Abstract
Objective
People with depressive symptoms typically report lower levels of exercise self-efficacy and are more likely to discontinue regular exercise than others, but it is unclear how depressive symptoms affect people’s exercise self-efficacy. Among potential sources of self-efficacy, engaging in the relevant behavior is the strongest (Bandura, 1997). Thus, we sought to clarify how depressive symptoms affect the same-day relation between engaging in exercise and self-efficacy during the initiation of regular exercise.
Methods
Participants (N=116) were physically inactive adults (35% reported clinically significant depressive symptoms at baseline) who initiated regular exercise and completed daily assessments of exercise minutes and self-efficacy for four weeks. We tested whether (a) self-efficacy differed on days when exercise did and did not occur, and (b) the difference was moderated by depressive symptoms. Mixed linear models were used to examine these relations.
Results
An interaction between exercise occurrence and depressive symptoms (p<.001) indicated that self-efficacy was lower on days when no exercise occurred, but this difference was significantly larger for people with high depressive symptoms. People with high depressive symptoms had lower self-efficacy than those with low depressive symptoms on days when no exercise occurred (p=.03), but self-efficacy did not differ on days when exercise occurred (p=.34).
Conclusions
During the critical period of initiating regular exercise, daily self-efficacy for people with high depressive symptoms is more sensitive to whether they exercised than for people with low depressive symptoms. This may partially explain why people with depression tend to have difficulty maintaining regular exercise.
Keywords: exercise self-efficacy, depressive symptoms, daily exercise, initiation of regular exercise
People have much to gain from regular exercise, such as improved sleep, increased cognitive function, and reduced risk for cardiovascular disease, and these benefits may be particularly important for people experiencing deficits in these areas due to depressive symptoms (Kubesch et al., 2003; Lett et al., 2004; Rethorst et al., 2013). Moreover, regular exercise can be an effective treatment for depression (Babyak et al., 2000; Dunn et al., 2005; Mather et al., 2002; Mota-Pereira et al., 2011; Rethorst, Wipfli, & Landers, 2009). Despite the myriad benefits of regular exercise, adults with depressive symptoms have lower exercise levels than other adults (Patten, Williams, Lavorato, & Eliasziw, 2009; Roshaneai-Moghaddam, Katon, & Russo, 2009) and those with depressive symptoms who initiate a regular exercise regimen are more likely than others to discontinue it (DiMatteo, Lepper, & Croghan, 2000; Teixeira et al., 2004). Therefore, it is important to understand the reasons why people with depressive symptoms have difficulty initiating and maintaining regular exercise.
Models of health behavior change, such as the Health Action Process Approach, Theory of Planned Behavior, and Social Cognitive Theory, emphasize the role of self-efficacy in successful behavior change (Ajzen, 1991; Bandura, 1997; Schwarzer, 1992). Self-efficacy is a person’s belief in his or her own ability to execute a specific behavior (Bandura, 1997), and it is central to behavior change because it guides what behaviors people choose to engage in and how people respond to obstacles and challenges in changing their behaviors (Bandura, 1997). Self-efficacy is known to be a predictor of successful initiation and maintenance of regular exercise (Rodgers, Hall, Blanchard, McAuley, & Munroe, 2002; Williams et al., 2008). Low self-efficacy may be one reason why people with depressive symptoms have difficulties initiating and maintaining regular exercise. People with depressive symptoms report lower self-efficacy than people without depressive symptoms across various behaviors (Bandura, 1998; Robinson-Smith, Johnston & Allen, 2000; Sacco et al., 2005). However, to date, it is unclear how depressive symptoms affect people’s self-efficacy while they attempt to initiate regular exercise.
Self-efficacy beliefs are based on perceptions of ability to execute the relevant behavior, as well as perceptions of specific task demands and situational circumstances. Self-efficacy is theorized to stem from four sources: personal experience, vicarious experience, social persuasion (e.g., verbal encouragement), and physiological factors (e.g., arousal such as increased heart rate can lead to perceived inefficacy). Among these different sources, personal experience in executing the relevant behavior is the strongest source of self-efficacy perceptions (Bandura, 1997). Consistent with the proposition that personal experience is the strongest source of self-efficacy, exercise self-efficacy has been shown to significantly increase following participation in exercise. This has been observed after individual bouts of exercise (McAuley, 1995; McAuley et al., 2011) and long-term interventions for regular exercise (Marcus, Selby, Niaura, & Rossi, 1992; McAuley, 1995). This pattern of self-efficacy increases following the execution of exercise is consistent across the literature among healthy adults (Keller, Fleury, Gregor-Holt, & Thompson, 1999). In contrast, self-efficacy has been shown to decrease when individuals do not adhere to regular exercise, especially during the initiation of regular exercise as a new behavior (Parschau, Richert, Koring, Lippke, & Schwarzer, 2012). These findings all demonstrate that perceptions of self-efficacy are sensitive to whether people are successfully engaging in exercise or not.
However, Bandura (1997) emphasized that it is not simply objective task execution that influences self-efficacy, but also the subjective perceptions and interpretations associated with the behavior. Subjective perceptions can be influenced by personal factors, such as differences in affective and cognitive processing. Depressive symptoms may be a personal factor that moderates the path between personal experience with exercise and self-efficacy. Depression is characterized by negative interpretations of experiences that are influenced by rumination, negative cognitions, and negative recall bias (Beck, 1991). The result of these negative interpretations is a tendency to minimize successes and exaggerate failures (Bandura, 1997).
There is evidence to suggest that depressive symptoms influence self-efficacy to exercise regularly, but the evidence does not specifically address how this occurs. For example, older adults with depressive symptoms who enrolled in a 10-week progressive resistance-training program did not report increases in self-efficacy despite regular participation in exercise and objective gains in physical capability (Singh et al., 1997). This finding suggests the possibility that people with depressive symptoms have difficulties deriving self-efficacy from successfully performing exercise. This would be consistent with evidence that depressive symptoms are associated with a tendency to minimize successes (Bandura, 1997; Beck, 1991). Alternatively, Conroy and colleagues (2007) analyzed cross-sectional data one year after an exercise intervention in order to understand lapses (i.e., two weeks of failed adherence) during the initiation of a regular exercise regimen. They found that participants with high depressive symptoms were more likely than other participants to never return to regular exercise after a lapse to inactivity. This suggests that not exercising for two weeks was more detrimental to regular exercise behavior for participants with high depressive symptoms than those with low symptoms. One possible explanation is that participants with depressive symptoms may have experienced greater decreases in self-efficacy when they did not exercise regularly. This would be consistent with evidence that depressive symptoms can also be associated with a tendency to exaggerate failures (Bandura, 1997; Beck, 1991). However, this possibility was not directly examined.
