Abstract
Background:
Quantitative magnetic resonance imaging (MRI) techniques, such as T2 and T2 star (T2*) mapping, have been used to evaluate ligamentous tissue in vitro and to identify significant changes in structural integrity of a healing ligament. These studies lay the foundation for a clinical study that uses quantitative mapping to evaluate ligaments in vivo, particularly the posterior cruciate ligament (PCL). To establish quantitative mapping as a clinical tool for identifying and evaluating chronic or acute PCL injuries, T2 and T2* values first must be determined for an asymptomatic population.
Purpose:
To quantify T2 and T2* mapping properties, including texture variables (entropy, variance, contrast, homogeneity), of the PCL in an asymptomatic population. It was hypothesized that biomarker values would be consistent throughout the ligament, as measured across 3 clinically relevant subregions (proximal, middle, and distal thirds) in the asymptomatic cohort.
Study Design:
Cross-sectional study; Level of evidence, 4.
Methods:
Unilateral knee MRI scans were acquired for 25 asymptomatic subjects with a 3.0-T MRI system using T2 and T2* mapping sequences in the sagittal plane. The PCL was manually segmented and divided into thirds (proximal, middle, and distal). Summary statistics for T2 and T2* values were calculated. Intra- and interrater reliability was assessed across 3 raters to 2 time points.
Results:
The asymptomatic PCL cohort had mean T2 values of 36.7, 29.2, and 29.6 ms in the distal, middle, and proximal regions, respectively. The distal PCL exhibited significantly higher mean, variance, and contrast and lower homogeneity of T2 values than the middle and proximal subregions (P < .05). T2* results exhibited substantial positive skew and were therefore presented as median and quartile (Q) values. Median T2* values were 7.3 ms (Q1-Q3, 6.8-8.9 ms), 7.3 ms (Q1-Q3, 7.0-8.5 ms), and 7.3 ms (Q1-Q3, 6.4-8.2 ms) in the distal, middle, and proximal subregions, respectively.
Conclusion:
This is the first study to identify T2 and T2* mapping values, and their texture variables, for the asymptomatic PCL. The distal third of the PCL had significantly greater T2 values than the proximal or middle thirds.
Clinical Relevance:
T2 and T2* values of the asymptomatic PCL can provide a baseline for comparison with acute and chronic PCL injuries in future studies.
Keywords: posterior cruciate ligament, MRI, T2 mapping, T2* mapping, asymptomatic
Although conventional magnetic resonance imaging (MRI) is an accurate qualitative tool for detecting acute posterior cruciate ligament (PCL) injuries, it is less reliable in the diagnosis of chronic injuries; the ligament may appear to be healed when it is in fact functionally deficient,26,31 and PCL stress radiographs are often necessary to determine the functional integrity of the PCL.13 Tewes et al31 reported that a number of PCLs presenting with chronic injury showed low intensity on conventional MRI. Furthermore, the functional status of the chronically injured PCL-deficient knees could not be determined using these conventional MRIs.31 Servant et al26 evaluated chronically injured PCLs in 10 knees with 7 experienced musculoskeletal radiologists reading the scans and determined an accuracy of diagnosing the PCL injury of 57% using conventional MRI. The authors postulate that healing in continuity may occur and produce an intact yet “lax” ligament in chronic PCL cases, lending diagnosis via conventional MRI difficult. Furthermore, in a study by Orakzai et al23 evaluating whether chronic PCL injuries heal in a lengthened state, they were able to identify, retrospectively, a cohort of 6 patients who were diagnosed unequivocally with a chronic PCL injury through physical examination but who had normal MRI readings. Although the primary purpose of the study was not to identify sensitivity of MRI in diagnosing a chronic PCL injury, it does highlight the current difficulties when relying on conventional MRI.23 However, with the advent of higher magnetic field strengths (ie, 3.0 T and investigational/early clinical use 7.0-T magnets) and new imaging protocols, MRI can now be used as a quantitative tool to measure tissue quality.8,18–21 This is done using biochemically sensitive imaging sequences to evaluate such parameters as water content, fibral alignment, and tissue density.21 Quantifiable results of the structural integrity and health of the PCL are needed to add to and support visual interpretation during clinical diagnosis and are especially needed in the case of a chronic injury.14,17
One such noninvasive quantitative MRI technique, T2 mapping, has demonstrated sensitivity to water content and the integrity of the proteoglycan collagen matrix of articular cartilage8,18 and has seen widespread use because it is readily available on most scanning platforms. T2 star (T2*) mapping, another technique, describes the transverse relaxation of an imaged tissue and has demonstrated similar sensitivity to water content and matrix organization. T2* values may be more sensitive in evaluating tissues such as ligaments and tendons with very rapid MRI signal decay and short time constant properties compared with the longer time constants typically evaluated with T2 mapping. Mamisch et al19 demonstrated that both T2 and T2* values reflect similar changes in osteoarthritic articular cartilage and seem to be well correlated. Biercevicz et al2 recently demonstrated the capabilities of volume and gray-scale values from high-resolution T2*-weighted MRI scans to predict the structural properties of the healing anterior cruciate ligament (ACL) or reconstruction graft in a porcine model. This study may be a critical step in the development of a noninvasive method to predict the structural properties of the healing ACL repair or graft. Interestingly, this technique may prove valuable as a surrogate outcome measure in preclinical animal and clinical studies.1,2 Biercevicz et al3 continued this work to analyze the T2* values of the intact PCL in harvested ovine knees after ACL transection surgery; however, the purpose of their study was to compare 2 mapping methodologies, and they did not report numerical results. In addition, quantitative MRI using T2 mapping has been used to measure structural variation and water content within a rabbit Achilles tendon under various loads.12,32 These studies have laid the foundation for a clinical study that uses quantitative mapping to evaluate the structural integrity of ligamentous tissue in vivo.
