Abstract
The intrinsic affinity of 8-phenylxanthine analogs at striatal A2-adenosine receptors is highly species dependent. [3H]XAC (8-[2-aminoethyl[amino[carbonyl[methyl[oxyphenyl]]]]]-1,3-dipropylxan-thine), although A1-selective in the rat brain, binds to A2 receptors in rabbit striatal membranes with sufficiently high affinity to serve as a radioligand. In the presence of 50 nM CPX (8-cyclopentyl-1,3-dipropylxanthine), an A1-selective antagonist added to eliminate binding to A1 receptors, [3H]XAC exhibits saturable, specific binding (70% of total) to A2 sites with a Kd of 3.8 nM and a Bmax of 1.23 pmol/mg protein. At 24°C, the association and dissociation rate constants were 0.13 min−1 nM−1 and 0.36 min−1, respectively. Binding was performed for 1 h, with non-specific binding defined in the presence of 100 μM NECA (N-ethylcarboxamidoadenosine). The potency order for antagonists against 1 nM [3H]XAC at rabbit A2-receptors was XAC ≈ Nα-Me-XAC ≫ CPX = XCC > 1,3-dipropyl-8-p-sulfophenylxanthine > PSPT. The relative potency order for agonists was CGS ≈ NECA > APEC [ = 2-(aminoethylaminocarbonyl-ethylphenylethylamino)-NECA] > PAPA-APEC > ADAC > R-PIA (N6-phenylisopropyladenosine) > S-PIA.
The ability to characterize central A2-adenosine receptors using an antagonist ligand that is chemically functionalized offers the possibility to design affinity labeling probes for this receptor subtype in the brain, similar to those antagonist probes already developed for A1-receptors. The results also suggest that affinity columns containing chemically immobilized XAC may be used for isolating central A2-adenosine receptors from rabbit striatum.
Adenosine, acting as a neuromodulator, inhibits the release of numerous stimulatory neurotransmitters, including glutamate, norepinephrine, serotonin and acetylcholine (Fredholm and Dunwiddie, 1988). Adenosine agonists acting in vivo via a central mechanism cause a dramatic depression of locomotor activity (Nikodijevic et al., 1990). 1,3-Dialkylxan-thines act as antagonists of adenosine at A1- and A2- adenosine receptor subtypes (Ramkumar et al., 1988). X A C (8-[4-[[[(2-aminoethyl)amino]carbonyl]-methyl]-oxy]phenyl]-1,3-dipropylxanthine), a xanthine amine congener (Jacobson et al., 1986), is a potent adenosine antagonist with an A1-selectivity ratio in the rat of 20- to 80-fold. [3H]XAC has been utilized as a radio-ligand at central A1-receptors, with a Kd value of 1.2 nM in rat cortex. At A2-receptors, [3H]XAC has been reported as a radioligand only in human plate-lets with a Kd-value of 12 nM (Ukena et al., 1986a). At rat striatal A2-receptors, [3H]XAC (at concentrations ≤5 nM) is not a satisfactory radioligand due to relatively low affinity and to non-specific binding to the glass fiber filters (J. W. Daly, personal communication).
A large species-dependent variation in the potencies of certain substituted purine derivatives at A1 and at A2 receptors has been noted. For example, a given 8-phenylxanthine or N6-phenyladenosine derivative may have affinities for central A1-receptors ranging over several orders of magnitude depending on species (Ukena et al., 1986b). Typically, the species-dependent order of potency for 8-phenylxanthines at A1-receptors is: calf > rat > guinea pig, human. At A2-receptors, the Ki-values for 1,3-diethyl-8-phenyl-xanthine (DPX) were shown to be at least an order of magnitude less in rabbit or human than in rat or bovine brain (Stone et al., 1988). Thus, the subtype selectivity of a given xanthine derivative is highly dependent on the species.
We have shown that XAC, like DPX, is of much greater affinity at A2-receptors in the rabbit than in the rat. This enhanced affinity allows the measurement of specific binding of [3H]XAC to rabbit striatal A2-receptors.