If depressive symptoms moderate the relation between exercise and self-efficacy, the moderation effect could be manifest in two different ways. First, depressive symptoms may dampen an increase in self-efficacy when people exercise (e.g., self-efficacy is lower for people with high depressive symptoms than those with low symptoms on days they exercise). Second, depressive symptoms may amplify a decrease in self-efficacy when people do not exercise (e.g., self-efficacy is lower for people with high depressive symptoms than those with low symptoms on days they do not exercise). Current evidence is unclear as to whether either or both effects occur for people with depressive symptoms (Singh et al., 1997; Conroy et al., 2007). Elucidating how these effects unfold on a daily basis would help specify why people with depressive symptoms have difficulties initiating regular exercise.
Self-efficacy and exercise both have unique features that would benefit from daily examination over time. First, self-efficacy is not a static construct; it varies according to a person’s experiences (Shiffman et al., 2000). Second, the first days and weeks of initiating regular exercise are a sensitive period in both the formation of self-efficacy (Bandura, 1997) and establishing regular exercise (Dishman, Ickes, & Morgan, 1980), thus it is likely that exercise self-efficacy will be particularly sensitive to daily influences during this critical phase. Third, regular exercise is a health behavior that does not necessitate daily adherence, unlike other health behaviors such as smoking cessation or medication adherence. Indeed, public health guidelines for physical activity recommend activity on most but not all days of the week (USDHHS, 2008). Consequently, for people initiating regular exercise, there will routinely be days on which they exercise and days on which they do not. Distinguishing between experiences that occur on days of exercise and days of no exercise is relevant to understanding the daily psychological processes that occur during the initiation phase of regular exercise.
Current Study
This study advances current research by examining how depressive symptoms moderate the relation between exercise and self-efficacy at the daily level. This is important for understanding disparities in regular exercise and self-efficacy levels for people with depressive symptoms, and it is also important for informing interventions designed to increase the maintenance of regular exercise (Dunton & Atienza, 2009). Using daily diary reports collected over 28 days from a sample of physically inactive adults initiating regular exercise, we tested whether exercise self-efficacy differed between days of exercise and days of no exercise and whether depressive symptoms moderated the same-day relation between exercise occurrence and self-efficacy. We did not have an a priori hypothesis about whether the moderation effect would be manifest in smaller increases in self-efficacy on days when exercise occurred or larger decreases in self-efficacy on days when no exercise occurred. In addition to examining the primary research question, we explored two secondary questions. First, we examined whether these relations differed by gender or body mass index (BMI) because both depressive symptoms and physical activity have been show to differ between men and women (Kessler et al., 2003; Troianao et al., 2008) and among different levels of BMI (Frank et al., 2004; Stunkard et al., 2003). Second, we examined whether depressive symptoms also moderated the effect of self-efficacy on the occurrence of exercise the next day.
Method
Participants
Participants (N = 119) from the Dallas-Fort Worth area consented to be part of a research study of the initiation of regular exercise. Three participants did not complete any measures beyond baseline and were therefore excluded from the present analyses. Among the sample included in analyses (N = 116), the mean age was 34.5 years, with a range of 18 to 61 years. The majority of participants were female (75.9%), the sample was racially and ethnically diverse (32.8% Hispanic, 42.2 % non-Hispanic White, 15.5% non-Hispanic Black, 5.2% Asian, and 2.6% Other), and had a mean body mass index (BMI) of 27.8 (SD = 5.7), with similar numbers of normal weight (BMI < 25.0; 36.2%), overweight (BMI = 25.0 – 29.9; 29.3%), and obese (BMI ≥ 30.0; 34.5%) individuals. Participants’ baseline metabolic equivalent (MET) levels of cardiorespiratory fitness (M= 8.39 METs, SD= 2.19, range 3.81–13.84) were consistent with the average levels found in other general adult populations (Stamatakis et al., 2013), but were below the expected average for regularly active adults (i.e., 10 METs; Jurca et al., 2005).
Inclusion Criteria
To be eligible, potential participants had to (a) be physically inactive (i.e., report less than 60 minutes of moderate-to-vigorous intensity activity per week over the last month [CDC, 2009]); (b) have internet access at home; (c) have access to exercise equipment or a location to exercise; (d) have a BMI ≤ 40; (e) not have cardiovascular, pulmonary, or metabolic disease, or any health problems that would create a high risk for injury due to increased exercise (ACSM, 2009); and (f) express interest in initiating regular exercise.
Procedure
All study materials and procedures were reviewed and approved by the Institutional Review Board at Southern Methodist University. The present data were collected during the first four weeks after initiating a self-directed exercise plan, in which participants completed daily and weekly diaries reporting their exercise levels and experiences with regular exercise. The present analyses focus on the daily diary data because we were interested in examining how depressive symptoms moderate the relation between the daily occurrence of exercise and exercise self-efficacy.
Pre-screen
Participants were recruited through advertisements in the Dallas-Fort Worth area (e.g., craigslist, flyers in local community centers). People who responded to study advertisements were screened for eligibility and for willingness and ability to complete daily questionnaires and increase their levels of regular exercise. Eligible participants were scheduled for an in-person baseline session.
Baseline Session
A trained research assistant (RA) began each baseline session with the informed consent process. Then cardiorespiratory fitness was estimated with a non-exercise model (Jurca et al., 2005). The RA then provided participants with information about the study and safely initiating regular exercise on their own. All exercise recommendations were based on current public health guidelines and were presented using the structure and content of Be Active Your Way: A Guide for Adults (see USDHHS, 2008), which included information about different types of exercise, suggestions for physical activities, and weekly calendars that were used to make exercise plans for 150 minutes per week of cardiovascular activity. Finally, participants completed a battery of questionnaires including the baseline self-efficacy scale and depression inventory.