Texture analysis methods such as gray-level co-occurrence matrix (GLCM) can be used to extract information about the local spatial arrangement of the T2 or T2* signal. Specific GLCM features, such as entropy, contrast, variance, and homogeneity, use the spatial relationships of similar gray tones to characterize the underlying structure of a given tissue and may provide a more comprehensive understanding of the ligament structure than solely mean T2 or T2* values.11,15,28,29
The purpose of this study was to measure the MRI biomarker T2 and T2* values, and their texture properties (entropy, variance, contrast, and homogeneity), of the PCL in a rigorously prescreened asymptomatic PCL cohort using clinically relevant subregions for analysis. It was hypothesized that the biomarker values would be consistent throughout the ligament, as measured across 3 clinically relevant subregions (proximal, middle, and distal thirds) in the asymptomatic cohort. T2 and T2* mapping values, including texture variables, are yet to be established in the PCL in vivo.
Methods
Subjects
This study was approved by the institutional review board of the Vail Valley Medical Center, and informed consent was obtained from all individual participants included in the study. Thirty asymptomatic volunteers were prospectively enrolled in this study (mean age, 39.8 ± 12.7 years; 16 females and 14 males). Subjects were deemed asymptomatic through an objective clinical examination, subjective score, and morphologic MR evaluation, together with a thorough physical examination of the knee by a fellowship-trained sports orthopaedic surgeon. The examination included an evaluation of limb alignment and range of motion, identification of any areas of pain or tenderness, and the following specific tests: Lachman test, drawer tests (anterior, anterolateral, posterior, and posterolateral), pivot and reverse pivot shift tests, and evaluation of medial and lateral joint opening. Exclusion criteria included symptoms (eg, pain, stiffness, and swelling exceeding mild levels) in the knee and/or hip of the imaged side, prior injury or surgery in the knee and/or hip of the imaged side, history of inflammatory arthritis or infection within the joint of interest, negative test results during the physical examination, and evidence of PCL pathology from a conventional morphological MRI examination read by a fellowship-trained musculoskeletal radiologist.
Image Acquisition
Unilateral knee images were acquired on a 3.0-T MRI system (Magnetom Verio; Siemens Healthcare). The asymptomatic knee for each volunteer was imaged using a 15-channel multielement phased-array knee coil (Quality Electrodynamics). Volunteers were positioned in the supine position with the knee of interest extended and firmly fixed and the joint space centered in the coil. The time delay between lying down for the MR session and the beginning of the first sequence was limited to less than 5 minutes in all volunteers.
A standard clinical MRI examination protocol with T2 mapping and T2* mapping sequences was used. The scanning protocol consisted of (1) a fat-suppressed proton density turbo-spin echo scan in the coronal plane, (2) a proton density turbo-spin echo scan in the coronal plane, (3) a fat-suppressed proton density turbo-spin echo scan in the sagittal plane, (4) a T2-weighted turbo-spin echo scan in the axial plane, (5) a multiecho spin-echo T2 mapping scan in the sagittal plane (repetition time/echo time, 2000/10.7-74.9 ms; voxel size, 0.6 × 0.6 × 2 mm; field of view, 80 mm; acquisition time, 4 minutes 44 seconds), and (6) a multiecho T2* mapping scan in the sagittal plane (repetition time/echo time, 575/3.25-34.52 ms; voxel size, 0.6 × 0.6 × 2 mm; field of view, 80 mm; acquisition time, 2 minutes 29 seconds). Mapping sequences were acquired at the end of the examination to ensure the maximum amount of unloading of the joint had been reached.
