Abstract
Background
This study aimed to investigate renal outcomes and their predictors in biopsy-proven hypertensive nephrosclerosis (HN) patients and to compare clinico-pathological characteristics and prognoses between benign nephrosclerosis (BN) and malignant nephrosclerosis (MN) patients.
Methods
Data for biopsy-proven HN patients were retrospectively analyzed. Renal survival rates and relationships between clinico-pathological characteristics and outcomes were assessed.
Results
A total of 194 patients were enrolled; the mean age at biopsy was 43.8 years, and male gender predominated (82.5 %). The median duration of hypertension was 5.0 years, and the mean systolic and diastolic blood pressures were 195 ± 37 and 126 ± 26 mmHg, respectively. The median serum creatinine (Scr) level, estimated glomerular filtration rate (eGFR), and proteinuria level were 1.61 mg/dl, 49.6 ml/min/1.73 m2, and 0.80 g/24 h, respectively. BN and MN were found by renal biopsy in 55.2 % and 44.8 % of patients, respectively. At biopsy, MN patients were younger, and had higher median Scr and proteinuria levels, higher incidences of anemia, hypertensive heart disease and hypertensive retinopathy, and worse renal outcomes than BN patients. During a median follow-up period of 3.0 years, 36 patients (18.6 %) reached end-stage renal disease (ESRD), and the 5- and 10-year cumulative renal survival rates for HN patients were 84.5 % and 48.9 %, respectively. A decreased baseline eGFR, an increased baseline proteinuria level, anemia, increased percentage of global glomerulosclerosis and tubular atrophy and interstitial fibrosis (TAIF) were independent predictors of future ESRD.
Conclusions
The clinico-pathological characteristics and prognoses were significantly different between the MN and BN patients. The renal outcomes of HN patients were independently associated with the baseline eGFR and proteinuria level, anemia, percentage of global glomerulosclerosis and TAIF.
Electronic supplementary material
The online version of this article (doi:10.1186/s12882-016-0254-2) contains supplementary material, which is available to authorized users.
Keywords: Hypertension, Benign nephrosclerosis, Malignant nephrosclerosis, Risk factors, Renal survival
Background
Hypertension is a worldwide public health challenge due to its high prevalence, occurring in up to 26 % of the adult population [1], and the concomitant risks of cardiovascular, cerebrovascular and kidney disease. Hypertensive nephrosclerosis (HN) is a common risk factor for end-stage renal disease (ESRD) in developed countries, accounting for 3.3-23.4 % of ESRD patients in Europe (according to the ERA-EDTA Registry Annual Report, 2011) and 30.5 % of ESRD patients in the US [2]. The incidence of ESRD is 2.7 per 100,000 person-years in the Chinese population [3]; thus, it is a great burden in this country because of the large population.
Usually, a diagnosis of HN is assigned based on clinical manifestations. Most studies of HN have focused on the clinical predictors of renal disease progression, including race [4, 5], blood pressure [6–9], renal dysfunction [elevated serum creatinine (Scr) level or decreased estimated glomerular filtration rate (eGFR)] [6, 10–13], proteinuria [10, 11], and concomitant cardiovascular disease [11, 14, 15]. Hypertension is divided into benign hypertension and malignant hypertension, and renal damage from these types of hypertension is categorized as benign nephrosclerosis (BN) and malignant nephrosclerosis (MN), respectively. Long-term renal outcomes are much worse in patients with malignant hypertension [16, 17] than in those with benign hypertension [4]. Renal biopsy is useful for the differential diagnosis between HN and primary glomerulonephritis with hypertension [18–20], but only a limited number of studies of biopsy-proven HN have focused on long-term renal outcomes and negative prognostic factors [10–13, 21, 22].
We analyzed data from adult Chinese patients with biopsy-proven HN to investigate the clinico-pathological characteristics and to evaluate the long-term renal survival rates and related risk factors for progression to ESRD. In addition, the clinico-pathological characteristics and prognoses between patients with BN and MN were compared.
Methods
Study population
The clinical and renal histopathological data of patients with biopsy-proven HN in Jinling Hospital, Nanjing, from January 2003 to June 2013 were retrospectively reviewed. During the study period, the total number of native kidney biopsies was 31594, from which 411 patients (1.3 %) with a diagnosis of HN were identified. The inclusion criteria were as follows: (1) evidence of hypertension before the detection of proteinuria, hematuria and/or impaired renal function; (2) evidence of HN on renal histology; (3) the lack of any clinical, immunological or histological evidence of other glomerular disease or systemic disorder, such as glomerulonephritis or diabetic nephropathy, and the lack of any defined cause of thrombotic microangiopathy such as hemolytic uremic syndrome or thrombotic thrombocytopenic purpura; (4) an age of between 18 and 65 years; (5) a follow-up period of ≥1.0 year or reaching ESRD within 1 year; and (6) the availability of adequate biopsies (≥8 glomeruli). A total of 194 patients were included in this study.
Indications for renal biopsies included proteinuria (>0.4 g/24 h), hematuria (urine sediment red cell count >100,000 cells/ml) and/or impaired renal function. Renal biopsies were performed after adequate blood pressure control was achieved.
Clinical and laboratory parameters
The baseline and follow-up data of the patients were obtained by retrospective chart review. The data included gender, age, family history of hypertension, hypertension duration, systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking status, Scr, and uric acid levels, anemia (male: hemoglobin < 120 g/L, female: hemoglobin < 110 g/L), elevated lactate dehydrogenase (LDH) (LDH > 240 U/L), thrombocytopenia (platelets < 100 × 109/L), 24-h urinary protein excretion, microscopic hematuria, hypertensive heart disease, cerebrovascular disease, and retinopathy (using the Keith-Wagener-Barker criteria). The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Mean arterial pressure (MAP) was defined as the diastolic pressure plus one third of the pulse pressure. For each patient, the highest blood pressure before biopsy and the time-average blood pressure during follow-up, which was defined as the ratio of the area under the curve of MAP during follow-up to the duration of the follow-up [23], were recorded. The number of antihypertensive medications taken during the follow-up period was collected, and oral antihypertensive drugs were categorized into the following classes: angiotensin-converting enzyme inhibitors (ACEIs) and/or angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), beta blockers (BBs), diuretics and others. Patients were censored at the start of renal replacement therapy or loss to follow-up.