Daily Diaries
Beginning the day after the baseline session, we sent daily email questionnaires to participants for 28 consecutive days in which they reported the amount of exercise they engaged in and their experiences during that day, including self-efficacy. Online survey software (Qualtrics, Inc.) was used to administer and manage the questionnaires. In the baseline session, participants reported what time they typically went to bed and questionnaires were sent one hour prior to that time. Participants were instructed to complete daily questionnaires each night, but the questionnaires were considered valid if they were completed by 12:00 P.M. the following day (Gable & Poore, 2008). Timely completion was verified by electronic timestamps.
There were 3,248 possible daily diaries to be collected during the study, and participants completed 72.2% of the diaries (2,345 diaries completed). This completion rate is similar to other studies using daily exercise assessments (e.g., Dunton, Atienza, Castro, & King, 2009 - 76% completion). The mean number of daily diaries completed was 20.22 (SD = 8.30, range=1–28).
Weekly Contact
Research staff contacted participants by telephone weekly to discuss exercise plans for the upcoming week. Brief telephone contact between study staff and participants has been shown to improve adherence to exercise plans (Castro & King, 2002). This weekly contact also allowed the staff to address any questions or concerns from the participant. Participants were compensated up to $120 for completing the study.
Measures
Outcome Variable
Exercise self-efficacy
Exercise self-efficacy was assessed daily using a single-item that was specific to participants’ regular exercise plan. Participants responded on a Likert-scale ranging from 0 (not at all) to 8 (extremely) to the question: “How confident are you today that you will be able to continue following your exercise plan?” The phrasing of the single item is consistent with Conner and Norman’s (2005) recommendation for a single-item measure of self-efficacy in daily diary studies, and the use of a single-item measure of self-efficacy is supported by previous research (Shiffman et al., 2000). To validate this single-item measure within the current dataset, we examined correlations between this item and a five-item self-efficacy measure (detailed below; Linde, Rothman, Baldwin, & Jeffery, 2006) that was collected at the end of each week. In the current sample, this five-item measure had alphas ranging from .85 to .89 across the four weeks. We correlated the five-item measure from the end of each week (i.e., Week 1, 2, 3, and 4) with the single-item daily measure that was collected on the same day (i.e., Day 7, 14, 21, and 28, respectively). The two measures were significantly correlated at each time point with rs ranging from .52 to .80, supporting the validity of the single item measure.
Predictor and Moderator Variables
Daily exercise
Participants reported each day whether they had engaged in any walking, moderate intensity, or vigorous intensity activity and the specific amount of time spent doing each type. These items were modified from the Behavioral Risk Factor Surveillance System (CDC, 2009) to reflect daily rather than weekly reporting and were collected each day for the 28-day period. Participants responded to the walking item (i.e., “Today did you walk for at least 10 minutes at a time at your normal walking pace, while at work, for recreation, to get to and from places, or for any other reason?”) before answering the moderate- and vigorous-intensity items to ensure that this type of incidental physical activity was not included in their reports of moderate- or vigorous-intensity activity.
To create a daily exercise variable, we dichotomized total daily minutes of moderate- or vigorous-intensity activity to capture the occurrence or absence of exercise each day. Specifically, each day was coded as either a “day of exercise,” defined as a day in which 10 or more minutes of activity was reported, or a “day of no exercise,” defined as a day in which less than 10 minutes of activity was reported. We used 10 minutes as the threshold because current public health guidelines indicate that activity should be for at least 10 minutes per bout (USDHHS, 2008), and participants were informed of this guideline prior to planning their exercise. Also, there is evidence that bout length does not have additive psychological benefits beyond 10 minutes (Reed & Ones, 2006).
Depressive symptoms
Depressive symptoms were assessed at baseline, using the 20-item Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977). The measure assesses depressive symptoms within the last week, with participants responding to each symptom’s frequency on a scale ranging from 0 (rarely) to 3 (most of the time). A score of 16 or more (out of a possible 60) is reflective of at least moderate depression. The measure has high internal consistency across a variety of populations (αs = 0.85 in community samples and 0.90 in psychiatric samples; Roberts & Vernon, 1983) and the internal consistency within our sample was good (α = 0.76).
Covariates
Baseline exercise self-efficacy
Baseline self-efficacy for regular exercise was assessed using a five-item exercise-specific self-efficacy measure (Linde et al., 2006). The same scale was used in the weekly questionnaires referenced previously. The measure assesses confidence in ability to adhere to exercise goals when difficulties arise. An example item is: “How confident are you that you would be able to follow your exercise plan when you are sore or tired?” Participants responded on a Likert scale ranging from 0 (not at all confident) to 8 (extremely confident). The internal consistency within our sample was good (α = 0.88).
BMI and cardiorespiratory fitness
Height and weight were measured in the baseline session in order to calculate BMI. Cardiorespiratory fitness was calculated using a non-exercise model which estimates a metabolic equivalent level based on the individual’s physical activity level, gender, age, BMI, and resting heart rate (see Jurca et al., 2005 for the formula). The RA measured resting heart rate (beats per minute) three times for each participant using a digital heart rate monitor and the average of the three measurements was used in the calculation of CRF. Height was measured using a wall-mounted height rod, and weight was measured using a digital weight scale. Participants’ physical activity level was self-reported by selecting the appropriate activity level category used in the model (e.g., “Inactive or little activity other than usual daily activities”).
Race
Participants self-reported the race and ethnicity with which they identify.
Data Analysis
We used multi-level modeling (MLM) to analyze the data because MLM allows for the inclusion of all subjects in the analysis, regardless of missing data, and is the recommended analysis method for longitudinal data (Hamer & Simpson, 2009). We began data analysis by selecting the most appropriate covariance matrix by comparing models on −2 log likelihood fit statistics. A Toeplitz covariance model was the best fit for our data and is theoretically reasonable because our MLM analysis involved evenly spaced, repeated measures and because we had no reason to suppose that the error structure was changing over time. We also used maximum likelihood estimation instead of restricted maximum likelihood estimation because our sample was sufficiently large.