Image Analysis
Segmentations of the PCL were derived manually using image analysis software (Mimics; Materialise) by 2 orthopaedic surgeons and 1 musculoskeletal radiologist at 2 different time points separated by 1 month to assess intra- and interrater reliability. The PCL segments were exported as a binary image series, and custom software was used to isolate the T2 and T2* values in those regions (MATLAB; Mathworks). To divide the PCL accurately and reproducibly into 3 subregions, 3-dimensional (3D) PCL volumes were created from the binary image series using custom software (MILXView; CSIRO), in which a marching cube surface of the PCL surface was extracted and smoothened using a windowed sinc function with passband of 0.1.30 A 3D centerline that followed the length of the ligament was extracted and used to divide the PCL into proximal, middle, and distal thirds (Figure 1). Summary statistics (median and quartiles, mean ± standard deviation) and texture variables (entropy, variance, contrast, and homogeneity) for T2 and T2* values, in each third of the ligament, were compiled for further analysis. Texture variables were measured on a slice-by-slice basis by creating a GLCM for each subregion.11 For each texture variable, the GLCM values were calculated at orientations of 0°, 45°, 90°, and 135° with an offset of 1 pixel,11,28 and the orientation values were then averaged.15,29
Figure 1.
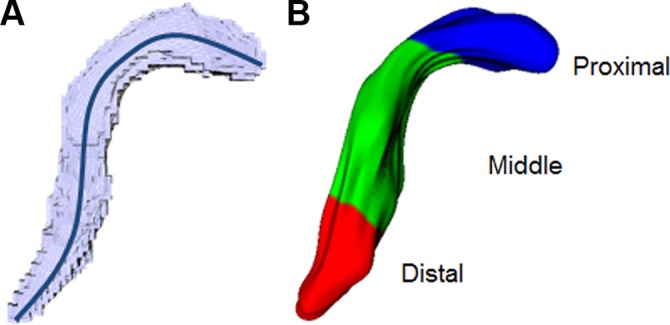
Posterior cruciate ligament (PCL) volumes were created from the binary image series for each subject. For each volume, (A) a 3-dimensional centerline that followed the length of the ligament was extracted, and (B) the centerline was divided to create proximal, middle, and distal thirds of the PCL.
Statistical Analysis
The primary goal of this study was to determine normative T2 and T2* values and texture variables for the PCL among asymptomatic subjects. Thorough summary statistics including the mean with its 95% confidence interval, minimum, and maximum were presented. To address the hypothesis that values would be consistent across the different subregions, repeated-measures (subregion) linear mixed-effects models were constructed using the MIXED procedure in SPSS. To reflect the possibility that subregions might correlate with one another in a heterogeneous manner, an unstructured covariance matrix was assumed. Pairwise comparisons among the 3 subregions were made with the Bonferroni method. Model assumptions and possible outliers were assessed using residual analysis.
Intra- and interrater reliability of median T2 values were assessed with the 2-way random effects, single-measures, intraclass correlation coefficient (ICC). All other analyses used data from the first segmentation round of the musculoskeletal radiologist. ICC values were interpreted as follows: ICC < 0.40, poor agreement; 0.40 < ICC < 0.75, fair to good agreement; ICC > 0.75, excellent agreement.10 Bonferroni-adjusted P values for subregion comparisons less than .05 were deemed significant, and all analyses were performed using SPSS Statistics, version 20 (IBM Corp).
Results
Of the 30 asymptomatic subjects enrolled in the study, 4 subjects were excluded because of prominent pulsation artifact issues from the popliteal artery that obscured the PCL in their MR images, and 1 subject was excluded for having a clinically silent chronic partial tear in the ligament that was identified on their clinical MRI during analysis. This left 25 subjects whose data were used in the final analysis, of which 11 were male and 14 were female. The mean age of the entire group was 40.6 ± 13.0 years; however, males (33.8 ± 9.1 years) were significantly younger than females (46.0 ± 13.4 years), t(22.6) = 2.702, P = .013. Because of this relationship, a formal analysis of association between quantitative imaging values and subject age and sex was not pursued.