Renal histopathology
The tissue for light microscopy was serially sectioned, using hematoxylin and eosin, periodic acid-Schiff, methenamine-silver, and Masson trichrome stains. Cryosections were stained with fluorescein isothiocyanate-conjugated rabbit anti-human immunoglobulin G (IgG), IgA, IgM, complement 3 (C3), and C1q. Paraffin sections were stained with fibrinogen. The tissue for electron microscopy was processed according to standard protocols. All biopsy slides were re-reviewed by two pathologists (Dr. Shaoshan Liang and Dr. Dandan Liang) without knowledge of the clinical outcomes. A senior pathologist (Dr. Caihong Zeng) reviewed the slides and made the final decision in cases of disagreement.
HN was divided into two pathological patterns, BN and MN. BN was characterized by arterial or arteriolar hyalinosis, intimal fibrosis, or medial hypertrophy. MN was characterized by fibrinoid necrosis (acute stage) or myointimal cell proliferation, usually with an “onion-skinning” appearance (chronic stage). Samples of concurrent MN and BN lesions were placed into the MN group. The arteries and arterioles were semiquantitatively evaluated for hyalinosis on a scale of 0–2+ (0, absent; 1+, present, nonocclusive of lumen; 2+, present, extensive, and/or impinging on lumen); intimal fibrosis on a scale of 0–4+ (0, no lesions; 1+, minimally recognizable intimal fibrosis; 2+, intimal fibrosis with <25 % luminal occlusion; 3+, intimal fibrosis with 26-50 % luminal occlusion; 4+, advanced lesions with >50 % luminal occlusion); and medial hypertrophy on a scale of 0–2+ (0, absent; 1+, minimal to mild; 2+, moderate to severe). The extents of global glomerulosclerosis, segmental glomerulosclerosis, and glomerular ischemia were expressed as percentages of the total glomeruli. The morphological classification of focal segmental glomerulosclerosis (FSGS) was based on the Columbia classification [24]. The severity of tubular atrophy and interstitial fibrosis (TAIF) was semiquantitatively scored as the percentage of the renal cortical area involvement.
Outcome
The primary outcome of this study was ESRD, which was defined as eGFR < 15 ml/min/1.73 m2, the initiation of chronic renal replacement therapy, or transplantation.
Statistical analysis
Normally distributed variables were expressed as the mean ± SD and compared using Student’s t-test. Non-parametric variables were expressed as medians [interquartile ranges (IQRs)] and compared using the Mann–Whitney-Wilcoxon test. Categorical variables were expressed as the number of positive cases (percentages) and compared using the Pearson χ2 test. The reproducibility of the pathology variables was evaluated using intraclass correlation coefficients (ICCs). Correlations between the pathology variables were analyzed using the Spearman test. The renal survival rates were estimated using the Kaplan-Meier method, and the log-rank test was used to assess the significance of differences in the Kaplan-Meier survival curves. The Cox proportional hazard model was used to explore the influences of the variables on the occurrence of ESRD. The pathological variables of poor reproducibility (ICC <0.40) were excluded from the model. The variables which were found to be significant (P < 0.05) by univariate analysis were included in the multivariate model using backward stepwise method. The assumption of Cox proportional hazards model were assessed using Schoenfeld residuals plots. All P values were two-tailed, and a P < 0.05 was considered statistically significant. All analyses were performed using SPSS 18.0 software for Windows (SPSS Inc., Chicago, IL, USA) and R software (version 3.2.1).
Results
Clinical features
This study included 194 patients who were predominantly male (82.5 %). The mean age at the time of biopsy was 43.8 ± 4.1 years. The median duration of hypertension was 5.0 years (IQR, 1.0-9.0). The mean SBP and DBP were 195 ± 37 and 126 ± 26 mmHg, respectively. The median Scr level was 1.61 mg/dl (IQR, 1.24-2.27), the median eGFR was 49.6 ml/min/1.73 m2 (IQR, 32.8-65.7), and the median proteinuria level was 0.80 g/24 h (IQR, 0.42-1.48). During a median follow-up period of 3.0 years (IQR, 1.8-4.3), 36 patients (18.6 %) developed ESRD (Table 1), and the 5- and 10-year cumulative renal survival rates after renal biopsy, calculated using Kaplan-Meier method, were 84.5 % and 48.9 %, respectively (Fig. 1). The average number of antihypertensive drugs taken during the follow-up period was 2.7 ± 1.3; and 88.7 % of the patients were treated with ACEIs/ARBs, 75.8 % were treated with CCBs, 49.0 % were treated with BBs, and 20.6 % were treated with diuretics.
Table 1.
