Abstract
Contemporary nutrition regime has focused the attention of the researchers on phytochemicals enriched spices to mitigate various oncological threats. Numerous chemopreventive strategies against malignancy have been developed considering the anticancer perspectives of allied nutraceutical constituents. Current evidences have proven an inverse association of spices with that of oncological incidences. The high antioxidant activity of spices derived bioactives triggers the free radicals scavenging ability at cellular level thereby alleviating various metabolic syndromes. Promising compounds including curcumin and curcuminoids (turmeric), limonene (cardamom), allicin, allyl isothiocyanate (garlic), cinnamic aldehyde, 2-hydroxycinnamaldehyde and eugenol (cinnamon), gingerol, zingiberone, zingiberene (ginger), dipropyle disulfides and quercetin (onion), piperidine piperine, limonene, α- and β-pinene (black pepper), crocetin, crocin and safranal (saffron) have been identified as chemopreventing agents against various malignancies. Chemopreventive properties of spices are mediated by functional bioactive ingredients that arrest the activity of cytochrome P450 and isozymes CYP 1A1, cyclooxygenase-2, reducing activator of transcription-3 (STAT-3) and signal transducer. They are closely associated with tumorigenesis activated by interleukin-6 (IL-6) receptors and epidermal growth factor (EGF) relate to an array of tumors. The bioactive constituents altering the expression of protein involved in cell cycle, activating caspases killer and suppressing Kappa-B activation. Alongside, they also restrain causative agents of cell structure damage as in lipid and protein membrane system and DNA that shifting healthy body towards cancerous state. Spices phytochemicals have established as carcinogenesis blockers by modulating cell proliferation pathways transformation, inflammation, metastasis etc. Furthermore, spices as functional ingredients may act as immune boosters and diminish inflammatory disorders. The current review is inevitably an affirmative approach in the development of novel guidelines against cancer by using dietary species to maintain good health.
Keywords: malignancy, allicin, cinnamic aldehyde, gingerol, dipropyle disulfides, piperidine piperine
Preamble and Historical Background
Spices and herbs are woven in the history of mankind since the early civilization. These fragrant commodities have compelled eminent traders of their time to voyage across the world to find spice producing lands. The herbs and spices were often grown due to their unique association with temples and daily rituals. The earliest gardens are reported to be grown by ancient Egyptian Christian and Islamic cultures. Marco Polo and Christopher Columbus navigated routes in the search of these pungent plants. In the late 14th century Vasco da Gama a Portuguese sailor, cruised around India and returned with jewels, ginger, pepper and cinnamon. Lately, wars were broken out over Indonesian Spice Island among the European nations that last for two centuries. It is hypothesized that the garlic was grown by semi itinerant tribes in Central Asia 10,000 years ago further introduced to India and Mediterranean basin. Onion natively belongs to central Asia and entered in Egypt via trade (Engeland, 1991[28]). Cinnamon is reported as one of the oldest spices of ancient Roman and Greek era. Comprehensive evidences also support its cultivation in China since 5000 years whilst in Egypt 2000 years BC (Etoh and Simon, 2002[31]). In modern era, strong nations have taken over the spice market; USA is one of the major buyers of condiments followed by Germany, Japan and France. To date, Asia is promising producer of a variety of spices and aromatic herbs. On global scale Brazil, Guatemala, Grenada and Jamaica are well reputed for their pepper, cardamom, nutmeg, ginger and allspice (Aggarwal and Shishodia, 2004[5]).
Generally, the terms spices and herbs are collectively used to explain a wide range of aromatic plants like cinnamon (bark), cloves (buds), chilies (fruit), mustard (seeds). Nonetheless, these are not clearly differentiated in various regions of the world. American Spice Trade Association (ASAT) has described spices as dried parts of plant primarily used for food seasoning. Likewise, United States Department of Agriculture (USDA) defines spices in broader terms with few exceptions i.e. any aromatic vegetable material in its intact, broken and ground form whose main role is seasoning of food other than providing nutritional benefits without the removal of volatile oils and flavoring compounds. Spices/herbs play an effective role in food, cosmetics and perfumery industry as well as raw material for modern medicines. Their antioxidative, antimicrobial and nutritional agents have direct impact on the sugar and salt reduction, texture improvement and spoilage protection of food. They have ability to mask, deodorize and flavor the edibles as well as imparting attractive colors to the end product. Generally, black pepper is available in dehydrated intact and powdered, pepper oil and pepper oleoresin forms. Similarly, cardamom fractions like oleoresin and oil are present in market. In case of ginger, products like candy, preserves, dehydrated ginger and turmeric in its powder, oil and oleoresin form can be assessed from spice market. Moreover, mixed powdered blends of various spices are one of the popular products used in various cuisines.
Concerted efforts have been made by the researchers to delineate the role of food ingredients against various provocative ailments for establishing better health. In present scenario, cancer is one of the major causes of mortality due to body resistance against cancer preventive tools like radio- and chemotherapy. In later stages, only a few tumor types are amenable to treatment therefore it is necessary to develop some therapeutic strategies through diet modulation. From ancient times, spices have been used as preservative as well as traditional medicine owing to their disease preventing ability. They are typically characterized as aromatic plant parts including seeds, roots, pods, leaves and bark that not only provide versatility in human diet but also contribute towards hedonic response (Lampe, 2003[50]; Basnet and Skalko-Basnet, 2011[14]). They are traditionally employed for the treatment of several ailments like dermatological disorders, viral infections, inflammation, parasitic diseases etc. Spices possess ample amount of phytochemicals that play a crucial role in the human body against malignancy as terpenes, phenylproponoids, diarylheptanoids, isothiocyanates and sulfur compounds are some promising candidates. Extensive research has been carried to explore the anticancer contemporary role of species based phytochemicals against various cancer cell lines including pancreatic, colon, breast and lung (Mueller et al., 2010[65]).
Recent development in the field of anti-cancer therapy has established novel strategies to treat malignancy i.e. chemoprevention. It is the use of particular synthetic or natural compounds to reduce, suppress or reverse the incidences of carcinogenesis. Various chemopreventive approaches associated with species have gained immense attention of the researchers due to their anti-oncological perspectives. In this context, bioactive constituents are being extracted using traditional plants as spices and herbs. Curcumin, the bioprotectant of turmeric has potential to improve the sensitivity of cancer cell against various drugs (Lim et al., 2001[57]). Likewise, polyacetylenes of celery leaves protects body from the development of tumors (Christensen and Brandt, 2006[24]). In case of star anise, phenylpropanoids of prenyl group plays a crucial role in the reduction of oncogenic activities (Padmashree et al., 2007[68]). Present review article is an effort to illuminate the anticancer perspectives of spices as they induce apoptosis in tumor cells along with inhibition of angiogenesis.
Botanical Classification of Promising Spices/Herbs
Date back, food plants were gathered from wild by hunters and aborigines that domesticated them as their dietary staples. In present era, novel technologies have been developed to determine native origin and ancestry of such wild plants. Majority of the commonly consumed spices belongs to phylum Magnoliophyta or Angiosperms i.e. flowering plants with seeds enclosed in ovary. According to taxonomic arrangement, genus Allium belongs to order Asparagales (class: Monocotyledons) including garlic, onion, chive etc. To date, almost 780 species are reported in genus Allium out of which 650 species have two more names used synonymously. Technically, Allium genus is perennial with underground bulbous swollen roots. The bulb part comprises of thickened leaf blades and true scales as prophylls in onion and thickened bladeless prophylls of garlic wrapped in dry skin. Garlic (Allium sativum) was originated from its wild ancestor Allium longicupis of central Asia. The term garlic came from Anglo-Saxon word “garleac” means spear owing to its leaves shape (Estes, 2000[30]). It has segmented cloves covered with white, purple or pinkish skin. Onion (Allium cepa) is herbaceous plant with edible bulb composed of large fleshy leaves that vary in color like red, purple, yellow, brown or white and cultivated as annual plant. It belongs to lily family “Liliaceae” that comprised of edible plants like garlic, leek, chive as well as ornamental including lily, tulip, hyacinth (Fritsch and Friesen, 2002[34]; Kuhl et al., 2004[47]; Friesen et al., 2006[33]; Brewster, 2008[19]).
The members of family Zingiberaceae have significant importance among the spices owing to their aromatic nature and therapeutic potential. They are distributed throughout the tropical and subtropical regions of Asia and Far East Asia. There are almost 150 species of Zingiberaceae amongst ginger (Zingiber officinale), turmeric (Curcuma longa), cardamom (Elettaria cardamomum) are some promising members. Ginger (Zingiber officinale) is perennial herb with 30 to 100 cm height usually grown as annual plant. Its underground tuber is rhizome used as major spice and propagating part. After 5 to 7 months of rhizome development, the young part is harvested manually however, the remained crop becomes dried ginger (Ravindran et al., 2004[74]). Likewise, turmeric (Curcuma longa) is also a rhizome herbaceous perennial plant of Zingiberaceae and has close resemblance with ginger with relatively broader leaves. It was domesticated as Curcuma aromatic in South Asia nonetheless, India is one of the largest producers especially Tamil Nadu region.
