Abstract
Despite significant progress in understanding of the potential of adenosine A1 receptor-based therapies in treatment of cerebral ischemia and stroke, very little is known about the effect of selective stimulation of adenosine A2A receptors on the outcome of a cerebrovascular arrest. In view of a major role played by adenosine A2 receptors in the regulation of cerebral blood flow, we have investigated the effect of both acute and chronic administration of the selective adenosine receptor agonist 2-[(2-aminoethylamino)-carbonylethylphenylethylamino]-5′-N-ethylcarboxoamidoadenosine (APEC) and antagonist 8-(3-chlorostyryl)caffeine (CSC) on the outcome of 10 min ischemia in gerbils. Acute treatment with APEC improved recovery of postischemic blood flow and survival without affecting neuronal preservation in the hippocampus. Acute treatment with CSC had no effect on the cerebral blood flow but resulted in a very significant protection of hippocampal neurons. Significant improvement of survival was present during the initial 10 days postischemia. Due to subsequent deaths of animals treated acutely with CSC, the end-point mortality (14 days postischemia) in this group did not differ statistically from that seen in the controls. It is, however, possible that the late mortality in the acute CSC group was caused by the systemic effects of brain ischemia that are not subject to the treatment with this drug. Chronic treatment with APEC resulted in a statistically significant improvement in all studied measures. Although chronic treatment with CSC improved postischemic blood flow, its effect on neuronal preservation was minimal and statistically insignificant. Mortality remained unaffected. The results indicate that the acute treatment with adenosine A2A receptor antagonists may have a limited value in treatment of global ischemia. However, since administered CSC has no effect on the reestablishment of postischemic blood flow, treatment of stroke with adenosine A2A receptor antagonists may not be advisable. Additional studies are necessary to elucidate whether chronically administered drugs acting at adenosine A2 receptors may be useful in treatment of stroke and other neurodegenerative disorders.
Keywords: Adenosine, Ischemia, Adenosine receptor, Therapy, (Gerbil)
1. Introduction
Treatment of cerebral ischemia with drugs acting at adenosine receptors was proposed over 10 years ago (Phillis and Wu, 1981). Experimental studies rapidly validated the original concept (reviewed by Rudolphi et al., 1992; Von Lubitz et al., 1995a) showing that both pre-and postischemic administration of adenosine A1 receptor agonists results in a significant protection against ischemic brain injury. Moreover, treatment with these drugs resulted in a substantial improvement of survival and reduction of neurological deficits caused by ischemia (Von Lubitz and Marangos, 1990; Von Lubitz et al., 1994a).
Recently, a number of studies showed that contrary to the protective effects of acute administration, chronic exposure to highly selective adenosine A1 receptor agonists causes aggravation of ischemia-related neuronal injury and reduction of survival (reviewed by Von Lubitz et al., 1995a). Similar findings were also reported in animal models of seizures (Adami et al., 1995; Von Lubitz et al., 1994b). The phenomenon of regimen-dependent reversal of the therapeutic effect has been described for adenosine A3 receptors as well (Von Lubitz et al., 1994c, 1995b). In view of current efforts aimed at the introduction of adenosine receptor-based therapies in treatment of several forms of neurodegenerative disorders (Von Lubitz et al., 1995a), further exploration of the effects of chronic exposure to agents acting at adenosine receptors becomes increasingly important.
The involvement of adenosine A2 receptors in regulation of the cerebral blood flow has been known for a long time (reviewed by Phillis, 1989; Wei and Kontos, 1993). Yet, no reports have been published on the effect of adenosine A2 receptor stimulation in the context of cerebral ischemia. Furthermore, only one paper describes the effect of the acute preischemic exposure to an A2 receptor antagonist (Gao and Phillis, 1994). Therefore, in order to further explore the possible therapeutic role of adenosine A2 receptors in treatment of cerebral ischemia, we have investigated the consequences of both acute and chronic preischemic exposure of these receptors to either an agonist or an antagonist.
2. Materials and methods
2.1. Animals
Female gerbils (70 g, Tumblebrook Farms, Brookfield, MA, USA) were used. Prior to the experiments, the method of Lee et al. (1984) was used to eliminate animals prone to spontaneous convulsions. Eighty gerbils were randomly selected from the screened population and divided into experimental groups. Each group used in the studies of postischemic survival consisted of 10 animals, whereas groups of 5 animals were used for the studies of postischemic cortical blood flow.
