Abstract
Hematopoietic stem cells (HSCs) form a rare population of multipotent stem cells, which give rise to all hematopoietic lineages. HSCs home to bone marrow niches and circulate between blood and bone marrow. Many factors, especially SDF1a, affect the circulation of HSCs, but these have not been fully recognized. SDF1a has been shown to bind CXCR7 in addition to CXCR4 and can also function as SDF1a/CXCR4 modulator. CXCR7 plays a role in HSCs homing via SDF1a gradient and is a mediator of CXCR4/SDF1a axis. This review describes the current concepts and questions concerning CXCR7/CXCR4/SDF1a axis as an important key in hematopoietic stem cells homing with particular emphasis on CXCR7 receptor. Homing of HSCs is an essential step for successful hematopoietic stem cell transplantation.
Keywords: CXCR7, CXCR4, CXCL12, HSCs homing
Introduction
Hematopoietic stem cells migrate through blood circulation and bone marrow niches. HSCs migration to bone marrow is an essential step in clinical stem cell engraftment. HSCs are a rare population of multipotent stem cells lodging in a specialized microenvironment within the bone marrow known as bone marrow niche. Homing of HSCs starts from the embryonic period, when HSCs migrate from the fetal liver to seed bone marrow via crosstalk between cytokines and adhesion molecules, especially SDF1a, CXCR4 and CXCR7 pathways (McGrath et al., 1999[33]; Gazitt, 2004[14]). SDF1a chemokine and its receptors have been implicated in survival, proliferation and trafficking of CD34+ cells (Lataillade et al., 2000[26]; Aiuti et al., 1997[1]; Mohle et al., 1998[35]; Wright et al., 2002[66]). CXCR4 was deemed to be the sole receptor for SDF1a but CXCR7 was later found to be another high affinity receptor for SDF1a (Balabanian et al., 2005[2]; Burns et al., 2006[6]). It is unclear whether the SDF1a/CXCR7 axis regulates HSCs homing, but there are evidences that CXCR7 modulates CXCR4/SDF1 responses (Hartmann et al., 2008[18]; Naumann et al., 2010[38]; Uto-Konomi et al., 2013[59]).
Bone Marrow Niches and Homing of HSCs
HSCs are multipotent stem cells capable of generating all hematopoietic lineages. The bone marrow cavities are the main residence for post-natal human hematopoiesis. HSCs must be maintained in a specific niche, which is critical for HSCs fate and hematopoiesis. HSCs niche, which is an inductive microenvironment, provides factors that maintain the hematopoietic stem cells and prevent their differentiation (Schofield, 1978[48]). Bone marrow niche maintains several functions of HSCs, including stem cell adhesion, self-renewal (Jones and Wagers, 2008[22]), apoptosis (Lin, 2002[29]) as well as homing and mobilization. HSCs physiologically leave the bone marrow and migrate between blood circulation and bone marrow niche (Wright et al., 2001[67]), a process termed mobilization and homing. Many agents have been reported to affect the homing of HSCs, most notably integrins (Watanabe et al., 1998[62]; Qian et al., 2006[43]), selectins (Sullivan et al., 2011[55]) and chemokines (Aiuti et al., 1997[1]). CXCR7/ CXCR4/SDF1a axis plays major roles in stem cell motility, which will be discussed later.