EXPERIMENTAL PROCEDURES
Materials
XAC, ADAC (N6-[4[[[4-[[[(2-aminoethyl)amino]carbonyl] methyl]anilino]carbonyl]methyl]phenyl]adenosine), NECA (N-ethylcarboxamidoadenosine), 8-p-sulfophenyl-xanthines (1,3-dimethyl and 1,3-dipropyl, abbreviated PSPT and DPSPX, respectively) and CPX (8-cyclopentyl-1,3-dipropylxanthine) were obtained from Research Bio-chemicals, Inc. (Natick, Mass.). N6-Cyclopentyladenosine was a gift from Dr Ray Olsson. [3H]XAC was obtained from Dr D. Ahern of Dupont NEN (Boston, Mass.). 2-Thio-CPX (8-cyclopentyl-1,3-dipropyl-2-thioxanthine), N,Nα-Me2-XAC (8-[2-dimethylaminoethyl[amino[carbonyl[methyl[oxy-phenyl]]]]]-1,3-dipropyl-xanthine), and the corresponding monomethyl XAC analog were synthesized by methods reported (Jacobsen et al., 1989a). m- and p-DITC-XAC isomers (1,3-dipropyl-8-[4-[[[[[2-[[[(isothiocyanatophenyl) amino] thiocarbonyl] amino] ethyl] amino] carbonyl] methyl] oxy]phenyl]xanthine) were synthesized as described (Jacobson et al., 1989b). APEC (2-[4-[2-[2-aminoethylamino-carbonyl]ethyl]phenyl] - ethylamino] - 5′ - N - ethylcarbox - amidoadenosine) and its Nα-p-aminophenylacetyl derivative, PAPA-APEC, were prepared as described (Jacobson et al., 1990). PD115,199 (8 - [2-dimethylaminoethyl[N-methyl-amino[sulfonyl[phenyl]]]]-1,3-dipropylxanthine) was the generous gift of Dr J. Bristol. Parke-Davis Warner Lambert. Ann Arbor Mich.
Preparation of striatal membranes
Rabbit striatal tissue (100 150 mg per brain) was isolated by dissection from whole rabbit brains, obtained frozen from Pel-Freeze Biologicals Co. (Rogers, Arkansas). Striatal membranes were homogenized in 20 vol of ice-cold 50 mM Tris, adjusted to pH 7.4 with hydrochloric acid, using a polytron (Kinematica, Gmbh, Luzerne, Switzerland) at a setting of 2–3 for 10 s. The membrane suspension was then centrifuged at 37,000 g for 20 min at 4°C. The pellet was resuspended in the above buffer solution, and the membranes were again homogenized and centrifuged. Finally the pellet was suspended in buffer (100 mg wet weight per ml) and stored frozen at −70°C until use. Protein was determined using the BCA protein assay reagents (Pierce Chemical Co., Rockford, Ill.), which is based on the complex with cuprous ions and bicinchoninic acid (Smith et al., 1985).
[3H]XAC binding
The binding of [3H]XAC to rabbit striatal membranes was measured in a total volume of 1 ml, each in a 13 × 100 mm glass tube. The unlabeled competing ligand or NECA (at a final concentration of 100 μM, for determination of non-specific binding) was dissolved in 25 μl of DMSO. To this solution was added 50 μl of 200 mM MgCl2, 50 μl of 1 μM CPX to eliminate binding to A1-adenosine receptors, 725 μl of 50 mM Tris*, at pH 7.4 at room temperature, and 100 μl of radioligand to produce a final concentration of 1 nM. Finally 100 μl of a striatal tissue suspension (final concentration of approx. 200 μg protein per ml), containing 3 IU/ml adenosine deaminase, Type VI from calf intestinal mucosa (Sigma, St Louis, Mo.), was added. The mixture was incubated with shaking for 60 min at 24°C. All assays were done in triplicate. Bound and free radioligand were separated by addition of 4 ml of ice-cold 50 nM Tris, pH 7.4, followed by rapid filtration using a Brandel Cell Harvester (Brandel, Gaithersburg, Md). The contents of each tube were filtered through a Whatman GF/B filter that had been soaked in a flat pan in buffer containing 0.3% polyethylene imine for 60 min. The filters were washed twice with 4 ml of ice-cold 50 mM Tris, pH 7.4. Each filter disk was added to 4 ml of scintillation fluid, vortexed and counted after 6 h.