Cardamom (Elettaria cardamomum) christened as “Queen of Spices" is one of the pleasantly aromatic spices of the Zingiberaceae family with evergreen erected thick stem 2 to 4 m tall perennial plant. Cardamom is ranked third most expensive spices after vanilla and saffron. It produces segmented aromatic pods or capsules with 15 to 20 seeds and is known as green or true cardamom. One of the cardamom types Amomum subulatum is generally recognized as black, Indian, Nepal or winged cardamom (Krishnan et al., 2005[46]; Reyes et al., 2006[75]).
The family Iridaceae has been documented for almost 80 genera and 1700 species with perennial herbs and shrubs as well as evergreen herbs. Saffron (Crocus sativus) a member of family Iridaceae is widely distributed throughout the southern Europe, Alps and Mediterranean region especially Irano-Turanian parts. Crocus sativus is grown in western Asia, generally used for seasoning and medicinal purposes (Saxena, 2010[77]). The genus Corcus is well adapted with environmental conditions and very low rainfall. Saffron has attained position of second most high priced spice after vanilla (Reyes et al., 2006[75]).
Black pepper (Piper nigrum) also known as “King of Spices” an important agricultural commodity is member of family Piperaceae. The black peppercorns are dried berries of woody stem creeper with oval leaves. The tiny flowers appear on pendulous spikes that transformed into spherical red fruit of 0.5 to 1 cm diameter. These are generally produced in South India. The word “pepper” was primarily derived from Sanskrit “pippali” however previously, in Latin “piper”, old English “pipor” and in German known as “pfeffer” (Dhanya et al., 2007[26]; Parthasarathy et al., 2007[70]; Bosland et al., 2012[18]). Cinnamon (Cinnamomum zeylanicum) is an ever green tropical tree of 10 to 15 m height with aromatic bark commonly consumed as spices. The true cinnamon Cinnamomum zeylanicum is originated from Sri Lanka thus previously known as Ceylon cinnamon nonetheless, Malagasy Republic and Seychelles are also major producers (Ranasinghe et al., 2002[73]; Jayaprakasha and Rao, 2011[41]). Star anise (Illicium verum) is a spice with star shaped pericarp native to northern Vietnam and southern China. It is a member of family Illiciaceae of order Illiciales with aromatic leaves and bisexual yellow axillary flowers that comprised of 7 to 15 carpels. However, fruit consists of 6 to 8 carpel joined centrally in a whorl. Each carpel of star anise fruit is rust colored, tough skinned and boat shaped with 1.25 inch length (Yong-xiu and Zheng, 2006[99]; Wang et al., 2011[94]). The comprehensive description regarding botanical classification of spices is given in Table 1(Tab. 1).
Table 1. Botanical classification of selected spices/herbs.
Phytochemicals in Spices/Herbs
Spices and herbs impart characteristic aroma, taste and color to the food product that mask undesirable odors. The volatile oils of spices/herbs are responsible for their particular flavor and smell used for the production of flavorants and colorants to intensify the appeal of edible products. The spices/herbs derived components are classified on the basis of their functional groups i.e. aldehydes, ketones, alcohols, amines, thiols, ethers, esters, terpenes constituting the volatile oils (Menon, 2000[61]). Garlic (Allium sativum) is a rich source of organosulfur compounds i.e. γ-glutamylcysteine and cysteine sulfoxide. Alliin (allylcysteine sulfoxide) constitutes almost 80 % of the cysteine sulfoxide of garlic.
When raw or crushed garlic is chopped an enzyme “allinase” is released that catalyzes the sulfonic acid formation from cysteine sulfoxides that on reaction with each other produces an unstable compound thiosulfinates or allicin (Table 2(Tab. 2)). The in vitro breakdown of allicin produces numerous fat soluble components; diallyl sulfide, diallyl disulfide (DADS) and diallyl trisulfide (DATS). Likewise, vinyldithiins, S-allylcysteine, ajoene, S-1-prpenylcysteine and S-allylmercaptocysteine are important constituents of garlic powder, oil and extracts (Amagase et al., 2001[7]).
Table 2. Active ingredients of spices.
Ginger (Zingiber officinale) is known for its distinctive flavor and odorous components that can be determined through steam volatile oils mainly monoterpene and sesquiterpene hydrocarbons and oxygenated monoterpene whilst, the pungency can be assessed by non-steam volatile compound i.e. gingerols (Ali et al., 2008[6]). The sesquiterpene hydrocarbons of ginger are zingeberene, farnesene, curcumene, b-sesquiphellandrene and bisabolene. The distinct sensory perception of ginger is also due to shogaols, a homologous of gingerols (Shukla and Singh, 2007[85]). Onion (Allium cepa) has been valued for their rich phytochemistry potentially beneficial for health. It has a combinational scheme of bioactive components i.e. flavonoids, fructans and organosulfurs. The organosulfurs are worked by their sulfur-oxygen and sulfur-sulfur linkages and produced on disruption of cell wall during cutting of onion. During their production, allinase synthesized S-alk(en)yl cysteine sulphoxides that further convert into cepaenes, thiosulfinates and lachrymatory factors. Structurally, flavonoids are polyphenols consisting of aromatic rings of C3 unit that defines subclasses like flavanones, flavonols and anthocyanins. The major flavonoids of onion include quercetin and kaempferol of subclass flavonol (Block et al., 1997[17]; Lancaster et al., 1998[51]).
In case of black pepper (Piper nigrum) major aroma producing components are volatile oils ranging from 2 to 5 % in berries. It constitutes β-pinene, α-pinene, 1-α-phellandrene, piperonal, limonene, dihydrocarveol, piperidine and β-caryophyllene. Almost 15 monoterpene hydrocarbons and 43 oxygenated components of monoterpenoid nature have been identified in volatile oil of black pepper including camphene, p-cymene, myrcene, limonene, borneol, cis-carveol, linalool, piperitone, geraniol, nerol, methyl geranate (Pino and Borges, 1999[71]; Bhardwaj et al., 2002[15]; Karsha and Lakshmi, 2010[43]).
India is one of the global leaders of turmeric production included further products like dehydrated turmeric, turmeric powder, curcuminoids and oleoresin. At industrial level, dried leaves and rhizomes of turmeric are used for the extraction of volatile oils from 5 to 6 and 1 to 1.5 %, respectively. The distinctive aroma of turmeric is imparted by its one of the major oils i.e. ar-turmerone. The turmeric oleoresin is organic extract generally used as coloring agent in various food items. The curcuminoids or curcumin is also used as coloring agent however, its acceptable dietary intake (ADI) is almost 0 to 1 mg/kg body weight per day.
Cinnamon (Cinnamomum cassia L.) is generally used as spices and recognized as folk herbal medication from number of centuries. Various in vitro and in vivo trials suggest that its phytochemicals have strong antioxidant, antiinflammatory, antimicrobial, antitumor, cholesterol lowering and immunomodulatory effects (Gruenwald et al., 2010[36]). The volatile oils from bark, leaves and roots have an array of monoterpene hydrocarbons as cinnamaldehyde, eugenol and camphor in various proportions. The bark portion contains almost 0.4 -2.8 % of volatile oils containing cinnamaldehdyde, caryophyllene, cinnamyl acetate, linalool and eugenol.
The chemical composition of cardamom varies on the basis of origin, nature and maturity stage. The seed contains 2 to 5 % of volatile components that describes its sweet and spicy taste. Additionally, the volatile oil contains α-pinene, α-phellandrene, β-pinene, myrcene, limonene, sabinene, 1,8-cineole, linalool, terpinolene, γ-terpinene, linalyl acetate, α-terpineol, terpinen 4-ol, α-terpinyl acetate, geraniol, citronellol, trans-nerolidol and methyl eugenol. Nonetheless, the peculiar aroma of cardamom is due to α-terpinyl acetate and 1,8-cineole.
Saffron has medicinally important bioactive components that also impart color and flavor to the food products. The color of saffron is owing to crocetin and crocin contents produced during saffron carotenoid degradation. Moreover, the characteristics flavor is produced as a result of safranal and glucoside oxidation (Gohari et al., 2013[35]). Star anise is one of the aromatic spices, gives burning and sweet flavor with pleasant odor due to aromatic oils. The major constituents of star anise estragole, aretrans-anethole and limonene. The phenylpropanoids of star anise may include phenylpropanoids 1-(4′-methoxyphenyl)-(1R, 2R and 1S, 2S)-propanediol] and [1-(4′-methoxyphenyl)-(1R, 2S and 1S, 2R)-propanediol.
