Abstract
We investigated the association between self-reported physical exercise and cortical thickness in a large sample of cognitively normal individuals. We also determined whether a combination of physical exercise and education had more protective effects on age-related cortical thinning than either parameter alone. A total of 1,842 participants were included in this analysis. Physical exercise was assessed using a questionnaire regarding intensity, frequency, and duration. Cortical thickness was measured using a surface-based method. Longer duration of exercise (≥1 hr/day), but not intensity or frequency, was associated with increased mean cortical thickness globally (P-value = 0.013) and in the frontal regions (P-value = 0.007). In particular, the association of exercise with cortical thinning had regional specificity in the bilateral dorsolateral prefrontal, precuneus, left postcentral, and inferior parietal regions. The combination of higher exercise level and higher education level showed greater global and frontal mean thickness than either parameter alone. Testing for a trend with the combination of high exercise level and high education level confirmed this finding (P-value = 0.001–0.003). Our findings suggest that combined exercise and education have important implications for brain health, especially considering the paucity of known protective factors for age-related cortical thinning.
Decline in cognitive function is associated with the normal aging process and has been termed ‘age-related cognitive decline.’ Although not fully understood, a large number of studies have revealed multiple risk factors contributing to age-related cognitive decline, including vascular risk factors/cardiovascular diseases1; inflammatory biomarkers2; malnutrition, especially vitamin B deficiency3; and lifestyle factors such as smoking, alcohol, and sleep problems4. However, there is also increasing evidence that lifestyle behaviors can mitigate age-related cognitive decline. Among these behaviors, education and physical exercise are generally accepted as protective factors against age-related cognitive decline.
Several studies have comprehensively described that education might increase cognitive reserve by increasing the number of neurons and synapses, which allows the brain to cope with age-related diseases using preexisting cognitive processes or enlisting compensatory approaches regardless of brain size5,6. Furthermore, in a recent study measuring cortical thickness, which is a promising marker of morphometric change in the brain during normal aging7, we demonstrated that higher level of education positively correlated with cortical thickness, and that these protective effects became more prominent with aging8.
Physical exercise is also known to have global effects on factors that influence general brain health9,10,11. There are several underlying mechanisms, including increased blood flow through vascularization and angiogenesis, activation of the immune system, and induction of neurotrophic factors. However, there have been few neuroimaging studies addressing the relationship between physical exercise and cortical thickness12,13,14. One previous study reported that higher level of physical exercise was associated with increased cortical thickness in the right prefrontal cortex14. However, these studies were conducted in selected and small patient samples. Moreover, there is a need to investigate which parameters of physical exercise, for example, intensity, frequency, or duration, have more beneficial effects on age-related cortical thinning. Considering the results of our previous study showing the effects of education on cortical thinning8, it would be reasonable to hypothesize that the combination of physical exercise and education would have more protective effects on age-related cortical thinning than either physical exercise or education alone.
In this study, we investigated the association between self-reported physical exercise and cortical thickness in a large sample of cognitively normal individuals. First, we explored which parameters of physical exercise –intensity, duration, or frequency, have more beneficial effects on age-related cortical thinning. Second, we determined whether a combination of physical exercise and education had more protective effects on age-related cortical thinning than either parameter alone.
Results
Comparisons of baseline characteristics according to exercise parameters
Table 1 summarizes the baseline characteristics of the 1,842 participants. In the study population, the mean (SD) age was 63.8 (6.9) years, ranging from 45 to 91 years; and the mean (SD) education length was 12.9 (4.2) years. In addition, the distributions of the dichotomized exercise groups were as follows: 681 (37.0%) patients were in the longer duration group (≥1 hr/day), 495 (26.9%) patients were in the higher intensity group (≥moderate intensity), and 549 (29.8%) patients were in the higher frequency group (≥5 days/week).
Table 1. Distribution of self-reported assessment of physical exercise and comparisons of the characteristics between the exercise duration groups.