Data analysis
IC50 values were computer-generated from competition binding data, using a non-linear regression formula on the GraphPAD program (Institute for Scientific Information), were converted to Ki values using a Kd value for [3H]XAC of 3.8 nM and the Cheng-Prusoff equation (Cheng and Prusoff, 1973).
RESULTS
[3H]XAC was tested as a radioligand at A2-aden-osine receptors in rabbit striatal membranes. Since XAC is not A2-selective, even in the rabbit, it was necessary to add a sufficient quantity of an A1-selective antagonist to selectivity block the A1 component. CPX (8-cyclopentyl-1,3-dipropylxanthine) was selected for this purpose and added to the incubation medium. CPX at a concentration of 50 nM was found to occupy 90% of the A1-receptors, but only 6.5% of the A2-receptors in rabbit striatum. A curve representing competition for [3H]XAC binding sites in the rabbit striatum (data not shown) fit a 2-site model when analyzed by a computer-assisted curve-fitting program (Scatfit). The Ki-value for CPX at the high affinity (A1) site was found to be 2.9±2.2 nM and at the low affinity (A2) site 250±144 nM. The high affinity component represented 46% of the combined, specific [3H]XAC binding.
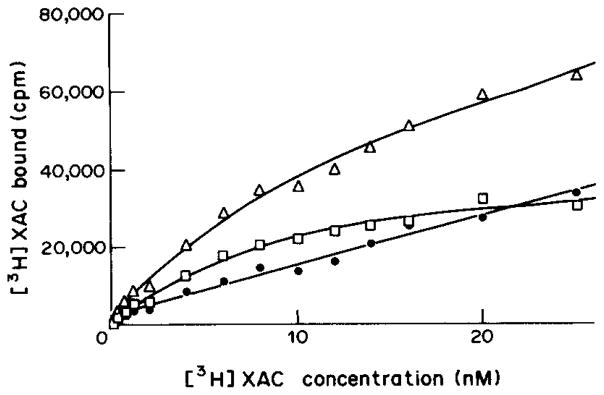
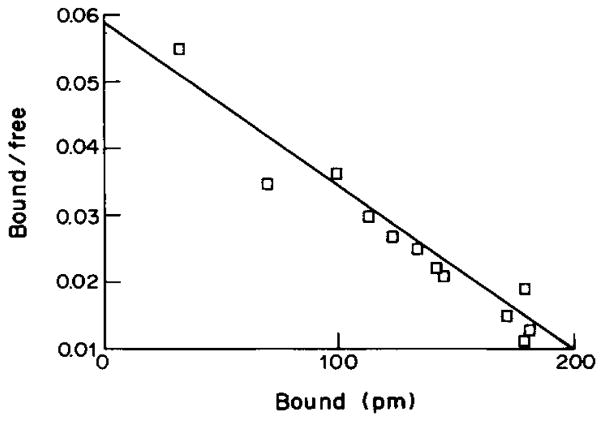
A representative saturation isotherm for specific [3H]XAC binding, in the presence of 50 nM CPX is shown in Fig. 1. The Scatchard plot of the data (Fig. 2) is linear indicating a homogeneous population of non-cooperative binding sites with a Kd value of 3.80±0.15 nM and a binding capacity (Bmax) of 1.23±0.17 pmol/mg protein. The amount of specific binding was approx. 70% of total binding.
Fig. 1.