Comparisons of the clinical features between the MN and BN groups
| Total patients (n = 194) |
BN group (n = 107) |
MN group (n = 87) |
P | |
|---|---|---|---|---|
| At time of biopsy | ||||
| Sex (male:female) | 160:34 | 81:26 | 79:8 | 0.006 |
| Age (y) | 43.8 ± 4.1 | 47.4 ± 10.3 | 39.4 ± 10.7 | <0.001 |
| Hypertension family history | 114(58.8 %) | 59(55.1 %) | 55(63.2 %) | 0.26 |
| Hypertension duration (y) | 5.0(1.0-9.0) | 6.0(2.4-10.0) | 3.0(0.2-7.0) | <0.001 |
| SBP (mmHg) | 195 ± 37 | 182 ± 32 | 213 ± 32 | <0.001 |
| DBP (mmHg) | 126 ± 26 | 119 ± 23 | 138 ± 25 | <0.001 |
| MAP (mmHg) | 150 ± 27 | 139 ± 24 | 163 ± 25 | <0.001 |
| Current smoker | 57(29.4 %) | 30(28.0 %) | 27(31.0 %) | 0.65 |
| Grades of retinopathy | ||||
| I-II (%) (n*) | 73(50.3 %)(145) | 44(58.7 %)(75) | 29(41.4 %)(70) | <0.001 |
| III-IV (%) (n*) | 42(29.0 %)(145) | 8(10.7 %)(75) | 34(48.6 %)(70) | |
| Hypertensive heart disease | 110(56.7 %) | 42(39.3 %) | 68(78.2 %) | <0.001 |
| Hypertensive cerebrovascular disease | 18(9.3 %) | 12(11.2 %) | 6(6.9 %) | 0.30 |
| Scr (mg/dl) | 1.61(1.24-2.27) | 1.35(1.07-1.60) | 2.27(1.74-3.14) | <0.001 |
| eGFR (ml/min/1.73 m2) | 49.6(32.8-65.7) | 60.5(48.9-76.5) | 34.5(24.2-46.8) | <0.001 |
| eGFR < 60 ml/min/1.73 m2 | 130(67.0 %) | 51(47.7 %) | 79(90.8 %) | <0.001 |
| Uric acid (μmol/l) | 455 ± 109 | 437 ± 111 | 482 ± 101 | 0.004 |
| Anemia | 36(18.6 %) | 10(9.3 %) | 26(29.9 %) | <0.001 |
| Elevated LDH | 5(2.6 %) | 0(0 %) | 5(5.7 %) | - |
| Thrombocytopenia | 1(0.5 %) | 0(0 %) | 1(1.1 %) | - |
| Proteinuria (g/24 h) | 0.80(0.42-1.48) | 0.72(0.38-1.34) | 0.89(0.58-1.57) | 0.02 |
| Microscopic hematuria | 46(23.7 %) | 31(29.0 %) | 15(17.2 %) | 0.06 |
| Follow-up | ||||
| No. of antihypertensive drugs | 2.7 ± 1.3 | 2.3 ± 1.1 | 3.3 ± 1.2 | <0.001 |
| Time-average MAP (mmHg) (n*) | 129 ± 13(157) | 124 ± 11(88) | 135 ± 14(69) | <0.001 |
| ESRD | 36(18.6 %) | 7(6.5 %) | 29(33.3 %) | <0.001 |
Data are presented as the medians (25th and 75th percentiles), the mean ± SD, or the number of positive cases (percentages). n* is the available n for a given value. Where not specified, the available n is the same as the total number of cases in the top row
BN, benign nephrosclerosis; MN, malignant nephrosclerosis; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean blood pressure; Scr, serum creatinine; eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; ESRD, end-stage renal disease
Fig. 1.
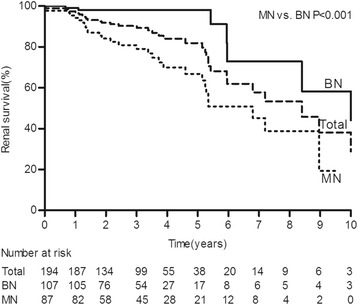
Kaplan-Meier renal survival curves for 194 patients with HN. The 5- and 10-year cumulative renal survival rates after biopsy were 84.5 % and 48.9 %, respectively, for 194 patients with HN. The 5- and 10-year cumulative renal survival rates after biopsy were 98.1 % and 58.3 %, respectively, for the BN group, and 66.8 % and 19.4 %, respectively, for the MN group (log-rank P < 0.001). HN, hypertensive nephrosclerosis; BN, benign nephrosclerosis; MN, malignant nephrosclerosis
Comparisons of clinical features between the MN and BN groups
MN was found in 87 patients (44.8 %), and BN was found in 107 (55.2 %). The comparisons of the clinical features between the BN and MN groups are presented in Table 1. The patients with MN were younger, had a higher male: female ratio, had a shorter duration of hypertension and had higher blood pressure. The MN group had higher incidences of hypertensive heart disease and hypertensive retinopathy than the BN group.
The patients with MN exhibited more severe renal injuries, as indicated by higher median Scr, mean serum uric acid and median proteinuria levels, and a higher incidence of anemia, compared with those with BN.
Although the patients with MN received more antihypertensive medications, the time-average MAP was higher, and more patients progressed to ESRD than those with BN (Table 1). The 5- and 10-year cumulative renal survival rates after biopsy were 98.1 % and 58.3 %, respectively, for the BN group, and 66.8 % and 19.4 %, respectively, for the MN group (log-rank P < 0.001) (Fig. 1).
Comparisons of histological features between the MN and BN groups
The patients with MN exhibited more severe medial hypertrophy, whereas those with BN exhibited a higher hyalinosis score. The intimal fibrosis score was not significantly different between the MN and BN groups. In general, the percentage of segmental glomerulosclerosis was significantly increased in the MN group compared with the BN group. Ninety patients presented with segmental glomerulosclerosis, 54 of which had the perihilar variant. And the MN group had a higher incidence of perihilar variant than the BN group (69.4 % vs. 48.8 %, P = 0.047). The percentage of ischemic glomeruli was significantly higher in the MN group than in the BN group, but the percentage of global glomerulosclerosis did not differ between two groups. The percentage of TAIF was significantly greater in the MN group than in the BN group (Table 2).
Table 2.
Comparisons of the histological features between the MN and BN groups
| Total patients (n = 194) |
BN group (n = 107) |
MN group (n = 87) |
P | |
|---|---|---|---|---|
| Hyalinosis | 1.09 ± 0.67 | 1.25 ± 0.57 | 0.90 ± 0.73 | <0.001 |
| Intimal fibrosis (n*) | 1.74 ± 1.18(189) | 1.79 ± 1.10(105) | 1.68 ± 1.28(84) | 0.52 |
| Medial hypertrophy | 0.69 ± 0.65 | 0.42 ± 0.55 | 1.01 ± 0.62 | <0.001 |
| Global glomerulosclerosis (%) | 30(18–46) | 32(18–45) | 28(18–50) | 0.63 |
| Segmental glomerulosclerosis (%) | 0(0–6) | 0(0–5) | 3(0–6) | 0.04 |
| Ischemic glomeruli (%) | 8(0–22) | 3(0–9) | 20(8–30) | <0.001 |
| TAIF (%) | 30(20–60) | 30(10–30) | 60(40–70) | <0.001 |
Data are presented as the medians (25th and 75th percentiles), the mean ± SD. n* is the available n for a given value. Where not specified, the available n is the same as the total number of cases in the top row
BN, benign nephrosclerosis; MN, malignant nephrosclerosis; TAIF, tubular atrophy/interstitial fibrosis
Histological features of MN lesions
Of the 87 patients in the MN group, the incidence of arteriolar involvement was 100 %, whereas that of arterial involvement was 44.0 %. Chronic MN lesions were observed in 94.3 % of the patients in the MN group with arteriolar (81 cases) and arterial (37 cases) involvement. Acute MN lesions were observed in 37.9 % of the patients in the MN group with arteriolar (33 cases) and arterial (1 case) involvement. Twenty-eight patients presented with both chronic and acute MN lesions. Only one case showed glomerular fibrinoid necrosis.