2.2. Drugs
The selective adenosine A2A receptor agonist 2-[(2-aminoethylamino)-carbonylethylphenylethylamino]-5′-N-ethylcarboxoamidoadenosine (APEC) and antagonist 8-(3-chlorostyryl)caffeine (CSC) were synthesized at this laboratory (Jacobson et al., 1993). Drugs were dissolved in a 20:80 v/v solution of Alkamuls (Rhône-Poulenc, Cranbury, NJ, USA) and saline and injected i.p. at 0.15 ml using a 25 gauge needle.
2.3. Treatment regimens
Controls
Controls were injected with the vehicle. The injections were made daily for 14 days, with the last injection taking place 15 min prior to ischemia.
Acute regimen
In the acute regimen, animals were injected with the vehicle for 13 days. On the 14th day, they were given either 0.1 mg/kg APEC or 1 mg/kg CSC administered 15 min prior to ischemia.
Chronic regimen
Chronic injections of either APEC (0.1 mg/kg) or CSC (1 mg/kg) were given once daily for 13 days. On day 14, animals were injected with the vehicle given 15 min preischemia.
2.4. Ischemia
Forebrain ischemia was induced for 10 min by simultaneous occlusion on both carotid arteries. During ischemia, the body/brain temperature of the animals was maintained within individual preischemic values by means of an infrared heating lamp and a heating blanket (Harvard Apparatus, South Natick, MA, USA). The details of both ischemia induction and temperature control have been published previously (Von Lubitz et al., 1994c).
2.5. Cortical blood flow measurements
Relative changes in the cortical blood flow were measured using a laser Doppler probe and monitor (Perimed, Piscataway, NJ, USA). In order to establish baseline values, blood flow monitoring was initiated 2 min prior to carotid occlusion. During the initial 2 min of ischemia, blood flow changes were monitored every 30 s. Subsequent intraischemic measurements were made at 5 min and immediately prior to the release of the occluding sutures. After ischemia, the measurements were made every 5 min for the initial 30, min and then every 15 min for the subsequent 90 min.
Techniques employed in the placement of the probe and the execution of blood flow measurements have been described elsewhere (Lin et al., 1993).
2.6. Postischemic survival
Survival and non-quantitative neurological assessment were determined each day for 14 days postischemia. Neurological assessment consisted of noting the presence of ptosis, hyperactivity and/or convulsive behavior, locomotor impairment (circling), and coma.
2.7. Neuronal survival
Fifteen days after ischemia, survivors in all groups were anesthetized with Nembutal (50 mg/kg). Transcardiac perfusion was made using saline flush (30 s) followed by 3.5% buffered paraformaldehyde (pH 7.4; 10 min). After removal, brains were sectioned on a freeze microtome and the sections were processed for light microscopy as described previously (Von Lubitz et al., 1994c). The extent of pathological changes in the hippocampus was determined by an investigator unaware of the treatment protocol.
2.8. Statistical analysis
Fisher’s exact test was used to analyze the end-point survival data, while statistical parameters of neuronal survival were determined using the Student-Newman-Keuls test. Due to the extensive exposure to anesthetics during measurements, animals used in the studies of the cortical blood flow were excluded from the analysis of survival and morphological data. The Student-Newman-Keuls test was also used for the analysis of the blood flow data.
3. Results
3.1. Postischemic blood flow
Acute regimen
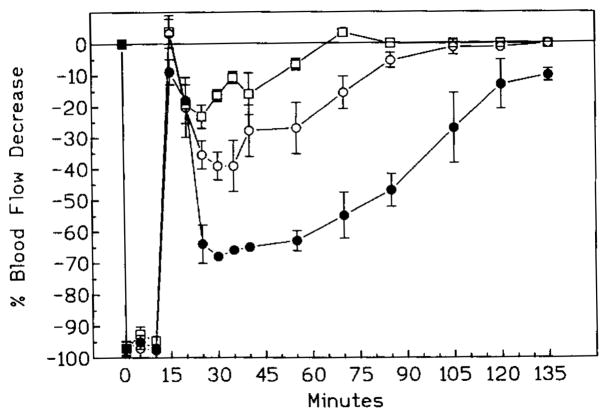
In all groups, occlusion of carotid arteries resulted in a rapid (20 s) decrease of the cortical blood flow by more than 97% (Fig. 1).