SDF1 alpha/CXCR4 axis
SDF1a is expressed in bone tissue and endothelial cells and can induce the expression of LFA1 and VLA4 adhesion molecules. However, the expression of CXCR4 in USSCs is decreased with the increase in passage number. There are two isoforms of SDF1a, and its gene is located on human chromosome 10 (Levesque et al., 2003[27]; Hartmann et al., 2008[18]; Watt and Forde, 2008[63]). In most reports, CXCL12-abundant reticular cells (CARs) and osteoblasts are introduced as the main sources of SDF1a in both osteoblastic and vascular niches (Jung et al., 2006[23]; Sugiyama et al., 2006[54]). Cytokines and hormones play a role in SDF1a expression. For instance, TNF-α, IL-1 beta, PTH and PDGF-BB stimulate SDF1a secretion in vitro. However, TGF-beta 1 rapidly reduces SDF1a expression in most osteoblastic cell lines (Jung et al., 2006[23]). CXCR-4 (also identified as fusin or CD184) is the receptor for SDF1a chemokine. SDF1a/ CXCR4 axis plays a role in HSCs homing and quiescence (Moriuchi et al., 1997[37]; Saini et al., 2010[46]). Ex vivo pretreatment of HSCs with high dose SDF1a reduces CD34+ cells engraftment, but low dose SDF1a increases their engraftment. These diverse effects are likely to be mediated by various second messenger systems sensitive to different concentrations of SDF1a (Plett et al., 2002[42]). CXCR4 has a tendency to form hetero- and homo oligomers, and such oligomerization could play a role in the allosteric regulation of CXCR4 signaling (Wu et al., 2010[68]). CXCR4 signaling is mediated through activation of G proteins, AKT, ERK and JAK-STAT signaling pathway (Hartmann et al., 2008[18]; Teicher and Fricker, 2010[57]). Obstruction of CXCR4-SDF1a axis pathway leads to proliferation of HSCs (Naumann et al., 2010[38]). In contrast, some experiments have reported that SDF1a enhances the repopulating potential of CD34+cells, and have suggested that the function of hematopoietic progenitor cells (HPCs) can be satisfactorily regulated through specific CXCR4 signaling (Plett et al., 2002[42]). In BM cells, CXCR4 regulates the homing and presentation of circulating functional SDF1a; therefore, these cells regulate the trafficking of HSCs together with CXCR4 positive HSCs. Immature HSCs express low levels of SDF1a that can interact with CXCR4 on stromal cells by secretion of this ligand, reversing the usual form of interaction and activation (Dar et al., 2006[10]). Stem cell factor (SCF) and IL-6 enhance CXCR4 on the CD34+ cells and improve stem cell engraftment (Peled et al., 1999[41]). CXCR4 knockout mice have defective B lymphopoiesis and myelopoiesis and die perinatally, showing intense defects in the hematopoietic tissue (Ma et al., 1998[32]). HIF-1 plays a role in SDF1a and CXCR4 expression, and improves the function of CXCR4. Drugs such as Plerixafor (AMD3100) that block CXCR4 or G-CSF which causes elastase release from neutrophils and SDF1a degradation lead to HSCs mobilization (Balabanian et al., 2008[3]; Rajagopal et al., 2010[44])
CXCR7 as a novel receptor for SDF1a
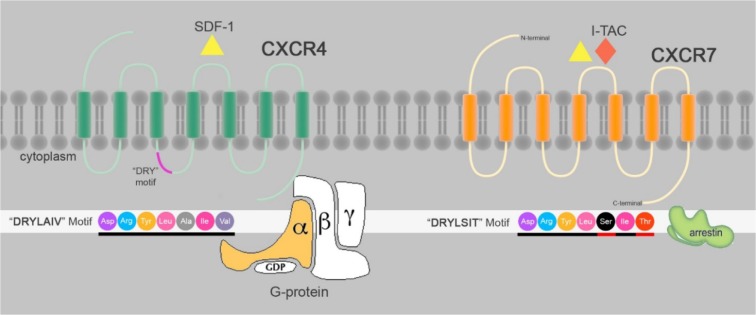
Although CXCR4/SDF1a was thought to be a single axis, the interaction of SDF1a with CXCR4 was questioned after Burns report (Burns et al., 2006[6]). He showed that small molecular inhibitors of CCX451, CCX751 and CXCL11 compete with SDF1a binding to CXCR4, with human cancer cell lines showing a contradictory pattern of CXCR4 expression and SDF1a binding. Due this observation, he suggested a new receptor for SDF1a, the orphan receptor RDC-1, which was named CXCR7 (Burns et al., 2006[6]). CXCR7 (RDC-1; CXC-CK) is a seven-transmembrane-spanning chemokine protein receptor (Heesen et al., 1998[19]) with characteristic structural features similar to other chemokine