROIs were then averaged to produce the mean cerebellar amino acid uptake. Three measures were used for tumors. First, the metabolically active volume (cm3) was calculated as the tumor volume containing pixels >110% of the mean cerebellar amino acid uptake. We derived this threshold from our earlier methodological study.13 It corresponds well with the visual impression of active WHO grade II gliomas on PET.14 Second, the tumor amino acid uptake (counts) was determined by placing a ROI over the tumor on the PET slice with the largest extent, which was normalized to the mean cerebellar uptake (mean T-to-CBL ratio, unit-less). Third, peak uptake ratios were calculated by averaging all counts from tumor voxels that exhibited ≥95% of the maximum tumor radioactivity. In participants without active tumor volume, ROIs were placed on the T2-weighted MR images and then transferred to the corresponding PET planes. In line with our previous study,10 we defined a metabolic treatment response as a reduction of ≥10% from the initial tumor volume (active PET volume) on at least 2 subsequent time points at least 4 weeks apart after the initiation of chemotherapy.
Molecular Genetics
1p/19q co-deletion was assessed by fluorescence in situ hybridization and MGMT promoter methylation by methylation-specific PCR. IDH1 status was determined by immunohistochemistry or gene sequencing.
Statistics
We used descriptive statistics to characterize the patient population. To test group-to-group differences, the Mann-Whitney U test for independent samples was applied. The association between baseline imaging parameters and PFS was assessed using the Spearman rank test. The prognostic value of the metabolically active tumor volume on PFS was assessed by receiver-operating-characteristic (ROC) curve analyses. Participants were divided into responders (PFS ≥12, 24, 36, 48, or 60 months) and nonresponders (PFS <12, 24, 36, 48, or 60 months). Decision cutoff was considered optimal when the product of paired values for sensitivity and specificity reached its maximum. Moreover, we determined the area under the ROC curve (AUC), its standard error, and level of significance as measures of diagnostic quality. In addition, we used uni- and multivariate analyses including logistic regression to test for associations of histology, PET and MRI response, and total dose of TMZ as well as 1p/19q co-deletion, IDH1, and MGMT status with PFS. PFS and OS were calculated from the start of chemotherapy. Analyses were performed using SigmaStat software (version 3.5; Systat Software, Inc.).
Ethics
Amino acid PET examinations are part of routine clinical investigations in WHO grade II glioma participants in Austria, Italy, Switzerland, and Germany. All participants gave written informed consent before each FET or MET PET investigation. Signed consent is not required to perform clinical PET scans in Switzerland. The local ethics committee of Innsbruck Medical University approved this retrospective study. In Italy, local ethics committee approval or patients' signed consent is not required for retrospective data evaluation.
Results
Thirty-three participants with WHO grade II astrocytoma (n = 6), oligodendroglioma (n = 20), or oligoastrocytoma (n = 7) were enrolled (21 male and 12 female, mean age: 37 ± 8 [SD] y, range: 20–53 y). Prior to chemotherapy, 29 participants underwent one, and 4 participants underwent 2 surgical procedures (gross total resection in 6, partial resection in 18, biopsy in 9 participants, Table 1). During the entire study, no participant received steroids, and no participant was treated with either previous radiotherapy or PCV (procarbazine-lomustine-vincristine) chemotherapy.
Chemotherapy
TMZ chemotherapy started 40 ± 36 months (range: 1–126 mo) after the last operation because of radiological progression. Chemotherapy was completed as scheduled in 17 participants and was interrupted because of participant request, toxicity, or progression in 1, 7, and 8 participants, respectively. Hematological adverse effects of CTCAE grades 1, 2, or 3 were noted in 5, 3, and 8 participants, respectively. Nonhematological adverse effects consisted of nausea in 8 participants (grades 1–3), fatigue in 6 (grades 2-3), anorexia in 2 (grades 1-2), pruritus in 2 (grade 2), and local skin infection/zoster in 2 (grades 2-3). No secondary neoplasias were observed after a median follow- up of 74 months.