Fig. 1.
Postischemic cortical blood flow in controls (black circles) and in animals treated acutely with either APEC (open circles) or CSC (open squares). Time measured from the moment of the occlusion which ends 10 min later. Note the swift increase of flow in the APEC group and virtually identical pattern of recovery in control and CSC groups.
Following removal of the occluding sutures, the flow increased transiently in both control and CSC group to within 10% below the baseline, followed by a rapid decrease by approximately 60% (CSC) to 70% (controls) below the baseline (Fig. 1). Subsequent recovery was slow and, at 2 h postischemia, the cortical blood flow in both control and CSC groups was still lower (P < 0.05) than prior to ischemia.
Contrary to the control and CSC groups, the cortical blood flow in the APEC group rose steadily following removal of the occluding sutures. At 30 min postischemia, the values reached the level of preischemic baseline and remained there during the rest of the monitoring period.
Chronic regimen
Intraischemic depression of the cortical blood flow in the chronically treated groups was identical to that seen in animals treated acutely (Fig. 2).
Fig. 2.
Postischemic cortical blood flow in animals treated chronically with either APEC (open circles) or CSC. The recovery of flow in both groups is significantly better (P < 0.05, Student-Newman-Keuls) than in the controls (black circles). For most of its course, recovery in the CSC group is also much better (P < 0.05, Student-Newman-Keuls) than in the APEC group.
Following ischemia, in both CSC and APEC groups there was a transient elevation of the cortical blood flow that reached the level of the preischemic baseline.
In the CSC group, the subsequent depression of cortical blood flow did not exceed 30%. One hour after ischemia the cortical flow in the animals treated chronically with CSC attained its preischemic value. No further changes were observed (Fig. 2).
At 15 min postischemia, the reduction of the cortical blood flow in the APEC group was significantly greater (P < 0.05) than in the CSC group (Fig. 2). Nonetheless, even at the peak of the postischemic depression, the flow in the APEC group was very significantly better (P < 0.01) than in the controls. Although the subsequent recovery was slow and its pattern resembled that of the control animals (Fig. 2), the CBF values in the APEC group reached the preischemic level at approximately 1.5 h after ischemia as opposed to over 2 h in the control group.
3.2. Recovery
Controls
Control animals recovered from postischemic coma within 30 min. Three hours later, all were fully mobile but displaying hyperactive behavior (i.e., aimless running, jumping and convulsive scratching). Moreover, all animals were prone to brief convulsing episodes induced by external stimuli (e.g., sudden noise). In 7 gerbils hyperactivity transformed into locomotor depression within 12–24 h postischemia, followed subsequently by coma of varying length and death at 24–48 h after ischemia. In the surviving controls, the hyperactive behavior abated 3–4 days postischemia. Ptosis was present in all survivors.
Acute treatment
Ptosis was absent in all animals treated acutely with CSC. Hyperactive behavior (running either across or along the periphery of the cage but no jumping or scratching) was evident for up to 36 h. However, in gerbils that were to die within the initial 5 days postischemia, hyperactivity was rapidly (5–12 h) transformed into locomotor depression lasting 12–72 h followed by coma and subsequent death.
During the initial 24 h of postischemic recovery, the behavior of the animals in the APEC group was strikingly similar to that seen in the controls (i.e., persistent hyperactivity, susceptibility to exogenously induced seizures, ptosis in 50% of the animals). In three gerbils, hyperactivity abated within 36 h. Following a brief period of immobility, all of these animals entered into coma and died within 48 h postischemia.
Chronic treatment
The course of neurological recovery of animals treated chronically with CSC was indistinguishable from that of the control animals (see Controls section above).
Apart from one gerbil which never regained consciousness, animals treated chronically with APEC recovered from postischemic coma within 30 min. Contrary to other groups, the periods of hyperactivity were intermittent with bursts of running or violent scratching of cage walls separated by periods of virtually normal behavior (i.e., cage exploration, grooming, eating or sleep). During the first 24 h postischemia, hyperactive periods lasted 15–30 min followed by up to 1 h of quiescence. During the following 24 h the quiet interval gradually increased until, at 48 h postischemia, the behavior of the group treated chronically with APEC was indistinguishable from that of normal, nonischemic gerbils.