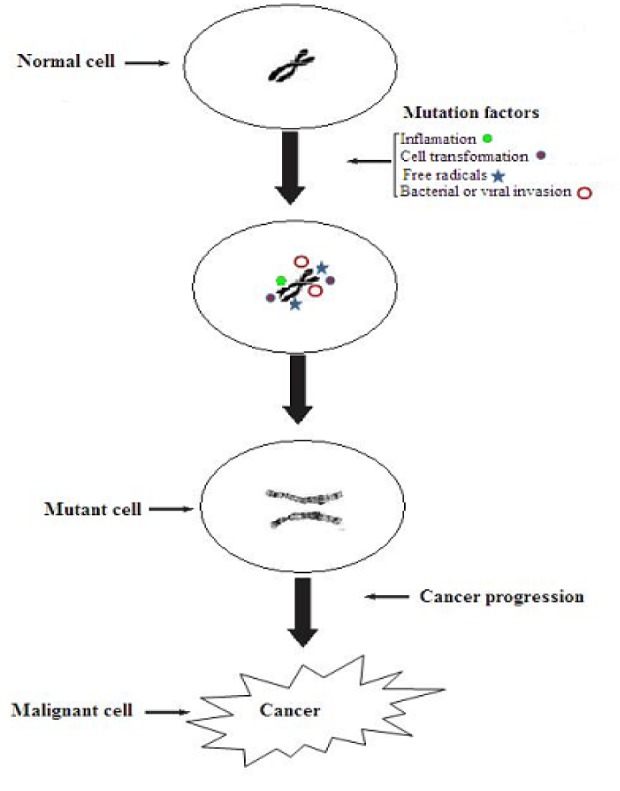
Chemoprevention is one of the highly practiced anti-oncological strategies emphasizing on synthetic or natural agents to restrict or delay neoplasia by inhibiting neoplastic inception (Sindhu et al., 2011[86]). It is extensively accepted that imbalance between oxidant and antioxidant produce harmful side effects including cancer, atherosclerosis, aging and ischemic injury. In this background, dietary antioxidants may contribute to protect cellular organelles including lipid membranes, DNA and various proteins from reactive oxygen species. Non-nutritive pungent phytochemicals of spices have chemopreventive action effective against carcinogenesis by inhibiting DNA mutagenesis and epigenesis. Extracts of a variety of spices have shown inhibiting action of lipid peroxidation. Additionally, xenobiotic enzymes i.e. drug metabolizing or biotransformation enzymes have considerable association with progression of neoplastic, toxic and mutagenic effects, resulting in carcinoma insurgence (Sengupta et al., 2004[81]; Shukla and Singh, 2007[85]).
Cancer Progression Mechanism
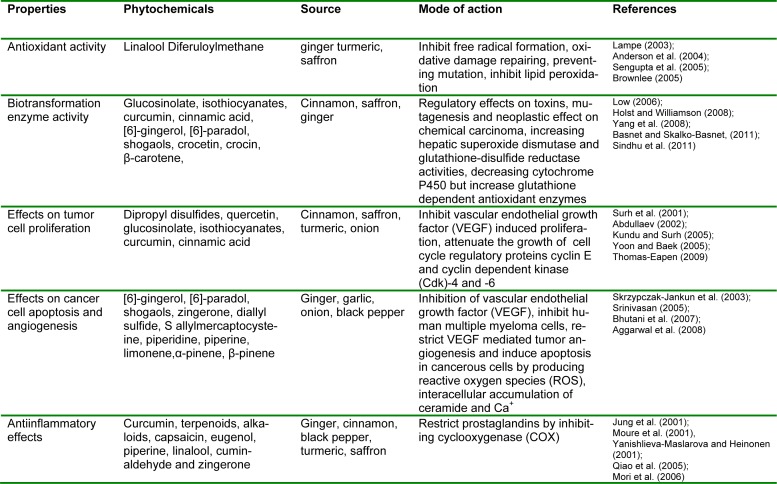
Accumulated evidences have explicated the cancer progression within the human body due to exposure of certain factors like environmental pollution, cigarette smoking and unhealthy dietary patterns. Chronic inflammation, a major cancer initiator has emerged out as one of the leading causes of cancer progression. In this scenario, cytokines [receptor activator for nuclear factor κ B ligand (RANKL) and tumor necrosis factor (TNF)], inflammatory enzymes of chemokines [urokinase plasminogen activator (uPA), matrix metalloproteinase (MMP), 5-lipoxygenase and cyclooxygenase-2], adhesion molecules and various growth factors have eventually been recognized as chronic inflammation markers. The inflammation enhancers are primarily influenced by signal transducer and activator of transcription-3 (STAT-3) however, nuclear transcription factor kappa B (NF-κB) is found in cytoplasm in its inactivated form by the action of anchorin domain containing proteins i.e. 1κBα, 1κBβ, 1κBγ, 1κBε, p105, p100 and B cell lymphoma protein-3 (Aggarwal and Shishodia, 2004[5]; Schoene et al., 2005[79]). These factors are activated by free radicals tumor promoters, carcinogens, X-rays and ultraviolet light. In activated form they induce about 200 genes expression that further cascade cellular transformation, metastasis and chemoresistance thereby causing cancer insurgence along with allied ailments as myocardial infarction, atherosclerosis, asthma, diabetes and inflammatory disorders (Thomas-Eapen, 2009[92]). Signal transducer and activator of transcription-3 (STAT-3), a member of STAT family closely linked with tumorigenesis is activated by epidermal growth factor (EGF) and interleukin-6 (IL-6) receptors and various growth factors thus leading to tumor growth. Alongside inflammation, tumor progression requires cell transformation, decreased antioxidant activity, viral or bacterial invasion, angiogenesis and metastasis etc. Free radicals present within the body due to oxidation related complications are leading cause of malignancy. It is evident that high production of reactive oxygen and nitrosative species causes oxidative and nitrosative stress, respectively (Baliga et al., 2011). Amongst, hydroxyl radical, superoxide anion radical, peroxynitrite (ONOO-), nitric oxide (NO) and hydrogen peroxide (H2O2) are causative agents of structural cell damage as in lipid and protein membranes system and DNA thus shifting healthy cells towards cancerous state as shown in Figure 1(Fig. 1).
Figure 1. Cancer progression in healthy cell.
Chemopreventive Perspectives of Some Selected Spices/Herbs
a. Garlic
Garlic (Allium sativum L.) is a multifaceted medicinal herb with antithrombotic, hypolipidemic, hypoglycemic, antiarthritic, antimicrobial and antitumor activities. Besides sulfurous compounds it also possessed arginine, oligosaccharides, flavonoids and selenium that may be responsible for maintaining good health (Ross et al., 2006[76]). Earlier, numerous research trials have explicated anti-oncogenic potential of garlic along with its various fractions as fresh extract and oil, aged garlic and organosulfur moieties. The anticancer activity of garlic is mainly due to sulfur containing constituents that have effect on various drug metabolizing enzymes, free radical scavenging activity and anti-oncogenesis (Table 3(Tab. 3)). It is noticed that extract from aged garlic has high free radical scavenging activity owing to high radical scavenging ability of its major compounds i.e. S-allylcysteine and S-allylmercapto-L-cysteine (Shon et al., 2004[82]). Additionally, organosulfur compounds of garlic especially S-allylcysteine disrupt the development of chemically induced tumors in various biological trials. Thus, garlic consumption may prevent from cancer insurgence (Thomson and Ali, 2003[93]).
Table 3. Mode of action of spices and their phytochemicals.
The rodent experimental studies have shown the antitumor aspects of garlic due to allyl derivatives that restrict carcinogenesis in esophagus, fore stomach, colon and mammary gland. Furthermore, these compounds modulate several metabolizing enzymes that stimulate cytochrome P450s or nullify glutathione S-transferases and also reduce DNA adducts in various tissues. Antiproliferative action has been discussed through cancer cell line studies, possibly facilitated by apoptosis induction and alteration in cell cycle thereby organosulfur constituents in garlic are reported as anticancer agents. However, clinical trials are still required to elaborate effectiveness dose with almost non toxic effect in humans (Omar and Al-Wabel, 2010[67]).
It is reported that progressed metastasized tumors are normally inoperable; therefore an attempt to control the oncogenic incidences through chemoprevention has appeared consistent with this notion. Presently, a substantial consideration is given to natural chemopreventive constituents that inhibit, retard or reverse the occurrence of carcinogenesis. In this context, numerous plants based dietary phenolics, are identified as anticarcinogenic and antimutagenic agents (Cerella et al., 2011[21]). Epidemiological and biological studies in cancer cell proliferation and animal modeling have proven anti-oncogenic behavior of garlic and its preparations, traditionally consumed for various human ailments around the world. Numerous processes have also explained the chemopreventive aspects of garlic based products. The DNA adducts inhibition, radical scavenging and antioxidative properties of garlic regulate cell apoptosis, proliferation and immunity. Moreover, phytochemicals of garlic modulate the cell signaling routes and arrest unwanted cell proliferation thus exhibit cancer preventive therapeutic potential (Shukla and Kalra, 2007[84]).
