Abstract
Since an intact membrane is required for normal cellular homeostasis, membrane repair is essential for cell survival. Human genetic studies, combined with the development of novel animal models and refinement of techniques to study cellular injury, have now uncovered series of repair proteins highly relevant for human health. Many of the deficient repair pathways manifest in skeletal muscle, where defective repair processes result in myopathies or other forms of muscle disease. Dysferlin is a membrane-associated protein implicated in sarcolemmal repair and also linked to other membrane functions including the maintenance of transverse tubules in muscle. MG53, annexins, and Eps15-homology domain (EHD)-containing proteins interact with dysferlin to form a membrane repair complex and similarly have roles in membrane trafficking in muscle. These molecular features of membrane repair are not unique to skeletal muscle, but rather skeletal muscle, due to its high demands, is more dependent on an efficient repair process. Phosphatidylserine and phosphatidylinositol 4, 5 bisphosphate, as well as Ca2+, are central regulators of membrane organization during repair. Given the importance of muscle health in disease and in aging, these pathways are targets to enhance muscle function and recovery from injury.
Keywords: repair, sarcolemma, injury, muscular dystrophy, recycling
Membrane Integrity Essential for Cell Survival
To preserve cellular integrity and structure, maintenance of the plasma membrane is required to sustain proper osmotic homeostasis and cell survival. This review will focus on the sarcolemma, the plasma membrane in striated muscle cells of cardiac and skeletal muscle. The unique shape and function of striated muscle is designed to accommodate marked cell shape changes that occur concomitant with contraction and to transmit force. Like other membranes, the sarcolemma is a lipid bilayer composed of individual phospholipids oriented in an impermeable sheet that relies on the cytoskeleton for structure and stability. Disruptions altering the lipid bilayer or the cytoskeleton result in membrane dysfunction and disease. Dystrophin is a cytoskeletal protein essential for sarcolemmal stability; loss of dystrophin leads to a fragile sarcolemma that is highly susceptible to contraction induced damage. Dysferlin, another protein whose loss is linked to muscle disease, impairs muscle membrane repair as well as other membrane trafficking functions. Many of the proteins that interact with dystrophin and dysferlin are also regulators of sarcolemmal membrane integrity and repair.
Plasma membrane disruption due to mechanical forces, and chemical or toxin onslaughts are common forms of cellular injury for many different mammalian cell types under normal physiological conditions (McNeil and Khakee, 1992; McNeil and Kirchhausen, 2005). Skeletal and cardiac muscle undergo extreme physiological daily stress and continually undergo bouts of membrane damage, requiring quick and efficient means of membrane repair. Additionally, epidermal cells, epithelial, and endothelial cells undergo membrane disruption and have evolved related mechanisms for cellular repair (Yu and McNeil, 1992). Lesions occurring in the plasma membrane are a major threat to the survival of cells as extracellular Ca2+ flows freely through the damaged membrane. Normally, Ca2+ levels are found at low levels within the cell. In muscle, increased intracellular Ca2+ triggers not only muscle contraction but sustained elevated intracellular Ca2+ elicits a number of downstream signaling cascades, including the activation of Ca2+-dependent proteases causing degradation of structural and mechanical proteins of the cell, ultimately leading to cell death. The mdx mouse model of muscular dystrophy lacks dystrophin, a multi-spectrin repeat containing protein, that stabilizes the sarcolemma (Bulfield et al., 1984). Dystrophin-deficient myofibers and cardiomyocytes are fragile; they display increased sarcolemmal disruption and leak, typically monitored with vital tracers and fluorescent dyes (Straub et al., 1997). The absence of dystrophin produces sarcolemmal fragility in a muscle that is thought to have normal repair mechanisms. Dystrophin binds to cytoskeletal actin and also to a complex of transmembrane proteins linked to extracellular matrix proteins (Constantin, 2014). Mutations in the transmembrane components of the dystrophin complex similarly lead to a fragile sarcolemma indicating that the entire complex is required for sarcolemmal stability (Hack et al., 2000).
Models of cell membrane repair
Efficient membrane resealing in mammalian cell membranes generally occurs within 10–30 seconds (McNeil and Steinhardt, 1997). Healthy cells, specifically myofibers, trigger a Ca2+-induced, phospholipid-dependent repair mechanism, originally thought to involve the fusion of intracellular vesicles at the site of damage (Bansal et al., 2003; Davis et al., 2002). In this model, elevated levels of Ca2+ at the site of injury induce rapid vesicular fusion at the site of damage. Through vesicle cycling, a repair patch forms at the site of membrane disruption and described as the “patch repair” hypothesis (McNeil and Khakee, 1992; McNeil and Kirchhausen, 2005). Piccolo and colleagues provided further evidence for the patch repair model using electron microscopy to document increased vesicle accumulation at the sarcolemma in muscle lacking dysferlin, a protein implicated in membrane repair (Piccolo et al., 2000). The authors suggested that the enrichment of vesicles on the cytoplasmic face of the sarcolemma, which were not seen in healthy cells, represented vesicular trapping due to delayed fusion (Piccolo et al., 2000). However, unlike in nerve terminal synaptic vesicle transmission, there is no direct evidence that muscle contains a reservoir of “pre-docked” vesicles beneath the plasma membrane, poised for fusion, in the event of a membrane lesion. Thus, the patch hypothesis has been challenged recently (see below). Notably, the activation and translocation of non-local vesicles to the site of damage is a slow process that would not prevent the rapid influx of intracellular Ca2+.