3.3. Survival
Controls
All of control gerbils which were destined to die expired within the initial 3 days postischemia and at 14 days postischemia 30% remained alive (Figs. 3 and 4).
Fig. 3.
Postischemic survival in controls (black circles) and animals treated acutely with either APEC (open circles) or CSC (open squares). Between day 5 and 10, survival in both APEC and CSC groups is statistically better than in the control group (P < 0.05, Fisher’s test). However, at the end-point, the significance is lost in the CSC group (see text for details).
Fig. 4.
Survival in animals treated chronically with either APEC (open circles) or CSC (open squares). While survival in the APEC group is significantly better than in the controls (black circles (P < 0.05)) beginning with day 4 postischemia, the difference between CSC and control groups has no statistical significance.
Acute treatment
In the APEC group, all deaths occurred between 24 and 48 h postischemia (Fig. 3). At the end of the monitoring period the survival in the APEC group was significantly better than among control animals (70% vs. 30%, P < 0.05).
Although there was no statistical significance, the initial mortality rate in animals treated acutely with CSC was numerically lower than in the two other groups (Fig. 3). Thus, only one animal died during the initial 2 days, and an additional three in the interval between the 2nd and the 5th day postischemia. During the period between day 2 and day 10, the survival of CSC treated animals was significantly better (P < 0.05) than in the control group. However, two additional animals died unexpectedly between day 10 and day 14 postischemia. Neither of these two animals showed any signs of a discernible neurological impairment and their behavior prior to death remained completely unremarkable. End-point mortality in the CSC group was 50% (no statistical significance compared to the controls).
Chronic treatment
As in the acute treatment, all deaths in the group treated chronically with APEC occurred within the initial 48 h postischemia (Fig. 4). However, in contrast to the acute treatment, only two animals died during that period, and the end-point survival was 80%.
Neither mortality rate nor the ultimate survival among animals treated chronically with CSC differed statistically from those of the control group (Fig. 4).
3.4. Histopathological changes in the hippocampus
At the end of the monitoring period (i.e. 14 days postischemia), control animals showed only 45% of hippocampal neurons with intact morphology (Fig. 5).
Fig. 5.
Survival of the hippocampal neurons following vehicle (black bar), and either acute CSC (shaded bar) or acute APEC (open bar). Acute treatment with CSC results in a significant (P <0.01, Student-Newman-Keuls test) improvement of hippocampal morphology.
Acute treatment
The number of surviving neurons in the acute APEC group (38%) did not differ statistically from that in the control group (45%, P < 0.3, Fig. 5). In contrast, neuronal preservation in the CSC group was very significantly higher than that seen in the control animals (90% vs. 45%, Fig. 5).
Chronic treatment
Compared to controls, animals treated chronically with APEC showed a significant improvement of neuronal preservation (Fig. 6 (85%, P < 0.01)). On the other hand, although in animals treated chronically with CSC the number of neurons with intact morphology was higher than in controls, the difference was statistically insignificant (60% vs. 45%, P < 0.07).
Fig. 6.
Neuronal survival in the hippocampus following chronic treatment with either CSC (shaded bar) or APEC (open bar). While treatment with CSC results in a numerical improvement vs. controls (black bar), there is no statistical significance. The number of morphologically intact neurons in the APEC group is, however, significantly better (P < 0.03, Student-Newman-Keuls test). Note that neuronal preservation following chronic treatment with APEC is statistically indistinguishable from that seen after acute exposure to CSC (Fig. 5).
4. Discussion
Excessive release of excitatory amino acids and hyperstimulation of their receptors during cerebrocirculatory arrest are among the chief causes of postischemic neuronal death (Choi and Rothman, 1990). Therefore, a wide range of therapies based on antagonism of excitotoxic phenomena have been either proposed or employed in experimental treatment of focal and global ischemia (Krieglstein, 1990). Among them, the concept of adenosine-based interventions is beginning to gain a rapid acceptance (Rudolphi et al., 1992; Von Lubitz and Marangos, 1992). Until recently, the conceptual basis of treatment of cerebral ischemia with adenosine rested solely on agents acting at adenosine A1 receptors whose acute stimulation has been shown to result in diminished release of excitatory amino acids, attenuation of NMDA receptor efficacy, and reduction of neuronal of neuronal excitability (reviewed by Phillis, 1989; Von Lubitz et al., 1995a). Moreover, although the neuroprotective effect of chronic exposure of the recently discovered adenosine A3 receptors (reviewed by Linden, 1994) to their agonist has been explored as well (Von Lubitz et al., 1994c, 1995b), virtually nothing is known about the postischemic outcome following stimulation of adenosine A2 receptors. This lack of knowledge is even more surprising in view of the involvement of the latter receptor subclass in regulation of the cerebral blood flow, i.e., a phenomenon both known for many years (reviewed by Phillis, 1989) and also of an unquestionable interest in treatment of cerebral ischemias.