Several population based biological trials have presented an indirect link between garlic intake and cancerous events. For example, an analysis of seven population based studies concluded that higher intake of garlic reduced the stomach and colorectal cancer risk. Similarly, European Prospective Investigation into Cancer and Nutrition (EPIC) reported garlic consumption reduce the colon cancer risk in both men and women of various countries. Meanwhile, women that consume higher amount of garlic depicted about 50 percent reduction in colon cancer incidences than that of those who consumed lesser amount (Fleischauer and Arab, 2001[32]).
b. Ginger
The Zingiber officinale Roscoe rhizome generally recognized as ginger, is one of the widely consumed spices/condiments. Numerous preclinical studies with several assay against carcinogens have confirmed Zingiber officinale and its fractions antineoplastic and chemopreventive activities (Table 3(Tab. 3)). The trials from in experimental mice during past decade have presented that ginger extract as well as its bioactive moieties i.e. [6]-dehydroparadol, [6]-paradol, [6]-gingerol and zerumbone have cancer preventive potential. For example, ethanolic extract of ginger exhibits chemopreventive effects in the SENCAR mice (Katiyar et al., 1996[44]). Ginger extracts attenuate the tumor growth, number, size and invasion induced by 7,12-dimethylbenz[a]anthracene (DMBA) and promoted by tissue plasminogen activator (TPA) and induced hyperplasia andepidermal edema that were reduced 44 and 56 %, correspondingly (Katiyar et al., 1996[44]).
Recently, the apoptotic role of fresh, dried and steamed gingers was explored against Hela cancer cell line. The outcomes presented antiproliferative behavior of steamed ginger during 4 h at 120 °C almost 1.5 to 2-folds greater as compared to fresh and dried ginger, respectively. The greater antiproliferative effect of the steamed ginger is due to twenty-two components that were characterized in it. Moreover, the reduced concentration of gingerols and elevated levels of shogaols participates in the improvement of steamed ginger anticancer potential. The study showed the association of heating mechanism with respective constituents and resultant anticancer action for the development, optimization processed of ginger extract for chemoprevention (Cheng et al., 2011[23]).
In an experimental trial, effects of extracted ginger constituents as 6-gingerol, curcumin and labdane-type diterpene on apoptosis induction and cell proliferation was studied in the cultured human T lymphoma Jurkat cells. It was noticed that galanals A and B extracted from Japanese ginger buds of myoga (Zingiber mioga Roscoe) had potent cytotoxic impact. Cell lines exposure to galanals reported apoptosis induction through caspase-3 activation and DNA fragmentation. The damage to mitochondrial pathway probably due to its involvement in galanal-induced apoptosis resulted through cells treatment with galanals induced mitochondrial cytochrome emancipation and trans membrane potential (DCm) alteration. The antiapoptotic Bcl-2 protein was downregulated through galanal treatment alongside augmentation of Bax expression. Thus, it was evident that fractions of ginger have potential anticancerous properties (Miyoshi et al., 2003[62]).
During a biological study, the active constituents of Chinese ginger rhizomes i.e. diarylheptanoids and gingerol-related compounds were isolated followed by the assessment of their apoptotic and cytotoxic activities against human promyelocytic leukaemia (HL-60). It was observed that phytochemical extracts of ginger have preventive action against cytotoxicity of HL-60 cells (IC50 < 50 M) that is associated with cell apoptosis (Wei et al., 2005[95]). In an in vitro, anti-inflammatory mechanism of isolated ginger constituents were assessed against U937 cells, followed by exposure to Escherichia coli (1 mg/mL) lipopolysaccharide (LPS) during the absence and presence of standard compounds and organic extracts present in ginger i.e. 6-, 8-, 10-gingerol or 6-shogaol for 24 h. The supernatant resultant adducts were gathered and analyzed for their TNF-α (tumor necrosis factor alpha) and PGE2 (prostaglandin E2) production ability through ELISA assays. Purposely, major components were identified in the organic extracts including 6-, 8-, 10-shogaols and -gingerols. The gingerol standards and organic extracts did not show any cytotoxic characteristics however, fractions with shogaols were cytotoxic at 20 mg/mL and above. The unrefined organic extracts of ginger had ability to inhibit lipopolysaccharide (LPS) induced PGE2 production. Additionally in a study, approximately thirty three fractions and subfractions were analyzed for their bioactivity prepared after column chromatography. It was concluded that extracts with either gingerols or shogaols were highly potent to inhibit LPS-induced PGE2 production. Standards or extracts with major gingerols are capable to inhibit LPS-induced COX-2 (cyclooxygenase) expression. Contrarily, shogaol extracts did not have any effect on it. Thus the resultant data demonstrated inhibiting action of PGE2 production at several sites (Lantz et al., 2007[53]).
In a study regarding oral squamous carcinoma cell lines, ginger extracts i.e. 6 and 10 paradol and 3, 6 and 10 dehydroparadol significantly showed anti-oncological behavior. It was observed that these components have an inverse relation with tumor promotion (Keum et al., 2002[45]). In another similar investigation, authors recognised autophagic cell death mechanism of 6-shagol in A549 cell line of lung cancer. Wei et al. (2005[95]) suggested that treatment with 6-gingerol for 32 consecutive weeks is effective against oncological insurgencies.
c. Onion
The search for therapeutic anticancer agents from plant based foods gained attention when epidemiological studies indicated an inverse association between the dietary species intake with that of cancer insurgence (Table 3(Tab. 3)). Accordingly, sodium n-propyl thiosulfate (NPTS), alk(en)yl thiosulfates and sodium 2-propenyl thiosulfate (2PTS) are natural constituents from onion and garlic with antitumor activity. These constituents inhibit in vitro proliferation of human tumorigenic cell lines i.e. kidney embryo cell line 293, colon adenocarcinoma cell line WiDr, and acute myelocytic leukemia (AML) cell line HL-60. Generally, sodium n-propyl thiosulfate (NPTS) has less activity to inhibit cell proliferation as that of 2PTS nonetheless, absent in WiDr cells that is sensitive to these compounds. The 2PTS and NPTS result in oxidative degeneration to HL-60 cell by inducing apoptosis. The apoptosis is almost proportional to the oxidative degeneration and cytotoxicity resulted from such compounds. The outcomes depicted that alk(en)yl thiosulfates possess antitumor properties by inducing of apoptosis through oxidative stress (Chang et al., 2004[22]).
Earlier, it is reported that onion intake significantly reduces the risk of colorectal cancer. In an experimental, diet containing 5 % dried onion of American Institute of Nutrition (AIN76) was tested against carcinogenesis initiators 2-amino-3-methylimidazo[4,5-f]quinoline, azoxymethane (AOM) and N-nitroso-N-methylurea. In a research, cancer initiation was assessed by providing AOM to rats, subsequently randomized to: 5 % onion diet, AIN76 diet, phytochemicals diet+propyl-disulfide, quercetine-glycosides and oligofructose, 1 % pluronic F68 diet; a potent chemopreventive PEG-like block-polymer used as positive control. Aberrant crypt foci (ACF) were noted at initiation and promotion after injecting carcinogen. The diet containing onion was given at starting stage that subsequently reduced AOM induced ACF (p = 0.03) as well as size of quinolone (IQ)-induced ACF. After initiation, onion based diet diminished both large ACF and number. Pluronic diet and phytochemicals limit growth of ACF in a similar way. In a rat model feeding trial, it was noticed that diet with 5 % onion significantly reduces carcinogenesis throughout the study duration (Tache et al., 2006[90]).
d. Black pepper
Piperine is one of the main constituents of black pepper (Piper nigrum), consumed as traditional medicinal therapy against various ailments from antique. Numerous experimentals have shown beneficial aspects of piperine like anti-tumor, antioxidant and anti-inflammatory aspects. The protective role of piperine was tested on tumor incursion and migration, possible process involved in HT-1080 cell line. It was assessed that piperine reduces PMA elevated MMP-9 (matrix metalloproteinase-9) expression at the mRNA, protein and transcriptional levels by curbing AP-1 and NF-κB activation without altering tissue inhibitor level i.e. metalloproteinase (TIMP)-1. The piperine may hinder PMA enhanced membrane type-1 MMP expression without varying the concentrations of TIMP-1 and TIMP-2 tissue inhibitors. Moreover, it also inhibits PMA based c-Jun nuclear translocation and NF-κB, upstream of PMA-induced MMP-9 invasion. Likewise, piperine extensively suppressed PMA induced phosphorylation of ERK (extracellular signal regulated kinases), dependent on PKC (protein kinase C) pathway. Conclusively, anti-invasive behavior of piperin was also reported due to ERK and PKC reduction and phosphorylation of AP-1 and NF-κB activation. Thereby, piperine showed usefulness as good anti-tumor medication in therapeutic approaches against metastasis of fibrosarcoma (Hwang et al., 2011[40])
During a trial, black pepper extract with pure piperine (98 %) was assessed through a double blind experimental design. The comparative bioavailability was assessed at a dose level 90 and 120 mg Q10 coenzyme provided in a single dose or in separate trials of 14 and 21 days at 5 mg piperine meanwhile, measuring alterations in plasma levels. The inter subject changes were diminished by careful selection of experimental volunteers to healthy men with coenzyme Q10 fasting values 0.30 and 0.60 mg/L, respectively. The resultant data of single and the 14 day trial showed lesser rise in coenzyme Q10 concentrations of plasma in control as compared to individuals getting coenzyme Q10 with piperine as a supplement (Badmaev et al., 2000[9]).