| Total | Exercise group |
|||
|---|---|---|---|---|
| Longer duration (≥1 hr/day) | Shorter duration (<1 hr/day) | P-value | ||
| Number (%) | 1842 (100) | 681 (37.0) | 1161 (63.0) | |
| Age, years | 63.8 (6.9) | 64.1 (6.5) | 63.7 (7.2) | 0.213 |
| Female, N (%) | 858 (46.6) | 321 (47.1) | 537 (46.3) | 0.714 |
| Education, years | 12.9 (4.2) | 12.6 (4.4) | 13.1 (4.1) | 0.022* |
| Hypertension, N (%) | 847 (46.0) | 302 (44.3) | 545 (46.9) | 0.281 |
| Diabetes mellitus, N (%) | 308 (16.7) | 97 (14.2) | 211 (18.2) | 0.029* |
| Hyperlipidemia, N (%) | 608 (33.0) | 219 (32.2) | 389 (33.5) | 0.553 |
| Ischemic heart disease, N (%) | 103 (5.6) | 29 (4.3) | 74 (6.4) | 0.056 |
| History of stroke, N (%) | 43 (2.3) | 20 (2.9) | 23 (2.0) | 0.190 |
| Familial history of stroke, N (%) | 397 (21.6) | 148 (21.7) | 249 (21.4) | 0.886 |
| Familial history of dementia, N (%) | 266 (14.4) | 95 (14.0) | 171 (14.7) | 0.646 |
| BMI, kg/m2 | 23.9 (2.6) | 23.8 (2.5) | 24.0 (2.7) | 0.143 |
| Height, cm | 162.7 (8.1) | 162.4 (8.2) | 162.9 (8.1) | 0.208 |
| Weight, kg | 63.6 (9.8) | 63.0 (9.5) | 63.9 (9.9) | 0.060 |
| ICV, cm3 | 1354.6 (123.5) | 1354.1 (119.9) | 1355.0 (125.6) | 0.882 |
| K-MMSE, points | 28.1 (1.8) | 28.1 (1.8) | 28.1 (1.8) | 0.481 |
| Exercise parameters | ||||
| Higher intensity, N (%) | 495 (26.9) | 259 (52.3) | 236 (47.7) | |
| Higher frequency, N (%) | 549 (29.8) | 322 (58.7) | 227 (41.3) | |
| Longer duration, N (%) | 681 (37.0) | |||
Chi-square and t-tests were performed to compare demographic variables between two exercise groups. Values are mean (SD) or number (%).
N: number, SD: standard deviation, BMI: body mass index, ICV: intracranial volume, K-MMSE: Korean mini mental status examination, *P < 0.05.
There were differences in demographics and/or vascular risk factors between the subgroups with higher exercise parameters and those with lower exercise parameters: education and diabetes mellitus varied by exercise duration; age, sex, education, ischemic heart disease, familial history of stroke, height, weight, and ICV varied by exercise intensity; age, education, history of stroke, height, weight, and K-MMSE varied by exercise frequency (Table 1 and Supplementary Table 1).
Relationships between exercise parameters and cortical thickness
In Model 1, subjects with longer durations of exercise had greater cortical thickness in the global and frontal regions than those with shorter durations (Table 2). On the other hand, there was no significant relationship between intensity or frequency of exercise and cortical thickness. When we simultaneously added the three exercise parameters to the independent variables in Model 2, longer exercise duration, but not intensity or frequency, was associated with cortical thickness globally (P-value = 0.013) and in the frontal regions (P-value = 0.007, Table 2). In sensitivity analyses, we performed additional analyses after excluding 140 participants who had a history of ischemic heart disease or stroke (Total N = 1,702). The new results were similar with previous one (Supplementary Table 2).
Table 2. Relationships between exercise parameters and mean cortical thickness.
| Global |
Frontal |
Temporal |
Parietal |
Occipital |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Model 1 | |||||||||||||||
| Duration | 0.011 | 0.005 | 0.023* | 0.013 | 0.005 | 0.011* | 0.010 | 0.007 | 0.168 | 0.008 | 0.006 | 0.192 | 0.006 | 0.006 | 0.305 |
| Intensity | −0.002 | 0.005 | 0.752 | 0.001 | 0.006 | 0.929 | 0.002 | 0.008 | 0.778 | −0.001 | 0.007 | 0.847 | −0.010 | 0.006 | 0.118 |
| Frequency | <0.001 | 0.005 | 0.970 | <0.001 | 0.006 | 0.976 | 0.013 | 0.008 | 0.095 | −0.005 | 0.007 | 0.490 | −0.006 | 0.006 | 0.342 |
| Model 2 | |||||||||||||||
| Duration | 0.013 | 0.005 | 0.013* | 0.015 | 0.006 | 0.007* | 0.007 | 0.008 | 0.349 | 0.011 | 0.007 | 0.107 | 0.010 | 0.006 | 0.102 |
| Intensity | −0.004 | 0.006 | 0.458 | −0.002 | 0.006 | 0.703 | <0.001 | 0.008 | 0.958 | −0.003 | 0.007 | 0.688 | −0.011 | 0.006 | 0.082 |
| Frequency | −0.004 | 0.006 | 0.497 | −0.004 | 0.006 | 0.479 | 0.011 | 0.008 | 0.183 | −0.008 | 0.007 | 0.277 | −0.008 | 0.006 | 0.226 |
Model 1, multiple linear regressions were performed after adjusted for age, sex, education (continuous), history of hypertension, diabetes mellitus, hyperlipidemia, ischemic heart disease, stroke, BMI, and ICV.
Model 2, multiple linear regressions were performed after further adjusted for the two exercise parameters not used in the analysis.