Apart from regulating cerebral blood flow, adenosine A2A receptors appear to be involved in the excitatory phenomena in the hippocampus (Sebastião and Ribeiro, 1992; Sebastião et al., 1995) and in the intraischemic release of glutamate and aspartate (O’Regan et al., 1992). Facilitation of neurotransmitter release appears to result from the interaction of adenosine A2 receptors and P-type calcium channels (Umemiya and Berger, 1994). Utilizing the concept of adenosine A2 receptor participation in excitatory neurotransmitter release, Gao and Phillis (1994) demonstrated that acute administration of a potent and weakly selective adenosine A2 receptor antagonist, CGS 15943, at 0.1 mg/kg prior to 5 min ischemia in gerbils results in a substantial attenuation of neural damage in the CA1 sector of the hippocampus and in a marked reduction of postischemic neurological deficit (hyperactivity).
Essentially, our present results confirm the findings of Gao and Phillis (1994) and demonstrate that acute treatment with 1 mg/kg of CSC, a highly selective A2A antagonist, leads to reduction of neurological impairment and a significant reduction of neuronal death in the hippocampus at 14 days following 10 min forebrain ischemia. The treatment also results in a significant attenuation of postischemic mortality during the initial 10 days of postischemic recovery but statistical significance is lost at the end-point (i.e., 14 days postischemia). It is, nonetheless, very likely that the late deaths of animals treated acutely with CSC are not related to the long-term failure of cerebroprotective actions of the drug but to systemic phenomena which, although evoked by ischemia, may not be directly related to the cerebral functions per se. The latter conclusion is supported by the fact that cerebral ischemia induces a wide range of lasting functional changes in several organ systems (e.g. liver, kidneys, adrenals, lung, etc. (Wexler, 1972)) which possibly require therapeutic support other than that offered by adenosine A2A receptor-based drugs.
Interestingly, while acute treatment with APEC results in a very rapid reestablishment of cortical blood perfusion at the preischemic level and in a significant improvement of survival, neuronal damage in the hippocampus does not differ statistically from that seen in the control group. In contrast, the protective effects of CSC were obtained despite the fact that the recovery of postischemic cortical blood flow was virtually identical to that of the controls. Other authors have also shown that the neuroprotective effects of agents acting either at the NMDA receptor complex (e.g. dizolcipine (MK-801), Stevens and Yaksh, 1990; Park et al., 1989; kynurenic acid, Seylaz et al., 1990), adenosine A1 receptors (Seylaz et al., 1990) or calcium channels (Hakim, 1986)) may be independent of postischemic blood flow in both focal (Hakim, 1986; Park et al., 1989) and global (Stevens and Yaksh, 1990; Seylaz et al., 1990) ischemia. It appears, therefore, that ‘hemodynamic protection’ (Nakayama et al., 1988) during the immediately postocclusive period may be less critical than prevention of the metabolic derangement of the ischemic brain tissue. Nonetheless, the importance of rapid normalization of the postischemic blood flow must not be underrated since several studies clearly show that a meaningful tissue protection, particularly in focal ischemia, can be obtained only when an adequate blood perfusion is reestablished within 2–4 h following the occlusive event (reviewed by Ginsberg, 1995).
In vitro and in vivo studies (Lupica et al., 1991; Abbracchio et al., 1992; Shi et al., 1993; Adami et al., 1995) indicate that chronic exposure of adenosine A2A receptors to agonists or antagonists does not result in changes of either receptor density or their dissociation constants. Moreover, contrary to a drastic reversal of effect reported by us in cases where adenosine A1 and A3 receptors were stimulated either acutely or chronically (Von Lubitz et al., 1994a,b,c), chronic exposure to either an agonist or antagonist of the A2 receptor results in a much blunted response reversal. Thus, although chronic treatment with CSC significantly improves reestablishment of postischemic blood flow (i.e., reversal of the acute treatment), it also results in a numerical (but not significant) improvement of neuronal preservation and an entirely unaffected postischemic mortality. On the other hand, and contrary to our expectations, chronic treatment with APEC significantly improves the outcome in all three of the studied measures.