Summary and texture variables for T2 were reasonably normally distributed. Table 1 presents means, 95% CIs, and extreme values for each variable and each subregion. The PCL had mean T2 values of 36.7 ms (95% CI, 32.7-40.6; range, 20.3-53.2) in the distal subregion, 29.2 ms (95% CI, 26.3-32.1; range, 19.2-46.3) in the middle subregion, and 29.6 ms (95% CI, 26.8-32.3; range, 19.7-48.0) in the proximal subregion (Figure 2). Pairwise comparisons within the mixed-effects models found that the distal PCL exhibited significantly higher mean, standard deviation, variance, and contrast of T2 values compared with both the middle and proximal subregions (each P < .05) (Figure 3). Additionally, the distal PCL had significantly greater median T2 values than the middle subregion (P < .001). Meanwhile, homogeneity was significantly lower in the distal PCL compared with middle (P < .001) and proximal PCL (P = .013), and entropy was significantly lower in the distal PCL than the middle PCL (P < .001). Furthermore, the middle subregion had significantly greater entropy and contrast than the proximal subregion (each P < .02). Residual analysis showed that all repeated-measures models fit the T2 data appropriately.
TABLE 1.
Summary of T2 Values and T2 Texture Variables by PCL Subregiona
| T2 Summary | Distal | Middle | Proximal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Sig. | Min, Max | Mean (95% CI) | Sig. | Min, Max | Mean (95% CI) | Sig. | Min, Max | |
| Median T2 | 26.9 (23.8, 30.1) | M | 15, 42.5 | 21.1 (19.6, 22.5) | D | 16, 29 | 22.6 (20.9, 24.3) | 16, 33.5 | |
| Mean T2 | 36.7 (32.7, 40.6) | M, P | 20.3, 53.2 | 29.2 (26.3, 32.1) | D | 19.2, 46.3 | 29.6 (26.8, 32.3) | D | 19.7, 48 |
| SD T2 | 30.7 (27, 34.4) | M, P | 12.2, 46.1 | 24.4 (21, 27.8) | D | 9.9, 42.3 | 21.9 (18.3, 25.5) | D | 8.5, 43.9 |
| Entropy | 3.2 (3, 3.4) | M | 2.5, 4.1 | 3.7 (3.5, 3.8) | D, P | 2.7, 4.3 | 3.4 (3.3, 3.6) | M | 2.7, 4 |
| Variance | 485.8 (382.8, 588.7) | M, P | 107.9, 990.4 | 316.2 (235.4, 396.9) | D | 67.6, 829 | 247.9 (175.5, 320.2) | D | 89.7, 866.2 |
| Contrast | 345.7 (259.8, 431.7) | M, P | 46.2, 898.6 | 211.5 (149.6, 273.4) | D, P | 25.1, 615.6 | 107.2 (76.4, 137.9) | D, M | 21.6, 352.5 |
| Homogeneity | 0.2 (0.2, 0.2) | M, P | 0.1, 0.3 | 0.2 (0.2, 0.2) | D | 0.1, 0.3 | 0.2 (0.2, 0.2) | D | 0.1, 0.3 |
aWithin the significant difference (Sig.) column: M, different from middle; P, different from proximal; D, different from distal (all P < .05). PCL, posterior cruciate ligament; Q, quartile; SD, standard deviation. Means are presented with 95% confidence limits of the mean in parentheses.
Figure 2.
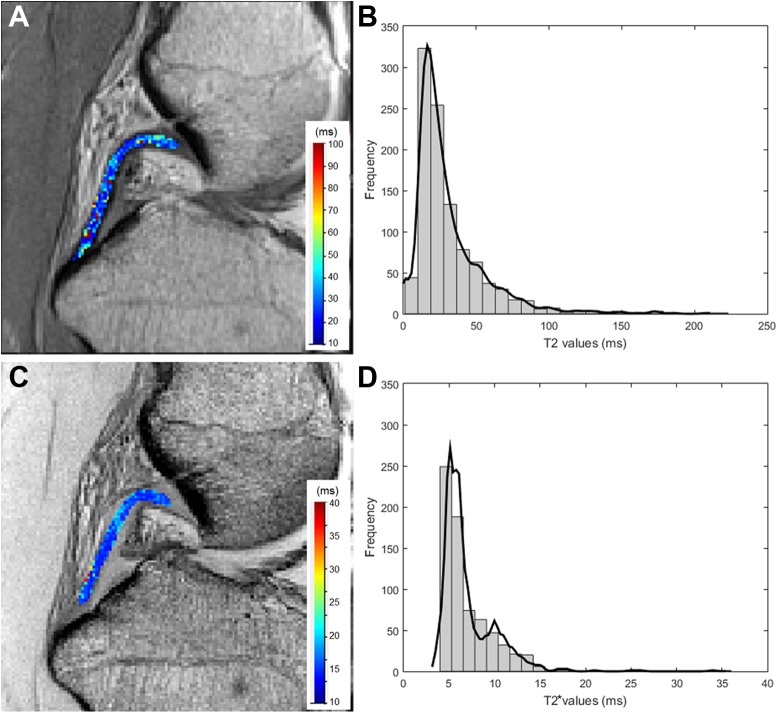
Example asymptomatic posterior cruciate ligament (PCL) showing (A) T2 mapping image slice, (B) histogram of T2 values for the entire PCL volume with a kernel distribution fit line, (C) T2* mapping image slice, and (D) histogram of T2* values for the whole PCL volume with a kernel distribution fit line.