Another repair model also maintains that membranes are repaired through a patching mechanism, but postulates that the vesicles responsible are not generic cellular vesicles, but instead are lysosomes. Experiments in cells including 3T3 fibroblasts, CHO cells, and NRK cells have shown that during mechanical damage, a subset of lysosomes are activated by the influx of Ca2+, translocate to the site of damage, and fuse to the membrane lesion forming a repair patch (Reddy et al., 2001; Rodriguez et al., 1997). However, live cell imaging of damaged zebrafish muscle with fluorescently labeled Lamp1, a lysosome associated membrane protein, did not find that Lamp1-positive vesicles translocated to the site of injury suggesting that lysosome-mediated repair may not be conserved among all cell types or types of damage (Roostalu and Strahle, 2012).
These data are consistent with a model where lysosomes may not play a direct role in membrane repair, but instead may serve a secondary role in maintaining muscle health during damage. The rise in intracellular Ca2+ during injury also activates lysosomal removal of potential toxins and cellular debris that, if insufficiently scavenged, may enhance membrane damage. Where injury was induced by viruses, bacteria, or chemical compounds, lysosomal scavenging is a critical element for mitigating injury. The lysosome model has been further refined by demonstrating that lysosomes secrete acid sphingomyelin, triggering vesicle endocytosis, to aid in the removal of toxins and likely facilitating membrane repair (Corrotte et al., 2013). Additionally, toxins are expelled along with other cytoplasmic contents in the form of macrovesicles or blebs bolstering membrane recovery and reducing intracellular Ca2+ levels (Babiychuk et al., 2011). However, these two models do not address the main mechanism of rapid membrane repair in response to contraction-induced damage in muscle or disruption of the plasma membrane in epithelial cell damage.
A more recent model suggests that repair of the disrupted sarcolemma relies on lateral recruitment of membrane to reseal the site of injury. McDade and colleagues used confocal imaging of fluorescently-labeled membrane to visualize membrane movement during laser damage. This method demonstrated that sarcolemma adjacent to the site of flowed towards the lesion to plug the site of injury (McDade et al., 2014). In this model, the plasma membrane is already positioned to diffuse into the lesion upon disruption, making the repair process and blockade of Ca2+ influx quick and efficient. This lateral recruitment model does not exclude the possibility that the patch hypothesis and/or the lysosome repair mechanism act in conjunction with lateral diffusion to repair the plasma membrane. However, the lateral recruitment model is attractive in that it does provide evidence for a model that correlates with the timing of repair required in cells to maintain Ca2+ homeostasis.
Modeling membrane repair
Several in vitro methods have been designed in multiple cell types to understand the process of membrane repair. These include mechanical injury methods such as cell scraping, in which cells are injured by scraping a razor blade or pipette across a field of cells (Reddy et al., 2001). Cultured cells are also injured by the addition of microscopic glass beads rolled over cells to induce mechanical damage (Reddy et al., 2001). Both glass bead rolling and scraping elicit membrane damage that lacks precision with regard to position of injury. Damage between cell cultures and within cultures can vary considerably. These methods are thought to resemble a mechanical-type injury creating large lesions and high levels of Ca2+ influx. As an alternative, laser-induced damage, a method by which a confocal laser burns a hole into the cell membrane and micro-needle puncturing, the act of inserting a fine point into the cell have been utilized (Bansal et al., 2003; Cai et al., 2009b; Swaggart et al., 2014). Laser-induced injury and cell puncturing allow for the location and dimension of damage to be precisely tuned, providing more reproducible results. The use of high heat during laser-induced damage may denature local proteins if not done appropriately and cell puncturing highly disorganizes the underlying cytoskeleton by pushing the membrane into the interior of the cell making real-time analysis of the damaged area more difficult. Despite these issues, laser injury and micro-needle damage have emerged as two methods that are utilized in combination with live-cell fluorescent imaging. Combining these methods allows the study of fluorescently-tagged protein trafficking in real-time, to assess timing, localization, and response to a variety of experimental manipulations (Cai et al., 2009a; Swaggart et al., 2014).