A possible source of the baffling results of chronic treatment with APEC may rest with the very recently reported affinity of APEC at not only adenosine A2A but at A3 rat receptors as well (Ki 50 nM, Kim et al., 1994). We have previously shown that chronic stimulation of adenosine A3 receptors is highly protective in the same model of cerebral ischemia as used in the present study (Von Lubitz et al., 1994c). Moreover, chronic treatment with the adenosine A3 receptor agonist IB-MECA administered at doses as low as 5 μg/kg results in a significant reduction of neurological deficits and in a significantly improved survival of 10 min ischemia in gerbils (manuscript in preparation). It is therefore possible that the impressive protection obtained with chronically administered APEC is the result of a protracted, low intensity chronic stimulation of adenosine A3 rather than A2 receptors alone. Several observations support this conclusion. First, chronic exposure to APEC produces a partial but statistically significant elevation of postischemic cerebral blood flow (as measured against controls) rather than its pronounced depression, as might be expected if a fully fledged regimen-dependent effect reversal mediated by adenosine A2A receptors were taking place. Second, the blood flow response induced by chronic treatment with APEC is virtually identical to that seen after chronic administration of an adenosine A3 receptor agonist (Von Lubitz et al., 1994c). Moreover, postischemic recovery in other measures (i.e., survival and neuronal preservation) are also indistinguishable when present results are compared to those reported for chronic administration of adenosine A3 receptor agonist prior to 10 min ischemia in gerbils (present study and Von Lubitz et al., 1994c). Unfortunately, direct confirmation of possible stimulation of adenosine A3 receptors by chronically administered APEC must await development of sufficiently selective adenosine A3 receptor antagonists.
As stated previously, compared to a striking regimen-dependent reversal of the therapeutic effect seen during acute vs. chronic stimulation of adenosine A1 or A3 receptors (Von Lubitz et al., 1994a,b,c), the divergence between acute and chronic treatment with either APEC or CSC is less clear. It is therefore probable that the blunted character of the reversal effect induced by chronic administration of either APEC or CSC represents a sum of individual actions elicited by stimulation or inhibition of receptors belonging both to adenosine (A2A, A3). Moreover, involvement of compensatory ‘downstream’ responses of non-adenosine types as a part of the effects of chronic administration of adenosine A2A receptor acting agents cannot be excluded either. It has been shown that prolonged exposure to the non-specific adenosine A1/A2 antagonist caffeine causes marked density changes in both adenosine A1 as well as non-adenosine receptor systems (e.g., cholinergic, adrenergic and GABAergic (Shi et al., 1993)). The likelihood of such complex interactions is strengthened even further by the fact that both the affinity and signal transduction at dopamine D2 receptors are modulated by the colocalized and coexpressed adenosine A2A receptors (reviewed by Ferré et al., 1992).
In conclusion, it appears that the acute treatment with adenosine A2 receptor antagonists may represent a potential venue for therapies aimed at the treatment of global cerebral ischemia as seen in cardiac arrest. Treatment of focal ischemia, on the other hand, is more questionable in view of its indifferent impact on the postischemic blood flow. Whether, in similarity to drugs acting at either adenosine A1or A3 receptors, chronic exposure of adenosine A2A receptors to their ligands may offer a suitable treatment for other degenerative brain pathologies requires further studies.
