Abstract
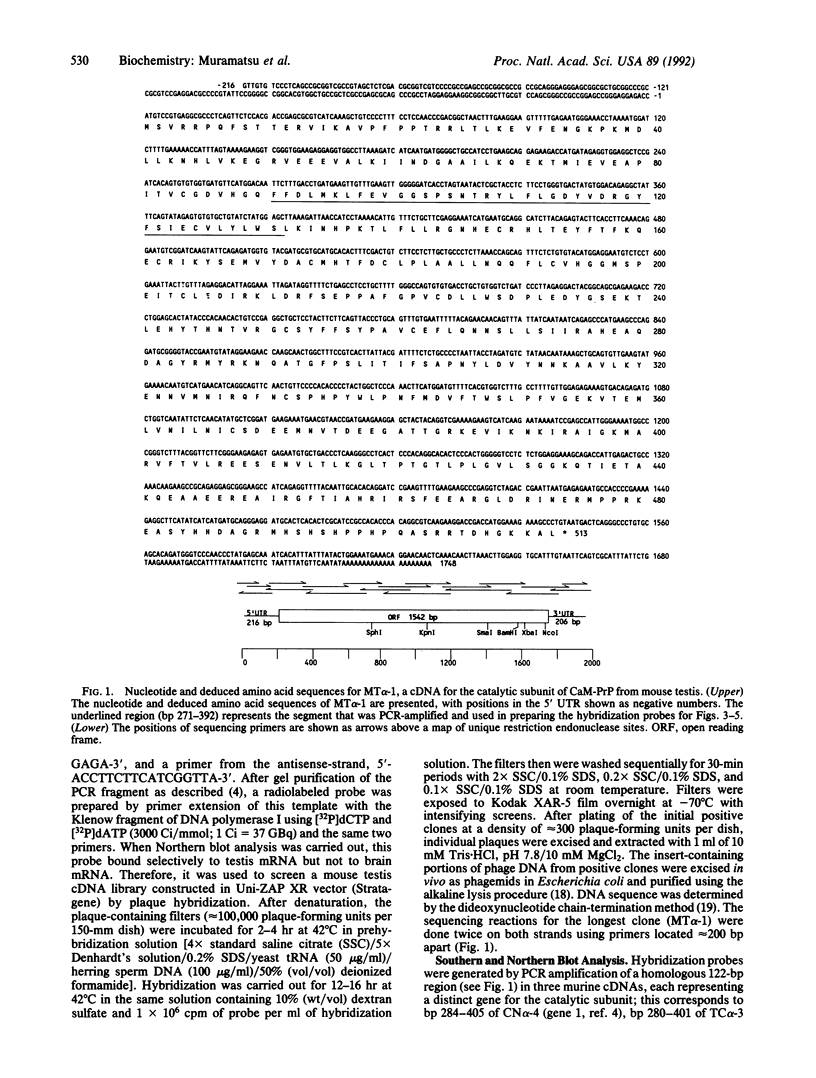
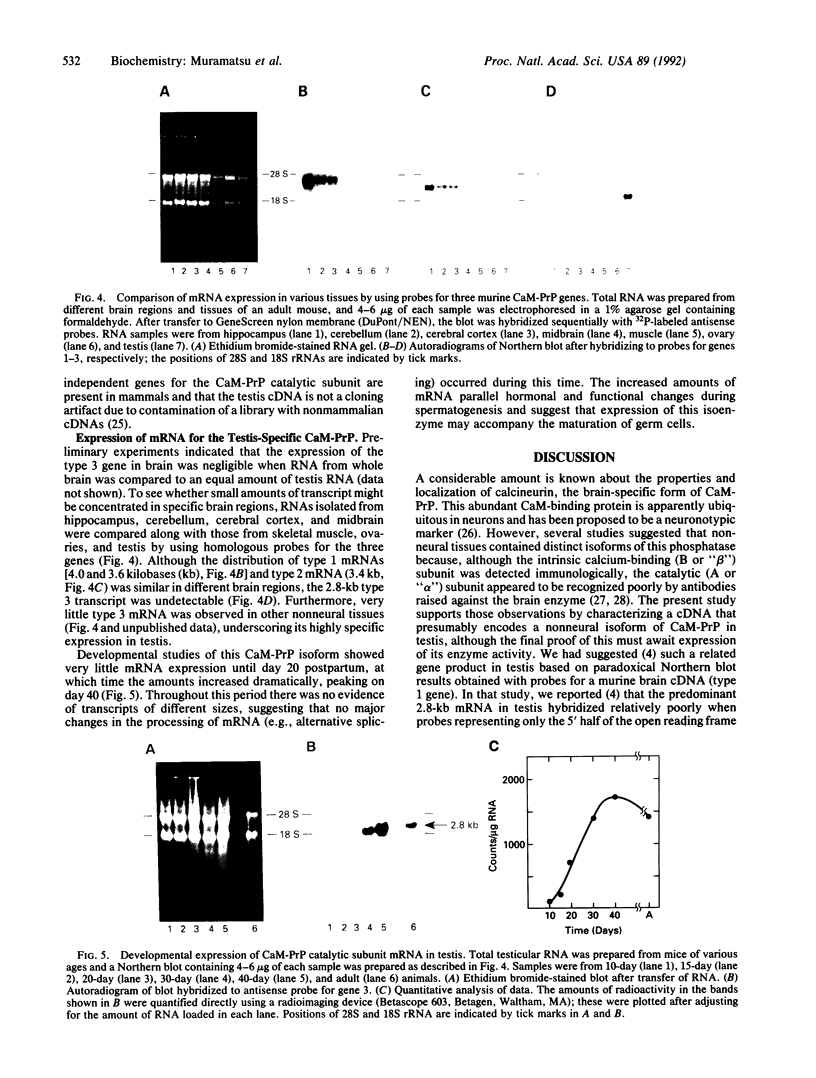
A unique isoform of the catalytic subunit of calmodulin-dependent protein phosphatase (CaM-PrP) was cloned from a murine testis library. The cDNA sequence of 1964 base pairs contained an open reading frame encoding a protein of 513 amino acids (Mr approximately 58,706), the predicted isoelectric point of which (pI 7.1) was much more basic than those of brain isoforms (pI 5.6-5.8). The deduced amino acid sequence was 77-81% identical to two other murine CaM-PrP genes and displayed a distinct Southern blot hybridization pattern, indicating that it was derived from a separate gene (type 3). High amounts of a 2800-nucleotide mRNA transcript were observed in testis, whereas mRNA species were not detectable in brain; thus, it seems likely that this CaM-PrP represents a nonneural isoenzyme. Measurements of CaM-PrP mRNA during testicular development showed a dramatic increase in expression during weeks 4-6, correlating with the later stages of spermatogenesis. These data suggest that this phosphatase isoform may be involved in germ-cell function and are consistent with the report of a flagellum-associated form of CaM-PrP that may regulate sperm motility [Tash, J. S., Krinks, M., Patel, J., Means, R. L., Klee, C. B. & Means, A. R. (1988) J. Cell Biol. 106, 1625-1633].
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler P. D. Calcium-dependent association of a protein complex with the lymphocyte plasma membrane: probable identity with calmodulin-calcineurin. J Cell Biol. 1985 Jul;101(1):207–216. doi: 10.1083/jcb.101.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S., Wolgemuth D. J., Hsu M. T., Darnell J. E., Jr Transcription and accurate polyadenylation in vitro of RNA from the major late adenovirus 2 transcription unit. Cell. 1982 Mar;28(3):575–584. doi: 10.1016/0092-8674(82)90212-4. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Da Cruz e Silva E. F., Hughes V., McDonald P., Stark M. J., Cohen P. T. Protein phosphatase 2Bw and protein phosphatase Z are Saccharomyces cerevisiae enzymes. Biochim Biophys Acta. 1991 Jun 13;1089(2):269–272. doi: 10.1016/0167-4781(91)90023-f. [DOI] [PubMed] [Google Scholar]
- Giri P. R., Higuchi S., Kincaid R. L. Chromosomal mapping of the human genes for the calmodulin-dependent protein phosphatase (calcineurin) catalytic subunit. Biochem Biophys Res Commun. 1991 Nov 27;181(1):252–258. doi: 10.1016/s0006-291x(05)81410-x. [DOI] [PubMed] [Google Scholar]
