Abstract
The potential to reverse diabetes has to be balanced against the morbidity of long-term immunosuppression associated with transplantation. For a patient with renal failure, the treatment of choice is often a simultaneous transplant of the pancreas and kidney or pancreas after kidney. For a patient with glycaemic instability, choices between a solid organ or islet transplant have to be weighed against benefits and risks of remaining on insulin. Results of simultaneous transplant of the pancreas and kidney transplantation are comparable to other solid-organ transplants, and there is evidence of improved quality of life and life expectancy. There is some evidence of benefit with respect to the progression of secondary diabetic complications in patients with functioning transplants for several years.
Keywords: Chronic diseases, endocrinology, evidence-based practice, surgery
Introduction
Diabetes is the pandemic disease of the modern era, with an estimated UK prevalence of over 5 million sufferers by 2025. Ten percent of these patients have type 1 diabetes mellitus with currently over 400,000 type 1 diabetes mellitus patients in the UK, of which 29,000 are children.1,2 Despite the prevalence, morbidities and associated significant financial burden, diabetes treatment options have changed little since the introduction of injectable insulin.
To date, over 40,000 pancreas transplants have been performed globally. It remains the only known method for restoring glycaemic control and thus curing type 1 diabetes mellitus. The procedure has been shown to reduce and in some cases reverse diabetic complications. Many type 1 diabetes mellitus patients have significant renal failure, so offering simultaneous pancreatic and kidney transplant, treats both problems with a single operation. Despite this, little is known about this life-altering procedure outside the specialty of transplantation.3,4
The aim of this review is to bring pancreatic transplantation out of the specialist realm, bridging the gap between primary and secondary care, informing all practitioners and non-specialists regardless of level and background about this important procedure, so that they feel better equipped to refer suitable patients for transplantation and counsel, and support them afterwards.
Methods
The authors independently assessed the articles according to pre-determined criteria (articles in English), and suitable articles were retrieved from inception to July 2015. The OVID interface was used searching the EMBASE and MEDLINE databases. Articles were summarised identifying key features to provide a reference database of 28 articles. Other sources of data included conference proceedings and guidelines, including unpublished data from our institution (West London Renal and Transplant Centre).
History
Experimental transplantation of the pancreas in animals began in the 1890s, where it was shown that solid-organ transplantation could cure diabetes. In 1893, an attempt was made to graft three pieces of sheep pancreas into the subcutaneous tissue of a diabetic child. Despite initial success, the patient died after 3 days because of severe ketoacidosis. The first successful human pancreatic transplant was performed in 1966 by Kelly and Lilihe at the University of Minnesota Hospital 3 years after the first kidney transplant.5,6
Progression of pancreatic transplantation was slow due to ineffective immunosuppression, issues with rejection and surgical complications. Early surgical ideas promoted the drainage of exocrine secretions into the bladder, using urinary amylase to monitor function. This, however, caused multiple complications ranging from chemical urethritis and stenosis to increasing risks of perforation, reflux pancreatitis and bladder malignancy.4,7
By the early 1990s, the introduction of cyclosporine and a change in surgical technique, which involved draining pancreatic secretions into small bowel using a duodenal conduit, provided an effective method resulting in better outcomes. Over 80% of procedures are currently being performed using enteric drainage with successful outcomes.4
Currently, there are four types of pancreatic transplant.