The model included daily self-efficacy scores as the dependent variable with daily exercise, baseline depressive symptoms, and their interaction as predictors. We also included baseline cardiorespiratory fitness, race, and baseline exercise self-efficacy as covariates. We included cardiorespiratory fitness and race to adjust for any effect the variables may have on the amount of exercise participants would complete or the confidence they would feel in their abilities. We included baseline self-efficacy to adjust for the effect that initial differences in self-efficacy might have on daily self-efficacy levels.
To assess whether depressive symptoms moderated the relation between exercise and daily self-efficacy, we used the following models. The level 1 component of the MLM model (which estimated self-efficacy as a function of daily exercise within individuals) was:
where i represents each individual subject and j represents the 28 daily assessments. Daily exercise was a dichotomous variable of the presence or absence of at least 10 minutes of moderate- to vigorous-activity reported each day. Note that because exercise and self-efficacy were both assessed at the end of the day, daily self-efficacy levels were reported after any exercise did or did not occur that day.
The level 2 portion of the model allowed for differences among individuals in the intercept and slope (from the level 1 model), as determined by individual characteristics (i.e., baseline depressive symptoms). The level 2 equations were:
where, again, i represents each individual subject and j represents the 28 daily assessments. Depressive symptoms are best analyzed continuously (see Haslam, 2003, for a review) and were therefore entered into this model as a z-scored, continuous predictor. Accordingly, we tested the following multilevel composite model:
Results
Descriptive Analyses
Descriptive statistics and correlations among the study variables are reported in Table 1. Participants reported an average of nearly 11 days of exercise over the four weeks, and the majority of reported exercise days included at least 30 minutes of exercise (68.2%; 581 of 852 reported days). In addition, baseline self-efficacy scores (see Table 1) suggest that participants reported moderate levels of confidence about their ability to adhere to regular exercise before initiating their regular exercise plan. The mean level of weekly activity for the sample (112.29 minutes/week) was under the public health guideline (i.e., ≥ 150 minutes/week), but participants’ exercise levels exceeded their baseline levels (< 60 minutes/week). These data suggest that many participants were actively engaged in changing their activity levels.
Table 1.
Descriptive statistics and correlations between study variables
| Variable | 1 | 2 | 3 | 4 | Mean | SD |
|---|---|---|---|---|---|---|
| 1. Average Daily SE | – | 5.56 | 1.88 | |||
| 2. Baseline SE | .37** | – | 4.58 | 1.70 | ||
| 3. Baseline Depressive Symptoms | −.16 | .11 | – | 14.55 | 10.18 | |
| 4. Total Days of Exercise | .42** | .10 | −.01 | – | 10.89 | 6.73 |
Note: SE=self-efficacy;
p < .001; possible range of scores for SE scales = 0 to 8, baseline depressive symptoms = 0 to 60, total days of exercise = 0 to 28
Participants reported a broad range of depressive symptom scores (range: 0–59). Forty-one participants (35.3%) scored 16 or above, indicating at least clinically significant levels of depressive symptoms (Lewinsohn, Seeley, Roberts & Allen, 1997). Average depressive symptom scores were lower and the range of scores was smaller for men (M=12.3, SD=7.4, range=2–31) than for women (M=15.21, SD=10.86, range=0–59). There was a fairly even distribution of participants with depressive symptoms across BMI levels, with slightly over a third of overweight (36.8%) and of obese (39.0%) participants reporting clinically significant depressive symptoms. Baseline depressive symptoms were not associated with baseline self-efficacy, daily exercise self-efficacy, or total days of exercise (see Table 1).
Depressive Symptoms Moderating the Daily Exercise-Self-Efficacy Relation
First, consistent with past research (Marcus, Selby, Niaura, & Rossi, 1992; McAuley, 1995; McAuley et al., 2011), we found that self-efficacy is higher on days exercise occurs compared to days on which it does not occur, b =0.37, p <.001, 95% CI [.29, .44]. We also found a significant interaction between exercise occurrence and depressive symptoms, b =0.15, p <.001, 95% CI [.07, .23]1, indicating that depressive symptoms moderated the relation between same-day exercise and self-efficacy. In order to understand the nature of this interaction, we centered the depressive symptoms variable alternately at two different values. First, the depressive symptoms variable was centered so that 0 = low depressive symptoms (i.e., one standard deviation below the mean; CES-D score = 4.37). As illustrated in Figure 1, this analysis showed that for participants with low depressive symptoms, daily exercise significantly predicted self-efficacy, b = .21, p < .001, 95% CI [.11, .32], such that self-efficacy was higher on days they exercised than on days they did not (d =.18). Next, we re-ran the model with depressive symptoms re-centered so that 0 = high depressive symptoms (i.e., one standard deviation above the mean; CES-D score = 24.73) and found the same effect, but stronger, for people with high depressive symptoms, b= .52, p <.001, 95% CI [.41, .64], d =.41.
Figure 1.
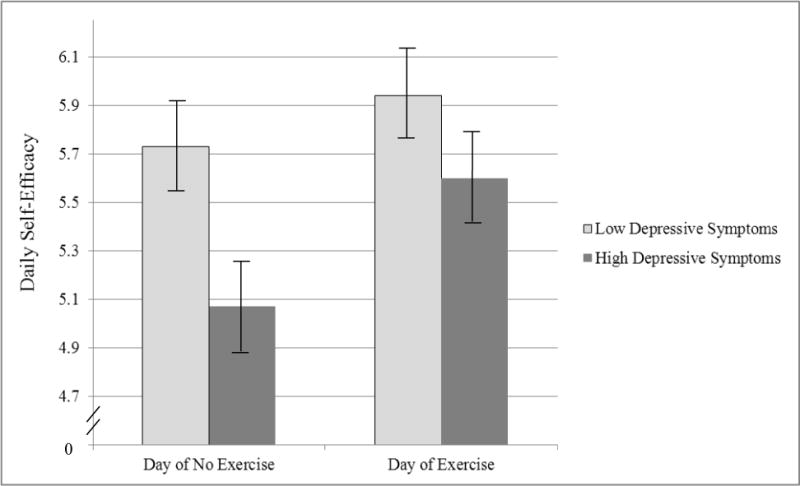
Mean daily self-efficacy for days in which exercise occurred compared to days in which exercise did not occur
Note. Possible range of scores for self-efficacy scale = 0 to 8; depressive symptoms centered at 4.37 and 24.73 (i.e., one standard deviation above and below the mean), with possible score range being 0 to 60; error bars reflect plus/minus one standard deviation.