B (SE): β value (standard error of the mean), *P < 0.05.
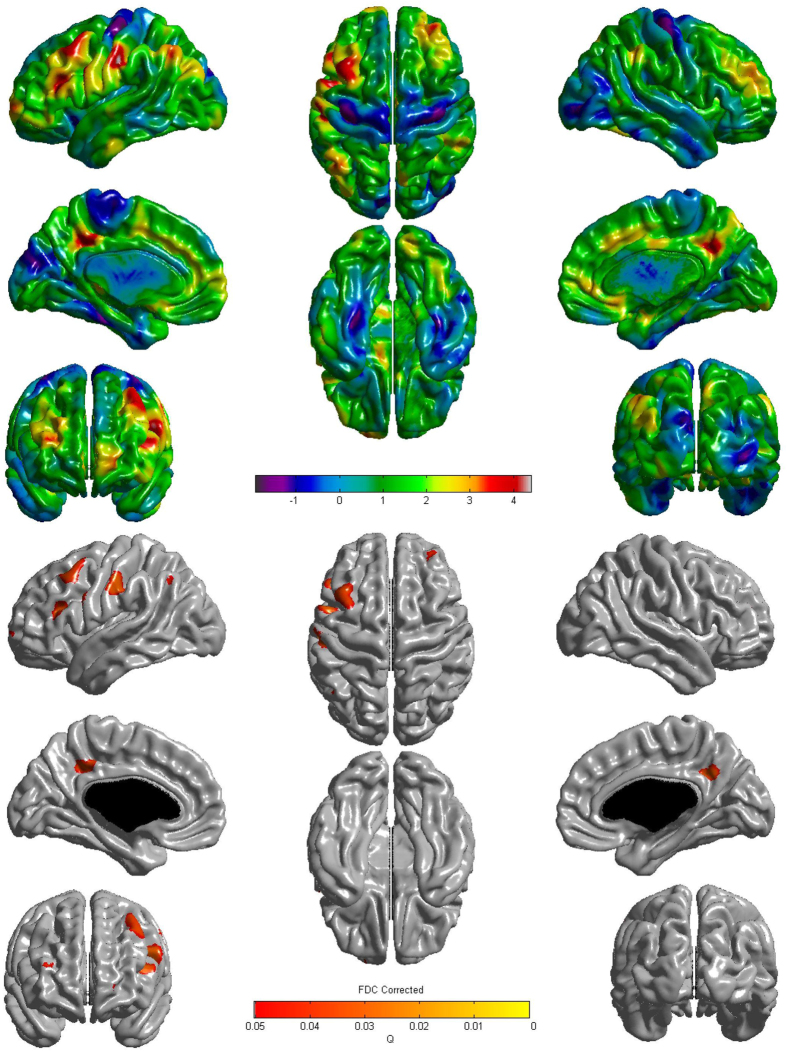
Application of the general linear model to vertex-wise cortical thickness showed that, in comparison to the shorter exercise duration group, the longer exercise duration group exhibited greater cortical thickness in the bilateral dorsolateral prefrontal, precuneus, left postcentral, and inferior parietal regions (Fig. 1).
Figure 1. Three-dimensional reconstruction for correlation between physical exercise and cortical thickness.
The association of longer duration of exercise with cortical thinning had regional specificity in the bilateral dorsolateral prefrontal, precuneus, left postcentral, and inferior parietal regions. The Q value denotes the FDR-corrected P value.
Relationships between duration of physical exercise, education level, and cortical thickness
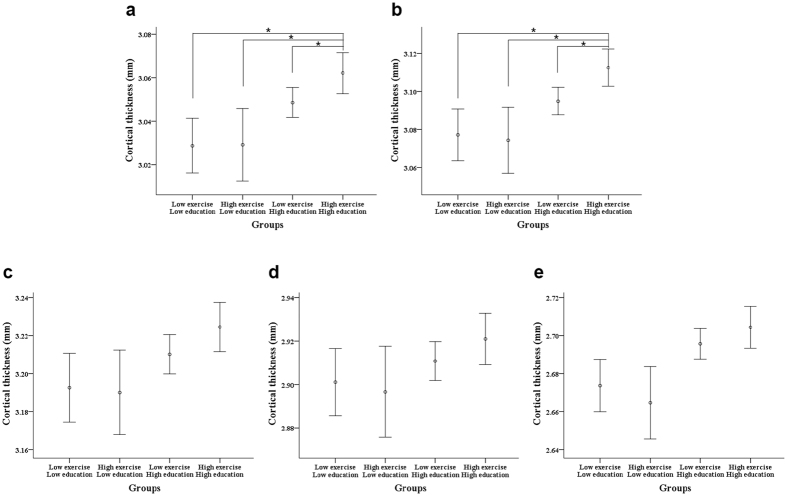
Figure 2 revealed that the group with a combination of higher exercise and higher education had greater global and frontal mean thickness than groups with only higher exercise, higher education, or lower exercise and lower education. Tests for linear trends across the combined exercise and education effects showed that combined higher exercise and higher education was associated with increased mean cortical thickness globally (p for linear trends = 0.003), and in the frontal region (p for linear trends = 0.001, Fig. 2).