Reproducibility was assessed statistically using ICCs, which are summarized in Table 3.
Table 3.
ICCs of the HN patients
| ICC | |
|---|---|
| Acute MN lesions | 0.66 |
| Chronic MN lesions | 0.63 |
| Hyalinosis | 0.57 |
| Intimal fibrosis | 0.64 |
| Medial hypertrophy | 0.40 |
| Global glomerulosclerosis (%) | 0.98 |
| Segmental glomerulosclerosis (%) | 0.94 |
| Ischemic glomeruli (%) | 0.71 |
| TAIF (%) | 0.75 |
Note: an ICC of less than 0.40 is poor reproducibility, 0.40-0.59 is reproducibility, 0.60-0.79 is substantial reproducibility, and 0.80-1 is outstanding reproducibility
MN, malignant nephrosclerosis; TAIF, tubular atrophy/interstitial fibrosis; ICC: intraclass correlation coefficient
Correlations between the pathology variables are shown in Additional file 1: Table S1.
Clinical and pathological predictors of ESRD
Table 4 shows the MN lesions and renal outcomes for the patients with preserved renal function vs. ESRD. The patients with chronic and acute MN lesions had a higher incidence of progression to ESRD than those with preserved renal function (77.8 % vs. 34.2 %, P < 0.001; 33.3 % vs. 13.3 %, P = 0.004, respectively). Based on the vessel size, the involvement of both arterioles and arteries in chronic MN lesions and the involvement of arterioles in acute MN lesions were associated with ESRD.
Table 4.
MN lesions and renal outcomes for patients with preserved renal function vs. ESRD
| Preserved renal function (n = 158) | ESRD (n = 36) | P | |
|---|---|---|---|
| MN | 58(36.7 %) | 29(80.6 %) | <0.001 |
| Chronic lesions | 54(34.2 %) | 28(77.8 %) | <0.001 |
| Arteriolar involvement | 53(33.5 %) | 28(77.8 %) | <0.001 |
| Arterial involvement (n*) | 22(14.3 %)(154) | 15(42.9 %)(35) | <0.001 |
| Acute lesions | 21(13.3 %) | 12(33.3 %) | 0.004 |
| Arteriolar involvement | 21(13.3 %) | 12(33.3 %) | 0.004 |
| Arterial involvement (n*) | 0(0 %)(154) | 1(2.9 %)(35) | - |
Data are presented as the number of positive cases (percentages). n* is the available n for a given value. Where not specified, the available n is the same as the total number of cases in the top row
MN, malignant nephrosclerosis; ESRD, end-stage renal disease
The univariate Cox regression analysis indicated that the baseline eGFR [hazard ratio (HR), 0.42, 95 % confidence interval (CI), 0.32-0.56 per 10 ml/min/1.73 m2 increase, P < 0.001], baseline proteinuria (HR, 2.27, 95 % CI, 1.70-3.02 per 1 g/24 h increase, P < 0.001), anemia (HR, 4.18, 95 % CI, 2.10-8.33, P < 0.001), hyperuricemia (HR, 2.31, 95 % CI, 1.08-4.93, P = 0.03), the percentage of global glomerulosclerosis (HR, 1.44, 95 % CI, 1.23-1.70 per 10 % increase, P < 0.001), percentage of segmental glomerulosclerosis (HR, 2.02, 95 % CI, 1.31-3.12 per 10 % increase, P = 0.001), percentage of TAIF (HR, 1.83, 95 % CI, 1.48-2.25 per 10 % increase, P < 0.001), and the presence of MN (HR, 5.51, 95 % CI, 2.27-13.33, P < 0.001) were associated with renal outcome (Table 5). These parameters were then considered in the multivariate Cox proportional hazards model, which showed that the baseline eGFR (HR, 0.55, 95 % CI, 0.39-0.77 per 10 ml/min/1.73 m2 increase, P < 0.001), baseline proteinuria (HR, 1.54, 95 % CI, 1.07-2.21 per 1 g/24 h increase, P = 0.02), anemia (HR, 2.28, 95 % CI, 1.04-4.99, P = 0.04), percentage of global glomerulosclerosis (HR, 1.33, 95 % CI, 1.12-1.58 per 10 % increase, P = 0.001) and percentage of TAIF (HR, 1.48, 95 % CI, 1.08-2.03, P = 0.02) were independent predictors of renal outcome (Table 6). Hyperuricemia, the percentage of segmental glomerulosclerosis and the presence of MN were not predictive of renal outcome independently.
Table 5.
Univariate Cox regression analyses of factors associated with renal survival
| HR (95 % CI) | P | |
|---|---|---|
| eGFRa | 0.42(0.32-0.56) | <0.001 |
| Proteinuriab | 2.27(1.70-3.02) | <0.001 |
| Anemia | 4.18(2.10-8.33) | <0.001 |
| Hyperuricemia | 2.31(1.08-4.93) | 0.03 |
| TAIFc | 1.83(1.48-2.25) | <0.001 |
| Global glomerulosclerosisc | 1.44(1.23-1.70) | <0.001 |
| Segmental glomerulosclerosisc | 2.02(1.31-3.12) | 0.001 |
| Presence of MN | 5.51(2.27-13.33) | <0.001 |
aper 10 ml/min/1.73 m2 increase; bper 1 g/24 h increase; cper 10 % increase
MN, malignant nephrosclerosis; TAIF, tubular atrophy/interstitial fibrosis; HR, hazard ratio; CI, confidence interval
Table 6.
Multivariate Cox regression analyses of factors associated with renal survival
| HR (95 % CI) | P | |
|---|---|---|
| eGFRa | 0.55(0.39-0.77) | <0.001 |
| Proteinuriab | 1.54(1.07-2.21) | 0.02 |
| Anemia | 2.28(1.04-4.99) | 0.04 |
| TAIFc | 1.48(1.08-2.03) | 0.02 |
| Global glomerulosclerosisc | 1.33(1.12-1.58) | 0.001 |
aper 10 ml/min/1.73 m2 increase; bper 1 g/24 h increase; cper 10 % increase
TAIF, tubular atrophy/interstitial fibrosis; HR, hazard ratio; CI, confidence interval
Discussion
The incidence of hypertension significantly increased from 1991–2009 in China [25], and accordingly, hypertension-induced renal damage also increased. Based on the renal biopsy registry of Jinling Hospital, Nanjing, the incidence of HN increased from 0.45 % in 1979–2002 [26] to 1.3 % in 2003–2013, which was similar to the findings of a recent report from Japan (1.3 %) [27]. Therefore, HN might become a common risk factor for ESRD in China.