To examine this interaction in another way, we re-ran the model centering the exercise variable in two ways. First, we centered the exercise predictor so that 0 = day of no exercise. As illustrated in Figure 1, we found that on days when exercise did not occur, depressive symptoms were significantly related to self-efficacy, b = −0.33, p =.03, 95% CI [−.62, −.03], indicating that self-efficacy was lower for people with higher depressive symptoms on days of no exercise than those with low depressive symptoms (d =.41). An increase in daily self-efficacy by .41 standard deviations would predict an average of 84.4 minutes more exercise over the course of the four weeks. Second, the exercise variable was centered so that 0 = day of exercise. In this case, we found that on days when exercise occurred, depressive symptoms were not related to self-efficacy, b= −0.17, p =.24, 95% CI [−.47, .12], indicating that levels of self-efficacy did not significantly differ at varying levels of depressive symptoms.
To examine whether these relations varied over time, we re-ran the model with time (i.e., Day 1–28) in the level 1 portion of the model. There was not a significant main effect of time, nor were any interactions that included time significant. This suggests that neither overall daily self-efficacy, nor the strength of the relations described above, changed over the 28-day period.
To explore one of our secondary questions, we tested whether the moderation effect differed by gender or BMI. We re-ran the models and added gender in one set of models and BMI in the other, along with the two-way and three-way interactions. We found a three-way interaction with gender, b =0.2, p =.02, 95% CI [.03, .49], indicating that depressive symptoms moderated the relation between exercise and self-efficacy for women, b =−.19, p <.001, 95% CI [−.28, −.10], but not for men, b =−.08, p =.48, 95% CI [−.29, .13]. We also found that the three-way interaction with BMI approached statistical significance, b=−.07, p =.052, 95% CI [−.14, .001], indicating that the moderation effect was present among those with lower BMIs (i.e., one standard deviation below the mean; BMI = 20.02), b=−.11, p =.03, 95% CI [−.20, −.01], but was even stronger for people with higher BMIs, (i.e., one standard deviation above the mean; BMI = 33.50), b=−.25, p <.001, 95% CI [−.37, −.13].
Aggregate Effect Over Time
The results of the moderation analyses indicate how the occurrence of exercise and depressive symptoms are associated with self-efficacy on a daily level. To examine the aggregate effect that this daily effect had over the 28-day period, we ran an additional analysis to examine how depressive symptoms and the total number of exercise days interacted to predict self-efficacy over time. In this analysis, daily self-efficacy was again the dependent variable. Predictors included total number of days of exercise (range 0–28), baseline depressive symptoms, and time, along with each of the two-way interactions and the three-way interaction. Covariates were again cardiorespiratory fitness, race, and baseline exercise self-efficacy. All variables were centered at their means. The three-way interaction (days of exercise × depressive symptoms × time) was not significant and was dropped from the model.
The results revealed a pattern that was consistent with the daily level findings. There were main effects for both baseline depressive symptoms, b = −.09, p < .001, and total exercise days, b = .69, p < .001, indicating that lower depressive symptoms and higher total exercise days predicted higher self-efficacy. However, these main effects were qualified by two interactions. First, the interaction between depressive symptoms and total exercise days was significant, b = .13, p < .001, 95% CI [.06, .20], indicating that the relation between total exercise days and self-efficacy was stronger for participants with higher depressive symptoms, b = .80, p < .001, 95% CI [.49, .89], d =.67, than for those with lower depressive symptoms, b = .54, p < .001, 95% CI [.43, .65], d =.38, (see Figure 2). Second, the interaction between time and total exercise days approached significance, b = .008, p = .07, indicating that self-efficacy levels remained stable over time for people who exercised at high levels, t(2545) = 0.88, p = .38, but decreased over time for people who exercised at low levels, t(2545) = −1.66, p = .09. Together, these two interactions suggest that aggregated over 28 days, a low number of days of exercise resulted in lower levels of self-efficacy, but this was especially true for those with high depressive symptoms.
Figure 2.
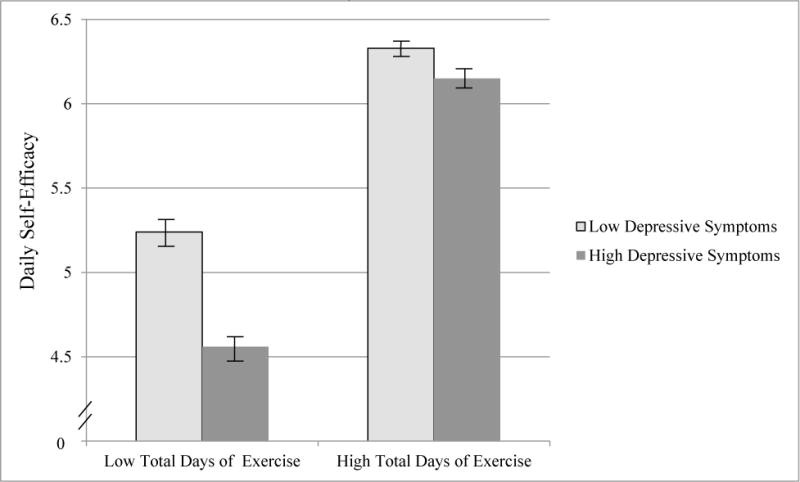
Mean daily self-efficacy for people with high and low depressive symptoms who exercised at different levels over the 28 days
Note. Possible range of scores for self-efficacy scale = 0 to 8; depressive symptoms centered at 4.37 and 24.73 (i.e., one standard deviation above and below the mean), with possible score range being 0 to 60; total days of exercise centered at 4.16 and 17.26 (i.e., one standard deviation above and below the mean), with possible score range being 0 to 28.
Do Depressive Symptoms Moderate the Effect of Self-Efficacy on Next-Day Exercise?
To explore the other secondary question, we tested whether depressive symptoms also moderated the effect of self-efficacy on the next-day occurrence of exercise. We ran a multilevel logistic regression model with next-day (day x+1) exercise as the dependent variable and daily self-efficacy (day x), baseline depressive symptoms, and their interaction as predictors, controlling for exercise on day x. We included baseline cardiorespiratory fitness and baseline exercise self-efficacy as covariates. We found that self-efficacy on day x predicted a greater likelihood of exercise on day x+1, OR=1.12, 95% CI [1.04, 1.21]. However, this relation was not moderated by depressive symptoms, OR=1.00, p =.43, 95% CI [.99, 1.01]. These findings indicate that self-efficacy on one day predicts the likelihood of exercising the next day, but that relation is not moderated by depressive symptoms.