The overexpression of permeability glycoprotein (P-gp) and multidrug resistant protein (MRP1) in cancer cells is an important process, leading to multidrug resistance (MDR) that damages chemotherapy effectiveness. The MRP1, P-gp, and BCRP are ATP-Binding Cassette (ABC) transporters that expel various lipophilic anti-oncogenic medicines thus protect cancerous cells. During an analysis, MDR reversal agents were assessed as inhibitor among various structural types of alkaloids including, piperine and piperidine alkaloid. The piperine has potential to work as anti-cancer drugs in resistant sublines as multidrug resistance A-549/DDP and MCF-7/DOX produced from A-549 and MCF-7 cell lines. The long term treatment of cancerous cells with piperine reduces corresponding ABC transporter genes transcription. The findings presented that piperine has potential to back MDR through multiple activities (Li et al., 2011[54]).
e. Turmeric
Turmeric is dried powdered rhizome of plant Curcuma longa, containing curcumin that is prime constituent having anticancer and antiinflammatory perspectives owing to its high antioxidant capacity. Curcumin referred as diferuloyl methane that is a hydrophobic polyphenol obtained from turmeric rhizome. It has potent potential to inhibit oncologial incidences of skin, fore stomach, colon, lungs and oral cavity and protect from several carcinogens and mutagens.
Extensive work has revealed its activities as cytokines releaser, antiinflammatory, immunomodulatory, antioxidant enhancing of the apoptotic and antiangiogenic. Curcumin is also considered as an initiator of radio- and chemoresistance. Various clinical trials of healthy volunteers showed anticancer aspects of curcumin along with gemcitabine (a nucleoside analog used for chemotherapy) use in treatment of pancreatic carcinoma patients. It has antiproliferative activity by enhancing the antitumoral aspects of gemcitabine. The preclinical and clinical data have shown curcumin chemopreventive activity (Bar-Sela et al., 2010[13]; Schaffer et al., 2011[78]).
Compelling evidences have enumerated chemopreventive aspects of turmeric at tissue levels when Swiss mice were fed with benzo(a)pyrene [B(a)P]. A decline in B(a)P-derived DNA adducts status was observed in lung, liver and fore stomach in rodents pretreated with various turmeric doses. It was observed that mice obtaining turmeric diet reduces 1 % B(a)P-induced activity of cytochrome P450 (CYP450), isozymes CYP 1A1 and 1A2 in target organs. Thereby, it was deducted that turmeric decreased the induction of phase-I enzymes (Thapliyal et al., 2002[91]).
In vitro, curcumin revealed as a potent anticancer agent that considerable depleting effect on blocks formation of lesions and tumor of melanoma B16F10 cells of brain and neck in experimental C57BL6 mice (Eberling et al., 2009[27]). Scientific evidences had shown that lesser solubility and rapid in vivo metabolism of curcumin is a prime reason of its effectiveness. Moreover, curcumin revealed marked effectiveness against melanoma surface antigen Muc18 when coupled with an antibody (Ab). This combination had near about 230 fold higher effectiveness against B16F10 cells elimination (Langone et al., 2011[52]).
f. Cinnamon
For example, cinnamon extracts have ability to interact with cancer and inflammation by inhibiting nitric oxide production and prostaglandin biosynthesis. In this context, cinnamon extracts inhibit the cyclooxygenase-2 (COX-2) activity in LPS-induced mouse macrophage RAW264.7 cells. Additionally, cinnamaldehyde and eugenol have ability to impede the activity of COX-2 in vitro in an enzymatic assay (Hong et al., 2002[39]).
Recently, antitumor properties of cinnamon have been meticulously related to its antioxidative and immunomodulatory aspects. In this regard, C. cassia and C. zeylanicum have prime importance for their potent oncological perspectives (Panickar et al., 2009[69]). Cinnamon extract has ability to improve insulin signaling that consequence to relegate diabetes, obesity and cancer complications. Epidemiological studies advocate the interaction of cinnamon polyphenols with decreasing incidences of cancer. In a trial, anticancer properties were explored of, polymeric water-soluble polyphenol components of cinnamon. For the purpose, myeloid cell lines including Jurkat, Wurzburg and U937 were exposed to higher concentrations of cinnamon aqueous extract (CE) for a period of 24 h. The cell cycle and growth spreading designs showed their behavior in a dose dependent manner to CE. Further, a rise in G2/M cells percentage was noted in respective cell in a way the extent of CE increased. It was observed that maximum CE dose for Wurzburg cells in G2/M was approximately 1.5 and 2.0 fold greater as compared to Jurkat and U937 cells, respectively. The Wurzburg cell line was deprived in CD45 phosphatase as well as tends towards inequities in signaling by kinase/phosphatase systems that stimulate growth. The resultant data suggested the capacity of CE to interact with phosphorylation/dephosphorylation signaling activities to lessen the cell proliferation by blocking G2/M phase of cell cycle (Schoene et al., 2005[79]).
It has been reported that phase I enzymes have an important place in activation of procarcinogens. In this context, Couturier et al. (2010[25]) assessed the anti-inflammatory role of cinnamon extract. Treatment with cinnamon extract inhibited maturation of major histocompatibility complex (MHCII) and antigen presenting cells (APCs) or integrin, alpha X (ITGAX) and dendritic cells (DCs) by suppressing expression of co-stimulatory molecules (B7.1, B7.2, ICOS-L), MHCII and cyclooxygenase (COX)-2. Cinnamon extract induced regulatory DCs (rDCs) that produce low levels of pro-inflammatory cytokines [interleukin (IL)-1β, IL-6, IL-12, interferon (IFN)-γ and tumor necrosis factor (TNF)-α] while expressing high levels of immunoregulatory cytokines (IL-10 and transforming growth factor-β). In addition, rDCs produced by cinnamon extract halted APC-dependent T-cell proliferation and converted CD4+ T cells into IL-10 high CD4+ T cells. Furthermore, oral administration of cinnamon extract inhibited development and progression of intestinal colitis by inhibiting expression of COX-2 and pro-inflammatory cytokines (IL-1β, IFN-γ and TNF-α), while enhancing IL-10 levels (Kwon et al., 2011[49]).
g. Cardamom
Imbalance in the production of reactive oxygen species (ROS) may lead toward oxidative stress that escort to various carcinogenic events (Table 3(Tab. 3)). Numerous phytochemicals, extracted from fruits, vegetables, spices and herbs showed exceptional chemopreventive effects against carcinogenesis through regulating redox status of cells during oxidative stress. Cardamom phytochemicals i.e. cineole and limonene have shown protective role against cancer progression. Thus, research related to cardamom phytochemicals and their bioavailability studies may lead to many patent applications (Acharya et al., 2010[3]).
Moreover, immunomodulatory aspects of aqueous extract were verified in vivo that significantly enhanced splenocyte proliferation in a synergistic and dose-dependent fashion. Enzyme-linked immunosorbent assay reveals that cardamom significantly suppresses T helper (Th)1 cytokine release by splenocytes. Conversely, Th2 cytokine release by splenocytes is significantly enhanced by cardamom. Previous trials suggested that cardamom extract exert anti-inflammatory role in experimental rats. Remarkably, cardamom extracts significantly enhance the cytotoxic activity of natural killer cells, indicating its anticancer potential (Majdalawieh and Carr, 2010[60]).
Cardamom extract exerts chemopreventive roles by retarding, inhibiting and reversing the multistep oncogenesis. The azoxymethane (AOM) induced colonic aberrant crypt foci (ACF) behavior of cardamom was assessed in Swiss Albino mice. Moreover, cardamom modulates the status of proliferation, modification of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression for apoptosis development. The concluding results suggested cardamom protective effects on experimentally induced colon carcinogenesis (Sengupta et al., 2005[80]). In another similar experiment, influence of essential oils of cardamom was probed to evaluate its inhibiting potential against hepatic carcinogen metabolizing enzymes (cytochrome P450, aryl hydrocarbon hydroxylase, and glutathione S-transferase) and acid-soluble sulfhydryl level. For the purpose, oil was fed by gavage at 10 µL/day for 14 days. Only nutmeg and zanthoxylum oils induced cytochrome P450 level significantly, whereas cardamom oil caused a significant reduction in its activity. However, aryl hydrocarbon hydroxylase activity was significantly enhanced only with ginger oil treatment, but contradict results were found with nutmeg oil treatments. Glutathione S-transferase activity and acid-soluble sulfhydryl were significantly elevated in all experimental groups involved in essential oils of cardamom, nutmeg, and zanthoxylum compared with controls. Generally, it is suggested that intake of essential oils alter host enzymes that is associated with activation and detoxication of xenobiotic invaded compounds, including chemical carcinogens and mutagens (Banerjee et al., 1994[12]). Moreover, essential oils from common spices like ginger, nutmeg, celery, cardamom, black pepper and cumin were assessed for their capacity to destroy DNA adducts formation through aflatoxin B1 in vitro in a microsomal enzyme-mediated reaction. The respective essential oils from all spices were found to inhibit adduct formation momentously in a dose dependent manner. The adduct formation appeared to be modulated through action on microsomal enzymes due to formation of activated metabolite of different oil. The resultant enzymatic modulation of chemical constituents of oils showed anticarcinogenic activity (Hashim et al., 1994[37]).