Figure 3.
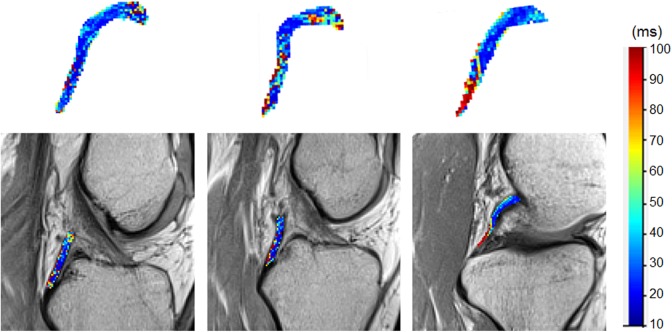
Examples of subjects in which the distal posterior cruciate ligament (PCL) exhibited significantly higher mean, standard deviation, variance, and contrast of T2 mapping values compared with both the middle and proximal subregions (P < .05). The upper row displays the 3-dimensional PCL volume with voxels color-mapped to the T2 values (ms), and the lower row shows the corresponding T2 mapping image slice with the T2 values overlaid.
Nearly all summary and texture variables for T2* exhibited a substantial positive skew. Thus, these variables are summarized in Table 2 with medians, quartiles, and extreme values. Residual analysis for the repeated-measures models of T2* values indicated a poor fit in all cases, and variable transformation did not mitigate the problem. As a consequence, we do not report statistical inference for subregion comparisons of T2* values.
TABLE 2.
Summary of T2* Values and T2* Texture Variables by PCL Subregiona
| T2* Summary | Distal | Middle | Proximal | |||
|---|---|---|---|---|---|---|
| Median (Q1, Q3) | Min, Max | Median (Q1, Q3) | Min, Max | Median (Q1, Q3) | Min, Max | |
| Median T2* | 6 (6, 7) | 5, 10 | 6 (6, 7) | 5, 9 | 6 (6, 7) | 5, 9 |
| Mean T2* | 7.3 (6.8, 8.9) | 5.8, 14.8 | 7.3 (7, 8.5) | 6.3, 14.7 | 7.3 (6.4, 8.2) | 5.7, 9.9 |
| SD T2* | 3.7 (2.6, 6) | 1.5, 17 | 3 (2.8, 4.9) | 2.2, 22.9 | 2.8 (2.1, 3.9) | 1.8, 12.6 |
| Entropy | 2 (1.3, 2.2) | 0.8, 3.2 | 2.1 (1.9, 2.5) | 1.1, 3.3 | 1.9 (1.4, 2.3) | 1, 3 |
| Variance | 6.9 (5.1, 12) | 1.9, 74.2 | 6.8 (4.6, 13.6) | 3.2, 115.1 | 4.9 (3, 9.8) | 1.9, 39.6 |
| Contrast | 4.6 (2.5, 9.3) | 0.7, 71.6 | 3.3 (2.3, 5.4) | 1, 191.2 | 2.6 (1.3, 4.5) | 0.9, 30.6 |
| Homogeneity | 0.6 (0.5, 0.7) | 0.4, 0.9 | 0.6 (0.5, 0.7) | 0.4, 0.8 | 0.6 (0.5, 0.7) | 0.4, 0.8 |
aPCL, posterior cruciate ligament; Q, quartile; SD, standard deviation. Medians presented with first (Q1) and third (Q3) quartiles in parentheses.
Intra- and interrater reliability was good to excellent (ICC, 0.7-0.9)10 for T2 and T2* values in all subregions, except for the interrater reliability for T2 values in the proximal subregion, which was fair to good (ICC, 0.597; 95% CI, 0.376-0.778).