Pancreas transplant alone: Primarily for type 1 diabetes mellitus with frequent and severe episodes of hypoglycaemia, who may be unaware, have impaired quality of life, or other issues that lead to non-compliance with insulin therapy. These patients tend to have adequate renal function and no uraemia. Patients with a glomerular filtration rate of 80–100 mL/min/1.73 m2 are unlikely to need a kidney transplant.8,9
Simultaneous pancreas–kidney transplant: Organs come from the same deceased donor. Simultaneous pancreas–kidney transplant indications have been adapted by the UK Transplant Kidney and Pancreas Advisory Group and include type 1 diabetics with end-stage renal failure requiring immediate dialysis or within 6 months.10
Pancreas after kidney transplant: Deceased donor pancreas transplant is performed after a previous, and different, living or deceased, donor kidney transplant. Pancreatic after kidney transplant is indicated for those patients who would qualify for a pancreas-alone transplant and those with a previously viable kidney allograft. The benefits include a reduced waiting time and reduced mortality rate when compared to simultaneous pancreas–kidney transplant patients.11
Simultaneous deceased donor pancreas and live donor kidney has the benefit of lower rate of delayed graft function than simultaneous pancreas–kidney transplant and significantly reduced waiting times, resulting in improved outcomes compared to patients waiting for an simultaneous pancreas–kidney transplant.10,12
Pancreas donation
The pancreas transplant waiting list in many countries is growing, with the NHS Blood and Transplant showing over 250 patients waiting for transplantation in March 2015. Donors with a body mass index greater than 35 kg/m2 have higher fatty infiltration of the pancreas, which is a risk factor for failure. Few absolute contraindications to donation exist, but donor obesity is the most common reason for organ refusal as pancreas steatosis is associated with graft pancreatitis and fistula formation, leading to poorer outcomes.3,4,13,14
The majority of pancreas grafts are retrieved from heart-beating deceased brain-dead donors, with an increase in numbers seen from non-heart-beating donors after circulatory death or from living donors. Overall, solid organs offered for pancreas transplantation remain underused due to the strict acceptance criteria of transplant units. The acceptance criteria include body mass index, age of donor, alcohol and lifestyle factors, and vary greatly between units.13,14
Procedure
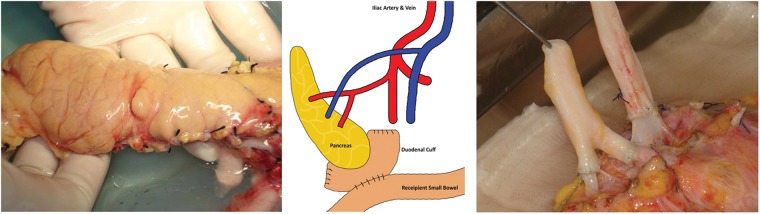
A pancreas, and a kidney if needed, from the same donor can be transplanted serially or simultaneously. The pancreas-alone procedure takes 3–4 h, while the combined procedure takes 6–8 h. The pancreas is placed on the right side of the abdomen and the kidney on the left. The native pancreas and kidney remain in place. The operation involves connecting the blood vessels of the new pancreas to the iliac vessels, which supply the lower limbs. As the pancreas has two arterial supplies, a ‘Y’ graft is created using donor arteries to allow both to be supplied from a single arterial anastomosis. In addition, a connection between the small intestine and the pancreas is made to drain the digestive juices that the pancreas produces; this is done using a piece of donor duodenum which is already joined to the pancreatic head. All of this is performed through either one incision in the abdomen or two along either groin15 (Figure 1).
Figure 1.
Solid organ pancreas with vessel extension and diagramatic representation of operation.
Current eligibility for transplant (Table 1) is strict, but invariably requires the patient to have insulin-controlled diabetes. NHS Blood and Transplant acknowledge that these criteria may exclude a minor subset of patients who would be appropriate for transplantation. These cases are assessed on an individual basis by the Pancreas Advisory Group exemptions panel after being approached by the local transplant centre. Age is not an absolute contraindication to surgery; however, the majority of patients are aged less than 65 years as potential to reverse diabetic complications reduces with age. Rejection rates remain lower in younger recipients, and older recipients have an increased rate of post-operative complication with longer length of hospital stay.4,13,15
Table 1.
Eligibility criteria for pancreatic transplantation.
| Transplant type | Eligibility |
|---|---|
| Pancreas transplant alone | • Insulin-treated type 2 diabetes mellitus with body mass index ≤30 kg/m2 or • Type 1 diabetes mellitus with at least two severe hypoglycaemic states within last 24 months and be specially assessed as having disabling hypoglycaemia |
| Simultaneous kidney pancreas transplant | • Pancreas transplant alone requirements and • Receiving dialysis or glomerular filtration rate <20 mL/min |
Various antibody induction therapies and complex immunosuppression regimes are used, which include CD25 antibody Basiliximab and Alemtuzumab that targets CD52, and are also used in the treatment of chronic lymphocytic leukaemia. The reduced ability to fight infection requires vigilance to avoid sepsis. Patients are often spared steroids where possible and discharged on Tacrolimus and Mycofenolate Mofetil, most commonly, to maintain immunosuppression. The common side effects of the immunosuppressant drugs include tremors, increased blood pressure, hair loss, mood changes and neutropenia.16,17 They will also usually be taking antimicrobial and antiviral agents for up to 6 months following transplant.