Saturation of [3H]XAC binding to rabbit striatal A2-adenosine receptors. Specific (□), non-specific (○) and total (△) binding were determined for 60 min at 24°C. Values are means of a typical experiment done in triplicate. The calculated Kd-value of 3.8 nM represents the mean of 3 separate experiments.
Fig. 2.
Scatchard plot for the binding of [3H]XAC to rabbit striatal A2-adenosine receptors (data same as for Fig. 1). A Kd-value of 3.8 nM and a Bmax value of 1.23 pmol/mg protein were determined.
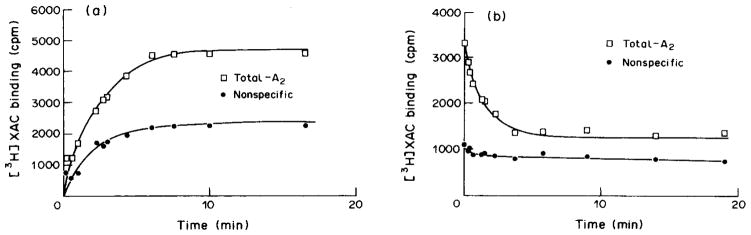
Association and dissociation kinetics for [3H]XAC were rapid and monophasic at 24°C (Fig. 3). The association rate constant, ka and the dissociation rate constant, kb, for [3H]XAC calculated from the least squares plot were 0.13 min−1 nM−1 and 0.36 min−1, respectivey, resulting in a Kd estimate of 2.77 nM. This value is in good agreement with the Kd of 3.8 nM determined from saturation experiments.
Fig. 3.
Association (a) and dissociation (b) curves for [3H]XAC binding to rabbit striatal A2-adenosine receptors. Total binding at A2-receptors (□) and non-specific binding (○) are shown. Concentration of the radioligand was 0.7 nM, in the presence of 50 nM CPX. Dissociation was initiated after 75 min by the addition of 100 μM N6-cyclopentyladenosine. Data were fit to exponential curves by weighted non-linear least squares curvefitting. The experiments were carried out twice with essentially identical results. Association and dissociation constants of 0.13 min−1 nM−1 and 0.36 min−1, respectively, were calculated from linear transformation of the data. The linear regression coefficients for association and dissociation plots were 0.09 and 0.824, respectively.
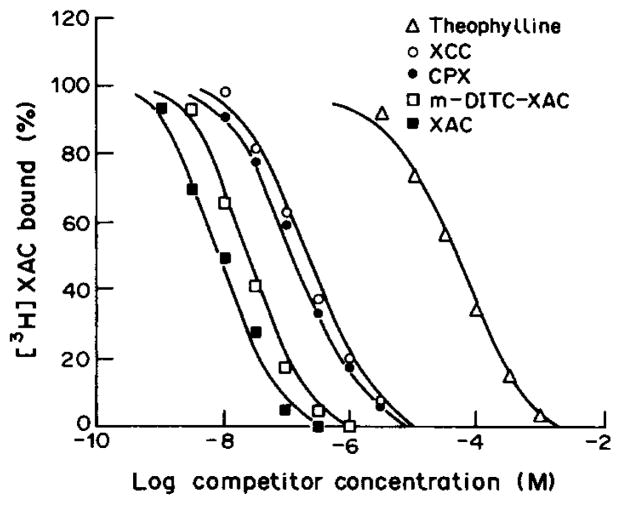
Some known agonists and antagonists at adenosine receptors competitively displaced specific [3H]XAC binding to rabbit striatal membranes as shown in Table 1. The rank order of potency expected for binding to rabbit A2-adenosine receptors was observed, i.e. for antagonists (Fig. 4) XAC ≈ Nα-Me - XAC ≫ CPX ≈ XCC > 1,3-dipropyl-8-p-sulfo-phenylxanthine > PSPT, and for agonists CGS-21680 ≈ NECA > APEC > PAPA-APEC > ADAC > R-PIA > S-PIA. The curves for antagonists were monophasic with slope factors approximately equal to 1. The agonist inhibition curves also fit mono-phasic analysis with correlation coefficients in the range of 0.9–1.0. The Ki values obtained for agonists were higher than those reported in rat brain using [3H]NECA as a radioligand, similar to the findings of Bruns et al. (1987) using another tritiated antagonist, PD115,199 at rat brain A2-adenosine receptors.