receptors (Figure 1(Fig. 1)). However, it is a decoy CXC chemokine receptor similar to Duffy Antigen of D6 (Graham, 2009[16]). Decoy chemokine family is characterized by modification of Asp-Arg-Tyr-Leu-Ala-Ile-Val (DRYLAIV) motif essential for G-protein mediated responses (Haraldsen and Rot, 2006[17]; Graham, 2009[16]). CXCR7 does not signal through G proteins, and is an atypical chemokine receptor (Rajagopal et al., 2010[44]). Previously, CXCR7 was wrongly thought to be a receptor for vasoactive intestinal peptide (VIP) (Heesen et al., 1998[19]). CXCR7 is involved in several biological processes, including embryonic development (Burns et al., 2006[6]), scavenging for SDF1a and CXCL11 (Naumann et al., 2010[38]), promotion of adhesion to activated endothelial cells, cell survival but not cell proliferation or tumor development (Naumann et al., 2010[38]; Tarnowski et al., 2010[56]; Kumar et al., 2012[25]). Like CXCR4, CXCR7 is highly conserved in mice and humans (Heesen et al., 1998[19]), so that CXCR7 -/- mice show different heart abnormalities and die at birth (Sierro et al., 2007[52]). CXCR7 binds to SDF1a and I-TAC (IFN-inducible T cell chemo attractant; CXCR11) (Balabanian et al., 2005[2]; Burns et al., 2006[6]). Like CXCR4, CXCR7 N-terminal domain binds to SDF1a (Veldkamp et al., 2008[61]). CXCR7 is expressed in activated endothelial cells, tumor cell lines, early fetal liver cells, transforming cells (Burns et al., 2006[6]), migrating primordium in zebrafish (Dambly-Chaudiere et al., 2007[9]), monocytes, human fetal heart, neuronal tissue, adult mouse cerebellum, mature B cells (Infantino et al., 2006[21]; Thelen and Thelen, 2008[58]), blood and lymphatic endothelial cells in human renal allografts (Neusser et al., 2010[39]). Nevertheless, the expression of CXCR7 on several cell types is controversial (Balabanian et al., 2005[2]; Burns et al., 2006[6]; Kumar et al., 2012[25]), and it is poorly expressed on human and mouse T-cells and CD34+ progenitors (Infantino et al., 2006[21]; Hartmann et al., 2008[18]; Tarnowski et al., 2010[56]) but the expression of its mRNA has been shown in PBL (Shimizu et al., 2000[51]). CXCR7 is required for activation of LFA-1 and VLA-4 on T-cells and CD 34+ cells under shear flow (Hartmann et al., 2008[18]) but the role of CXCR7 in chemotaxis and cell migration has been a subject of debate. Some reports have suggested that CXCR7 induces T-cell migration (Balabanian et al., 2005[2]; Kumar et al., 2012[25]). In contrast, other experiments showed that CXCR7 is not involved in SDF1a/ CXCR4/CXCR7 mediated T-cell and CD34+ mobilization (Burns et al., 2006[6]; Hartmann et al., 2008[18]; Naumann et al., 2010[38]; Tarnowski et al., 2010[56]). Like CXCR4, CXCR7 is a co-receptor for several HIV and SIV strains (Shimizu et al., 2000[51]).The human CXCR7 gene was mapped to chromosome 2, closely linked to CXCR1, CXCR2, and CXCR4 genes (Balabanian et al., 2005[2]; Thelen and Thelen, 2008[58]). CXCR7 circulates between the cell and endosomal membrane in presence or absence of ligand, slightly reduced only after long term incubation with SDF1a and CXCL11, leading to ligand vanishing due to internalization. In contrast, after encounter with ligand, CXCR4 undergoes down regulation and ligand disappears to some extent. DYR sequence deletion does not affect the clearance of SDF1a but C-terminal deletion inhibits SDF1a internalization and wiping out (Naumann et al., 2010[38]).
Figure 1. Schematic structure of CXCR4 and CXCR7: CXCR4 and CXCR7 are 7TMRs that share binding to SDF1a. CXCR7 also binds to I-TAC. “DRYLAIV” is a common motif in GPCRs modified to “DRYLSIT” motif essential for G-protein responses. SDF1a/CXCR7 interactions recruit Beta-arrestin but not G-protein.