Imaging
All participants from Italy were imaged with MET-PET (n = 9). Participants from all other countries were imaged with FET-PET (n = 24). One-hundred twenty-five PET scans and 125 MRI scans were available for evaluation. On MRI, 14 participants achieved an objective response (9 minor responses, 5 partial responses) (Table 1). Baseline tumor volumes on T2-weighted MRI ranged from 4.3–450.3 cm3 (75.7 ± 103.9 cm3). Among those 30 participants with baseline active tumor defined by PET, volumes ranged from 1.4–269.9 cm3 (51.2 ± 51.2 cm3). In line with previous work,11 we found similar baseline mean tumor uptake ratios for MET (1.43 ± 0.27) and FET (1.42 ± 0.20). For histological subtypes, the following numbers were found: T-to-CBL ratios were 1.43 ± 0.24 in oligodendrogliomas, 1.43 ± 0.27 in oligoastrocytomas, and 1.28 ± 0.14 in astrocytomas. Peak T-to-CBL ratios were 2.34 ± 2.29 in oligodendrogliomas, 2.49 ± 1.99 in oligoastrocytomas, and 1.80 ± 1.58 in astrocytomas. We found no active tumor volume at baseline in 3 participants. In these patients, the median T-to-CBL ratio was 1.04 ± 0.02.
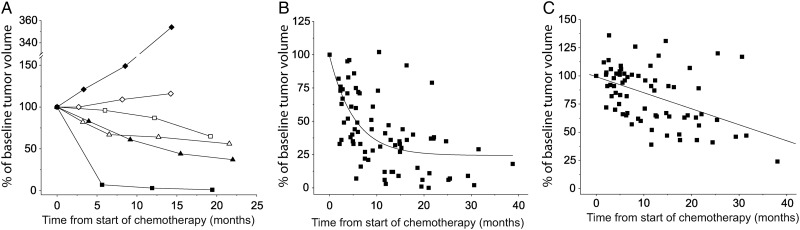
We identified 25 metabolic responders and 5 nonresponders. Examples of individual PET and MRI responses are presented in Figure 1A. Metabolic responses during chemotherapy were best described by an exponential time course, which yielded a reduction of 25% after 2.3 months (Fig. 1B). In contrast, MRI responses on T2-weighted images of the same participants were delayed, followed a linear decrease, and showed a volume reduction of 25% at 16.8 months after initiation of chemotherapy (Fig. 1C). During chemotherapy, the mean and peak T-to-CBL ratios in PET responders were reduced in 7 participants by 10%–21%. The remaining participants showed decreases of <7%, most likely indicating that successful chemotherapy reduced the spatial extent of tumor burden (ie, the active tumor volume) but not the histology or WHO tumor grade. In contrast to the responses of the active tumor volume, T-to-CBL ratios did not follow a linear or exponential fit (data not shown).
Fig. 1.
Time course of imaging responses. (A) Examples of individual responses as measured with PET (filled symbols) and MRI (open symbols). Square: oligoastrocytoma (#12); triangle: oligodendroglioma (#16); diamond: astrocytoma (#6). (B and C) Data points represent pooled volume changes measured for the whole cohort of metabolic responders during chemotherapy before progression (ie, participants were censored at the last PET or MRI prior to progression. Black lines correspond to exponential fit for PET data (B) (y = 74.61 * exp(−x/158.86) + 24.31) and linear fit for MRI data (C) (y = 99.27–0.05 * x), respectively. Values on the y-axis represent percent volume changes compared with baseline (ie, before the start of chemotherapy).
In PET, nonresponding participants' active tumor volumes increased within 6 months up to 354% from baseline. The mean T-to-CBL ratios increased by 7%–11%, and the peak T-to-CBL ratios increased by 14%–36%. All tumors without active volume remained metabolically inactive during chemotherapy, and T-to-CBL ratios remained in a range between 96% and 103% from baseline.