Table 1.
Affinity, expressed as Ki values in nM, of adenosine receptor agonists and antagonists at central A2-adenosine receptors in rabbit (vs [3H]XAC) and in rat (vs [3H]NECA) striatum, unless noted
| Compound | Ki (nM) at rabbit A2-adenosine receptors | Ki (nM) at rat A2-adenosine receptors | Ratio‡ |
|---|---|---|---|
| Antaqonists | |||
| (1) N,Nα-Me2-XAC | 5.3±1.2 | 5.0 | 0.94 |
| (2) Nα-Me XAC | 7.0±0.63 | 9.3* | 1.33 |
| (3) p-DITC-XAC | 7.2±1.6 | 321 | 46 |
| (4) XAC | 7.5±0.46 | 63 | 8.4 |
| (5) m-DITC-XAC | 15.6±1.6 | 343 | 22 |
| (6) N-Ac-XAC | 21.1±2.9 | 530 | 25 |
| (7) 2-thio-CPX | 30.7±5.9 | 314 | 10.2 |
| (8) CPX | 179±11.8 | 340 | 1.90 |
| (9) XCC | 196±31 | 2200 | 11.2 |
| (10) DPSPX | 277±79.2 | 11,000* | 39.7 |
| (11) PSPT | 30,090±1504 | 15,300 | 0.51 |
| (12) Theophylline | 38,250±244 | 25,300 | 0.66 |
| (13) 8-p-Sulfophenylcaffeine | > 100,000 | 150,000* | |
| Agonists | |||
| (14) NECA | 70.8±5.2 | 10.3 | 0.15 |
| (15) CGS21680 | 79.1±4.7 | 15 | 0.19 |
| (16) APEC | 169±34.7 | 5.7 12† |
0.034 |
| (17) PAPA-APEC | 279±52.0 | 28† | 0.10 |
| (18) ADAC | 860±48.5 | 210 | 0.24 |
| (19) R-PIA | 3216±253 | 124 410† |
0.039 |
| (20) S-PIA | 4688±159 | 1820 3020† |
0.39 |
Binding of [3H]XAC (1.0 nM) was carried out for 60 min at 24°C, in the presence of 0.05 M Tris buffer at pH 7.4, 10 mM MgCl2 and 50 mM CPX. Ki values are the mean ± SD of at least 3 experiments done in triplicate. Ki values in the rat are from Bruns et al. (1986), Daly and Jacobson (1989), Jacobson el al. (1989a, 1989c) and Shamim et al. (1989). ND = not determined.
Versus NECA stimulation of adenylate cyclase in rat PC 12 pheochromocytoma cell membranes.
Versus binding of [3H]CGS21680 in rat striatum.
Ratio of Ki-values at rat A2-receptors vs Ki-values at rabbit A2-receptors.
Fig. 4.
Inhibition of binding of [3H]XAC to rabbit striatal A2-adenosine receptors by various adenosine receptor ligands. The binding was carried out at 24°C for 60 min. [3H]XAC was present at a concentration of 1.0 nM in 0.05 M tris buffer at pH 7.4 in the presence of 10 mM MgCl2 and 50 mM CPX.
XAC itself was a very potent inhibitor of [3H]XAC binding with a Ki value of 7.5 nM. This value is in good agreement with the Kd value derived from saturation experiments with [3H]XAC. Certain derivatives of XAC (structures shown in Table 2), particularly compounds 1–3, were also very potent, with Ki values in the range of 10−8 M at rabbit A2-adenosine receptors. p-DITC-XAC, 3, has been shown to be an irreversible inhibitor of A1-adenosine receptors of bovine brain. we are currently examining whether there exists irreversible binding of 3 and 5 at rabbit A2-receptors based on the high affinity.