Discussion
The most important finding of the study was providing a baseline of quantitative T2 biomarker values and texture parameters for an asymptomatic PCL population, serving as a basis for comparison with acute and chronic PCL injuries in the future. Using this methodology, we may be able to detect earlier changes in ligamentous tissue and/or aid in the diagnosis of ligament injury.
Significant differences in T2 mapping values and texture parameters were also observed between clinically relevant subregions of the asymptomatic PCL, suggesting that there are natural variations in the tissue properties of the PCL in an asymptomatic population. Textural parameters are used to characterize the spatial variation of intensity obtained in images. In this study, we report the parameters (entropy, variance, contrast, homogeneity) obtained from T2/T2* mapping of asymptomatic PCLs. Each parameter provides a measure of different textural information based on the work by Haralick et al.11 Entropy provides a measure of heterogeneity (or randomness) in the region being considered, with high values representing tissue with a diverse range of T2/T2* samples. Variance provides a measure of the variation of intensity around the mean and is especially useful for identifying changes within mostly homogenous regions. Contrast provides a measure between regions, with a high value often representing regions with 2 or more tissue types that have more similar T2/T2* samples. Homogeneity provides a measure of the uniformity in the T2/T2* map, with a high value in regions with mostly very similar T2/T2* values. The application of these parameters in characterizing the T2/T2* signal of the PCL has not been previously reported; however, in other clinical applications, measures such as entropy have been found to increase with disease process (eg, lesions in breast tissue,22 cartilage T2 changes in osteoarthritis,4–6 and intervertebral disc herniation20). It has also been suggested in the literature that greater T2 values, as well as greater variance, contrast, entropy, and heterogeneity, are indicative of more “complex” tissue, which could be related to degenerative changes.22 In the PCL, we found greater T2 values and greater variance, contrast, and heterogeneity in the distal region of the ligament (see Figure 3), indicating an area with more complexity to the tissue. Future work using histology for comparison is needed to clarify these changes identified in the distal subregion of the PCL and to determine the clinical significance of the textural results in relation to the PCL.
Much like T2 mapping, T2* mapping is sensitive to the interactions between water molecules and macromolecular concentration and the structure of the extracellular matrix. However, T2* analysis has been suggested to be more appropriate for imaging fast relaxing tissues with short time constant values and highly organized collagenous structures,7,16,34 where T2 value evaluation may not be as sensitive. Biercevicz et al2 reported that T2* significantly predicted maximum load, yield load, and linear stiffness of the healing ACL in porcine specimens. We suspect that several features of the T2* scale found in our data have contributed to the failure to fit our chosen models: (1) effect of magic angle, (2) substantial positive skew and/or possible outliers, (3) a possible floor effect within the T2* scale, and (4) potential effects of the variable sex and age of our asymptomatic population.
While T2* has been suggested to be more applicable for the shorter time constant values found in tendons and ligaments, use in the PCL may be influenced by the magic angle effect. This effect introduces artifactual signal for short echo time images such as T2* images (but less effect on long echo time images such as T2 images27) when ordered collagen structures are at an angle of 55° to the orientation of the main magnetic field of the MRI scanner. Magic angle effect may be difficult to avoid in the PCL because of the substantial curvature. Significant differences in T2* values have been reported with magic angle for the collateral ligaments of the equine distal interphalangeal joint33 and for the Achilles tendon.9 Du et al9 found that mean T2* values increased from 1.94 ms when orientated at 0° relative to the main magnetic field to 15.25 ms when orientated at 55°. Due to the curve of the PCL, the magic angle effect may be present and influence the results. The lowest echo time (TE) acquired for the T2* scan was 3.25 ms. Novel sequences such as zero echo time (ZTE) and ultrashort echo time (UTE) MRI may help to mitigate this issue by acquiring the lowest TE down to 0 ms and should be evaluated in future studies. Furthermore, future work to analyze the existence of skew in the T2* mapping results could help clarify the clinical utility of this biomarker for analyzing ligament tissue.