References
- Abbracchio MP, Fogliatto G, Paoletti AM, Rovati GE, Cattabeni F. Prolonged in vitro exposure of rat brain slices to adenosine analogues: selective desensitization of adenosine A1 but not A2 receptors. Eur J Pharmaeol Mol Pharmacol. 1992;227:317. doi: 10.1016/0922-4106(92)90010-s. [DOI] [PubMed] [Google Scholar]
- Adami M, Bertorelli R, Ferri N, Foddi MC, Ongini E. Effects of repeated administration of selective adenosine A1 and A2 receptor agonists on pentylenetetrazole-induced convulsions in the rat. Eur J Pharmacol. 1995;294 doi: 10.1016/0014-2999(95)00557-9. in press. [DOI] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemk neuronal death. Annu Rev Neurosci. 1990;13:171. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fuxe K, Von Euler G, Johansson B, Fredholm BB. Adenosine-dopamine interactions in the brain. Neuroscience. 1992;51:501. doi: 10.1016/0306-4522(92)90291-9. [DOI] [PubMed] [Google Scholar]
- Gao Y, Phillis JW. CGS 15943, an adenosine A2 receptor antagonist, reduces cerebral ischemic injury in the Mongolian gerbil. Life Sci. 1994;3:PL 61. doi: 10.1016/0024-3205(94)00889-2. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. The concept of the therapeutic window – a synthesis of critical issues. In: Moskowitz MA, editor. Proceedings of the 19th Princeton Conference on Cerebrovascular Disease. 1995. in press. [Google Scholar]
- Hakim AM. Cerebral acidosis in focal ischemia. II. Nimodipine and verapamil normalize cerebral pH following middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1986;6:676. doi: 10.1038/jcbfm.1986.123. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Nikodijević O, Padgett WL, Gallo-Rodriguez C, Maillard M, Daly JW. 8-(3-chlorostyryl)caffeine (CSC) is a selective A2 adenosine antagonist in vitro and in vivo. FEBS. 1993;323:141. doi: 10.1016/0014-5793(93)81466-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, Ji X-d, Sidiqqi SM, Olah ME, Stiles GL, Jacobson KA. 2-substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem. 1994;21:3614. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein J. Pharmacology and drug therapy of cerebral ischemia. In: Schurr A, Rigor B, editors. Cerebral Ischemia and Resuscitation. CRC Press; Boca Raton: 1990. p. 347. [Google Scholar]
- Lee RJ, Bajorek JG, Lomax P. Similar anticonvulsive, but unique behavioural effects of opioid agonists in the seizure-sensitive Mongolian gerbil. Neuropharmacology. 1984;5:517. doi: 10.1016/0028-3908(84)90024-8. [DOI] [PubMed] [Google Scholar]
- Lin RCS, Matesic DF, McKenzie RJ, Devlin TM, Von Lubitz DKJE. Neuroprotective activity of dimer of 16, 16′-dimethyl-15-dehydroprostaglandin B1 (di-Calciphor) in cerebral ischemia. Brain Res. 1993;606:130. doi: 10.1016/0006-8993(93)91580-l. [DOI] [PubMed] [Google Scholar]
- Linden J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol Sci. 1994;15:298. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Berman RF, Jarvis MF. Chronic theophylline treatment increases adenosine A1 but not adenosine A2 receptor binding in the rat brain – an autoradiographic study. Synapse. 1991;9:95. doi: 10.1002/syn.890090203. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Ginsberg MD, Dietrich WD. (S)-Emopamil, a novel calcium channel blocker and serotonin S2 antagonist, markedly reduces infarct size in rats with middle cerebral artery occlusion. In: Krieglstein J, editor. Pharmacology of Cerebral Ischemia 1988. Wissenschaftliche Verlagsgesellschaft; Stuttgart: 1988. p. 125. [DOI] [PubMed] [Google Scholar]
- O’Regan MH, Simpson RE, Perkins LM, Phillis JW. The selective adenosine A2 receptor agonist CGS 21680 enhances excitatory transmitter amino acid release from the ischemic rat cerebral cortex. Neurosci Lett. 1992;138:169. doi: 10.1016/0304-3940(92)90498-v. [DOI] [PubMed] [Google Scholar]
- Park CK, Nehls DG, Teasdale GM, McCulloch J. Effect of NMDA antagonist MK-801 on local cerebral blood flow in focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1989;9:617. doi: 10.1038/jcbfm.1989.88. [DOI] [PubMed] [Google Scholar]
- Phillis JW. Adenosine in control of cerebral circulation. Cerebrovasc Brain Metab Rev. 1989;1:26. [PubMed] [Google Scholar]