Molecular Genetic Data
Information on IDH1 status and LOH1p/19q was available for 26 participants each, and MGMT promoter methylation was available for 24 participants (Table 1). Twenty-two (85%) tumors showed IDH1 mutation, 13 (50%) had 1p/19q co-deletion, and 20 (83%) had MGMT promoter methylation. PET responders exhibited IDH1 mutation in 17 (77%) participants, 1p19q co-deleted tumors in 11 (85%) participants, and MGMT promoter methylation in 17 (85%) participants.
Seizures
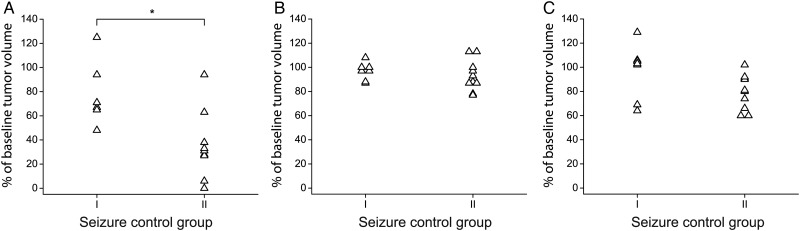
Twenty-two participants presented with seizures before chemotherapy. Tumor size on MRI, active PET tumor volume, and tumor uptake ratios at baseline were balanced between the 2 seizure response groups (Table 2). During chemotherapy, antiepileptic drug doses were changed to account for low drug serum concentration or for persisting seizures in 5 participants, and were stable in 17 participants. No participant experienced an increase in seizure frequency during chemotherapy. Nine participants experienced seizure frequency reductions by 0%–50% and 6 participants by >50%, while 7 participants were seizure-free. Among the 17 participants under stable antiepileptic drug doses, TMZ reduced the active tumor volumes by 22% ± 27% in group I participants and by 64% ± 28% in group II participants (Fig. 2A, P = .012, Mann-Whitney test). In contrast, neither T-to-CBL ratio changes nor volume changes on T2-weighted MRI images corresponded with seizure frequency reductions (Fig. 2B and C).
Table 2.
Baseline characteristics according to seizure frequency reduction groups
| Group I | Group II | |
|---|---|---|
| n | 9 | 13 |
| Tumor localization | ||
| Frontal (n) | 4 | 5 |
| Temporal (n) | 0 | 6 |
| Parietal (n) | 5 | 2 |
| Tumor size on MRIa | 57 ± 96 | 60 ± 57 |
| Active PET tumor volumea | 51 ± 90 | 47 ± 40 |
| Mean T-to-CBL uptake ratio | 1.34 ± 0.32 | 1.40 ± 0.16 |
| Peak T-to-CBL uptake ratio | 2.37 ± 1.40 | 2.30 ± 0.48 |
Patient groups refer to the maximal percent reduction of seizure frequency during chemotherapy: group I = 0–50%, II = 51–99% or seizure-free.
aVolumes are presented in cm3. T-to-CBL = tumor-to-cerebellum uptake ratio (unit-less) (mean ± SD).
Fig. 2.
Seizure control and imaging responses. Best imaging responses on active PET volume (A), mean T-to-CBL uptake ratio (B), MRI T2 lesion size (C) in patients under stable antiepileptic drug doses (n = 17). Seizure control group I = reduction of seizure frequency by 0%–50%, seizure control group II = reduction >50% or seizure-free patients during chemotherapy. Figure 2A: *P = .012 (Mann-Whitney U test).
Survival
At the time of last follow-up, 25 participants were alive, 7 had died, and one was lost to follow-up. Twenty-six participants progressed at 33 ± 22 months (range: 6–85 mo) after the start of chemotherapy, while 7 participants were free from progression at 42 ± 22 months (range: 6–68 mo). Progression was characterized by neurological deterioration in 23 participants, by increase of the T2 lesion in 24 participants, and by appearance of new contrast enhancement in 14 participants, respectively. Nine participants underwent re-operation for progressive tumor (Table 1). Of the progressing participants, 7 died 45 ± 19 months (range: 21–75 mo) after starting chemotherapy.