Hypertension is categorized as benign hypertension and malignant hypertension, and the renal damage caused by these types of hypertension is classified as BN and MN. MN was observed in 44.8 % of 194 patients with biopsy-proven HN in the present study, which was nearly identical to that reported by Caetano et al. (43 %) [28] and was higher than those reported by other studies (10.3-12.8 %) [20, 29]. The patients in our study were Chinese, whereas those in the other studies were Caucasian or African American. In Caetano’s study [28], the average blood pressure was lower than that in the present study (SBP/DBP 183/117 mmHg vs. 195/126 mmHg), whereas the Scr level was higher (3.28 mg/dl vs. 1.61 mg/dl). In Fogo’s study [20], the average eGFR was higher than that in the present study (51.1 ml/min/1.73 m2 vs. 49.6 ml/min/1.73 m2). The different incidences of MN in biopsy-proven HN may be associated with variations in ethnicity and patient inclusion criteria.
In the present study, the MN group was younger, displayed higher SBP and DBP levels, had higher median Scr, mean uric acid and median proteinuria levels, and had higher incidences of hypertensive heart disease and retinopathy at baseline than the BN group. In Caetano’s series [28], the MN group was younger, displayed higher DBP and Scr levels, and had a higher incidence of hypertensive retinopathy than the BN group, whereas the uric acid and proteinuria levels were not different between the two groups. In Bohle’s series [29], the MN group was younger and displayed higher SBP and DBP, and Scr and proteinuria levels than the BN group. In Ratschek’s series [30], the MN group displayed higher SBP and DBP and Scr levels, whereas no difference in age was observed. Thus, the previous and present studies show that patients with MN are younger, have a higher Scr level and a higher incidence of hypertensive retinopathy, and have variable uric acid and proteinuria levels compared with those with BN.
In the MN group, 5 patients had elevated LDH and 1 patient had thrombocytopenia at the time of biopsy. In previous retrospective studies of patients with malignant hypertension, 27-44 % of patients have presented with thrombotic microangiopathy [16, 31, 32]. Microangiopathic hemolysis and thrombocytopenia have been reported to resolve within 3–21.7 days and 3–5 days, respectively, in patients with controlled blood pressure [31, 33]. The low incidences of elevated LDH and thrombocytopenia in this study may have been due to the fact that the patients were receiving antihypertensive therapies and had controlled blood pressure on admission to our hospital.
Two pathological patterns based on the vascular lesion, BN and MN, have been described as HN. MN is characterized by fibrinoid necrosis (acute stage) and myointimal cell proliferation, usually with an “onion-skinning” appearance (chronic stage). In the present study, the incidence of chronic-stage MN lesions was as high as 94.3 % in the MN group, whereas that of acute-stage MN lesions was low (37.9 % in the MN group). Furthermore, most patients with acute-stage MN lesions coexisted with chronic-stage MN lesions. These findings are similar to those of previous reports [30, 34]. The acute-stage MN lesions occurred during the more early stage of severe hypertension [34]. All these patients had to achieve adequate blood pressure control before biopsy because of the potential hazard of bleeding complications. The low incidence of acute-stage lesions might have been due to the severe hypertension in the more emergent patients serving as a contraindication of renal biopsy. Segmental fibrinoid necrosis of the glomerular tufts was perceived to be one of the characteristic lesions associated with malignant hypertension [35]. Nevertheless, glomerular fibrinoid necrosis was found in only one case in our study, and it was thus hard to evaluate its relationship with malignant hypertension.
The patients with MN presented with more severe histological lesions than those with BN. In agreement with previous studies [29, 30], the MN group exhibited a higher percentage of ischemic glomeruli (MN vs. BN, 20 % vs. 3 %). MN lesions led to marked narrowing or even occlusion of the lumen and caused the formation of ischemic glomeruli. Furthermore, the percentage of segmental glomerulosclerosis in the MN group was higher (MN vs. BN, 3 % vs. 0 %) and was predominantly of the perihilar type. These data may reflect the fact that the hyperfiltration status of the remaining nephron units was more severe in the MN group than in BN group [36]. The extent of TAIF was significantly higher in the MN group in our study, and previous studies have demonstrated that tubular atrophy and interstitial fibrosis are associated with reduced flow in peritubular capillaries, resulting in hypoxic damage [37, 38]. The MN lesions caused narrowing of the lumen and reduced renal blood flow, leading to hypoxia and ultimately resulting in TAIF. Thus, we speculated that the MN lesions caused the devastating ischemic alterations that resulted in ischemia of glomeruli, segmental glomerulosclerosis, and TAIF. The MN group showed a higher medial hypertrophy score than the BN group in the present study, similar to the results of the study by Caetano [28]. Furthermore, our data showed that hyalinosis was more frequent in the BN group than in the MN group.
HN can lead to ESRD, as shown in Table 7, which summarizes the patient outcomes and prognostic indicators in our study as well as those in previous reports. In the present study, the 5- and 10-year cumulative renal survival rates after biopsy were 98.1 % and 58.3 %, respectively, for the BN group. The 5- and 10-year renal survival rates of BN patients were respectively 80 % and 72 % in Norway [10], 56 % and 35 % in the UK [12], and 35.9 % and 23.6 % in Germany [13]. The patients in our study were younger and had lower baseline Scr levels than those in other studies. In addition, differences in ethnicity and changes in treatment strategies in recent decades might have contributed to the better renal outcomes observed in our study compared with previous studies. With the development of modern antihypertensive drugs, the prognosis of clinical malignant hypertension has improved. We reported the long-term renal outcome of biopsy-proven MN In this study, showing that during a median follow-up period of 3.0 years, 33.3 % of the patients progressed to ESRD in the MN group. Yu et al. reported that a series of MN patients, in which more severe renal involvement was observed, had a worse renal outcome (45.9 % patients progressed to ESRD) during a mean follow-up period of 2.5 years than the present study [39] (Table 7).