Complications
As in all surgery, there is a risk of bleeding, occurring in approximately 5% of patients. The most common cause of non-immunogenic graft failure is thrombosis with a reported incidence in literature of 10–35%, but the appropriate use of anticoagulants such as Heparin, Dextran or Epoprostenol aims to reduce the rate of thrombosis.18
Other risks include enteric anastomotic leak, graft pancreatitis, pancreatico-enteric fistula and intra-abdominal sepsis. These may increase length of stay in hospital and require further intervention. Further risks are similar to other surgical complications associated with large operations.15 Careful observation, regular imaging, use of anticoagulants and antimicrobial agents all minimise complications through prophylaxis and early detection. Optimising patient and organ selection are also important for success.
Cardiac morbidity and postoperative infections are the most common issues faced by recipients. Coronary artery disease should be treated early, with routine angiogram and echocardiograms becoming part of the preoperative assessment as 30% of asymptomatic patients with type 1 diabetes mellitus have substantial coronary stenosis on angiography.19
Rejection rates are in the range of 5–25% depending on what immunosuppressive regime is used, with Tacrolimus and Mycofenolate Mofetil being the most common agents used to maintain immunosuppression. Acute rejection is an important risk factor for developing chronic rejection (10% for pancreas transplant alone and 4% for simultaneous pancreas–kidney transplant). Treatment with Methylprednisolone or antithymocyte globulin is commonly used in this instance. This said, more than 90% of pancreas rejection episodes are reversible in the absence of hyperglycaemia. But once hyperglycaemia occurs, it is associated with a low probability of reversal of rejection, making monitoring of blood sugar an important.4,16
Patient survival
Patient survival has now reached more than 96% at 1 year after transplant, and more than 83% at 5 years after transplant, with the longest surviving graft recorded as simultaneous pancreas–kidney transplant 26 years, 24 years pancreas after kidney and 23 years for pancreas transplant alone.4
Pre-emptive simultaneous pancreas–kidney transplant recipients have better survival outcomes compared to those already established on dialysis. The main advantage of simultaneous pancreas–kidney transplant apart from it being a cost-effective option is the overall success rate of grafts as acute rejection is easier to detect in both organs using serum creatinine as a marker.4 Acute rejection rates are similar for simultaneous pancreas–kidney transplant and pancreas after kidney, estimated at 4% and 4.3%, respectively.15,26
Simultaneous pancreas–kidney transplant has been shown to increase observed versus expected lifespan, when compared to kidney transplant alone. Simultaneous pancreas–kidney transplant recipients had the highest longevity, 23.4 years compared with 20.9 years for live kidney transplant and 12.8 years for deceased donor kidney transplant.15,26
Evidence shows pancreas after kidney recipients have improved patient and kidney graft survival as a result of pancreas transplantation, with significantly higher glomerular filtration rates seen in pancreas after kidney patients compared with kidney transplant alone.11
Patients often experience significant improvements in quality of life.20 A greater satisfaction with life, health, social and sex life, having more feelings of control and independence and better perceptions of social and mental health have been documented.20 There also seems to be a strong consensus that immunosuppressive regimes are easier to manage than type 1 diabetes mellitus.21
Biological improvement
The Diabetes Control and Complications Trial showed pancreas transplantation lowers HbA1c to within normal limits even after 10 years, with mean HbA1c at 6 years being 42 mmol/L compared to 55 mmol/L of those being treated by intensified insulin regimes, thus restoring glycaemic control, with several studies reporting improvement of lipid metabolism.22
The established lesions of diabetic nephropathy had been considered to be irreversible, but in 1998, Floretto et al.23 published a series of eight type 1 diabetic patients with advance nephropathy who underwent pancreas transplantation, having serial biopsies at time of transplant, 5 years and 10 years post-transplant.
The diabetic glomerulopathy lesions, unchanged at 5 years post-pancreas transplantation, significantly improved after 10 years, with complete normalisation of glomerular structure, reduction in thickness of basement membrane and improved creatinine clearance in most patients, suggesting the human kidney has the potential to remodel glomerular and tubular structures promoting healing.23
Pancreas transplantation restores glucagon secretion, returns hepatic glucose production to normal and improves lipid profiles. This translates into clinical improvements with respect to diabetic nephropathy, neuropathy, gastroparesis, retinopathy (microvascular and macrovascular disease), cardiac function and sexual function.24
Patient follow-up
Regular examination and blood are an important part of the post-hospital period, both in the outpatient and primary care setting. In the community, vigilant practice and effective communication with the transplant centre can help with diagnosing complications such as sepsis and rejection. Monitoring of blood sugars and amylase levels is essential as sharp rises in these markers are signs of problems. Having a low threshold for the treatment for infection, appropriate vaccinations during travel and the assessing patients promptly are essential in avoiding hospital stays. Drug interactions and poly-pharmacy are another important issue practitioners need to be aware of when managing patients.