Neither the mean nor the peak T-to-CBL ratio nor the magnitude of active tumor volumes prior to chemotherapy was predictive for PFS (P = .551, P = .748, and P = .622, respectively). In participants with active tumor volume (n = 30), the median PFS was not significantly different between metabolic responders (34.3 ± 21.8 mo, range: 6–85 mo) and nonresponders (27.6 ± 27.2 mo, range: 6–67 mo) (P = .605). In contrast, ROC analysis yielded a decrease of the active tumor volume of ≥80.5% as an optimal cutoff for the prognostication of a PFS ≥60 months (sensitivity 67%, specificity 90%, accuracy 83%, AUC 0.78 ± 0.02, P = .018). For the prognostication of a PFS ≥48 months, a decrease of the active tumor volume of ≥64.5% was the optimal threshold (sensitivity 70%, specificity 58%, accuracy 62%, AUC 0.74 ± 0.11, P = .037). The prognostication of a PFS ≥36 months (P = .154), ≥24 months (P = .369), and ≥12 months (P = .057), respectively, was not significant.
The PFS in participants without active tumor volume was 29.1 ± 12.4 months (range: 17–41 mo). None of the following parameters correlated with PFS, either on univariate or multivariate analysis: histology (oligodendroglial/oligoastrocytic vs astrocytic tumor, P = .988), PET (responder vs nonresponder, P = .734), MRI (objective response [RANO], P = .276), total TMZ dose (P = .100), IDH1 status (P = .088), 1p/19q co-deletion (P = .331), MGMT promoter methylation status (P = .672) (P values shown for univariate analysis). In addition group-to-group comparisons (imaging responses) did not show differences between tumors with or without IDH mutation, between tumors with or without co-deletion 1p19q, or between tumors with methylated or unmethylated MGMT promotor (Mann-Whitney U test, P > .5 for all comparisons). This may, in part, be explained by the small subgroups compared within this test.
Discussion
In line with previous reports on patients with diffuse cerebral WHO grade II gliomas, we have observed that chemotherapy responses on MRI occur with variable delay from the initiation of treatment.8,9 We noticed a 25% tumor volume reduction on MRI after 16.8 months, whereas a 25% reduction of the metabolically active tumor volume on PET was observed as early as 2.3 months after initiation of chemotherapy. We excluded participants with enhancing tumors on MRI because blood-brain barrier disruption contributes to nonspecific imaging results through passive radiotracer influx into the tumor. Thus, we can assume that the amino acid PET signal in our participants is specific for active amino acid uptake,15,16 which for MET and FET is largely mediated by the L-amino acid transport system.17 Accordingly, TMZ chemotherapy appears to downregulate active amino acid transport in WHO grade II gliomas as an early response indicator. Altogether, 83% of our participants with active tumor volume were PET responders following exposure to TMZ. The long delay between PET and MRI responses may reflect that amino acid transporters represent a sensitive nutritional part of tumor cell metabolism, whereas signal abnormalities on MRI T2-weighted sequences, which may relate to tumor cell densities, edema, and alteration of the extracellular matrix, respond slowly to cytotoxic therapy.
Apart from tumor control, TMZ chemotherapy resulted in improved seizure control. To the best of our knowledge, this is the first study showing that seizure control corresponds to the magnitude of active tumor volume reductions on amino acid PET. Of note, seizure control was neither paralleled by changes in amino acid uptake ratios nor by decreases of tumor volumes as measured by MRI. The latter is in line with recent observations in patients with WHO grade II gliomas treated with either radiotherapy18 or TMZ.7 Thus, improved seizure control may not rely exclusively on reduced compression of neuronal structures by the tumor but also on the reduction of magnitude and spatial extent of local metabolic disequilibrium. The mechanisms behind improved seizure control may involve the glutamatergic system. Gliomas release glutamate as an excitatory neurotransmitter and activate neuronal glutamate receptors.19,20 FET uptake is increased during status epilepticus in the cortex adjacent to gliomas and resolves after cessation of epileptic activity.21 Whether transport of both types of amino acids, which use different transport systems under physiological conditions, relates to each other in gliomas remains to be evaluated.