Table 2.
Structures of XAC derivatives assayed for affinity at rabbit A2-adenosine receptors
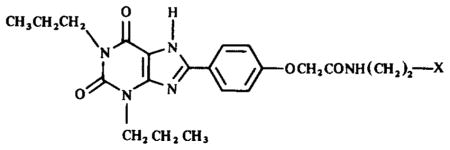
| |
|---|---|
| X = | Compound |
| NH2 | 4 |
| NHCH3 | 2 |
| N(CH3)2 | 1 |
| NHCOCH3 | 6 |
|
|
3 |
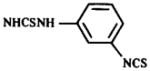
|
5 |
The enhancement of affinity of xanthines at rabbit vs rat A2-adenosine receptors is indicated by ratios of Ki values in Table 1. Previously, Stone et al. (1988) comparing inhibition of [3H]NECA binding at striatal A2-receptors in rat vs rabbit found ratios of Kis of 10.2 and 8.1 for 8-phenyltheophylline and 1,3-diethyl-8-phenylxanthine, respectively. The ratio of Ki-values for XAC (an 8-phenylxanthine analog) at rat and at rabbit brain A2-receptors was 8.4. This was in contrast to the ratio for CPX (an 8-cycloalkyl analog) of 1.9. The irreversibly-binding adenosine antagonists, meta-and para-isomers of DITC-XAC, showed an even greater enhancement of affinity at rabbit brain A2-receptors, with ratios of 22 and 46, respectively. Even 8-phenylxanthines such as XCC, 9, and DPSPX, 10, containing negatively charged groups, showed enhancement of affinity similar to XAC, which contains a positively charged amino group. Thus, it seems that the species-dependent enhancement of affinity at A2-adenosine receptors in rabbit is most pronounced for 8-phenylxanthines. The enhancement appears to be dependent on the presence of 1,3-dipropyl groups, as well as on the nature of the 8-phenyl substitutent. Compound 11 (ratio of 0.5) is identical to compound 10 (ratio of 40) except for methyl vs propyl groups at the 1- and 3-positions. 8-p-Sulfophenylcaffeine, similar in structure to PSPT, yet nearly inactive at rat adenosine receptors (Shamim et al., 1989), failed to displace [3H]XAC from rabbit A2-adenosine receptors.
Curiously, the gain in potency observed at the rat A2-receptor upon N-methylation of XAC, e.g. analogs 1 and 2, was not observed in the rabbit striatum. The Ki-value for another dimethylamino xanthine derivative, PD115,199, vs [3H]XAC binding was found to be 3.28±0.86 nM.
DISCUSSION
It would be desirable to have a readily available adenosine antagonist radioligand for A2-adenosine receptors in the striatum, the brain region in which the greatest amount of A2-receptor sites are found, to complement studies that are being carried out with adenosine agonists. Comparative studies of agonist and antagonist binding have already been performed at central A1-adenosine receptors.
Several agonist-derived, tritiated and iodinated radioligands have been used to characterize the binding properties and molecular structure of A2-adenosine receptors of striatum. A binding assay based on [3H]NECA, a non-selective agonist, requires the addition of a selective A1-ligand (N6-cyclopentyladenosine) to the medium to selectively occupy the A1-sites (Bruns et al., 1986). CGS21680 (Hutchison et al., 1989, 1990; Jarvis et al., 1989) and an iodinatable functionalized congener related to it, PAPA-APEC (Barrington et al., 1989; Jacobson et al., 1989c), are high affinity agonist radioligands that are selective for A2-receptors. There are only two reports of adenosine antagonists, namely XAC and PD115,199, as radioligands at A2-adenosine receptors (Ukena et al., 1986a; Bruns et al., 1987). Both XAC and PD115,199 bind to A2-receptors under the proper circumstances, but neither ligand is A2-selective.