Figure 2. Mean cortical thickness in four groups was classified according to exercise and education.
(a) Global region, (b) Frontal region, (c) Temporal region, (d) Parietal region, (e) Occipital region. ‘o’ denotes mean cortical thickness, error bars shows the 95% confidence interval (CI). *P < 0.05.
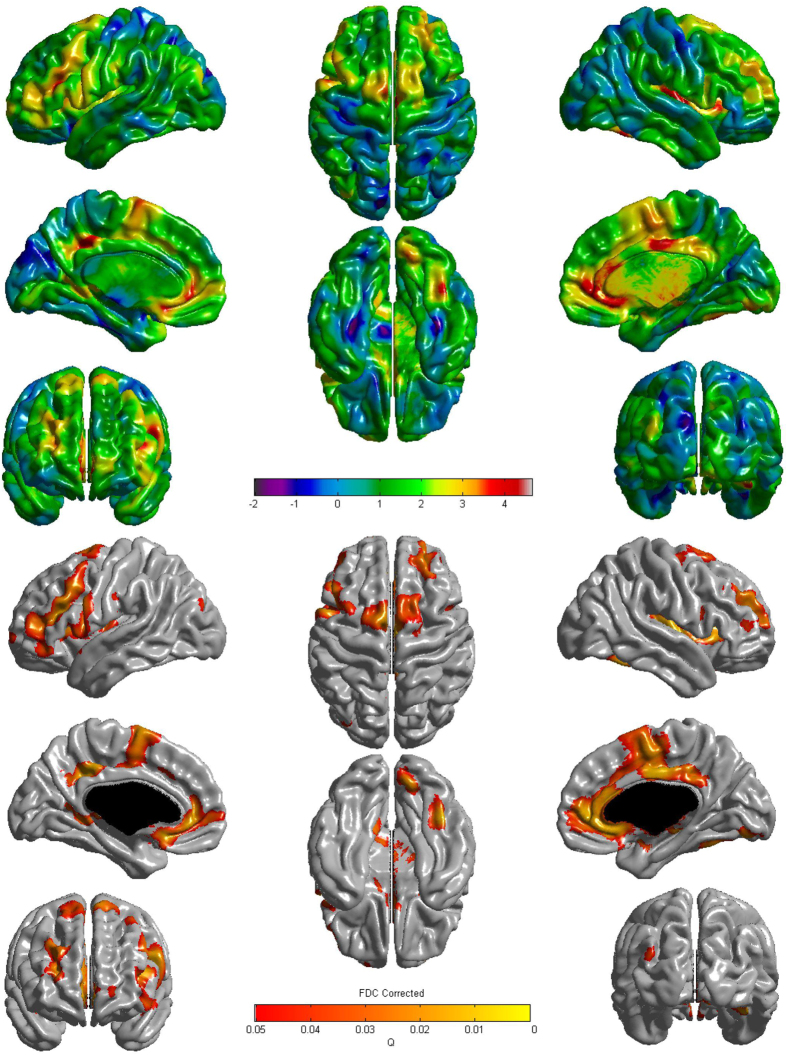
Regional differences in cortical thickness in patients with longer exercise duration (≥1 hr/day) and higher education (≥12yrs) were reflected as greater thickness in the anterior and posterior cingulate, insular, and supplementary motor regions, as well as in the bilateral dorsolateral prefrontal, precuneus, and inferior parietal regions, compared with the groups with shorter exercise duration (<1 hr/day) and lower education (<12yrs, Fig. 3).
Figure 3. Regional differences in cortical thickness between the group with higher educatation level and higher exercise duration and the group with lower education level and lower exercise duration.
The association of lower education level and lower exercise duration with cortical thinning had more extensive regional specificity in the bilateral dorsolateral prefrontal, supplementary motor, anterior and posterior cingulate, precuneus, and insular regions. The Q value denotes the FDR-corrected P value.
Discussion
We report new evidence of a relationship among exercise, education, and cortical thickness in cognitively normal individuals. Our major findings were as follows. First, longer duration of exercise (≥1 hr/day), but not the frequency or intensity of exercise, was correlated with increased cortical thickness in the bilateral dorsolateral prefrontal cortex, precuneus, left postcentral gyrus, and inferior parietal regions. Second, a combination of higher exercise and higher education was associated with greater global and frontal mean thickness than either exercise or education alone. Furthermore, tests for trends across combinations of high exercise and high education confirmed this finding. Taken together, our findings suggest that combined exercise and education has important implications in brain health, especially considering the paucity of known protective factors for age-related cortical thinning.