h. Saffron
Saffron (Crocus sutivus) is dry stigma containing tetraterpenes class of phytochemicals having principal components i.e. glycoside picrocrocin that crystallized and produce aldehyde and glucose by hydrolysis yielding characteristic bitter flavor to saffron (Abdullaev and Espinosa-Aguirre, 2004[2]). Chemical profiling revealed that crocetin and crocin as characteristic compounds of saffron responsible for cytotoxic ability on tumoral cells and inhibit the carcinomas of colon, skin and soft tissue tumor in combination with vitamin E and Cysteine. Furthermore, this combination retard cisplantin induced toxicity in rats modeling system (Abdullaev, 2002[1]). The governing mechanism behind anticancer activity is attributed to the polyene dicarboxylic acid in saffron. Similarly, glycosyl ester of crocetin also has antitumor activity. In an investigation, saffron constituents i.e. picrocrocin, crocetin, crocin and safranal were analyzed for their cytotoxic ability by animal trial. For the purpose, hela cells were treated with 0.8 and 3 mM of crocin, safranal and picrocrocin, respectively. However, crocin exhibited less cytoplasm pyknotic nuclei and cell shrinkage, showing apoptosis as compared with other two components. Thereby, crocin has inhibitory effect on growth cancerous cell thus it may act as cancer therapeutic agent (Escribano et al., 1995[29]). In a similar research, saffron extract containing dimethyl-crocetin was evaluated against human leukemia and murine tumor cell lines and noted that saffron extract reduced ascites tumor development and increased the life expectancy of mice up to 45-120 %. Additionally, it also prolongs progression of papilloma development and decreased squamous cell carcinoma. It was noted that it has also ability to decrease soft tissue sarcoma in treated mice. Furthermore, extract also inhibit nucleic acids synthesis in carcinoma development and thus concluded that dimethyl-crocetin interrupts interaction of DNA (Nair et al., 1995[66]). In an in vitro trial, Abdullaev (2002[1]) examined cytotoxic activity of saffron extract in colony forming assay and showed antimutagenic and noncomutagenic behavior. Furthermore, saffron extract showed a dose dependent inhibition in HeLa cells.
Kashmiri saffron was investigated for its in vitro and in vivo xenograft growth inhibition by isolated crocin. It was reported that crocin reduced cell viability in diffusion limited aggregation (DLA) cells with dose dependent activity. Moreover, animals administrated with spice extract treatment before induction of cancer showed 58 % increase in lifespan, whereas, about 95.6 % reduction of solid tumor in crocin treated animals was observed after 31st day of subsequent to tumor inoculation. Crocin also showed momentous impact on hematological aspects especially hemoglobin concentration and lymphocyte count (Bakshi et al., 2009[10]).
i. Star anise
Star anise (Illicium verum) closely resembles anise in flavour, obtained from the star-shaped pericarp, potentially used as therapeutic spice. Star anise has potent ability to combat numerous cancerous events. Free radical generation is one of the leading causes of numerous pathological states as cancer, diabetes mellitus, stroke, hypercholesterolemia etc., thus resulted in cellular DNA damage and initiate oncogenesis. Star anise is used as condiment in various cuisines and reported as anti-tumor potency against N-nitrosodiethylamine (NDEA) initiated and phenobarbital (PB) promoted hepato-carcinogenesis. In an experimental rodent modeling, a dose of NDEA at 200 mg/kg bw intraperitoneally as carcinogens initiator, subsequently promoted by 0.05 % PB in drinking water for 14 successive weeks. The NDEA treatment increased liver weight of rats afterwards, reduced by the treatment of star anise. A significant reduction was noticed in nodule incidence and nodule multiplicity. Moreover, star anise also reduced nodule volume and size. The long term treatment with star anise significantly lowers the lipid peroxidation (LPO) in erythrocytes and liver. The treatment also restored erythrocyte and liver superoxide dismutase (SOD) activities to a normal level in carcinogenic rodents. However, liver catalase (CAT) activity was elevated in treated groups. The erythrocyte CAT activity was augmented in star anise treated rats during initiation and promotion stages only. The level of liver glutathione (GSH) was significantly elevated in treated groups. However, the erythrocyte GSH level was dropped in rats treated with NDEA and PB that was due to star anise, which assisted in raising erythrocyte GSH. The erythrocyte and liver glutathione-S-transferase (GST) activity was increased in all the groups treated with PB and NDEA. The treatment with star anise significantly reduced the level of GST (Yadav and Bhatnagar, 2007[96]).
Numerous star anise derived products as powder, alcoholic or water soluble extracts and essential oil have documented for antioxygenic potential. Additionally, these fractions have potential to reduce oncological events, oxidative damage and elevate phase II enzymes level. Moreover, various contemporary pharmacological evidences have put forward chemopreventive activities of essential oil, polysaccharides and shikimic acid derived from star anise (Li et al., 2006[56]; Liu et al., 1997[58]; Li et al., 2008[55]).
In a clinical trial, star anise polysaccharide extracts were characterized for their antitumor perspectives. Star anise containing monosaccharide i.e. arabinose, xylose and glucose showed antitumor properties in in vivo study. Pharmacological aspects of these monosaccharide revealed Sarcoma 180 tumor growth inhibition in mice at 30.92 % dose. Conclusively, star anise reduces the tumor burden, lowers oxidative stress and increases level of phase II enzymes that may contribute to its anti-carcinogenic potential (Shu et al., 2010[83]).
Conclusion
Spices are used to make food scrumptious alongside exert beneficial physiological health effects. Further, they are also probed as therapeutic agents due to their indelible association with human as food adjunct thereby gained nutraceutical status. Numerous animal and human studies have delineated the chemopreventive role of spices owning to their phytochemicals. Liberal consumption of spices is associated with higher antioxidant status thus helpful against oxidative stress. Spices are attributed as contemporary chemopreventive tool to mitigate malignancy thus extend life expectancy. Such mechanism based therapies using functional dietary ingredients provide better understanding to the scientists against cancer insurgence. In toto, spices based on phytochemicals are becoming popular as antiproliferative agent, cancer cell growth suppressors and angiogenesis inhibitor thus strengthened their futuristic chemopreventive behavior.
References
- 1.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron and medicinal phenolic substances. Exp Biol Med. 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 2.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemopreventive trials. Cancer Detect Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Acharya A, Das I, Singh S, Saha T. Chemopreventive properties of indole-3-carbinol, diindolylmethane and other constituents of cardamom against carcinogenesis. Recent Patents Food Nutr Agric. 2010;2:166–177. doi: 10.2174/2212798411002020166. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B, Anand P. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74:1560–1569. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Shishodia S. Suppersion of the nuclear factor-κB activation pathway by spice-derived phytochemicals. Ann NY Acad Sci. 2004;1030:434–441. doi: 10.1196/annals.1329.054. [DOI] [PubMed] [Google Scholar]
- 6.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol. 2008;46:409–20. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 7.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955–962. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004;52:65–70. doi: 10.1021/jf034916b. [DOI] [PubMed] [Google Scholar]
- 9.Badmaev V, Majeed M, Prakash L. Piperine derived from black pepper increases the plasma levels of coenzyme Q10 following oral supplementation. J Nutr Biochem. 2000;11:109–113. doi: 10.1016/s0955-2863(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 10.Bakshi HA, Sam S, Feroz A, Ravesh Z, Shah GA, Sharma M. Crocin from Kashmiri saffron (Crocus sativus) induces in vitro and in vivo xenograft growth inhibition of Dalton’s lymphoma (DLA) in mice. Asian Pac J Cancer Prev. 2009;10:887–890. [PubMed] [Google Scholar]
- 11.Baliga MS, Haniadka R, Pereira MM, D’Souza JJ, Pallaty PL, Bhat H, et al. Update on the chemopreventive effects of ginger and its phytochemicals. Crit Rev Food Sci Nutr. 2011;51:499–523. doi: 10.1080/10408391003698669. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee S, Sharma R, Kale RK, Rao AR. Influence of certain essential oils on carcinogen-metabolizing enzymes and acid-soluble sulfhydryls in mouse liver. Nutr Cancer. 1994;21:263–269. doi: 10.1080/01635589409514324. [DOI] [PubMed] [Google Scholar]