CXCR7 Recruitment of beta-arrestin but not G-protein
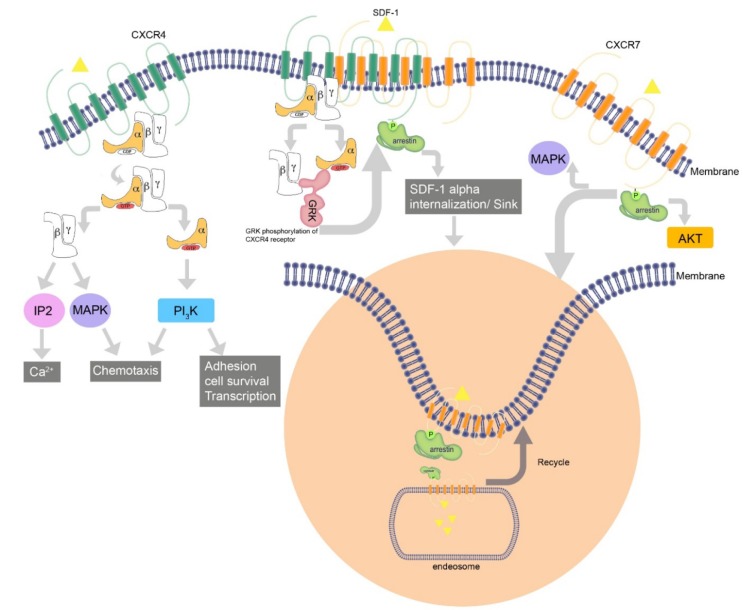
Seven-transmembrane receptors (7TMRs) signal through G proteins and β-arrestins (Figure 2(Fig. 2)). The latter function as multipurpose adapters controlling receptor signaling, desensitization and trafficking. Commonly, there is a balance between G protein and beta-arrestin-mediated pathways. Researches revealed that CXCR7 does not couple to G proteins and cannot lead to GTP hydrolysis or calcium mobilization; nevertheless, it does trigger MAP kinases cascade through beta-arrestins (Rajagopal et al., 2010[44]). CXCR7 inhibition does not affect the degree or kinetics of CXCL12-triggered Akt or ERK1/2 phosphorylation. However, one study showed that CXCR7-SDF1a interaction activetes ERK protein and AKT in T-cells, which is Giα independent (Kumar et al., 2012[25]). In contrast, ERK and Akt activation is completely dependent upon CXCR4-triggered Gi signaling due to complete inhibition of it by AMD3100 and Gi- inhibitor PTX. Similar to other chemokines, ligand binding to CXCR7 regulates downstream signaling pathways. Unlike other chemokines, decoy receptor CXCR7 fails to activate G-proteins responses but it activates beta-arrestin pathway. Upon binding CXCR7, CXCL12 activates beta-arrestin2 and mediates pERK formation, which is activated by G-protein. β-arrestin depletion reduces the migration of rVSMC in response to I-TAC (Rajagopal et al., 2010[44]).
Figure 2. CXCR7, CXCR4 and CXCR7/CXCR4 hetrodimerization signaling pathways.
On the other hand, a research showed that co-expression of CXCR4 and CXCR7 in the same cell leads to strong SDF1a-induced intracellular calcium signaling. If CXCR7 sequesters arrestin2 away from CXCR4, this may eliminate signal-regulating attributes of the beta-arrestins, and allow for improved signaling through CXCR4 (Zabel et al., 2009[69]). It has been revealed that CXCR7 cooperates with beta-arrestin in a ligand and dose independent mode, which leads to ligand internalization. CXCR7 recruitment of beta-arrestin is associated with ERK phosphorylation in a short period of time, which is then distributed in cytoplasmic vesicles in comparison with G protein-mediated activation of pERK, which takes longer time and is dispensed to cytoplasm and nucleus (Rajagopal et al., 2010[44]). Recruitment of beta-arrestin is dependent upon C-terminal end of the receptor. SDF1a initiates beta-arrestin2 association with both CXCR4 and CXCR7.
Beta-arrestin2 may create a distinct intracellular signaling pathway that can participate in cross-talk between CXCR7 and CXCR4.
Heterodimerization of CXCR7 with CXCR4 changes the comparatively different orientation between CXCR4 and Giα, decreasing SDF1a mediated G protein activation.CXCR7 has an interaction with inactive G protein Gai1; nevertheless, cross-regulation of CXCR4 via G protein sequestration by CXCR7 has not been confirmed (Zabel et al., 2009[69]). 7TMRs include two groups: A and B receptors based on communication with beta-arrestin molecules (Moore et al., 2007[36]). Class A7TMRs have a temporary interaction with beta-arrestin and recycle quickly to the cell membrane from early endosomes, binding beta-arrestin2 versus beta-arrestin1 with high affinity. Class B 7TMRs show inverse properties and bind to both beta-arrestin 1 and 2. CXCR7 seems to have characteristics of both class A and class B 7TMRs (Luker et al., 2008[30]). It has considerably higher associations with beta-arrestin 2 similar to class A receptors, and there are complexes of CXCR7 and beta-arrestin2 that continue to increase over time as would be expected for a class B receptor (Chakera et al., 2008[7]). Noticeably, in the absence of SDF1a and under baseline situations, the interaction between CXCR7 and beta-arrestin2 is higher compared to control pairs of CXCR7 and c-fos or FKBP12 (Cheng et al., 2000[8]; Chakera et al., 2008[7]; Luker et al., 2009[31]). Luker et al. (2009[31]) reported that depletion of beta-arrestin in mouse embryonic fibroblasts reduces but does not eliminate the accumulation of SDF1a, suggesting that CXCR7 possibly uses another distinct pathway for uptake of SDF1a. Agents directed against CXCR4 such as AMD3100, which are used in saturating concentrations, could stimulate the association between CXCR7 and beta-arrestin2 in cells expressing both receptors.