Islet cell transplantation
Currently, implantation remains a technically much simpler procedure, avoiding the complications and risks associated with solid-organ transplant. However, the refinement and isolation of islet cells have been a limiting factor, with studies quoting from 300,000 to 750,000 islets required for 70% of patients to be insulin independent. Some studies suggest around 1 million islets are needed for an average islet transplantation. Up to six purified donor pancreases are required to generate 1 million islets, which becomes an issue when donor organs are in short supply.25
A major difference between islets and pancreas transplantation is that the primary goal of current islet trials is not insulin independence but reduced incidence and severity of hypoglycaemic events and reduction in insulin requirement, which is a difficult concept to digest in view of the sensitisation effect, as several doses of islets may need to be delivered in order to achieve full insulin independence.26
Future of pancreatic transplantation
Segmental pancreatic transplant from live donors has been reported in the USA. The success of this concept potentially bypasses the issues surrounding organ shortage, in the same way live kidney transplant and live segmental liver transplant have provided further options for patients on the waiting list.27
As technology advances, new devices to improve blood glucose monitoring and insulin therapy are being developed to reduce the risk of hypoglycaemia. Continuous glucose monitoring through a closed-loop pump are aiming to become an artificial pancreas although currently are still not yet refined.26,28
Advances over the last decade suggest that generating functional beta-cells from stem cells is achievable. However, there are aspects of beta-cell development including the signalling pathways that instruct endocrine progenitor cells to differentiate into mature and functional beta-cells which remain poorly understood. These ideas combined with ideas of biological printing using the extracellular matrix as a scaffold to recreate a new biological pancreas are exciting, and although have their challenges may change the way transplant is performed.28
Conclusion
Pancreatic transplantation is growing and has quickly become the gold standard of care for patients with type 1 diabetes mellitus and renal failure. Significant improvements in quality of life and life expectancy make pancreatic transplant a viable and economically feasible intervention. It remains the most effective method of establishing and maintaining euglycaemia, halting and potentially reversing complications associated with diabetes.
Being better informed about the benefits and risks of this procedure allows doctors to advise, inform and refer patients for transplant and to manage the challenges their patients go on to face.
A patient’s view
I have been type 1 diabetic since the age of 7 years, although it was only with the onset of my renal failure, that it became more troublesome.
I found the idea of curing diabetes exciting and found the surgical process fairly easy to manage and recover from.
The pancreatic transplant was a real life- changer not only for me but, more importantly, for my friends and family.
My sugar control was good but my parents and partner always worried it might drop and something might happen to me and I would be unable to get help.
My employers also, I think, were always con cerned that my sugar might drop at work. Having to always worry and think about blood sugars is hard and was imprinted on me from an early age.
I have lived with the dichotomy of keeping tight control on my blood sugars so as not to shorten my life but also to make sure I do not let anyone down.
Much more than not having to inject or check blood sugar, the removal of this stress on me, my employers and my friends and family has improved my life significantly. I wish more people knew how to get access to treatment, and more GPs were aware of it.
Information for patients
Declarations
Competing interests
None declared
Funding
None declared
Ethical approval
Written informed consent for publication was obtained from the patient.
Guarantor
VP
Contributorship
SD initiated the concept. SD and YO performed the literature search, with GE and VP reviewing results. SD wrote the first draft of the manuscript, with all authors equally contributing to the script and GE and VP acting as supervising consultants.
Acknowledgements
None
Provenance
Not commissioned; peer-reviewed by Luigi Bonavina and Elroy Weledji
References
- 1.http://www.diabetes.org/diabetes-basics/diabetes-statistics/ (2013, accessed 12 February 2016).
- 2.https://www.diabetes.org.uk/Global/Homepage/News/FINAL%20NPDA%20Report%202014%20FOR%20WEB.pdf (accessed 12 February 2016).