- 13.Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem. 2010;17:190–197. doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- 14.Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302:645–650. doi: 10.1124/jpet.102.034728. [DOI] [PubMed] [Google Scholar]
- 16.Bhutani M, Pathak AK, Nair AS, Kunnumakkara AB, Guha S, Sethi G, et al. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res. 2007;13:3024–3032. doi: 10.1158/1078-0432.CCR-06-2575. [DOI] [PubMed] [Google Scholar]
- 17.Block E, Gulati H, Putman D, Sha D, You N, Zhao SH. Allium chemistry: synthesis of 1-[alk (en) ylsulfinyl] propyl alk (en) yl disulfides (cepaenes), antithrombotic flavorants from homogenates of onion (Allium cepa) J Agric Food Chem. 1997;45:4414–4422. [Google Scholar]
- 18.Bosland PW, Votava EJ. Peppers: vegetable and spice capsicums. 2nd. Wallingford, Oxfordshire: Cabi; 2012. (Crop Production Science in Horticulture, Book 22). [Google Scholar]
- 19.Brewster JL. Onions and other vegetable allium. In: Brewster JL, editor. The classification, origins, distribution and economic importance of the major vegetable crops. 2nd. Wallingford, Oxfordshire: CABI; 2008. [Google Scholar]
- 20.Brownlee M. The pathobiology of diabetic complications. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 21.Cerella C, Dicato M, Jacob C, Diederich M. Chemical properties and mechanisms determining the anti-cancer action of garlic-derived organic sulfur compounds. Anticancer Agents Med Chem. 2011;11:267–271. doi: 10.2174/187152011795347522. [DOI] [PubMed] [Google Scholar]
- 22.Chang HS, Yamato O, Yamasaki M, Ko M, Maede Y. Growth inhibitory effect of alk(en)yl thiosulfates derived from onion and garlic in human immortalized and tumor cell lines. Cancer Lett. 2004;223:47–55. doi: 10.1016/j.canlet.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Cheng XL, Liu Q, Peng YB, Qi L-W, Li P. Steamed ginger (Zingiber officinale): changed chemical profile and increased anticancer potential. Food Chem. 2011;129:1785–1792. [Google Scholar]
- 24.Christensen LP, Brandt K. Bioactive polyacetylenes in food plants of the Apiaceae family: occurrence, bioactivity and analysis. J Pharm Biomed Anal. 2006;41:683–693. doi: 10.1016/j.jpba.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 25.Couturier K, Batandier C, Awada M, Hininger-Favier I, Canini F, Anderson RA, et al. Cinnamon improves insulin sensitivity and alters the body composition in an animal model of the metabolic syndrome. Arch Biotechnol Biophys. 2010;501:158–161. doi: 10.1016/j.abb.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Dhanya K, Kizhakkayil J, Syamkumar S, Sasikumar B. Isolation and amplification of genomic DNA from recalcitrant dried berries of black pepper (Piper nigrum L.) - a medicinal spice. Mol Biotechnol. 2007;37:165–168. doi: 10.1007/s12033-007-0044-y. [DOI] [PubMed] [Google Scholar]
- 27.Eberling JL, Kells AP, Pivirotto P, Beyer J, Bringas J, Federoff HJ, et al. Functional effects of AAV2-GDNF on the dopaminergic nigrostriatal pathway in parkinsonian rhesus monkey. Hum Gene Ther. 2009;20:511–518. doi: 10.1089/hum.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engeland RL. Growing great garlic. Okanogan, WA: Filaree Prod; 1991. [Google Scholar]
- 29.Escribano J, Alonso GL, Coca-Prados M, Fernandez JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1995;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- 30.Estes JW. Staple foods: domesticated plants and animals: garlic. In: Kiple KF, Ornelas KC, editors. The Cambridge world history of food. Cambridge: Cambridge University Press; 2000. p. 256. [Google Scholar]
- 31.Etoh T, Simon PW. Diversity, fertility and seed production of garlic. Allium Crop Science: recent advances. Wallingford, Oxfordshire: CABI; 2002. pp. 101–118. [Google Scholar]
- 32.Fleischauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. J Nutr. 2001;131:1032–1040. doi: 10.1093/jn/131.3.1032S. [DOI] [PubMed] [Google Scholar]
- 33.Friesen N, Fritsch RM, Blattner FR. Phylogeny and new intrageneric classification of Allium L. (Alliaceae) based on nuclear rDNA ITS sequences. Aliso. 2006;22:372–395. [Google Scholar]
- 34.Fritsch RM, Friesen N. Evolution, domestication and taxonomy. Allium Crop Science: recent advances. Wallingford, Oxfordshire: CABI; 2002. pp. 5–30. [Google Scholar]
- 35.Gohari AR, Saeidnia S, Mahmoodabadi MK. An overview on saffron, phytochemicals, and medicinal properties. Pharmacogn Rev. 2013;7(13):61. doi: 10.4103/0973-7847.112850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruenwald J, Freder J, Armbruester N. Cinnamon and health. Crit Rev Food Sci Nutr. 2010;50:822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 37.Hashim S, Aboobaker VS, Madhubala R, Bhattacharya RK, Rao AR. Modulatory effects of essential oils from spices on the formation of DNA adduct by aflatoxin B1 in vitro. Nutr Cancer. 1994;21:169–175. doi: 10.1080/01635589409514314. [DOI] [PubMed] [Google Scholar]
- 38.Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19:73–82. doi: 10.1016/j.copbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Hong CH, Hur SK, Oh OJ, Kim SS, Nam KA, Lee SK. Evaluation of natural products on inhibition of indcible cycloxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J Ethnopharmacol. 2002;83:153–159. doi: 10.1016/s0378-8741(02)00205-2. [DOI] [PubMed] [Google Scholar]
- 40.Hwang YP, Yun HJ, Kim HG, Han EH, Choi JH, Chung YC, et al. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKCα/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol Lett. 2011;203:9–19. doi: 10.1016/j.toxlet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Jayaprakasha GK, Rao LJM. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit Rev Food Sci Nutr. 2011;51:547–562. doi: 10.1080/10408391003699550. [DOI] [PubMed] [Google Scholar]
- 42.Jung MY, Kang HJ, Moon A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001;165:139–45. doi: 10.1016/s0304-3835(01)00426-8. [DOI] [PubMed] [Google Scholar]
- 43.Karsha PV, Lakshmi OB. Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian J Nat Prod Resour. 2010;1:213–215. [Google Scholar]
- 44.Katiyar SK, Agarwal R, Mukhtar H. Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale rhizome. Cancer Res. 1996;56:1023–1030. [PubMed] [Google Scholar]
- 45.Keum Y-S, Kim J, Lee KH, Park KK, Surh Y-J, Lee JM, et al. Induction of apoptosis and caspase-3 activation by chemopreventive [6]-paradol and structurally related compounds in KB cells. Cancer Lett. 2002;177:41–47. doi: 10.1016/s0304-3835(01)00781-9. [DOI] [PubMed] [Google Scholar]
- 46.Krishnan S, Bhosale R, Singhal RS. Microencapsulation of cardamom oleoresin: Evaluation of blends of gum arabic, maltodextrin and a modified starch as wall materials. Carbohydr Polym. 2005;61:95–102. [Google Scholar]
- 47.Kuhl J, Cheung F, Yuan Q, Martin W, Zewdie Y, McCallum J, et al. A unique set of 11,008 onion expressed sequence tags reveals expressed sequence and genomic differences between the monocot orders Asparagales and Poales. Plant Cell. 2004;16:114–125. doi: 10.1105/tpc.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kundu JK, Surh YJ. Breaking the relay in deregulated cellular signal transduction as a rationale for chemoprevention with anti-inflammatory phytochemicals. Mutat Res. 2005;591:123–46. doi: 10.1016/j.mrfmmm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Kwon HK, Hwang JS, Lee CG, So JS, Sahoo A, Im CR, et al. Cinnamon extract suppresses experimental colitis through modulation of antigen-presenting cells. World J Gastroenterol. 2011;17:976–986. doi: 10.3748/wjg.v17.i8.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lampe JW. Spicing up a vegetarian diet: chemopreventive effects of phytochemicals. Am J Clin Nutr. 2003;78:579S–583S. doi: 10.1093/ajcn/78.3.579S. [DOI] [PubMed] [Google Scholar]
- 51.Lancaster JE, Martin LS, William MR. Differential hydrolysis of alk (en) yl cysteine sulphoxides by alliinase in onion macerates: flavour implications. J Sci Food Agric. 1998;78:367–372. [Google Scholar]
- 52.Langone P, Debata PR, Dolai S, Curcio GM, Inigo JDR, Raja K, et al. Coupling to a cancer cell‐specific antibody potentiates tumoricidal properties of curcumin. Int J Cancer. 2012;131:E569–E578. doi: 10.1002/ijc.26479. [DOI] [PubMed] [Google Scholar]
- 53.Lantz RC, Chen GJ, Sarihan M, Solyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14:123–128. doi: 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Li S, Lei Y, Jia Y, Li N, Wink M, Ma Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine. 2011;19:83–87. doi: 10.1016/j.phymed.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 55.Li SG, Wang DG, Tian W, Wang XX, Zhao JX, Liu Z, et al. Characterization and anti-tumor activity of a polysaccharide from Hedysarum polybotrys Hand.-Mazz. Carbohydr Polym. 2008;73:344–50. [Google Scholar]
- 56.Li W, Cui SW, Kakuda Y. Extraction, fractionation, structural and physical characterization of wheat b-d-glucans. Carbohydr Polym. 2006;63:408–16. [Google Scholar]
