Recent clinical studies have highlighted the tremendous potential for immune checkpoint blockade to elicit effective antitumor responses in patients with advanced cancers.1,2 Anti–programmed cell death protein 1 (PD-1) blockade has demonstrated efficacy and regulatory approval in the treatment of refractory melanoma, non-small cell lung cancer, and renal cell carcinoma.3–7 Despite the remarkable clinical responses achieved to date in historically recalcitrant patient populations, only a subset of patients treated with single modality immune checkpoint inhibitors are converted to long-term responders to medical treatment. Combinatorial approaches that target multiple recently identified immune checkpoint pathways have a strong rationale for clinical development, and early studies exploring blockade of cytotoxic T lymphocyte antigen (CTLA)–4 in combination with PD-1 inhibition have demonstrated increased clinical responses in patients with metastatic melanoma.8,9 However, the myriad of possible combinatorial approaches amongst the now more than 15 immune checkpoint pathways identified as potentially relevant in antitumor immunity, and the increased toxicity sometimes observed when immune checkpoint blocking antibodies are combined, makes systematic evaluation of combinatorial approaches a major challenge in the clinical setting. Wei and colleagues10 have demonstrated an innovative approach to targeting multiple immune checkpoint pathways using micro(mi)RNAs that are delivered systemically to immune cells in tumor-bearing hosts.
In an elegant preclinical study, investigators led by Dr Amy Heimberger at MD Anderson Comprehensive Cancer Center selected miR-138 as a candidate for modulating immune checkpoint activity based on prediction algorithms demonstrating that the microRNA was a likely target for both PD-1 and CTLA-4 genes (Fig. 1). The investigators demonstrate that miR-138 delivered by complexing with Lipofectamine 2000 downregulates expression of PD-1 and CTLA-4 in CD4 + T cells as well as decreases expression of Forkhead box protein 3 (FoxP3) in regulatory T cells. Furthermore, intravenous delivery of miR-138 liposomal complexes inhibited the growth of intracranial glioma cells (GL261) in vivo through an immune mediated mechanism and prolonged survival in treated animals. Importantly, a scrambled miRNA control did not affect gene expression in T cells nor modulate antitumor activity, demonstrating the specificity of the miR-138 in modulating antitumor immunity. These findings are of considerable relevance and interest due to the potential for modulating entire immune networks via multiple genes using a miRNA approach and the relative ease of synthesis and intravenous delivery of miRNAs. Also the demonstration that systemic delivery of miRNA liposomes targeting the immune system can modulate antitumor growth within the CNS is an important precedent for exploiting the readily accessible hematologic compartment within tumor-bearing hosts as a target for drug delivery compared with the relatively sequestered compartment in which invasive glioma cells reside.
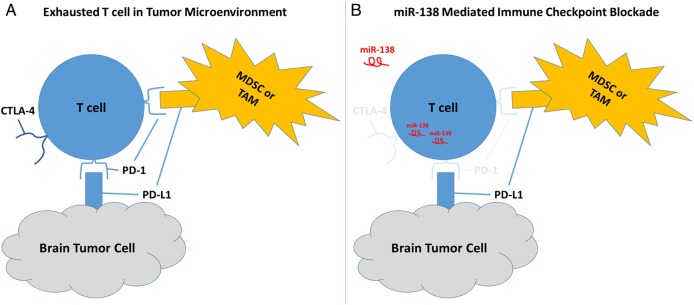
Fig. 1.
(A) T cells within the tumor microenvironment and tumor draining lymph nodes encounter immune checkpoint inhibition through interactions with tumor cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs). Depicted is the expression of programmed death ligand 1 (PD-L1) on tumor cells and myeloid-lineage suppressor cells engaging the PD-1 receptor on T cells leading to immune suppression. T cells expressing the inhibitory receptor, CTLA-4, also encounter their regulatory ligands expressed on myeloid cells within the tumor microenvironment and lymph nodes. (B) The delivery of miR-138 to T cells inhibits expression of the PD-1 receptor, thus rendering the T cell resistant to inhibition by PD-L1 on the surface of tumor cells, MDSCs, or TAMs. The miR-138 mediated downregulation of the PD-1 receptor may allow for effective maintenance of antitumor immunity in the treated host. MiR-138 delivery was shown to also downregulate expression of CTLA-4 and FoxP3 (not shown), leading to the stimulation of effective antitumor immunity in the GL261 glioma model.
The study by Wei et al10 demonstrates an innovative and promising approach to the immunologic treatment of CNS tumors. These findings also trigger important questions regarding the ultimate translational impact of this approach. It would be important to know whether glial tumors with a more invasive and less immunogenic phenotype than the GL261 model are amenable to such treatment. The pharmacokinetics of the miRNA-liposomal delivery strategy, duration of in vivo effects within T cells, and the optimal dosing strategy are all important parameters for exploration in future drug development. While Lipofectamine was an effective delivery vehicle for eliciting antitumor treatment effects, the exploration of other liposomal formulations and perhaps even targeted carrier molecules to more effectively transfect lymphocytes would improve clinical efficacy. Additionally, there are likely several other genes besides PD-1, CTLA-4, and FoxP3 that are directly or indirectly modulated by miR-138 delivery. An understanding of the network of genes that are modulated by this approach and their effects on immunologic phenotype would be important to ascertain. MiRNAs coordinately regulate multiple genes, can elicit global changes in gene expression through interactions that affect ribosomal RNA biogenesis, and can modulate gene expression through classical and nonclassical targets in the untranslated regions of RNA and DNA.11 Thus, while one gains the potential for combinatorial effects using a miRNA therapeutic strategy, there is a coordinate loss of the target specificity that is characteristic of monoclonal antibody blockade. It is therefore very likely that genes other than those that were the focus within the current study are modulated by the delivery of miR-138 and may, in fact, be major contributors to the observed antitumor activity. It is feasible that counterproductive changes or compensatory immunosuppressive mechanisms are also induced by this approach, and thus further mechanistic exploration is warranted. Lastly, while the inherent advantages of being able to achieve combinatorial immune checkpoint blockade with a single miRNA are novel and of considerable interest, a comparative analysis of the miRNA approach with monoclonal antibody blocking approaches would be of great interest.
The findings reported by Wei et al clearly lay the foundation for a very exciting and innovative approach to immune modulation for the treatment of brain tumors and likely many other cancers.
References
- 1.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–5309. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 7.Hamid O, Robert C, Daud A et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270–1271. [DOI] [PubMed] [Google Scholar]
- 9.Postow MA, Chesney J, Pavlick AC et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei J, Nduom EK, Kong LY et al. MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro Oncol. 2016;18(5):601–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12(11):847–865. [DOI] [PMC free article] [PubMed] [Google Scholar]