- Goto S., Matsukado Y., Mihara Y., Inoue N., Miyamoto E. An immunocytochemical demonstration of calcineurin in human nerve cell tumors. A comparison with neuron-specific enolase and glial fibrillary acidic protein. Cancer. 1987 Dec 15;60(12):2948–2957. doi: 10.1002/1097-0142(19871215)60:12<2948::aid-cncr2820601217>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Guerini D., Klee C. B. Cloning of human calcineurin A: evidence for two isozymes and identification of a polyproline structural domain. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9183–9187. doi: 10.1073/pnas.86.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Perrino B. A., Soderling T. R. Identification of an autoinhibitory domain in calcineurin. J Biol Chem. 1990 Feb 5;265(4):1924–1927. [PubMed] [Google Scholar]
- Hosey M. M., Borsotto M., Lazdunski M. Phosphorylation and dephosphorylation of dihydropyridine-sensitive voltage-dependent Ca2+ channel in skeletal muscle membranes by cAMP- and Ca2+-dependent processes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3733–3737. doi: 10.1073/pnas.83.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 1. Classification and substrate specificities. Eur J Biochem. 1983 May 2;132(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Ito A., Hashimoto T., Hirai M., Takeda T., Shuntoh H., Kuno T., Tanaka C. The complete primary structure of calcineurin A, a calmodulin binding protein homologous with protein phosphatases 1 and 2A. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1492–1497. doi: 10.1016/0006-291x(89)91148-0. [DOI] [PubMed] [Google Scholar]
- Jean-Faucher C., Berger M., de Turckheim M., Veyssiere G., Jean C. Developmental patterns of plasma and testicular testosterone in mice from birth to adulthood. Acta Endocrinol (Copenh) 1978 Dec;89(4):780–788. doi: 10.1530/acta.0.0890780. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Balaban C. D., Billingsley M. L. Differential localization of calmodulin-dependent enzymes in rat brain: evidence for selective expression of cyclic nucleotide phosphodiesterase in specific neurons. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1118–1122. doi: 10.1073/pnas.84.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Giri P. R., Higuchi S., Tamura J., Dixon S. C., Marietta C. A., Amorese D. A., Martin B. M. Cloning and characterization of molecular isoforms of the catalytic subunit of calcineurin using nonisotopic methods. J Biol Chem. 1990 Jul 5;265(19):11312–11319. [PubMed] [Google Scholar]