We now show that [3H]XAC binds to A2-adenosine receptors in the rabbit brain, unlike in the rat brain, where it is A1-selective and unsatisfactory as an A2-radioligand. Displacement of binding by other adenosine ligands occurs with a rank order of potency expected for A2-adenosine receptors. The rabbit striatal A2-receptor labeled by [3H]XAC appears to be a high affinity (A2a) type, consistent with characterization of the corresponding brain region in rat using [3H]NECA. This is indicated by the relatively high affinity for substituted adenosine agonists, such as NECA and CGS21680. Ki values derived for displacement of [3H]XAC by adenosine agonists are somewhat higher than previously determined Ki-values using agonist radioligands (Bruns et al., 1986; Jarvis et al., 1989). This is to be expected, since we are using agonists to compete against an antagonist radioligand. These apparent Ki values may represent a combination of high and low affinity states for agonist.
In order to accomplish affinity labeling of central A2-adenosine receptors with an antagonist ligand, we desired to identify a functionalized congener (having a chemically derivatizable pendant chain) which binds to the receptor. Derivatives of XAC have been used as spectroscopic and chemical affinity probes at A1-adenosine receptors (Stiles and Jacobson, 1988; Jacobson et al., 1987, 1989b). An agarose affinity column containing an immobilized form of XAC was used successfully to concentrate A1-adenosine receptors from bovine brain (Olah et al., 1989) and purify to homogeneity (Nakata, 1989) the rat brain A1-adenosine receptor. Based on our results, it is expected that a similar XAC affinity column might be used to isolate from rabbit brain the A2-adenosine receptor, which has not yet been isolated from any species. The results of Stone et al. (1988) indicate that the human striatal A2-adenosine receptor resembles the rabbit receptor in having a much enhanced affinity for 8-phenyl substituted xanthines. Therefore, antagonist molecular probes developed for rabbit A2-adenosine receptors will also likely be of use in human tissue.
The ability to characterize central A2-adenosine receptors using an antagonist ligand that is chemically functionalized offers the possibility to design affinity labeling probes for this receptor subtype in the brain, similar to those antagonist probes already developed for A1-adenosine receptors. The results suggest that affinity columns containing chemically immobilized XAC may be used for isolating rabbit central A2-adenosine receptors.
Acknowledgments
We are most grateful to Research Bio-chemicals Incorporated for financial support.
Footnotes
The addition of 0.01% CHAPS (3-[(cholamidopropyl)di-methylammonio]-1-propanesulfonate) to the Tris buffer solution was later found to enhance specific [3H]XAC binding.
References
- Barrington WW, Jacobson KA, Williams M, Hutchison AJ, Stiles GL. Identification of the A2 adenosine receptor binding subunit by photoaffinity crosslinking. Proc natn Acad Sci USA. 1989;86:6572–6576. doi: 10.1073/pnas.86.17.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RF, Lu GH, Pugsley TA. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Molec Pharmac. 1986;29:331–346. [PubMed] [Google Scholar]
- Bruns RF, Fergus JH, Badger EW, Bristol JA, Santay LA, Hays SJ. PD 115,199: an antagonist ligand for adenosine A2 receptors. Naunyn-Schmiedeberg’s Arch Pharmac. 1987;335:64–69. doi: 10.1007/BF00165038. [DOI] [PubMed] [Google Scholar]
- Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzyme reaction. Biochem Pharmac. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Daly JW, Jacobson KA. Adenosine Receptors in the Nervous System. Taylor & Francis; London: 1989. Molecular probes for adenosine receptors; pp. 41–52. [Google Scholar]
- Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmac Sci. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Hutchison AJ, Webb RL, Oei HH, Ghai GR, Zimmerman M, Williams M. CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J Pharmac exp Ther. 1989;251:47–55. [PubMed] [Google Scholar]