Discussion
Consistent with evidence that personal experience with the relevant behavior is an important source of self-efficacy (Bandura, 1997), we found that daily self-efficacy was higher on days when people exercised than on days when they did not. Moreover, we found that this effect was stronger for people with higher depressive symptoms. People with higher depressive symptoms reported lower levels of self-efficacy on days when they did not exercise than those with lower depressive symptoms, but differences in depressive symptoms did not significantly predict self-efficacy levels on days when people exercised. We observed a similar pattern when looking at the aggregate effect over the 28 days. These findings suggest that, in the context of the daily process of initiating regular exercise, exercise self-efficacy is more sensitive to the absence of exercise on a given day for people with higher levels of depressive symptoms than it is for those with lower depressive symptoms. This effect may be particularly strong for women and those with high BMI. Moreover, the fact that depressive symptoms did not moderate the effect of self-efficacy on next-day exercise suggests that this moderation effect is specific to the same-day relation between exercise and self-efficacy and does not reflect a generalized effect of depressive symptoms on the interrelations among these variables.
In interpreting the findings, it is important to note that people who differed in levels of depressive symptoms did not differ in baseline levels of self-efficacy or in the total number of days on which they exercised over the 28-day period. These observations help support the conclusion that the self-efficacy of people with depressive symptoms is particularly sensitive to the absence of daily exercise during the initiation of regular exercise. First, the findings cannot be attributed to larger increases in self-efficacy among participants with higher depressive symptoms on days when they exercised. Second, lower self-efficacy on days of no exercise is not merely an artifact of participants with higher depressive symptoms exercising for fewer days.
The current findings are consistent with theoretical work on self-efficacy and depression (Bandura, 1997). Bandura suggested that depression arises from a dysfunctional self-evaluation system and is defined by a sense of inefficacy to reach highly desired goals, which is further reinforced by dysfunctional thinking patterns that discount success and exaggerate failure, consistent with other common conceptualizations of depression (Beck, 1991). Bandura also specifically theorized that the exaggeration of failure was a stronger tendency than the discounting of success (Bandura, 1997). Although we did not collect data on participants’ perceptions of success and failure in adhering to their exercise plan, the results could be seen as consistent with Bandura’s proposition if we consider the occurrence or absence of exercise as proxies for success and failure. Future research could test this possibility with clearer measures of success and failure.
Similarly, future research may explore other psychological factors that influence daily fluctuations in exercise self-efficacy. For example, additional research is needed to understand why the moderation effect was stronger among women and people high in BMI. It is possible that gender and BMI are related to the subjective perceptions and interpretations associated with regular exercise, particularly when depressive symptoms are high. It is also possible that these groups may be prone to more rumination, negative cognitions, and negative recall bias (Beck, 1991), or perhaps physiological factors (Bandura, 1997) related to weight or gender lower self-efficacy. Further, future research may examine if people with depressive symptoms report even larger self-efficacy losses on days they do not exercise among those for whom improved fitness is a highly desired goal. This may clarify if self-efficacy processes for people with depression become more dysfunctional when goals are more highly desired.
The pattern of results is also consistent with the research regarding exercise interventions for the treatment of depression. Specifically, though the antidepressant effects of exercise are not clearly understood, increased self-efficacy is proposed to be a mediator between exercise and decreased depressive symptoms (Craft & Perna, 2004; DeBoer et al., 2012). Increased self-efficacy is theorized to be a mechanism for reducing depression because it effectively increases positive behaviors (Bandura, 1997; Zeiss, Lewinsohn & Muñoz, 1979). Thus, it is not surprising that depressive symptoms did not moderate the effect of self-efficacy on next-day exercise.
Though our results are consistent with previous findings that regular exercise has the ability to improve self-efficacy in people with depressive symptoms (Craft, 2005), our findings counter the research of Singh and colleagues (1997), where self-efficacy did not increase along with decreases in depression for older adults. It is important to note that Singh’s participants were all 65 or older. Older adults have been found to have trouble increasing their exercise self-efficacy (McAuley et al., 2011), likely due to the predisposition of older adults to view their physical abilities as deteriorating, which would bias them against seeing improvements or increasing confidence in their physical abilities. The current study included a younger and much larger range of ages (18–61; mean = 34.5), and our findings suggest that adults with depressive symptoms can experience high levels of self-efficacy on days they exercise. One practical implication of these results is that people with depressive symptoms who are attempting to initiate a habit of regular exercise might avoid having days of no exercise. An intervention may encourage people with depressive symptoms to exercise every day, even if only briefly, in order to maintain their self-efficacy for regular exercise. Such an exercise intervention should also provide support for self-efficacy maintenance when failures and lapses occur by providing basic skills of goal setting (e.g., making specific, measurable, time-bound goals; allowing for trial and error) and teaching cognitive restructuring to correct the cognitive distortions that could be decreasing self-efficacy. Future research should also seek to determine the efficacy of such interventions in populations with a range of depressive symptoms.
The study had several limitations that warrant mention. First, we used a community sample in which only one third of participants reported clinically significant depressive symptoms and, of those, few participants reported severe depressive symptoms. Therefore, the observed pattern of results may not be generalizable to populations diagnosed with severe depression. For example, past literature would lead us to expect that participants with high depressive symptoms would exercise less than participants with low depressive symptoms (DiMatteo, Lepper & Croghan, 2000), but this was not the case in our sample. The current subsample of people with depressive symptoms may have been more self-motivated and able to increase their exercise levels than people with more severe depression. This is likely, considering that this was a self-selected sample of people who were interested in increasing their physical activity levels. Second, we assessed depressive symptoms only at baseline, so we are unable to demonstrate how fluctuations in depressive symptoms were associated with self-efficacy throughout the four weeks. Third, the majority of our participants were women, the average depressive symptom scores for men were lower than for women, and only five men in our sample reported clinically significant depressive symptoms. These imbalances may explain why there were gender differences in how depressive symptoms influenced self-efficacy. It will be important to replicate these findings in a sample that includes men and women with a range of depressive symptoms before concluding whether the gender effect reflects unique characteristics of this sample or a true gender difference. Finally, the present data is from only the first four weeks of exercise initiation, which is a short amount of time for assessing regular exercise levels over time. For example, the four-week timespan may not have been sufficient time to observe differences in regular exercise levels for people with high and low depressive symptoms. Also, self-efficacy is only one of several psychological and environmental factors, like social support, that impact exercise behavior (Wendel-Vos, Droomers, Kremers, Brug & Van Lenthe, 2007). Future research should seek to clarify the longer-term impact of these self-efficacy processes in exercise behaviors for people with depressive symptoms.