Our first major finding was that longer duration of exercise (≥1 hr/day), but not intensity or frequency, was significantly associated with increased mean cortical thickness globally and in the frontal regions. Furthermore, the effects of longer duration of exercise remained significant after adjustments for the other two exercise parameters (intensity and frequency). To the best of our knowledge, the question of which exercise parameters have more beneficial effects on age-related cortical thinning remains unsettled15,16,17,18. A previous study suggested that moderate-intensity exercise was associated with better cognitive performance regardless of frequency15, while another study showed that the beneficial effects of physical exercise on memory were independent of exercise intensity16. Several studies have also suggested that the amount of exercise is related with GM volume17,18, although they did not discriminate the effects of duration or frequency of exercise. Therefore, our findings suggest that the duration of exercise is a more influential parameter on cortical thickness than are other parameters such as frequency and intensity.
Most regional differences in cortical thinning related to longer exercise duration were noted in the bilateral dorsolateral prefrontal precuneus and inferior parietal regions. Our findings were generally consistent with previous studies showing that increased exercise was associated with increased GM volume in the prefrontal and posterior cingulate regions14,16,17. The dorsolateral prefrontal region is a key region of the brain associated with executive functions such as working memory, cognitive flexibility, and planning19,20,21. In addition, the precuneus has a central role in highly integrated tasks, including episodic memory retrieval, visuospatial processing, and self-consciousness22. The inferior parietal region is known to play a key role in various cognitive functions such as attention, language, and visuospatial and action-related functions23,24. These regions are also known to be susceptible to age-related deterioration or Alzheimer’s disease (AD)10,25,26,27. Therefore, our results suggest that physical exercise is associated with protective effects on age- or AD-related cortical thinning.
Our second major finding was that a combination of higher exercise and higher education had greater effects on mean thickness globally and in the frontal regions than did either parameter alone. Tests for trends across combinations of high exercise and high education confirmed this finding. To the best of our knowledge, there have been no studies investigating the combined effects of education and exercise on cortical thinning. However, our findings are supported by a few randomized controlled trials that have suggested that a combination of education and physical exercise more highly enhanced cognitive function than did either parameter alone28,29,30. Increased cortical thickness related to the combination of exercise and education was observed more frequently than the increased thickness observed with exercise. In particular, increased thickness of the anterior and posterior cingulate, insular, and supplementary motor regions are reported to be related to education level31,32. The mechanisms through which the combined effects of physical exercise and education on age-related cortical thinning become greater than those of either parameter alone are unknown. However, the effects of education are known to be mediated by increased neural reserve5, while the effects of exercise might be explained by vascularization or angiogenesis10,11. Previous studies have shown that neurovascular decoupling is also an important mechanism that can explain age-related cognitive impairment or AD33. It is therefore reasonable to suggest that exercise and education increase vascularization and neural reserve, which in turn lead to enhanced neurovascular coupling, eventually resulting in more beneficial effects than either parameter alone.
The strengths of this study include the large sample size and sophisticated measurements of cortical thickness. However, some limitations should be considered when interpreting the results. First, we used self-reported physical exercise in the questionnaire; hence, recall bias is inherent. Interventional studies would help to more precisely assess physical exercise. Second, our participants were recruited from individuals seeking a comprehensive preventive health exam not covered by national medical insurance, which might limit the generalizability of this study to the general population. Third, the association between duration of exercise and cortical thickness is modest, but the association remained even when other risk factors known to affect brain structures were included in models. Finally, our study was designed to be cross-sectional, precluding claims of causality. Nevertheless, it is noteworthy that this is the first study showing the combined effects of education and exercise on age-related cortical thinning.
Methods
Study participants
We studied 2,217 participants who attended a preventative medical check-up, which included an assessment of cognitive function and dementia status, at the Health Promotion Center of the Samsung Medical Center (Seoul, Korea) from July 2009 to December 2014. Of these participants, 1,959 have been described in a previous study on the effects of education on cortical thinning8. All study participants underwent brain magnetic resonance imaging (MRI), including three-dimensional volume images, as a part of their dementia assessment. We excluded the following participants from this study: 16 participants who were younger than 45 years of age; 88 participants with significant cognitive impairment defined by Mini-Mental Status Examination (MMSE) scores below the 16th percentile in age-, sex-, and education-matched norms or through an interview conducted by a qualified neurologist; and 227 participants with missing data on demographics (N = 189), anthropometric variables (N = 9), or history of hypertension (N = 14), diabetes mellitus (N = 5), or hyperlipidemia (N = 10). We also excluded 44 participants with unreliable analyses of cortical thickness due to head motion, blurring of the MRI, inadequate registration to a standardized stereotaxic space, misclassification of tissue type, or inexact surface extraction. The final sample size was composed of 1,842 participants. Apolipoprotein E (APOE) genotyping was performed in 438 (23.8%) of the 1,842 participants in this study.