Some limitations of this study need to be identified. One limitation was the disparity in sex and age within our asymptomatic population, in particular having an increased number of younger males and older females. It is currently unknown whether there is an age or sex effect on asymptomatic ligament properties, particularly in terms of quantitative biomarkers. An age-related decrease in the diameter of collagen fibrils and increase in collagen fibril concentration has been identified in the human PCL25; however, further study is needed to determine whether these changes would affect ligament integrity. Additionally, it should be noted that imaging of the PCL for longitudinal evaluations with follow-up scans should be done, with special attention paid to the patient positioning during MR image acquisition. Varying degrees of extension experienced at the knee joint when supine in the MRI scanner may have a direct effect on the curve of the PCL. Any potential discrepancies created by patient positioning and its effect on the subregion analysis were minimized by the use of a 3D centerline during postprocessing of the PCL imaging data. A clinically applicable T2* sequence was utilized in this study to evaluate the short T2 species of the PCL. To maintain resolution and coverage, the lowest TE possible was 3.25 ms. In future work, even shorter time constant value techniques, such as UTE T2* or pointwise encoding time reduction with radial acquisition (PETRA) T2*, could be used to evaluate ligament tissue for comparison.24,34
Conclusion
The results of this study provide a baseline of quantitative T2 and T2* biomarker values and texture parameters for a rigorously screened asymptomatic PCL population. High ICC values were found for intra- and interrater reliability. Significantly greater T2 values were found in the distal PCL subregion relative to the proximal and middle regions. These results can serve as a basis for comparison with acute and chronic PCL injuries in future studies utilizing the presented methodology. T2 mapping produced more consistent results than T2* mapping, which exhibited a substantial positive skew, and future work is recommended to determine the feasibility of T2* mapping of ligament tissue.
Acknowledgment
The authors thank Erin Lucas for her assistance in study design and Bill Brock for his assistance in MRI scan acquisition.
Footnotes
One or more of the authors has declared the following potential conflict of interest: R.F.L. is a consultant for and receives royalties from Arthex, Ossur, and Smith & Nephew.
References
- 1. Biercevicz AM, Miranda DL, Machan JT, Murray MM, Fleming BC. In situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. Am J Sports Med. 2013;41:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biercevicz AM, Murray MM, Walsh EG, Miranda DL, Machan JT, Fleming BC. T2* MR relaxometry and ligament volume are associated with the structural properties of the healing ACL. J Orthop Res. 2014;32:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biercevicz AM, Walsh EG, Murray MM, Akelman MR, Fleming BC. Improving the clinical efficiency of T2* mapping of ligament integrity. J Biomech. 2014;47:2522–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blumenkrantz G, Stahl R, Carballido-Gamio J, et al. The feasibility of characterizing the spatial distribution of cartilage T(2) using texture analysis. Osteoarthritis Cartilage. 2008;16:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM. Spatial analysis of magnetic resonance T1ρ and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys. 2009;36:4059–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011;65:1184–1194. [DOI] [PubMed] [Google Scholar]
- 7. Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29:1433–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chou MC, Tsai PH, Huang GS, et al. Correlation between the MR T2 value at 4.7 T and relative water content in articular cartilage in experimental osteoarthritis induced by ACL transection. Osteoarthritis Cartilage. 2009;17:441–447. [DOI] [PubMed] [Google Scholar]
- 9. Du J, Pak BC, Znamirowski R, et al. Magic angle effect in magnetic resonance imaging of the Achilles tendon and enthesis. Magn Reson Imaging. 2009;27:557–564. [DOI] [PubMed] [Google Scholar]
- 10. Fleiss JL. The Design and Analysis of Clinical Experiments. New York, NY: Wiley; 1986. [Google Scholar]
- 11. Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Sys Man Cyber SMC. 1973;3:610–621. [Google Scholar]
- 12. Helmer KG, Wellen J, Grigg P, Sotak CH. Measurement of the spatial redistribution of water in rabbit Achilles tendon in response to static tensile loading. J Biomech Eng. 2004;126:651–656. [DOI] [PubMed] [Google Scholar]