- 57.Lim GP, Chu T, Yang F, Beech W, Frantschy SA, Cole GM. The curry spice curcumin reduces oxidative damage of amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–7. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu LM, Guo ZD, Zhang J. Study on composition of supercritical CO2 fluid extract of Illicium verum Hook. f. J Instrumental Anal. 1997;16(4):24–26. [Google Scholar]
- 59.Low DT. A reason to season: the therapeutic benefits of spices and culinary herbs. Explore (NY) 2006;2:446–9. doi: 10.1016/j.explore.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Majdalawieh AF, Carr RI. In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum) J Med Food. 2010;13:371–381. doi: 10.1089/jmf.2009.1131. [DOI] [PubMed] [Google Scholar]
- 61.Menon AN. Studies on the volatiles of cardamom (elleteria cardamomum) J Food Sci Technol. 2000;37:406–408. [Google Scholar]
- 62.Miyoshi N, Nakamura Y, Ueda Y, Abe M, Ozawa Y, Uchida K, et al. Dietary ginger constituents, galanals A and B, are potent apoptosis inducers in human T lymphoma jurkat cells. Cancer Lett. 2003;199:113–119. doi: 10.1016/s0304-3835(03)00381-1. [DOI] [PubMed] [Google Scholar]
- 63.Mori A, Lehmann S, O′Kelly J, Kumagai T, Desmond JC, Pervan M, et al. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006;66:3222–9. doi: 10.1158/0008-5472.CAN-05-0087. [DOI] [PubMed] [Google Scholar]
- 64.Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H, et al. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. [Google Scholar]
- 65.Mueller M, Hobiger S, Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010;122:987–996. [Google Scholar]
- 66.Nair SC, Kurumboor SK, Haseqawa JH. Saffron chemoprevention in biology and medicine: a review. Cancer Biother. 1995;10:257–264. doi: 10.1089/cbr.1995.10.257. [DOI] [PubMed] [Google Scholar]
- 67.Omar SH, Al-Wabel NA. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm J. 2010;18:51–58. doi: 10.1016/j.jsps.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Padmashree A, Roopa N, Semwal AD, Sharma GK, Agathian G, Bawa AS. Star-anise (Illicium verum) and black caraway (Carum nigrum) as natural antioxidants. Food Chem. 2007;104:59–66. [Google Scholar]
- 69.Panickar KS, Polansky MM, Anderson RA. Cinnamon polyphenols attenuate cell swelling and mitochondrial dysfunction following oxygen-glucose deprivation in glial cells. Exp Neurol. 2009;216:420–427. doi: 10.1016/j.expneurol.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 70.Parthasarathy VA, Sasikumar B, Nair RR, George KJ. Black pepper: botany and horticulture. Horticultural Reviews - Westport Then New York. 2007;33:173–266. [Google Scholar]
- 71.Pino JA, Borges P. Los componentes volátiles de las especias. I. Métodos de obtención y de análisis. Alimentaria. 1999;301:39–45. [Google Scholar]
- 72.Qiao S, Li W, Tsubouchi R, Haneda M, Murakami K, Yoshino M. Involvement of peroxynitrite in capsaicin-induced apoptosis of C6 glioma cells. Neurosci Res. 2005;51:175–83. doi: 10.1016/j.neures.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 73.Ranasinghe L, Jayawardena B, Abeywickrama K. Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et LM Perry against crown rot and anthracnose pathogens isolated from banana. Lett Appl Microbiol. 2002;35:208–211. doi: 10.1046/j.1472-765x.2002.01165.x. [DOI] [PubMed] [Google Scholar]
- 74.Ravindran PN, Babu KN, Shiva . Ginger: the genus Zingiber. In: Ravindran PN, Babu KN, editors. Botany and crop improvement of ginger. Boca Raton, FL: CRC Press; 2004. pp. 15–85. [Google Scholar]
- 75.Reyes T, Luukkanen O, Quiroz R. Small cardamom - precious for people, harmful for mountain forests: possibilities for sustainable cultivation in the East Usambaras, Tanzania. Mt Res Dev. 2006;26:131–137. [Google Scholar]
- 76.Ross SA, Finley JW, Milner JA. Allyl sulfur compounds from garlic modulte aberrant crypt formation. J Nutr. 2006;136:852–854. doi: 10.1093/jn/136.3.852S. [DOI] [PubMed] [Google Scholar]
- 77.Saxena RB. Botany, taxonomy and cytology of Crocus sativus series. Ayu. 2010;31:374. doi: 10.4103/0974-8520.77153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schaffer M, Schaffer PM, Zidan J, Sela GB. Curcuma as a functional food in the control of cancer and inflammation. Curr Opin Clin Nutr Metabol Care. 2011;14:588–597. doi: 10.1097/MCO.0b013e32834bfe94. [DOI] [PubMed] [Google Scholar]
- 79.Schoene NW, Kelly MA, Polansky MM, Anderson RA. Water-soluble polymeric polyphenols from cinnamon inhibit proliferation and alter cell cycle distribution patterns of hematologic tumor cell lines. Cancer Lett. 2005;230:134–140. doi: 10.1016/j.canlet.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 80.Sengupta A, Ghosh S, Bhattacharjee S. Dietary cardamom inhibits the formation of azoxymethane-induced aberrant crypt foci in mice and reduces COX-2 and iNOS expression in the colon. Asian Pac J Cancer Prev. 2005;6:118–122. [PubMed] [Google Scholar]
- 81.Sengupta A, Ghosh S, Bhattacharjee S, Das S. Indian food ingredients and cancer prevention-an experimental evaluation of anticarcinogenic effects of garlic in rats colon. Asian Pac J Cancer Prev. 2004;5:126–132. [PubMed] [Google Scholar]
- 82.Shon MY, Choi SD, Kahng GG, Nam SH, Sung NJ. Antimutagenic, antioxidant and free radical activity of ethyl acetate extracts from white, yellow and red onions. Food Chem Toxicol. 2004;42:659–666. doi: 10.1016/j.fct.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Shu X, Liu X, Fu C, Liang Q. Extraction, characterization and antitumor effect of the polysaccharides from star anise (illicium verum Hook. f.) J Med Plants Res. 2010;4:2666–2673. [Google Scholar]
- 84.Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167–181. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 85.Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Sindhu S, Chempakam B, Leela NK, Bhai RS. Chemoprevention by essential oil of turmeric leaves (Curcuma longa) on the growth of Aspergillus flavus and aflatoxin production. Food Chem Toxicol. 2011;49:1188–1192. doi: 10.1016/j.fct.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 87.Skrzypczak-Jankun E, Zhou K, McCabe NP, Selman SH, Jankun J. Structure of curcumin in complex with lipoxygenase and its significance in cancer. Int J Mol Med. 2003;12:17–24. [PubMed] [Google Scholar]
- 88.Srinivasan K. Role of spices beyond food flavoring: nutraceuticals with multiple health effects. Food Rev Int. 2005;21:167–188. [Google Scholar]
- 89.Surh YJ, Na HK, Lee JY, Keum YS. Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16(Suppl):S38–41. doi: 10.3346/jkms.2001.16.S.S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tache S, Ladam A, Corpet DE. Chemoprevention of aberrant foci in the colon of rats by dietary onion. Eur J Cancer. 2006;43:454–458. doi: 10.1016/j.ejca.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 91.Thapliyal R, Deshpande SS, Maru GB. Mechanism(s) of turmeric-mediated protective effects against benzo(a)pyrene-derived DNA adducts. Cancer Lett. 2002;175:79–88. doi: 10.1016/s0304-3835(01)00675-9. [DOI] [PubMed] [Google Scholar]
- 92.Thomas-Eapen NE. Turmeric: the intriguing yellow spice with medicinal properties. Explore. 2009;5:114–115. doi: 10.1016/j.explore.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 93.Thomson M, Ali M. Garlic (Allium sativum): a review of its potential use as an anti-cancer agent. Curr Cancer Drug Targets. 2003;3:67–81. doi: 10.2174/1568009033333736. [DOI] [PubMed] [Google Scholar]
- 94.Wang GW, Hu WT, Huang BK, Qin LP. Illicium verum: A review on its botany, traditional use, chemistry and pharmacology. J Ethnopharmacol. 2011;136:10–20. doi: 10.1016/j.jep.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 95.Wei QY, Ma JP, Cai YJ, Yang L, Liu ZL. Cytotoxic and apoptotic activities of diarylhepatanoids and gingerol-related compounds from the rhizome of Chinese ginger. J Ethnopharmacol. 2005;102:177–184. doi: 10.1016/j.jep.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 96.Yadav AS, Bhatnagar D. Chemo-preventive effect of star anise in N-nitrosodiethylamine initiated and Phenobarbital promoted hepato-carcinogenesis. Chem Biol Interact. 2007;169:207–214. doi: 10.1016/j.cbi.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 97.Yang RY, Lin S, Kuo G. Content and distribution of flavonoids among 91 edible plant species. Asian Pac J Cancer Prev. 2008;17(Suppl 1):275–9. [PubMed] [Google Scholar]
- 98.Yanishlieva-Maslarova NN, Heinonen M. Sources of natural antioxidants. In: Pokorny J, Yanishlieva N, Gordon M, editors. Antioxidants in food. Boca Raton, FL: CRC Press; 2001. pp. 210–249. [Google Scholar]
- 99.Yong-xiu SU, Zheng LI. Study of climatic division approach for Illicium verum planting based on GIS in Guangxi. [J]. J Fujian College Forestry. 2006;4:014. (Ger). [Google Scholar]
- 100.Yoon JH, Baek SJ. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med J. 2005;46:585–96. doi: 10.3349/ymj.2005.46.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]