Standard protocol approvals, registrations, and patient consents
This study was approved by the Institutional Review Board at Samsung Medical Center. In addition, all methods were carried out in accordance with the approved guidelines. The requirement for participant’s consent was waived since we used retrospective de-identified data collected during health exam visits.
Measurements
Health screening exams were conducted by trained personnel according to the standard protocol. Health screening exams included a questionnaire, physical exam, laboratory analyses, and brain MRI. Information regarding this screening program has been previously described in detail34. The questionnaire data included questions on medical history and medication use. To precisely evaluate the level of formal education achieved by the participants, we collected information on completed education (elementary school, middle school, high school, college, and graduate school) and the total duration of education.
Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or use of antihypertensive medication. Quality control procedures were performed in accordance with the Korean Association of Laboratory Quality Control. A patient was considered to have diabetes mellitus if he or she was prescribed diabetes medication or had a fasting blooding sugar level ≥126 mg/dl.
Physical exercise questionnaire
Physical exercise was assessed using the modified Korean version of International Physical Activity Questionnaire 7 (IPAQ-7)35,36,37. The questionnaire was a subjective measure that asked participants to recall their physical activity from the previous 7 days. It was separated into 3 sections and consisted of (1) the intensity (vigorous/moderate/light/very light/none), (2) the frequency (per week, more than 5 days/3–4 days/1–2 days/none), and (3) the duration (per day, more than 60 min/40–60 min/20–40 min/less than 20 min) of physical activity. Exercise intensity was presented in terms of the following activities; vigorous (e.g., aerobic exercise or playing soccer), moderate (e.g., cycling or mountain climbing), light (e.g., walking more than 10 min or doing housework), and very light or none (e.g., walking less than 10 min or no exercise).
For analysis, we dichotomized all subscales of the questionnaire following the current Physical Activity Guidelines for Adults38 which is moderate-intensity aerobic physical activity to 300 minutes (5 hours) each week. Then we used the cut-off, one hour per day, 5 days per week, and moderate intensity. Finally, three exercise parameters were created based on predefined variables: an intensity group (dichotomized as “higher” vs. “lower” based on moderate intensity), a frequency group (dichotomized as “higher” vs. “lower” based on 5 days per week), and a duration group (dichotomized as “longer” vs. “shorter” based on one hour per day).
Brain MRI scans
All participants underwent neurological and neuropsychological examination, MMSE, and 3D volumetric brain MRI scan. An Achieva 3.0-Tesla MRI scanner (Philips, Best, the Netherlands) was used to acquire 3D T1 Turbo Field Echo (TFE) MRI data from 2,310 participants using the following imaging parameters: sagittal slice thickness, 1.0 mm with 50% overlap; no gap; repetition time of 9.9 ms; echo time of 4.6 ms; flip angle of 8°; and matrix size of 240 × 240 pixels reconstructed to 480 × 480 over a field view of 240 mm.
Radiologists initially inspected all MRI images for evidence of brain tumors, lobar infarctions (except lacunar infarctions), and hemorrhages (observed as low intensity areas in T2-weighted images).
T1-weighted MR images were automatically processed using the standard Montreal Neurological Institute image processing software (CIVET) to measure cortical thickness. The software has been well-validated and extensively described elsewhere including aging/atrophied brain studies39,40,41,42,43. In summary, native MRI images were first registered into a standardized stereotaxic space using an affine transformation44. Non-uniformity artifacts were corrected using the N3 algorithm, and the registered and corrected volumes were classified as white matter, gray matter (GM), cerebrospinal fluid, and background using an artificial neural net classifier45. The surfaces of the inner and outer cortices were automatically extracted by deforming a spherical mesh onto the gray/white boundary of each hemisphere using the Constrained Laplacian-Based Automated Segmentation with Proximities algorithm, which has also been well-validated and extensively described elsewhere46,47.
Cortical thickness was calculated as the Euclidean distance between the linked vertices of the inner and outer surfaces after applying an inverse transformation matrix to cortical surfaces and reconstructing them in the native space47,48. To control for brain size, we computed ICV using classified tissue information and a skull mask acquired from the T1-weighted image49. ICV was defined as total volume of GM, white matter (WM), and cerebrospinal fluid (CSF), with consideration of voxel dimension. Classified GM, WM, CSF, and background within the mask were transformed back into individual native space.
To compare the thicknesses of corresponding regions among the participants, the thicknesses were spatially registered on an unbiased iterative group template by matching the sulcal folding pattern using surface-based registration involving sphere-to-sphere warping50,51. For global and lobar regional analyses, we used the lobe-parcellated group template that had been previously divided into frontal, temporal, parietal, and occipital lobes using SUMA (http://afni.nimh.nih.gov)48. Average values of thickness of the whole vertex in each hemisphere and lobar region were used for global analysis.