Table 7.
Renal outcomes and prognostic indicators for patients with biopsy-proven HN in the present study and in previous reports
| Case numbers | Sex (male:female) | Age (y) | Scr (eGFR) | Proteinuria (g/24 h) | BP (mmHg) | Duration of follow-up (y) | Renal outcomes | Predictors of renal survival | |
|---|---|---|---|---|---|---|---|---|---|
| Takebayashi [21] | 590 | 2.5:1 | 56.5 | 1.90(ND) | ND | 154(SBP) 87(DBP) |
10.1 | 49.2 % of 345 cases reached endpoint event (Scr ≥3 mg/dl) | Poor or no control of BP, global glomerulosclerosis (>40 %), presence of collapsed glomeruli and/or segmental glomerulosclerosis |
| Marcantoni [22] | 62a | 1.3:1 | 58.7 | 3.40(ND) | 1.8 | 105 ± 3(MAP) | 1.9 | 47.8 % of 23 cases reached ESRD | ND |
| Wehrmann [13] | 170 | 5.0:1 | ND | ND | ND | ND | ND | 5- and 10-year renal survival rates were 35.9 % and 23.6 %, respectively | Baseline Scr |
| Vikse [10] | 102 | 2.0:1 | 55.4 | 1.87(ND) | 0.4 | 156 ± 28(SBP); 92 ± 14(DBP) |
11.7 | 5- and 10-year renal survival rates were 80 % and 72 %, respectively | Baseline Scr, proteinuria |
| Norris [11] | 1094a | 1.6:1 | 54.6 | ND(46.4) | 0.3 | 150 ± 24(SBP); 96 ± 14(DBP) |
3.9 | 13.1 % reached endpoint event (50 % or 25 ml/min per 1.73 m2decline in GFR or ESRD) | Baseline proteinuria, GFR, Scr,urea nitrogen, phosphorus |
| Dasgupta [12] | 60 | 2.0:1 | 58.0 | 2.79(36.0) | 2.3 | 179 ± 25(SBP); 105 ± 15(DBP) |
6.7 | 5- and 10-year renal survival rates were 56 % and 35 %, respectively | Baseline Scr, mean SBP and DBP during follow-up (univariate analysis), DBP during follow-up (multivariate analysis) |
| Yu [39] | 61 | 9.2:1 | 32.0 | 6.33(ND) | 3.46 | ND | 2.5 | 45.9 % reached ESRD | ND |
| This study | 194 | 4.7:1 | 43.8 | 1.61(49.6) | 0.80 | 195 ± 37(SBP); 126 ± 26(DBP) |
3.0 | 5- and 10-year renal survival rates were 84.5 and 48.9 %, respectively | Baseline eGFR, proteinuria, anemia, the percentage of global glomerulosclerosis and TAIF |
aMost cases underwent renal biopsies
ND, no data; BP, blood pressure; MAP, mean blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MN, malignant nephrosclerosis; Scr, serum creatinine; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; TAIF, tubular atrophy/interstitial fibrosis
The clinical parameters showed significant prognostic value for patients with HN. Our study confirmed the baseline eGFR and proteinuria level as independent prognostic factors for progression to ESRD [10–13, 40]. In addition, anemia was found to be an independent prognostic factor for renal outcome. The serum uric acid level was higher in the MN group than in the BN group in our study. However, the presence of hyperuricemia, which predicted outcome in univariate Cox analysis, lost its prognostic value in multivariate Cox analysis. In the African American Study of Kidney Disease (AASK) cohort [11], the baseline hematocrit level was significantly associated with the risk for a GFR event or ESRD after adjustments for age, gender, baseline proteinuria, and baseline GFR, whereas no association was observed between serum uric acid level and renal outcome, which was similar to the findings of our study. Further prospective studies are required to confirm whether hyperuricemia is associated with renal outcomes for patients with HN in the Chinese population. Several studies have indicated that an increase in baseline blood pressure [7, 8], treatment-resistant hypertension [6, 9], and increases in SBP and DBP during follow-up [12, 16] are associated with an unfavorable outcome. In the present study, baseline blood pressure was not associated with renal outcome; however, these data are limited by the retrospective nature of this study. Prospective studies are needed to confirm the optimal blood pressure control strategy for patients with HN. Additionally, several studies have indicated that concomitant cardiovascular disease is associated with renal outcome [11, 14, 15]; however, no such associations were observed in the current series of patients.
Some pathological parameters were identified to predict renal outcome. TAIF was one of the prognostic factors identified in the present study that has also been reported as a factor for other renal diseases [41]. Global glomerulosclerosis also exhibited a significant negative prognostic influence in our study. Interestingly, the optimal cutoff of the percentage of global glomerulosclerosis for predicting renal outcome obtained by receiver operating characteristic (ROC) curve analysis was 40 % (data not shown), which was nearly the same as the value obtained from a series of patients with biopsy-proven BN in Japan, in which global glomerulosclerosis (>41 %) at biopsy was found to be an indicator of poor prognosis [21]. These findings suggest that global glomerulosclerosis has a negative prognostic impact on patients with both BN and MN. Correlation analysis of pathology variables demonstrated that there was a strong correlation between the presence of MN and the percentage of TAIF (r = 0.643). Thus, the presence of MN predicted the risk for progression to ESRD in univariate Cox analysis, but lost its prognostic value in multivariate Cox analysis.
The limitations of this study must be recognized. The major limitation in this study is its retrospective nature. We cannot rule out the influence of selection bias because some patients, especially those only very mild cases, were unwilling to undergo renal biopsy when HN was diagnosed according to the clinical criteria. In addition, data on patient deaths were incomplete and the candidate factors associated with death were not assessed in this study.
Conclusions
Our results indicated that patients with MN and BN exhibited significantly different clinico-pathological characteristics. The MN group presented with more severe renal involvement and higher incidences of hypertensive heart disease and retinopathy, received more antihypertensive medications, and had poorer renal outcomes than the BN group. A decreased baseline eGFR, an increased baseline proteinuria level, anemia, increased percentage of global glomerulosclerosis and TAIF were associated with unfavorable renal outcomes in the patients with biopsy-proven HN.