- Phillis JW, Wu PH. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16:187. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Adenosine and brain ischemia. Cerebrovasc Brain Metab Rev. 1992;4:346. [PubMed] [Google Scholar]
- Sebastião AM, Ribeiro JA. Evidence for the presence of A2 adenosine receptors in the rat hippocampus. Neurosci Lett. 1992;138:41. doi: 10.1016/0304-3940(92)90467-l. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Cunha RA, Correia-de-Sá P, De Mendonca A, Ribeiro JA. Role of A2a receptors in the hippocampus and motor nerve endings. In: Belardinelli L, Pelleg A, editors. Adenosine and Adenine Nucleotides: From Molecular Biology to Integrative Physiology. Kluwer Academic Publishers; Boston: 1995. p. 251. [Google Scholar]
- Seylaz J, Boulu RG, Charbonné R, Corréze JL, Lekieffre D, Le Peillet E, Lhoste JM, Meric P, Mispelter J, Pinard E, Plotkine M, Roucher P, Roussel S, Tiffon B. Monitoring of cerebral high-energy metabolites and blood flow associated with histopathology and behavior analysis in transient forebrain ischemia: a useful tool in pharmacology. In: Krieglstein J, Oberpiechler H, editors. Physiology of Cerebral Ischemia 1990. Wissenschaftliche Verlagsgesellschaft; Stuttgart: 1990. p. 255. [Google Scholar]
- Shi D, Nikodijevic O, Jacobson KA, Daly JW. Chronic caffeine alters the density of adenosine, adrenergic, cholinergic, GABA, and serotonin receptors and calcium channels in mouse brain. Cell Mol Neurobiol. 1993;13:247. doi: 10.1007/BF00733753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MK, Yaksh TL. Systematic studies on the effects of the NMDA receptor antagonist MK-801 on cerebral blood flow and responsivity, EEG, and blood-brain barrier following complete reversible cerebral ischemia. J Cereb Blood Flow Metab. 1990;10:77. doi: 10.1038/jcbfm.1990.10. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Activation of adenosine A1 and A2 receptors differentially modulates calcium channels and glycinergic synaptic transmission in rat brainstem. Neuron. 1994;13:1439. doi: 10.1016/0896-6273(94)90429-4. [DOI] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Marangos PJ. Cerebral ischemia in gerbils: postischemic administration of cyclohexyladenosine and 8-sulphophenyl-theophylline. J Mol Neurosci. 1990;2:53. doi: 10.1007/BF02896926. [DOI] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Marangos PJ. Self-defense of the brain: adenosinergic strategies in neurodegeneration. In: Marangos PJ, Lal H, editors. Emerging Strategies in Neuroprotection. Birkhauser; Boston: 1992. p. 151. [Google Scholar]
- Von Lubitz DKJE, Lin RC-S, Melman N, Ji X-d, Carter MF, Jacobson KA. Chronic administration of adenosine A1 receptor agonist or antagonist in cerebral ischemia. Eur J Pharmacol. 1994a;256:161. doi: 10.1016/0014-2999(94)90241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Paul IA, Ji X-d, Carter M, Jacobson KA. Chronic adenosine A1 receptor agonist and antagonist: effect on receptor density and N-methyl-D-aspartate induced seizures in mice. Eur J Pharmacol. 1994b;253:95. doi: 10.1016/0014-2999(94)90762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Lin RCS, Popik P, Carter MF, Jacobson KA. Adenosine A3 receptor stimulation and cerebral ischemia. Eur J Pharmacol. 1994c;263:59. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Carter MF, Beenhakker M, Lin RC-S, Jacobson KA. Adenosine: a prototherapeutic concept in neurodegeneration. In: Trembly B, Slikker B Jr, editors. Neuroprotective Agents, Proceedings of II International Conference. New York Academy of Sciences; New York: 1995a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lubitz DKJE, Carter MF, Deutsh SI, Lin RCS, Mastropaolo J, Meshulam Y, Jacobson KA. The effects of adenosine A3 receptor stimulation on seizures in mice. Eur J Pharmacol. 1995b;275:23. doi: 10.1016/0014-2999(94)00734-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei EP, Kontos HA. Cerebrovascular flow regulation by adenosine. In: Phillis JW, editor. The Regulation of Cerebral Blood Flow. CRC Press; Boca Raton: 1993. p. 281. [Google Scholar]
- Wexler BC. Pathophysiological responses to acute cerebral ischemia in the gerbil. Stroke. 1972;3:71. doi: 10.1161/01.str.3.1.71. [DOI] [PubMed] [Google Scholar]