The main limitation of our study is its retrospective nature and small sample size. Moreover, there was heterogeneity of TMZ regimens and PET and MRI scanners, and molecular data were not available for all participants. The variability of TMZ regimens reflects the current lack of evidence-based recommendations regarding the optimal regimen and duration of chemotherapy.22 However, based on our results, we assume that the time delay between amino acid PET and MRI response may be clinically meaningful. Individual PET-guided determination of chemotherapy duration in patients showing an early metabolic response and response plateau could reduce the risk of toxicity and lower treatment burden and costs. Moreover, one should be cautious about overtreating patients with alkylating chemotherapy, which can exert a mutagenic effect influencing the risk of malignant transformation.23,24 In conclusion, here we have characterized the patterns of chemotherapy response on amino acid PET in a cohort of patients with diffuse cerebral WHO grade II gliomas and report a positive correlation between active tumor volume responses, PFS, and seizure control. A prospective study should validate our findings including MRI, dynamic amino acid PET,25 and molecular markers to determine whether personalized PET-guided management has impact on survival. With regard to the choice of amino acid PET tracer, FET currently seems to be the most promising.26 For the purpose of assessing response to chemotherapy, FET uptake should be quantified as metabolically active volume. This may represent a useful approach to patients with WHO grade II gliomas, a rare disease with substantial variation of the clinical course and treatment response.
Funding
None declared.
Acknowledgments
The authors thank Gabriele Stoffels, MD (Research Center Jülich, Institute 4 of Neuroscience and Medicine) for assistance in the patient studies.
Conflict of interest statement. Prof. U. Roelcke has received honoraria for advisory board participation from MSD and Roche. No conflict of interest. PD Dr. Matthias Wyss reports no disclosures. Dr. M. Nowosielski reports no disclosures. Dr. R. Rudà has received honoraria for advisory board participation from Roche and Italfarmaco. No conflict of interest. PD Dr. P. Roth has received honoraria for advisory board participation from MSD, Roche, and Molecular Partners and honoraria for lectures from Novartis and Medac. No conflict of interest. Dr. S. Hofer reports no disclosures. PD Dr. N. Galldiks reports no disclosures. Dr. F. Crippa reports no disclosures. Prof. M. Weller has received research grants from Acceleron, Actelion, Alpinia Institute, Bayer, Isarna, MSD, Merck Serono, PIQUR, and Roche and honoraria for lectures or advisory board participation or consulting from Celldex, Immunocellular, Isarna, Magforce, MSD, Merck Serono, Pfizer, Roche, and Teva. No conflict of interest. Prof. R. Soffietti has received honoraria for advisory board participation from MSD, Roche, Novartis, and Abbvie. No conflict of interest.