- 13. Jackman T, LaPrade RF, Pontinen T, Lender PA. Intraobserver and interobserver reliability of the kneeling technique of stress radiography for the evaluation of posterior knee laxity. Am J Sports Med. 2008;36:1571–1576. [DOI] [PubMed] [Google Scholar]
- 14. Johannsen AM, Anderson CJ, Wijdicks CA, Engebretsen L, LaPrade RF. Radiographic landmarks for tunnel positioning in posterior cruciate ligament reconstructions. Am J Sports Med. 2013;41:35–42. [DOI] [PubMed] [Google Scholar]
- 15. Joseph GB, Baum T, Carballido-Gamio J, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls-data from the osteoarthritis initiative. Arthritis Res Ther. 2011;13:R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koff MF, Shah P, Pownder S, et al. Correlation of meniscal T2* with multiphoton microscopy, and change of articular cartilage T2 in an ovine model of meniscal repair. Osteoarthritis Cartilage. 2013;21:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaPrade RF, Wentorf FA, Fritts H, Gundry C, Hightower CD. A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy. 2007;23:1341–1347. [DOI] [PubMed] [Google Scholar]
- 18. Liess C, Lüsse S, Karger N, Heller M, Glüer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10:907–913. [DOI] [PubMed] [Google Scholar]
- 19. Mamisch TC, Hughes T, Mosher TJ, et al. T2 star relaxation times for assessment of articular cartilage at 3 T: a feasibility study. Skeletal Radiol. 2012;41:287–292. [DOI] [PubMed] [Google Scholar]
- 20. Mayerhoefer ME, Stelzeneder D, Bachbauer W, et al. Quantitative analysis of lumbar intervertebral disc abnormalities at 3.0 Tesla: value of T(2) texture features and geometric parameters. NMR Biomed. 2012;25:866–872. [DOI] [PubMed] [Google Scholar]
- 21. McRobbie DW, Moore EA, Graves MJ, Prince MR. MRI From Picture to Proton. 2nd ed Cambridge, England: Cambridge University Press; 2007. [Google Scholar]
- 22. Nie K, Chen JH, Yu HJ, Chu Y, Nalcioglu O, Su MY. Quantitative analysis of lesion morphology and texture features for diagnostic prediction in breast MRI. Acad Radiol. 2008;15:1513–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orakzai SH, Egan CM, Eustace S, Kenny P, O’Flanagan SJ, Keogh P. Correlation of intra-articular osseous measurements with posterior cruciate ligament length on MRI scans. Br J Radiol. 2010;83(985):23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qian Y, Williams AA, Chu CR, Boada FE. High-resolution ultrashort echo time (UTE) imaging on human knee with AWSOS sequence at 3.0 T. J Magn Reson Imaging. 2012;35:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sargon MF, Doral MN, Atay OA. Age-related changes in human PCLs: a light and electron microscopic study. Knee Surg Sports Traumatol Arthrosc. 2004;12:280–284. [DOI] [PubMed] [Google Scholar]
- 26. Servant CT, Ramos JP, Thomas NP. The accuracy of magnetic resonance imaging in diagnosing chronic posterior cruciate ligament injury. Knee. 2004;11:265–270. [DOI] [PubMed] [Google Scholar]
- 27. Shiomi T, Nishii T, Myoui A, Yoshikawa H, Sugano N. Influence of knee positions on T2, T*2, and dGEMRIC mapping in porcine knee cartilage. Magn Reson Med. 2010;64:707–714. [DOI] [PubMed] [Google Scholar]
- 28. Soh L, Tsatsoulis C. Texture analysis of SAR sea ice imagery using gray level co-occurrence matrices. IEEE Trans Geosci Remote Sens. 1999;37:780–795. [Google Scholar]
- 29. Surowiec RK, Lucas EP, Fitzcharles EK, et al. T2 values of articular cartilage in clinically relevant subregions of the asymptomatic knee. Knee Surg Sports Traumatol Arthrosc. 2014;22:1404–1414. [DOI] [PubMed] [Google Scholar]
- 30. Taubin G, Zhang T, Golub G. Optimal surface smoothing as filter design In: Buxton B, Cipolla R, eds. Computer Vision–ECCV’96. Proceedings of the 4th European Conference on Computer Vision (Lecture Notes in Computer Science, Vol. 1064). Berlin, Germany: Springer; 2005:283–292. [Google Scholar]
- 31. Tewes DP, Fritts HM, Fields RD, Quick DC, Buss DD. Chronically injured posterior cruciate ligament: magnetic resonance imaging. Clin Orthop Relat Res. 1997;335:224–232. [PubMed] [Google Scholar]
- 32. Wellen J, Helmer KG, Grigg P, Sotak CH. Spatial characterization of T1 and T2 relaxation times and the water apparent diffusion coefficient in rabbit Achilles tendon subjected to tensile loading. Magn Reson Med. 2005;53:535–544. [DOI] [PubMed] [Google Scholar]
- 33. Werpy NM, Ho CP, Kawcak CE. Magic angle effect in normal collateral ligaments of the distal interphalangeal joint in horses imaged with a high-field magnetic resonance imaging system. Vet Radiol Ultrasound. 2010;51:2–10. [DOI] [PubMed] [Google Scholar]
- 34. Williams A, Qian Y, Golla S, Chu CR. UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage. 2012;20:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]