Ethics approval
The study was approved by the Ethics Committee of Jinling Hospital. No additional administrative permissions were necessary in order to access the clinical and renal histopathological data.
Data availability statement
All data underlying the findings are within the paper and the supporting information file (Additional file).
Acknowledgements
This work was supported by Clinical Research Program of Jiangsu Province (No. BL2012007). We thank Professor Yang Zhao at the School of Public Health, Nanjing Medical University for his kind help in the statistical analysis of this paper.
Abbreviations
- ACEIs
angiotensin-converting enzyme inhibitors
- ARBs
angiotensin receptor blockers
- BBs
beta blockers
- BN
benign nephrosclerosis
- CCBs
calcium channel blockers
- CI
confidence interval
- CKD-EPI
chronic kidney disease epidemiology collaboration
- DBP
diastolic blood pressure
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- FSGS
focal segmental glomerulosclerosis
- HN
hypertensive nephrosclerosis
- HR
hazard ratio
- ICC
intraclass correlation coefficient
- IQR
interquartile range
- LDH
lactate dehydrogenase
- MAP
mean arterial pressure
- MN
malignant nephrosclerosis
- ND
no data
- ROC
receiver operating characteristic
- SBP
systolic blood pressure
- Scr
serum creatinine
- TAIF
tubular atrophy and interstitial fibrosis
Additional file
Correlations between pathology variables. (DOC 45 kb)
Footnotes
Competing interests
All authors declare that they have no competing interests.
Authors’ contributions
SL carried out the clinico-pathological studies and drafted the manuscript. WL, HC and FX participated in patient inclusion and demographic data collection. CZ, SL and DL participated in the renal pathology studies. WL and SL participated in statistical analysis. ZL and CZ contributed to the conception and design of the study and revised the manuscript. H-PC helped to revise the manuscript. All authors read and approved the final manuscript.
Contributor Information
Shaoshan Liang, Email: southerns007@126.com.
Weibo Le, Email: leweibo@gmail.com.
Dandan Liang, Email: ldd1111@hotmail.com.
Hao Chen, Email: chenhao_nju@sina.com.
Feng Xu, Email: sanyueweifeng@126.com.
Huiping Chen, Email: chenhuiping@medmail.com.cn.
Zhihong Liu, Email: zhihong--liu@hotmail.com.
Caihong Zeng, Phone: +86-25-80860218, Email: zengch_nj@hotmail.com.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)70151-3. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System, USRDS 2013 Annual Data Report . Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 3.Reynolds K, Gu D, Muntner P, Kusek JW, Chen J, Wu X, Duan X, Chen CS, Klag MJ, Whelton PK, et al. A population-based, prospective study of blood pressure and risk for end-stage renal disease in China. J Am Soc Nephrol. 2007;18(6):1928–1935. doi: 10.1681/ASN.2006111199. [DOI] [PubMed] [Google Scholar]
- 4.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997;277(16):1293–1298. doi: 10.1001/jama.1997.03540400043029. [DOI] [PubMed] [Google Scholar]
- 5.Shulman NB, Ford CE, Hall WD, Blaufox MD, Simon D, Langford HG, Schneider KA. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The Hypertension Detection and Follow-up Program Cooperative Group. Hypertension. 1989;13(5 Suppl):I80–93. doi: 10.1161/01.HYP.13.5_Suppl.I80. [DOI] [PubMed] [Google Scholar]
- 6.Tanner RM, Calhoun DA, Bell EK, Bowling CB, Gutierrez OM, Irvin MR, Lackland DT, Oparil S, McClellan W, Warnock DG, et al. Incident ESRD and treatment-resistant hypertension: the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis. 2014;63(5):781–788. doi: 10.1053/j.ajkd.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41(6):1341–1345. doi: 10.1161/01.HYP.0000069699.92349.8C. [DOI] [PubMed] [Google Scholar]
- 8.Perry HM, Jr, Miller JP, Fornoff JR, Baty JD, Sambhi MP, Rutan G, Moskowitz DW, Carmody SE. Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension. 1995;25(4 Pt 1):587–594. doi: 10.1161/01.HYP.25.4.587. [DOI] [PubMed] [Google Scholar]
- 9.De Nicola L, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, Nappi F, Conte G, Minutolo R. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. J Am Coll Cardiol. 2013;61(24):2461–2467. doi: 10.1016/j.jacc.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 10.Vikse BE, Aasarod K, Bostad L, Iversen BM. Clinical prognostic factors in biopsy-proven benign nephrosclerosis. Nephrol Dial Transplant. 2003;18(3):517–523. doi: 10.1093/ndt/18.3.517. [DOI] [PubMed] [Google Scholar]
- 11.Norris KC, Greene T, Kopple J, Lea J, Lewis J, Lipkowitz M, Miller P, Richardson A, Rostand S, Wang X, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol. 2006;17(10):2928–2936. doi: 10.1681/ASN.2005101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasgupta I, Porter C, Innes A, Burden R. "Benign" hypertensive nephrosclerosis. QJM. 2007;100(2):113–119. doi: 10.1093/qjmed/hcl139. [DOI] [PubMed] [Google Scholar]
- 13.Wehrmann M, Bohle A. The long-term prognosis of benign nephrosclerosis accompanied by focal glomerulosclerosis and renal cortical interstitial fibrosis, designated so-called decompensated benign nephrosclerosis by Fahr, Bohle and Ratscheck. Pathol Res Pract. 1998;194(8):571–576. doi: 10.1016/S0344-0338(98)80047-2. [DOI] [PubMed] [Google Scholar]
- 14.McClellan WM, Langston RD, Presley R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol. 2004;15(7):1912–1919. doi: 10.1097/01.ASN.0000129982.10611.4C. [DOI] [PubMed] [Google Scholar]
- 15.Marin R, Gorostidi M, Fernandez-Vega F, Alvarez-Navascues R. Systemic and glomerular hypertension and progression of chronic renal disease: the dilemma of nephrosclerosis. Kidney Int Suppl. 2005;99:S52–56. doi: 10.1111/j.1523-1755.2005.09910.x. [DOI] [PubMed] [Google Scholar]