CXCR7 Interference with SDF1a/CXCR4 Axis responses
CXCR7 expression in B-cells is inversely correlated with the activation of CXCR4 (Levoye et al., 2009[28]). CXCR7 cannot intrinsically cause the signal to be transmitted. CXCR7 receptors in breast tumor cell line MCF-7 do not cause increased cytoplasmic Ca2+ or cell migration in response to SDF1a (Burns et al., 2006[6]), but Ca2+ mobilization and chemotaxis is triggered in transformed T-cell line expressing CXCL4/SDF1a. Although CXCR7 is not directly involved in migration of HSCP (hematopoietic stem cell progenitors) (Tarnowski et al., 2010[56]), its modulatory role in CXCR4/SDF1 axis has been mentioned in literature.
CXCR7 can also act as a sink for SDF1a and regulates chemokine gradients (Naumann et al., 2010[38]; Hoffmann et al., 2012[20]). Hartmann et al. (2008[18]) suggested two pools of CXCR7 in CD34+ cells: the first expressed on cell surface and the second intracellular, the localization of which is associated with early endosomes. CXCR7 induces SDF1a internalization and degradation (Naumann et al., 2010[38]). CXCR4 heterodimerization with CXCR7 selectively impairs the ability of CXCR4 to activate G proteins after ligand binding, and alters CXCL12-CXCR7 axis mediated calcium responses and chemotaxis of T-cells (Levoye et al., 2009[28]).
CXCR7 response is up-regulated by VLA-4 and LFA-1
The retention of adult HSCs in the bone marrow niche is controlled by several factors such as adhesion receptors, including integrins. Very late activation antigen-4 (VLA-4; α4β1) and lymphocyte function-associated antigen-1 (LFA-1; αLβ2) are integrin molecules involved in hematopoietic cell trafficking (Milner and Campbell, 2002[34]). LFA-1 and VLA-4 are expressed in HSCs (Williams et al., 1991[64]). Activation of LFA-1 and VLA-4 on lymphocytes is inhibited by PTX. SDF1a- triggers the activation of integrin through G protein-coupled chemokine receptor CXCR4, which is inactivated by PTX (Hartmann et al., 2008[18]). CXCR7 effects SDF1a-CXCR4-triggered activation of LFA-1 and VLA-4 in T-cells as well as CXCR4 and CXCR7 uptake, which is necessary for LFA-1 and VLA-4 activation in T-cells on TNF-α activated HUVEC (Hartmann et al., 2008[18]).The majority of CXCL12-triggered lymphocytes in inflammatory endothelial tissue are inhibited by CXCR7 blocking (Hartmann et al., 2008[18]). Moreover, antagonism of CXCR7 attenuates chronic hypoxia-induced pulmonary vascular adhesion (Sartina et al., 2012[47]). According to these investigations, high expression of integrin is shown to be efficient for homing and retention of lymphocytes (Gorfu et al., 2009[15]), macrophages (Patel et al., 1998[40]) and HSCs (Watanabe et al., 1998[62]; Fibbe et al., 2000[12]; Kronenwett et al., 2000[24]; Scott et al., 2003[50]; Rettig et al., 2012[45]). CXCR7 is required for integrin activation through CXCR4/SDF1a pathway, and CXCR7/SDF1a is a likely modulatory axis regulating CXCR4/SDF1a-mediated HSCs homing. Transfected CXCR7 positive cells are adhered to the activated HUVECs considerably higher than intact cells (Burns et al., 2006[6]). TNFα and IL-1β promote de novo CXCR7 expression on endothelial cells (Burns et al., 2006[6]).