- Kincaid R. L., Nightingale M. S., Martin B. M. Characterization of a cDNA clone encoding the calmodulin-binding domain of mouse brain calcineurin. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8983–8987. doi: 10.1073/pnas.85.23.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Takayama H., Billingsley M. L., Sitkovsky M. V. Differential expression of calmodulin-binding proteins in B, T lymphocytes and thymocytes. Nature. 1987 Nov 12;330(6144):176–178. doi: 10.1038/330176a0. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno T., Takeda T., Hirai M., Ito A., Mukai H., Tanaka C. Evidence for a second isoform of the catalytic subunit of calmodulin-dependent protein phosphatase (calcineurin A). Biochem Biophys Res Commun. 1989 Dec 29;165(3):1352–1358. doi: 10.1016/0006-291x(89)92752-6. [DOI] [PubMed] [Google Scholar]
- McPartlin A. E., Barker H. M., Cohen P. T. Identification of a third alternatively spliced cDNA encoding the catalytic subunit of protein phosphatase 2B beta. Biochim Biophys Acta. 1991 Feb 16;1088(2):308–310. doi: 10.1016/0167-4781(91)90069-x. [DOI] [PubMed] [Google Scholar]
- Merat D. L., Hu Z. Y., Carter T. E., Cheung W. Y. Bovine brain calmodulin-dependent protein phosphatase. Regulation of subunit A activity by calmodulin and subunit B. J Biol Chem. 1985 Sep 15;260(20):11053–11059. [PubMed] [Google Scholar]
- Mukai H., Chang C. D., Tanaka H., Ito A., Kuno T., Tanaka C. cDNA cloning of a novel testis-specific calcineurin B-like protein. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1325–1330. doi: 10.1016/0006-291x(91)91718-r. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmanoff M. K., Goldman B. D., Ginsburg B. E. Developmental changes in serum luteinizing hormone, follicle stimulating hormone and androgen levels in males of two inbred mouse strains. Endocrinology. 1977 Jan;100(1):122–127. doi: 10.1210/endo-100-1-122. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Nairn A. C. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- Tash J. S., Krinks M., Patel J., Means R. L., Klee C. B., Means A. R. Identification, characterization, and functional correlation of calmodulin-dependent protein phosphatase in sperm. J Cell Biol. 1988 May;106(5):1625–1633. doi: 10.1083/jcb.106.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Wallace R. W., Whitaker J. N., Cheung W. Y. Immunocytochemical localization of calmodulin and a heat-labile calmodulin-binding protein (CaM-BP80) in basal ganglia of mouse brain. J Cell Biol. 1980 Jan;84(1):66–76. doi: 10.1083/jcb.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N., Furuyama S., Wang J. H. Demonstration of calmodulin-stimulated phosphatase isozymes by monoclonal antibodies. J Biol Chem. 1990 May 15;265(14):8170–8175. [PubMed] [Google Scholar]