- Hutchison AJ, Williams M, deJesus R, Oei HH, Ghai GR, Webb RL, Zoganas HC, Stone GA, Jarvis MF. 2-Arylalkylaminoadenosine 5′-uronamides: a new class of highly selective adenosine A2 receptor agonists. J med Chem. 1990;33:1919–1924. doi: 10.1021/jm00169a015. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Ukena D, Kirk KL, Daly JW. [3H]Xanthine amine congener of 1,3-dipropyl-8-phenylxanthine: an antagonist radioligand for adenosine receptors. Proc natn Acad Sci USA. 1986;83:4089–4093. doi: 10.1073/pnas.83.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Ukena D, Padgett W, Kirk KL, Daly JW. Molecular probes for extracellular adenosine receptors. Biochem Pharmac. 1987;36:1697–1707. doi: 10.1016/0006-2952(87)90056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Kiriasis L, Barone S, Bradbury BJ, Kammula U, Campagne JM, Daly JW, Neumeyer JL, Pfleiderer W. Sulfur-containing xanthine derivatives as selective antagonists at A1-adenosine receptors. J med Chem. 1989a;32:1873–1879. doi: 10.1021/jm00128a031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Barone S, Kammula U, Stiles GL. Electrophilic derivatives of purines as irreversible inhibitors of A1-adenosine receptors. J med Chem. 1989b;32:1043–1051. doi: 10.1021/jm00125a019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Barrington WW, Pannell LK, Jarvis MF, Ji X-D, Williams M, Hutchison AJ, Stiles GL. Agonist-derived molecular probes for A2-adenosine receptors. J molec Recgn. 1989c;2:170–178. doi: 10.1002/jmr.300020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Schutz R, Hutchison AJ, Do E, Sills MA, Williams M. [3H]CGS 21680, an A2 selective adenosine receptor agonist directly labels A2 receptors in rat brain tissue. J Pharmac exp Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- Nakata H. Purification of the A1-adenosine receptor from rat brain membranes. J biol Chem. 1989;264:16545–16551. [PubMed] [Google Scholar]
- Nikodijevic O, Daly JW, Jacobson KA. Characterization of the locomotor depression produced by an A2-selective adenosine agonist. FEBS Lett. 1990;261:67–70. doi: 10.1016/0014-5793(90)80638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah ME, Jacobson KA, Stiles GL. Affinity chromatography of the bovine cerebral cortex A1 adenosine receptor. FEBS Lett. 1989;257:292–296. doi: 10.1016/0014-5793(89)81555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar V, Pierson G, Stiles GL. Adenosine receptors: clinical implications and biochemical mechanisms. Prog Drug Res. 1988;32:195–247. doi: 10.1007/978-3-0348-9154-7_7. [DOI] [PubMed] [Google Scholar]
- Shamim MT, Ukena D, Padgett WL, Daly JW. Effects of 8-phenyl and 8-cycloalkyl substituents on the activity of mono-, di-, and trisubstituted alkylxanthines with substitution at the 1-, 3-, and 7-positions. J med Chem. 1989;32:1231–1237. doi: 10.1021/jm00126a014. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Analyt Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stiles GL, Jacobson KA. High affinity acylating antagonists for the A1 adenosine receptor: identification of binding subunit. Molec Pharmac. 1988;34:724–728. [PMC free article] [PubMed] [Google Scholar]
- Stone GA, Jarvis MF, Sills MA, Weeks B, Snowhill EW, Williams M. Species differences in high-affinity adenosine A2 binding sites in striatal membranes from mammalian brain. Drug Dev Res. 1988;15:31–46. [Google Scholar]
- Ukena D, Jacobson KA, Kirk KL, Daly JW. A [3H]amine congener of 1,3-dipropyl-8-phenyl xanthine—a new radioligand for A2 adenosine receptors of human platelets. FEBS Lett. 1986a;199:269–274. doi: 10.1016/0014-5793(86)80493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena D, Jacobson KA, Padgett W, Ayala C, Shamim MT, Kirk KL, Daly JW. Species differences in structure-activity relationships of adenosine agonists and xanthine antagonists at brain A1 adenosine receptors. FEBS Lett. 1986b;209:122–128. doi: 10.1016/0014-5793(86)81096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]