Conclusion
SDF1a/CXCR4 axis is regulated by many factors (Forde et al., 2007[13]; Williams et al., 2008[65]). According to previous content, CXCR7 is an important modulation factor. CXCR7 binds I-TAC and SDF1a, both of which can compete for binding to it. However, CXCR4 is a specific receptor for SDF1a (Burns et al., 2006[6]). I-TAC/CXCR7-medaited responses may interfere and compete with CXCR7-SDF1a mediated interaction (Burns et al., 2006[6]). CXCR4 expression is observed on HSCs and other cells. In contrast to CXCR4, CXCR7 is expressed on embryonic HSCs and cancer transformed cells. There is evidence that primitive erythrocytes express CXCR7 (Berahovich et al., 2010[4]) and traffic in embryonic CXCR7+ liver cells at E11-E13 in response to SDF1a (Burns et al., 2006[6]), so CXCR7 is likely to play a role in processes regulated by SDF1a. CXCR7 expression is up-regulated in some hematopoietic neoplasms like human B lymphoma (Burns et al., 2006[6]). CXCR7 blocking by antibody reduces the arrest of HSCs on immobilized VCAM-1 (Hartmann et al., 2008[18]). Zebra fish lateral-line primordium migration can be mediated only via CXCR7 (Valentin et al., 2007[60]). In addition, CXCR4 and CXCR7 are able to form heterodimer and homodimers and regulate CXCL12-promoted homing of HSCs (Levoye et al., 2009[28]). Some observations have demonstrated miRNA-mediated post translational regulation with CXCR7 and SDF1a expression (Staton et al., 2011[53]). Mutation in Von Hippel-Lindau (VHL), which degrades HIF1, leads to overexpression of CXCR4 (Burger and Kipps, 2006[5]). CXCR7 promoter contains two hypoxia-responsive elements (Tarnowski et al., 2010[56]). CXCR7 expression is up-regulated by hypoxia in human microvascular endothelial cells (Schutyser et al., 2007[49]; Esencay et al., 2013[11]). Overall, CXCR7/ SDF1a axis suggested a new migration path in homing of HSCs, and can be useful for therapeutic purposes.
References
- 1.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 3.Balabanian K, Levoye A, Klemm L, Lagane B, Hermine O, Harriague J, et al. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J Clin Invest. 2008;118:1074–1084. doi: 10.1172/JCI33187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berahovich RD, Zabel BA, Penfold ME, Lewen S, Wang Y, Miao Z, et al. CXCR7 protein is not expressed on human or mouse leukocytes. J Immunol. 2010;185:5130–5139. doi: 10.4049/jimmunol.1001660. [DOI] [PubMed] [Google Scholar]
- 5.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 6.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakera A, Seeber RM, John AE, Eidne KA, Greaves DR. The duffy antigen/receptor for chemokines exists in an oligomeric form in living cells and functionally antagonizes CCR5 signaling through hetero-oligomerization. Mol Pharmacol. 2008;73:1362–1370. doi: 10.1124/mol.107.040915. [DOI] [PubMed] [Google Scholar]
- 8.Cheng ZJ, Zhao J, Sun Y, Hu W, Wu YL, Cen B, et al. Beta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. J Biol Chem. 2000;275:2479–2485. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- 9.Dambly-Chaudiere C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Development Biol. 2007;7:23. doi: 10.1186/1471-213X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Esencay M, Sarfraz Y, Zagzag D. CXCR7 is induced by hypoxia and mediates glioma cell migration towards SDF-1alpha. BMC Cancer. 2013;13:347. doi: 10.1186/1471-2407-13-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fibbe WE, Pruijt JF, van Kooyk Y, Figdor CG, Opdenakker G, Willemze R. The role of metalloproteinases and adhesion molecules in interleukin-8-induced stem-cell mobilization. Semin Hematol. 2000;37:19–24. doi: 10.1016/s0037-1963(00)90085-4. [DOI] [PubMed] [Google Scholar]
- 13.Forde S, Tye BJ, Newey SE, Roubelakis M, Smythe J, McGuckin CP, et al. Endolyn (CD164) modulates the CXCL12-mediated migration of umbilical cord blood CD133+ cells. Blood. 2007;109:1825–1833. doi: 10.1182/blood-2006-05-023028. [DOI] [PubMed] [Google Scholar]
- 14.Gazitt Y. Homing and mobilization of hematopoietic stem cells and hematopoietic cancer cells are mirror image processes, utilizing similar signaling pathways and occurring concurrently: circulating cancer cells constitute an ideal target for concurrent treatment with chemotherapy and antilineage-specific antibodies. Leukemia. 2004;18:1–10. doi: 10.1038/sj.leu.2403173. [DOI] [PubMed] [Google Scholar]