Conclusion
During the critical first month of initiating regular exercise, self-efficacy for people with higher depressive symptoms is more sensitive to whether exercise occurred than for people with lower depressive symptoms. These findings extend existing research by suggesting that people with depressive symptoms may have greater difficulty sustaining regular exercise due to experiencing comparatively low levels of self-efficacy on the days when they do not exercise. The findings are consistent with theoretical work on self-efficacy and depression and suggest that intervention components may need to be tailored to maintain exercise self-efficacy of people with depressive symptoms.
Footnotes
Portions of this research were presented at the annual meeting of the Society of Behavioral Medicine, March 2013, San Francisco, CA.
We ran three alternative variations of the model and found that the pattern of results did not change. First, we ran the present model with a 30-minute threshold (rather than a 10-minute threshold) for defining days of exercise and observed the same interaction effect, b =0.09, p =.03. Second, we ran the model with exercise as a continuous variable rather than a dichotomous variable and the interaction between daily exercise minutes and depressive symptoms remained significant, b = .0001, p = .04. Third, we ran the model to control for daily walking, in order to adjust for any effects of this type of incidental physical activity and observed the same interaction effect, b =0.15, p <.001. In addition, there was no main effect of walking on self-efficacy, p=.37.
References
- Ajzen I. The theory of planned behavior. Organizational behavior and human decision processes. 1991;50(2):179–211. [Google Scholar]
- American College of Sports Medicine. ACSM Guidelines for Exercise Testing and Prescription. 8th. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2009. [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Krishnan KR. Exercise treatment for major depression: Maintenance of therapeutic benefit at 10 months. Psychosomatic Medicine. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- Bandura A. Health promotion from the perspective of social cognitive theory. Psychology and Health. 1998;13:623–649. [Google Scholar]
- Beck AT. Cognitive therapy: A 30-year retrospective. American Psychologist. 1991;46:368–75. doi: 10.1037//0003-066x.46.4.368. [DOI] [PubMed] [Google Scholar]
- Castro CM, King AC. Telephone-assisted counseling for physical activity. Exercise & Sport Sciences Reviews. 2002;30:64–68. doi: 10.1097/00003677-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention CDC. Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services; 2009. [Google Scholar]
- Conner M, Norman P. Predicting Health Behaviour: Research and Practice with Social Cognition Models. Buckingham: Open University Press; 2005. pp. 163–196. [Google Scholar]
- Conroy MB, Simkin-Silverman LR, Pettee KK, Hess R, Kuller LH, Kriska AM. Lapses and psychosocial factors related to physical activity in early post-menopause. Medicine & Science in Sports and Exercise. 2007;39:1858–1866. doi: 10.1249/mss.0b013e318137388d. [DOI] [PubMed] [Google Scholar]
- Craft LL. Exercise and clinical depression: examining two psychological mechanisms. Psychology of Sport and Exercise. 2005;6:151–171. [Google Scholar]
- Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Primary Care Companion to the Journal of Clinical Psychiatry. 2004;6(3):104–111. doi: 10.4088/pcc.v06n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer L, Powers M, Utschig A, Otto M, Smits J. Exploring exercise as an avenue for the treatment of anxiety disorders. Expert Review of Neurotherapeutics. 2012;12:1011–1022. doi: 10.1586/ern.12.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Ickes W, Morgan WP. Self-motivation and adherence to habitual physical activity. Journal of Applied Social Psychology. 1980;10:115–132. [Google Scholar]
- Dunn A, Trivedi M, Kampert J, Clark C, Chambliss H. Exercise treatment for depression: efficacy and dose response. American journal of preventive medicine. 2005;28(1):1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Dunton GF, Atienza AA. The need for time-intensive information in healthful eating and physical activity research. Journal of the American Dietetic Association. 2009;109:30–35. doi: 10.1016/j.jada.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Dunton GF, Atienza AA, Castro CM, King AC. Using ecological momentary assessment to examine antecedents and correlates of physical activity bouts in adults age 50+ years: A pilot study. Annals of Behavioral Medicine. 2009;38:249–255. doi: 10.1007/s12160-009-9141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L, Andresen M, Schmid T. Obesity relationships with community design, physical activity, and time spent in cars. American journal of preventive medicine. 2004;27(2):87. doi: 10.1016/j.amepre.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Gable SL, Poore J. Which thoughts count? Algorithms for evaluating satisfaction in relationships. Psychological Science. 2008;19(10):1030–1036. doi: 10.1111/j.1467-9280.2008.02195.x. [DOI] [PubMed] [Google Scholar]
- Hamer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. American Journal of Psychiatry. 2009;166:639–641. doi: 10.1176/appi.ajp.2009.09040458. [DOI] [PubMed] [Google Scholar]
- Haslam N. Categorical versus dimensional models of mental disorder: The taxometric evidence. Australian and New Zealand Journal of Psychiatry. 2003;37:696–704. doi: 10.1080/j.1440-1614.2003.01258.x. [DOI] [PubMed] [Google Scholar]
- Jurca R, Jackson AS, LaMonte MJ, Morrow JR, Blair SN, Wareham NJ, Laukkanen R. Assessing cardiorespiratory fitness without performing exercise testing. American Journal of Preventive Medicine. 2005;29:185–193. doi: 10.1016/j.amepre.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Keller C, Fleury J, Gregor-Holt N, Thompson T. Predictive ability of social cognitive theory in exercise research: An integrated literature review. The Online Journal of Knowledge Synthesis for Nursing. 1999;6:19–31. [PubMed] [Google Scholar]