- 3.Gruessner AC and Gruessner RWG. Pancreas transplant outcomes for United States and non United States cases as reported to the United Network for Organ Sharing and the International Pancreas Transplant Registry as of December 2011. Clin Transpl 2012:23–40. [PubMed]
- 4.Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233: 463–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams P. Notes on diabetes treated with extract and by grafts of sheep’s pancreas. Br Med J 1894; 2: 1303–1304. [Google Scholar]
- 6.Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61: 827–837. [PubMed] [Google Scholar]
- 7.Hickey DP, Bakthavatsalam R, Bannon CA, O’Malley K, Corr J, Little DM. Urological complications of pancreatic transplantation. J Urol 1997; 157: 2042–2048. [PubMed] [Google Scholar]
- 8.Scalea JR, Butler CC, Munivenkatappa RB. Pancreas transplant alone as an independent risk factor for the development of renal failure: a retrospective study. Transplantation 2008; 86: 1789–1794. [DOI] [PubMed] [Google Scholar]
- 9.Odorico JS, Voss B, Munoz DR. Kidney function after solitary pancreas transplantation. Transplant Proc 2008; 40: 513–515. [DOI] [PubMed] [Google Scholar]
- 10.Rayhill SC, D’Alessandro AM, Odorico JS. Simultaneous pancreas-kidney transplantation and living related donor renal transplantation in patients with diabetes: is there a difference in survival? Ann Surg 2000; 231: 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruessner AC, Sutherland DE, Dunn DL. Pancreas after kidney transplants in posturemic patients with type I diabetes mellitus. J Am Soc Nephrol 2001; 12: 2490–2499. [DOI] [PubMed] [Google Scholar]
- 12.Farney AC, Cho E, Schweitzer EJ. Simultaneous cadaver pancreas living-donor kidney transplantation: a new approach for the type 1 diabetic uremic patient. Ann Surg 2000; 232: 696–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthusamy AS, Vaidya A. Expanding the donor pool in pancreas transplantation. Curr Opin Organ Transplant 2011; 16: 123–127. [DOI] [PubMed] [Google Scholar]
- 14.http://www.odt.nhs.uk/pdf/pancreas_selection_policy.pdf (accessed 12 February 2016).
- 15.Sollinger HW, Odorico JS, Knechtle SJ, D’Alessandro AM, Kalayoglu M, Pirsch JD. Experience with 500 simultaneous pancreas-kidney transplants. Ann Surg 1998; 228: 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arbogast H, Malaise J, Fernandez-Cruz L, Saudek F. EURO SPK Group. Tacrolimus compared with ciclosporin microemulsion in primary simultaneous pancreas-kidney (SPK) transplantation: 3 year results of the EURO-SPK trial. Am J Transplant 2008; 5: 267–267. [Google Scholar]
- 17.Stegall MD, Simon M, Wachs ME, Chan L, Nolan C, Kam I. Mycophenolate mofetil decreases rejection in simultaneous pancreas-kidney transplantation when combined with tacrolimus or cyclosporine. Transplantation 1997; 64: 1695–1700. [DOI] [PubMed] [Google Scholar]
- 18.Drachenberg CB, Papadimitriou JC, Farney A. Pancreas transplantation: the histologic morphology of graft loss and clinical correlations. Transplantation 2001; 71: 1784–1791. [DOI] [PubMed] [Google Scholar]
- 19.La Rocca E, Fiorina P, Di C. Cardiovascular outcomes after kidney-pancreas and kidney-alone transplantation. Kidney Int 2001; 60: 1964–1971. [DOI] [PubMed] [Google Scholar]
- 20.Adang EM, Engel GL, van Hooff JP, Kootstra G. Comparison before and after transplantation of pancreas-kidney and pancreas-kidney with loss of pancreas—a prospective controlled quality of life study. Transplantation 1996; 62: 754–758. [DOI] [PubMed] [Google Scholar]
- 21.Milde FK, Hart LK, Zehr PS. Pancreatic transplantation. Impact on the quality of life of diabetic renal transplant recipients. Diabetes Care 1995; 18: 93–95. [DOI] [PubMed] [Google Scholar]
- 22.Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014; 37: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fioretto P, Steffes M, Sutherland D, Goetz F, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Eng J Med 1998; 339: 69–75. [DOI] [PubMed] [Google Scholar]
- 24.Robertson RP, Sutherland DE, Kendall DM, Teuscher AU, Gruessner RW, Gruessner A. Metabolic characterization of long-term successful pancreas transplants in type I diabetes. J Investig Med 1996; 44: 549–555. [PubMed] [Google Scholar]
- 25.Ryan EA, Lakey JR, Rajotte RV. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 2001; 50: 710–719. [DOI] [PubMed] [Google Scholar]
- 26.Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud 2011; 8: 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruessner RW, Sutherland DE. Simultaneous kidney and segmental pancreas transplants from living related donors – the first two successful cases. Transplantation 1996; 61: 1265–1268. [DOI] [PubMed] [Google Scholar]
- 28.Yagi H, Soto-Gutierrez A, Kitagawa Y. Whole-organ re-engineering: a regenerative medicine approach in digestive surgery for organ replacement. Surg Today 2013; 43: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]