- 15.Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009;9:836–850. doi: 10.2174/156652409789105525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham GJ. D6 and the atypical chemokine receptor family: novel regulators of immune and inflammatory processes. Eur J Immunol. 2009;39:342–351. doi: 10.1002/eji.200838858. [DOI] [PubMed] [Google Scholar]
- 17.Haraldsen G, Rot A. Coy decoy with a new ploy: interceptor controls the levels of homeostatic chemokines. Eur J Immunol. 2006;36:1659–1661. doi: 10.1002/eji.200636327. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann TN, Grabovsky V, Pasvolsky R, Shulman Z, Buss EC, Spiegel A, et al. A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12-triggered integrin activation but not in chemokine-triggered motility of human T lymphocytes and CD34+ cells. J Leukoc Biol. 2008;84:1130–1140. doi: 10.1189/jlb.0208088. [DOI] [PubMed] [Google Scholar]
- 19.Heesen M, Berman MA, Charest A, Housman D, Gerard C, Dorf ME. Cloning and chromosomal mapping of an orphan chemokine receptor: mouse RDC1. Immunogenetics. 1998;47:364–370. doi: 10.1007/s002510050371. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann F, Muller W, Schutz D, Penfold ME, Wong YH, Schulz S, et al. Rapid uptake and degradation of CXCL12 depend on CXCR7 carboxyl-terminal serine/threonine residues. J Biol Chem. 2012;287:28362–28377. doi: 10.1074/jbc.M111.335679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Infantino S, Moepps B, Thelen M. Expression and regulation of the orphan receptor RDC1 and its putative ligand in human dendritic and B cells. J Immunol. 2006;176:2197–2207. doi: 10.4049/jimmunol.176.4.2197. [DOI] [PubMed] [Google Scholar]
- 22.Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 23.Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts;a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Kronenwett R, Martin S, Haas R. The role of cytokines and adhesion molecules for mobilization of peripheral blood stem cells. Stem Cells. 2000;18:320–330. doi: 10.1634/stemcells.18-5-320. [DOI] [PubMed] [Google Scholar]
- 25.Kumar R, Tripathi V, Ahmad M, Nath N, Mir RA, Chauhan SS, et al. CXCR7 mediated Gialpha independent activation of ERK and Akt promotes cell survival and chemotaxis in T cells. Cell Immunol. 2012;272:230–241. doi: 10.1016/j.cellimm.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Lataillade JJ, Clay D, Dupuy C, Rigal S, Jasmin C, Bourin P, et al. Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95:756–768. [PubMed] [Google Scholar]
- 27.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113:6085–6093. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 29.Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3:931–940. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- 30.Luker KE, Gupta M, Luker GD. Imaging CXCR4 signaling with firefly luciferase complementation. Anal Chem. 2008;80:5565–5573. doi: 10.1021/ac8005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luker KE, Gupta M, Steele JM, Foerster BR, Luker GD. Imaging ligand-dependent activation of CXCR7. Neoplasia. 2009;11:1022–1035. doi: 10.1593/neo.09724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGrath KE, Koniski AD, Maltby KM, McGann JK, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 34.Milner R, Campbell IL. The integrin family of cell adhesion molecules has multiple functions within the CNS. J Neurosci Res. 2002;69:286–291. doi: 10.1002/jnr.10321. [DOI] [PubMed] [Google Scholar]
- 35.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- 36.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 37.Moriuchi M, Moriuchi H, Turner W, Fauci AS. Cloning and analysis of the promoter region of CXCR4, a coreceptor for HIV-1 entry. J Immunol. 1997;159:4322–4329. [PubMed] [Google Scholar]
- 38.Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, et al. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PloS One. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neusser MA, Kraus AK, Regele H, Cohen CD, Fehr T, Kerjaschki D, et al. The chemokine receptor CXCR7 is expressed on lymphatic endothelial cells during renal allograft rejection. Kidney Int. 2010;77:801–808. doi: 10.1038/ki.2010.6. [DOI] [PubMed] [Google Scholar]
- 40.Patel SS, Thiagarajan R, Willerson JT, Yeh ET. Inhibition of alpha4 integrin and ICAM-1 markedly attenuate macrophage homing to atherosclerotic plaques in ApoE-deficient mice. Circulation. 1998;97:75–81. doi: 10.1161/01.cir.97.1.75. [DOI] [PubMed] [Google Scholar]
- 41.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 42.Plett PA, Frankovitz SM, Wolber FM, Abonour R, Orschell-Traycoff CM. Treatment of circulating CD34(+) cells with SDF-1alpha or anti-CXCR4 antibody enhances migration and NOD/SCID repopulating potential. Exp Hematol. 2002;30:1061–1069. doi: 10.1016/s0301-472x(02)00880-9. [DOI] [PubMed] [Google Scholar]