- Kessler R, Berglund P, Demler O, Jin R, Wang PS. The epidemiology of major depressive disorder. Journal of the American Medical Association. 2003;289(3):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kubesch S, Bretschneider V, Freudenmann R, Weidenhammer N, Lehmann M, Spitzer M, Grön G. Aerobic endurance exercise improves executive functions in depressed patients. Journal of Clinical Psychiatry. 2003;64:1005–1012. doi: 10.4088/jcp.v64n0905. [DOI] [PubMed] [Google Scholar]
- Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosomatic medicine. 2004;66:305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiological Studies-Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- Linde JA, Rothman AJ, Baldwin AS, Jeffery RW. The impact of self-efficacy on behavior change and weight change among overweight participants in a weight loss trial. Health Psychology. 2006;25:282–291. doi: 10.1037/0278-6133.25.3.282. [DOI] [PubMed] [Google Scholar]
- Marcus B, Selby V, Niaura R, Rossi J. Self-efficacy and the stages of exercise behavior change. Research Quarterly for Exercise and Sport. 1992;63:60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- Mather AS, Rodriguez C, Guthrie MF, McHarg AM, Reid IC, McMurdo MT. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder. The British Journal Of Psychiatry. 2002;180(5):411–415. doi: 10.1192/bjp.180.5.411. [DOI] [PubMed] [Google Scholar]
- McAuley E. Exercise in middle-aged adults: Self-efficacy and self-presentational outcomes. Preventive Medicine. 1995;24:319–328. doi: 10.1006/pmed.1995.1053. [DOI] [PubMed] [Google Scholar]
- McAuley E, Mailey EL, Mullen SP, Szabo AN, Wójcicki TR, White SM, Kramer AF. Growth trajectories of exercise self-efficacy in older adults: Influence of measures and initial status. Health Psychology. 2011;30:75–83. doi: 10.1037/a0021567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota-Pereira J, Silverio J, Carvalho S, Ribeiro JC, Fonte D, Ramos J. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. Journal of psychiatric research. 2011;45(8):1005–1011. doi: 10.1016/j.jpsychires.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Parschau L, Richert J, Koring M, Lippke S, Schwarzer R. Changes in social-cognitive variables are associated with stage transitions in physical activity. Health Education Research. 2012;27:129–140. doi: 10.1093/her/cyr085. [DOI] [PubMed] [Google Scholar]
- Patten SB, Williams JV, Lavorato DH, Eliasziw M. A longitudinal community study of major depression and physical activity. General Hospital Psychiatry. 2009;31:571–5. doi: 10.1016/j.genhosppsych.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Radloff LF. The CES-D scale: a self-report depression scale for research in the general public. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reed J, Ones DS. The effect of acute aerobic exercise on positive activated affect: A meta-analysis. Psychology of Sport and Exercise. 2006;7:477–514. [Google Scholar]
- Rethorst CD, Sunderajan P, Greer TL, et al. Does exercise improve self-reported sleep quality in non-remitted major depressive disorder? Psychological Medicine. 2013;43(4):699–709. doi: 10.1017/S0033291712001675. [DOI] [PubMed] [Google Scholar]
- Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Medicine. 2009;39(6):491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Vernon SW. The Center for Epidemiologic Studies Depression Scale: Its use in a community sample. American Journal of Psychiatry. 1983;140:41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- Robinson-Smith G, Johnston MV, Allen J. Self-care self-efficacy, quality of life, and depression after stroke. Archives of Physical Medicine and Rehabilitation. 2000;8:460–4. doi: 10.1053/mr.2000.3863. [DOI] [PubMed] [Google Scholar]
- Rodgers WM, Hall CR, Blanchard CM, McAuley E, Munroe KJ. Task and scheduling self-efficacy as predictors of exercise behavior. Psychology & Health. 2002;17:405–16. [Google Scholar]
- Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. General Hospital Psychiatry. 2009;31:306–315. doi: 10.1016/j.genhosppsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Sacco WP, Wells KJ, Vaughan CA, Friedman A, Perez S, Matthew R. Depression in adults with type 2 diabetes. Health Psychology. 2005;24:630–634. doi: 10.1037/0278-6133.24.6.630. [DOI] [PubMed] [Google Scholar]
- Schwarzer R. Self-efficacy in the adoption and maintenance of health behaviors: Theoretical approaches and a new model. Hemisphere; Publishing Corp: 1992. [Google Scholar]
- Shiffman S, Balabanis M, Paty J, Engberg J, Gwaltney CJ, Paton SM. Dynamic effects of self-efficacy on smoking lapse and relapse. Health Psychology. 2000;19:315–23. doi: 10.1037//0278-6133.19.4.315. [DOI] [PubMed] [Google Scholar]
- Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 1997;52:27–35. doi: 10.1093/gerona/52a.1.m27. [DOI] [PubMed] [Google Scholar]
- Stamatakis E, Hamer M, O’Donovan G, Batty GD, Kivimaki M. A non-exercise testing method for estimating cardiorespiratory fitness. European heart journal. 2013;34(10):750. doi: 10.1093/eurheartj/ehs097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biological psychiatry. 2003;54(3):330–337. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- Teixeira PJ, Going SB, Houtkooper LB, Cussler EC, Metcalfe LL, Lohman TG. Pretreatment predictors of attrition and successful weight management in women. International Journal of Obesity Related Metabolic Disorders. 2004;28:1124–1133. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- Troiano R, Berrigan D, Dodd K, Mâsse L, McDowell M. Physical activity in the US measured by accelerometer. Medicine and science in sports and exercise. 2008;40(1):181. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services USDHHS. Be Active Your Way: A Guide for Adults. Washington, DC: 2008. [Google Scholar]
- Wendel-Vos W, Droomers M, Kremers S, Brug J, Van Lenthe F. Potential environmental determinants of physical activity in adults: a systematic review. Obesity reviews. 2007;8(5):425–440. doi: 10.1111/j.1467-789X.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- Williams DM, Lewis BA, Dunsiger S, Whiteley JA, Papandonatos GD, Marcus BH. Comparing psychosocial predictors of physical activity adoption and maintenance. Annals of Behavioral Medicine. 2008;36:186–194. doi: 10.1007/s12160-008-9054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