References
- 1.Smits A, Duffau H. Seizures and the natural history of World Health Organization Grade II gliomas: a review. Neurosurgery. 2011;68(5):1326–1333. [DOI] [PubMed] [Google Scholar]
- 2.Bourne TD, Schiff D. Update on molecular findings, management and outcome in low-grade gliomas. Nat Rev Neurol. 2010;6(12):695–701. [DOI] [PubMed] [Google Scholar]
- 3.Weller M, Weber RG, Willscher E et al. . Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129(5):679–693. [DOI] [PubMed] [Google Scholar]
- 4.Lashkari HP, Saso S, Moreno L, Athanasiou T, Zacharoulis S. Using different schedules of Temozolomide to treat low grade gliomas: systematic review of their efficacy and toxicity. J Neurooncol. 2011;105(2):135–147. [DOI] [PubMed] [Google Scholar]
- 5.van den Bent MJ, Wefel JS, Schiff D et al. . Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 6.Rudà R, Bello L, Duffau H, Soffietti R. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14(suppl 4):iv55–iv64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koekkoek JA, Dirven L, Heimans JJ et al. . Seizure reduction in a low-grade glioma: more than a beneficial side effect of temozolomide. J Neurol Neurosurg Psychiatry. 2015;86(4):366–373. [DOI] [PubMed] [Google Scholar]
- 8.Ricard D, Kaloshi G, Amiel-Benouaich A et al. . Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61(5):484–490. [DOI] [PubMed] [Google Scholar]
- 9.Peyre M, Cartalat-Carel S, Meyronet D et al. . Prolonged response without prolonged chemotherapy: a lesson from PCV chemotherapy in low-grade gliomas. Neuro Oncol. 2010;12(10):1078–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyss M, Hofer S, Bruehlmeier M et al. . Early metabolic responses in temozolomide treated low-grade glioma patients. J Neurooncol. 2009;95(1):87–93. [DOI] [PubMed] [Google Scholar]
- 11.Grosu AL, Astner ST, Riedel E et al. . An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys. 2011;81(4):1049–1058. [DOI] [PubMed] [Google Scholar]
- 12.Mikolajczyk K, Szabatin M, Rudnicki P, Grodzki M, Burger C. A JAVA environment for medical image data analysis: initial application for brain PET quantitation. Med Inform (Lond). 1998;23(3):207–214. [DOI] [PubMed] [Google Scholar]
- 13.Wyss MT, Hofer S, Hefti M et al. . Spatial heterogeneity of low-grade gliomas at the capillary level: a PET study on tumor blood flow and amino acid uptake . J Nucl Med. 2007;48(7):1047–1052. [DOI] [PubMed] [Google Scholar]
- 14.Hutterer M, Nowosielski M, Putzer D et al. . [18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 2013;15(3):341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergstrom M, Ericson K, Hagenfeldt L et al. . PET study of methionine accumulation in glioma and normal brain tissue: competition with branched chain amino acids. J Comput Assist Tomogr. 1987;11(2):208–213. [DOI] [PubMed] [Google Scholar]
- 16.Roelcke U, Radu E, Ametamey S, Pellikka R, Steinbrich W, Leenders KL. Association of rubidium and C-methionine uptake in brain tumors measured by positron emission tomography. J Neurooncol. 1996;27(2):163–171. [DOI] [PubMed] [Google Scholar]
- 17.Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J. 2003;373(Pt 1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudà R, Magliola U, Bertero L et al. . Seizure control following radiotherapy in patients with diffuse gliomas: a retrospective study. Neuro Oncol. 2013;15(12):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckingham SC, Campbell SL, Haas BR et al. . Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groot M, Reijneveld JC, Aronica E, Heimans JJ. Epilepsy in patients with a brain tumour: focal epilepsy requires focused treatment. Brain. 2012;135(Pt 4):1002–1016. [DOI] [PubMed] [Google Scholar]
- 21.Hutterer M, Krenn Y, Kunz A et al. . Increased cerebral amino acid uptake during and after epileptic disorders mimic brain tumor in 18F-FET PET [abstract]. Neuro Oncol. 2014;16(suppl 5):v146. [Google Scholar]
- 22.Soffietti R, Baumert BG, Bello L et al. . Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17(9):1124–1133. [DOI] [PubMed] [Google Scholar]
- 23.Johnson BE, Mazor T, Hong C et al. . Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2013;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baehring JM, Marks PW. Treatment-related myelodysplasia in patients with primary brain tumors. Neuro Oncol. 2012;14(5):529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thon N, Kunz M, Lemke L et al. . Dynamic 18F-FET PET in suspected WHO grade II gliomas defines distinct biological subgroups with different clinical courses. Int J Cancer. 2015;136(9):2132–2145. [DOI] [PubMed] [Google Scholar]
- 26.Galldiks N, Langen KJ, Pope WB. From the clinician's point of view - What is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol. 2015;17(11):1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]