- 43.Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–3510. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- 44.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, et al. Beta-arrestin- but not G protein-mediated signaling by the "decoy" receptor CXCR7. Proc Natl Acad Sci USA. 2010;107:628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26:34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem. 2010;285:15566–15576. doi: 10.1074/jbc.M110.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sartina E, Suguihara C, Ramchandran S, Nwajei P, Rodriguez M, Torres E, et al. Antagonism of CXCR7 attenuates chronic hypoxia-induced pulmonary hypertension. Pediatr Res. 2012;71:682–688. doi: 10.1038/pr.2012.30. [DOI] [PubMed] [Google Scholar]
- 48.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 49.Schutyser E, Su Y, Yu Y, Gouwy M, Zaja-Milatovic S, Van Damme J, et al. Hypoxia enhances CXCR4 expression in human microvascular endothelial cells and human melanoma cells. Eur Cytokine Netw. 2007;18:59–70. doi: 10.1684/ecn.2007.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimizu N, Soda Y, Kanbe K, Liu HY, Mukai R, Kitamura T, et al. A putative G protein-coupled receptor, RDC1, is a novel coreceptor for human and simian immunodeficiency viruses. J Virol. 2000;74:619–626. doi: 10.1128/jvi.74.2.619-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staton AA, Knaut H, Giraldez AJ. miRNA regulation of Sdf1 chemokine signaling provides genetic robustness to germ cell migration. Nature Genetics. 2011;43:204–211. doi: 10.1038/ng.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan C, Chen Y, Shan Y, Hu Y, Peng C, Zhang H, et al. Functional ramifications for the loss of P-selectin expression on hematopoietic and leukemic stem cells. PloS One. 2011;6:e26246. doi: 10.1371/journal.pone.0026246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarnowski M, Liu R, Wysoczynski M, Ratajczak J, Kucia M, Ratajczak MZ. CXCR7: a new SDF-1-binding receptor in contrast to normal CD34(+) progenitors is functional and is expressed at higher level in human malignant hematopoietic cells. Eur J Haematol. 2010;85:472–483. doi: 10.1111/j.1600-0609.2010.01531.x. [DOI] [PubMed] [Google Scholar]
- 57.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 58.Thelen M, Thelen S. CXCR7, CXCR4 and CXCL12: an eccentric trio? J Neuroimmunol. 2008;198:9–13. doi: 10.1016/j.jneuroim.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 59.Uto-Konomi A, McKibben B, Wirtz J, Sato Y, Takano A, Nanki T, et al. CXCR7 agonists inhibit the function of CXCL12 by down-regulation of CXCR4. Biochem Biophys Res Commun. 2013;431:772–776. doi: 10.1016/j.bbrc.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 60.Valentin G, Haas P, Gilmour D. The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr Biol. 2007;17:1026–1031. doi: 10.1016/j.cub.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 61.Veldkamp CT, Seibert C, Peterson FC, De la Cruz NB, Haugner JC, 3rd, Basnet H, et al. Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci Signal. 2008;1:ra4. doi: 10.1126/scisignal.1160755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe T, Dave B, Heimann DG, Jackson JD, Kessinger A, Talmadge JE. Cell adhesion molecule expression on CD34+ cells in grafts and time to myeloid and platelet recovery after autologous stem cell transplantation. Exp Hematol. 1998;26:10–18. [PubMed] [Google Scholar]
- 63.Watt SM, Forde SP. The central role of the chemokine receptor, CXCR4, in haemopoietic stem cell transplantation: will CXCR4 antagonists contribute to the treatment of blood disorders? Vox Sang. 2008;94:18–32. doi: 10.1111/j.1423-0410.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 64.Williams DA, Rios M, Stephens C, Patel VP. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature. 1991;352:438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- 65.Williams DA, Zheng Y, Cancelas JA. GTPases and regulation of hematopoietic stem cell localization. Methods Enzymol. 2008;439:365–393. doi: 10.1016/S0076-6879(07)00427-2. [DOI] [PubMed] [Google Scholar]
- 66.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 68.Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zabel BA, Wang Y, Lewen S, Berahovich RD, Penfold ME, Zhang P, et al. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol. 2009;183:3204–3211. doi: 10.4049/jimmunol.0900269. [DOI] [PubMed] [Google Scholar]