Statistical analysis
For demographic comparison, Chi-square and t-tests were performed between two groups. To evaluate the relationship of physical exercise and cortical thickness, we used multiple linear regression analysis models. In Model 1, the three exercise parameters were analyzed separately with regard to mean cortical thickness after controlling for age, sex, education (continuous), ICV, vascular risk factors (hypertension, diabetes mellitus, hyperlipidemia, and BMI), ischemic heart disease, and history of stroke. Model 2 was further adjusted for the two exercise parameters not used in the analysis.
In order to determine whether the combination of higher exercise and higher education had more beneficial effects on age-related cortical thinning than only higher exercise or higher education, we stratified our subjects into four groups: lower exercise (<1 hr/day) and lower education (<12yrs) (N = 256), higher exercise (≥1 hr/day) and lower education (<12yrs) (N = 184), lower exercise (<1 hr/day) and higher education (≥12yrs) (N = 905), and higher exercise (≥1 hr/day) and higher education (≥12yrs) (N = 497). In order to evaluate tests for linear trends across the combined physical exercise and education groups, we also entered four groups of exercise and education as continuous variables into Model 2. SPSS 20 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
For cortical thickness analyses, we used a MATLAB-based toolbox (available free online at the University of Chicago website: http://galton.uchicago.edu/faculty/InMemoriam/worsley/research/surfstat/). Diffusion smoothing with a full-width half-maximum of 20 mm was used to blur each cortical thickness map, leading to increased signal-to-noise ratio and statistical power40. In order to analyze the localized differences and the statistical map of cortical thickness on the surface model, linear regression was performed vertex-by-vertex after controlling for age, sex, education, ICV, vascular risk factors, ischemic heart disease, history of stroke, and the two exercise parameters not used in the analysis. The resulting statistical maps were thresholded using a false discovery rate (FDR)52 with a q value of 0.05 after pooling the P-values from regressions analysis.
Additional Information
How to cite this article: Lee, J. S. et al. Combined effects of physical exercise and education on age-related cortical thinning in cognitively normal individuals. Sci. Rep. 6, 24284; doi: 10.1038/srep24284 (2016).
Supplementary Material
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C2768); the Korean Science and Engineering Foundation (KOSEF) NRL program funded by the Korean government (MEST; 2011-0028333); Korea Ministry of Environment (MOE) as the Environmental Health Action Program (2014001360002); Korean Neurological Association (KNA-15-MI-08); and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2015R1C1A2A01053281).
Footnotes
Author Contributions J.S.L. contributed to study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis, and draft and revision of the manuscript for content. H.Y.S., J.L., Y.K.J., N.-Y.J., P.C., J.-J.Y., J.M.L., M.K., Y.J.K., H.J.K., K.-C.P. and D.L.N. contributed to acquisition of data and interpretation of data. S.W.S. contributed to study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis, revision of the manuscript for content, and study supervision. All authors report no disclosure.
References
- Qiu C. & Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol. 12, 267–277 (2015). [DOI] [PubMed] [Google Scholar]
- Schram M. T. et al. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 55, 708–716 (2007). [DOI] [PubMed] [Google Scholar]
- Morris M. C. et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 62, 641–645 (2005). [DOI] [PubMed] [Google Scholar]
- Deary I. J. et al. Age-associated cognitive decline. Br Med Bull. 92, 135–152 (2009). [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 11, 1006–1012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology. 43, 13–20 (1993). [DOI] [PubMed] [Google Scholar]
- van Velsen E. F. et al. Brain cortical thickness in the general elderly population: the Rotterdam Scan Study. Neurosci Lett. 550, 189–194 (2013). [DOI] [PubMed] [Google Scholar]
- Kim J. P. et al. Effects of education on aging-related cortical thinning among cognitively normal individuals. Neurology 85, 806–812 (2015). [DOI] [PubMed] [Google Scholar]
- Vivar C., Potter M. C. & van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. 15, 189–210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. I., Gildengers A. G. & Butters M. A. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 15, 99–108 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E. et al. The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. Am J Neuroradiol. 30, 1857–1863 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G., Zhang Y., Jiang T. & Luo J. Increased cortical thickness in sports experts: a comparison of diving players with the controls. Plos One. 6, e17112; 10.1371/journal.pone.0017112 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheewe T. W. et al. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol. 23, 675–685 (2013). [DOI] [PubMed] [Google Scholar]
- Walhovd K. B., Storsve A. B., Westlye L. T., Drevon C. A. & Fjell A. M. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol Aging 35, 1055–1064 (2014). [DOI] [PubMed] [Google Scholar]
- Geda Y. E. et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 67, 80–86 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscheweyh R. et al. Physical activity and memory functions: an interventional study. Neurobiol Aging 32, 1304–1319 (2011). [DOI] [PubMed] [Google Scholar]