- 16.Amraoui F, Bos S, Vogt L, van den Born BJ. Long-term renal outcome in patients with malignant hypertension: a retrospective cohort study. BMC Nephrol. 2012;13:71. doi: 10.1186/1471-2369-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez R, Morales E, Segura J, Ruilope LM, Praga M. Long-term renal survival in malignant hypertension. Nephrol Dial Transplant. 2010;25(10):3266–3272. doi: 10.1093/ndt/gfq143. [DOI] [PubMed] [Google Scholar]
- 18.Zucchelli P, Zuccala A. Progression of renal failure and hypertensive nephrosclerosis. Kidney Int Suppl. 1998;68:S55–59. doi: 10.1046/j.1523-1755.1998.06814.x. [DOI] [PubMed] [Google Scholar]
- 19.Zarif L, Covic A, Iyengar S, Sehgal AR, Sedor JR, Schelling JR. Inaccuracy of clinical phenotyping parameters for hypertensive nephrosclerosis. Nephrol Dial Transplant. 2000;15(11):1801–1807. doi: 10.1093/ndt/15.11.1801. [DOI] [PubMed] [Google Scholar]
- 20.Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, Glassock R. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. AASK Pilot Study Investigators. Kidney Int. 1997;51(1):244–252. doi: 10.1038/ki.1997.29. [DOI] [PubMed] [Google Scholar]
- 21.Takebayashi S, Kiyoshi Y, Hisano S, Uesugi N, Sasatomi Y, Meng J, Sakata N. Benign nephrosclerosis: incidence, morphology and prognosis. Clin Nephrol. 2001;55(5):349–356. [PubMed] [Google Scholar]
- 22.Marcantoni C, Ma LJ, Federspiel C, Fogo AB. Hypertensive nephrosclerosis in African Americans versus Caucasians. Kidney Int. 2002;62(1):172–180. doi: 10.1046/j.1523-1755.2002.00420.x. [DOI] [PubMed] [Google Scholar]
- 23.Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, Liu Z. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27(4):1479–1485. doi: 10.1093/ndt/gfr527. [DOI] [PubMed] [Google Scholar]
- 24.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43(2):368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Liang Y, Liu R, Du S, Qiu C. Trends in incidence of hypertension in Chinese adults, 1991–2009: The China Health and Nutrition Survey. Int J Cardiol. 2014. [DOI] [PMC free article] [PubMed]
- 26.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66(3):920–923. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, Tsuruya K, Kiyomoto H, Iida H, Sasaki T, et al. Japan renal biopsy registry and Japan kidney disease registry: committee report for 2009 and 2010. Clin Exp Nephrol. 2013;17(2):155–173. doi: 10.1007/s10157-012-0746-8. [DOI] [PubMed] [Google Scholar]
- 28.Caetano ER, Zatz R, Saldanha LB, Praxedes JN. Hypertensive nephrosclerosis as a relevant cause of chronic renal failure. Hypertension. 2001;38(2):171–176. doi: 10.1161/01.HYP.38.2.171. [DOI] [PubMed] [Google Scholar]
- 29.Bohle A, Wehrmann M, Greschniok A, Junghans R. Renal morphology in essential hypertension: analysis of 1177 unselected cases. Kidney Int Suppl. 1998;67:S205–206. doi: 10.1046/j.1523-1755.1998.06748.x. [DOI] [PubMed] [Google Scholar]
- 30.Ratschek M, Ratschek E, Bohle A. Decompensated benign nephrosclerosis and secondary malignant nephrosclerosis. Clin Nephrol. 1986;25(5):221–226. [PubMed] [Google Scholar]
- 31.Akimoto T, Muto S, Ito C, Takahashi H, Takeda S, Ando Y, Kusano E. Clinical features of malignant hypertension with thrombotic microangiopathy. Clin Exp Hypertens. 2011;33(2):77–83. doi: 10.3109/10641963.2010.503303. [DOI] [PubMed] [Google Scholar]
- 32.van den Born BJH, Honnebier UPF, Koopmans RP, van Montfrans GA. Microangiopathic hemolysis and renal failure in malignant hypertension. Hypertension. 2004;45(2):246–251. doi: 10.1161/01.HYP.0000151620.17905.ee. [DOI] [PubMed] [Google Scholar]
- 33.Nzerue C, Oluwole K, Adejorin D, Paueksakon P, Fremont R, Akatue R, Faulkner M. Malignant hypertension with thrombotic microangiopathy and persistent acute kidney injury (AKI) Clinical kidney journal. 2014;7(6):586–589. doi: 10.1093/ckj/sfu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitcock JA, Johnson JG, Hatch FE, Acchiardo S, Muirhead EE, Brown PS. Malignant hypertension in blacks. Malignant intrarenal arterial disease as observed by light and electron microscopy. Hum Pathol. 1976;7(3):333–346. doi: 10.1016/S0046-8177(76)80043-3. [DOI] [PubMed] [Google Scholar]
- 35.Laszik ZG, Silva FG. Hemolytic Uremic Syndrome, Thrombotic Thrombocytopenic Purpura, and Other Thrombotic Microangiopathies. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Hepinstall's Pathology of the Kidney. 6. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 701–764. [Google Scholar]
- 36.Luke RG. Hypertensive nephrosclerosis: pathogenesis and prevalence. Essential hypertension is an important cause of end-stage renal disease. Nephrol Dial Transplant. 1999;14(10):2271–2278. doi: 10.1093/ndt/14.10.2271. [DOI] [PubMed] [Google Scholar]
- 37.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17(1):17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 38.Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:S74–78. [PubMed] [Google Scholar]
- 39.Yu XJ, Yu F, Song D, Wang SX, Song Y, Liu G, Zhao MH. Clinical and renal biopsy findings predicting outcome in renal thrombotic microangiopathy: a large cohort study from a single institute in China. The Scientific World Journal. 2014;2014:680502. doi: 10.1155/2014/680502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gudbrandsson T, Hansson L, Herlitz H, Andren L. Malignant hypertension--improving prognosis in a rare disease. Acta medica Scandinavica. 1979;206(6):495–499. doi: 10.1111/j.0954-6820.1979.tb13553.x. [DOI] [PubMed] [Google Scholar]
- 41.Zeng CH, Le W, Ni Z, Zhang M, Miao L, Luo P, Wang R, Lv Z, Chen J, Tian J, et al. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult chinese patients. Am J Kidney Dis. 2012;60(5):812–820. doi: 10.1053/j.ajkd.2012.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the findings are within the paper and the supporting information file (Additional file).