- Erickson K. I. et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology 75, 1415–1422 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. I. et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108, 3017–3022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey A. K., Koenigs M. & Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex 49, 1195–1205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 7, 134–140 (2003). [DOI] [PubMed] [Google Scholar]
- Pochon J. B. et al. The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cereb Cortex 11, 260–266 (2001). [DOI] [PubMed] [Google Scholar]
- Cavanna A. E. & Trimble M. R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583 (2006). [DOI] [PubMed] [Google Scholar]
- Singh-Curry V. & Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia 47, 1434–1448 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S. et al. Organization of the human inferior parietal lobule based on receptor architectonics. Cereb Cortex 23, 615–628 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N. et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15, 1676–1689 (2005). [DOI] [PubMed] [Google Scholar]
- Weinstein A. M. et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. 26, 811–819 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarouf C. L. et al. Biochemical assessment of precuneus and posterior cingulate gyrus in the context of brain aging and Alzheimer’s disease. Plos One 9, e105784, 10.1371/journal.pone.0105784 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D. E. et al. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 173, 797–804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatil E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Front Aging Neurosci. 5, 8, 10.3389/fnagi.2013.00008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styliadis C., Kartsidis P., Paraskevopoulos E., Ioannides A. A. & Bamidis P. D. Neuroplastic Effects of Combined Computerized Physical and Cognitive Training in Elderly Individuals at Risk for Dementia: An eLORETA Controlled Study on Resting States. Neural Plast. 2015, 172192; 10.1155/2015/172192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaza-Urquijo E. M. et al. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage 83, 450–457 (2013). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Education increases reserve against Alzheimer’s disease–evidence from structural MRI analysis. Neuroradiology 54, 929–938 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 5, 347–360 (2004). [DOI] [PubMed] [Google Scholar]
- Park H. Y. et al. Lung function, coronary artery calcification, and metabolic syndrome in 4905 Korean males. Respir Med. 104, 1326–1335 (2010). [DOI] [PubMed] [Google Scholar]
- Chun M. Y. Validity and reliability of korean version of international physical activity questionnaire short form in the elderly. Korean J Fam Med. 33, 144–151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. Y., Choi Y. H. & Song Y. M. Metabolic Syndrome in Korean Cancer Survivors and Family Members: A Study in a Health Promotion Center. Nutr Cancer 67, 1075–1082 (2015). [DOI] [PubMed] [Google Scholar]
- Sung J. et al. Relationship between aerobic fitness and progression of coronary atherosclerosis. Heart Vessels Sep 23, 10.1007/s00380-015-0745-2 (2015). [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services., 2008 Physical Activity Guidelines for Americans. Available at: http://health.gov/paguidelines/guidelines (2008) Accessed: 23/03/2016.
- Kabani N., Le Goualher G., MacDonald D. & Evans A. C. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage 13, 375–380 (2001). [DOI] [PubMed] [Google Scholar]
- Lerch J. P. & Evans A. C. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 24, 163–173 (2005). [DOI] [PubMed] [Google Scholar]
- Lerch J. P. et al. Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb Cortex 15, 995–1001 (2005). [DOI] [PubMed] [Google Scholar]
- Lee J. K. et al. A novel quantitative cross-validation of different cortical surface reconstruction algorithms using MRI phantom. Neuroimage 31, 572–584 (2006). [DOI] [PubMed] [Google Scholar]
- Singh V. et al. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer’s disease. Brain 129, 2885–2893 (2006). [DOI] [PubMed] [Google Scholar]
- Collins D. L., Neelin P., Peters T. M. & Evans A. C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 18, 192–205 (1994). [PubMed] [Google Scholar]
- Sled J. G., Zijdenbos A. P. & Evans A. C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17, 87–97 (1998). [DOI] [PubMed] [Google Scholar]
- MacDonald D., Kabani N., Avis D. & Evans A. C. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage 12, 340–356 (2000). [DOI] [PubMed] [Google Scholar]
- Kim J. S. et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage 27, 210–221 (2005). [DOI] [PubMed] [Google Scholar]
- Im K. et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage 31, 31–38 (2006). [DOI] [PubMed] [Google Scholar]
- Smith S. M. Fast robust automated brain extraction. Hum Brain Mapp. 17, 143–155 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins S., Evans A. C., Collins D. L. & Whitesides S. Tuning and comparing spatial normalization methods. Med Image Anal. 8, 311–323 (2004). [DOI] [PubMed] [Google Scholar]
- Lyttelton O., Boucher M., Robbins S. & Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage 34, 1535–1544 (2007). [DOI] [PubMed] [Google Scholar]
- Genovese C. R., Lazar N. A. & Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15, 870–878 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.