In muscle, electroporation can be used to introduce plasmids to overexpress fluorescently-tagged proteins of interest in live muscle. Following electroporation, muscle cells are isolated and available for damage assays (DiFranco et al., 2009). Tagged proteins of interest can then be observed before, during, and after repair. The development of high-resolution confocal microscopy including structured illumination microscopy (SIM), stochastic optical reconstruction microscopy (STORM), and stimulated emission depletion (STED), allows for high resolution imaging of repair structures (Jaiswal et al., 2014; Swaggart et al., 2014). The utility of the method is its relative speed. However, the use of electroporation introduces injury to the recipient cell and this can be accompanied by a repair response (Roche et al., 2011). Most investigators allow 7–14 days between electroporation and laser induced injury, but even this interval may be insufficient to allow for full recovery. Like other methods, electroporation results in varying levels of plasmid transduction and therefore protein expression. Protein overexpression itself can induce toxic effects on cells from ER stress and other methods. Thus, while this is a highly useful method, electroporation, overexpression and laser injury all have experimental caveats that must be considered when interpreting results.
Membrane Repair and Human Myopathy
Ferlins
Muscular dystrophy occurs when muscle degeneration exceeds muscle regeneration. Loss-of-function mutation in the gene encoding dysferlin, also known as Fer1L1, result in Limb Girdle Muscular Dystrophy 2B (LGMD2B), Miyoshi Myopathy (MM), or Distal Anterior Compartment Myopathy. LGMD2B is an autosomal recessive form of muscular dystrophy that is most often associated with muscle weakening of distal muscles presenting in humans in the second decade of life (Bashir et al., 1998; Liu et al., 1998). Cardiac function is generally preserved with dysferlin mutations (Kuru et al., 2004). Creatine kinase (CK) is an enzyme localized in the interior of cells that can leak from the sarcolemma into the circulation during membrane damage and is a commonly used marker of muscle degeneration. With loss of dysferlin, serum CK is extremely elevated (10–40,000 U/I compared to normal 100 U/l) consistent with increased leak of muscle contents into the serum. Muscle biopsies often reveal inflammatory infiltrate, and the can lead to the misdiagnosis of polymyositis, an autoimmune disorder (Nguyen et al., 2007). Dysferlin is the best-studied member of the ferlin family, and multiple mouse models have been generated that recapitulate human disease (Bansal et al., 2003; Bittner et al., 1999; Demonbreun et al., 2010a; Demonbreun et al., 2014; Ho et al., 2004; Lostal et al., 2010).
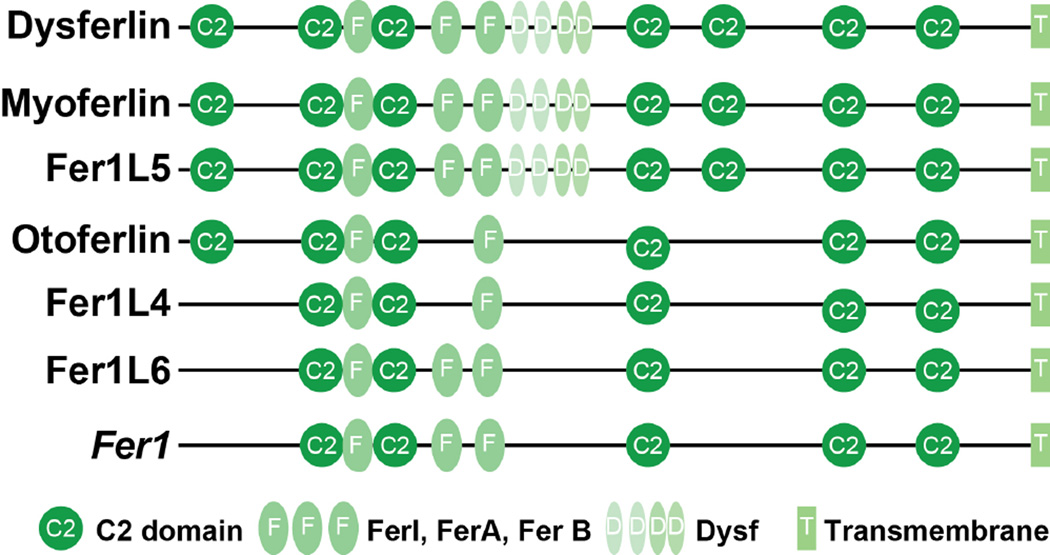
Dysferlin is 230 kDa protein that belongs to the ferlin family of proteins. These proteins are highly similar in structure with each containing a carboxyl-terminal transmembrane domain, which anchors the protein, and up to seven C2 domains. At the time of its discovery, the only other known ferlin protein was the C. elegans protein fer1, and fer1 was linked to membrane fusion defects that impaired fertilization (Achanzar and Ward, 1997). Fer1 contains multiple C2 domains like dysferlin. C2 domains are Ca2+ sensitive phospholipid binding domains of about 130 amino acids in length. The C2 domains in dysferlin have high relationship to those in the fusion protein synaptotagmin (Bansal and Campbell, 2004; Bansal et al., 2003; Davis et al., 2002). Synaptotagmins generally contain two C2 domains and mice-null for synaptotagmin 7, develop autoimmune myositis with defective membrane resealing properties (Chakrabarti et al., 2003). The amino-terminal C2 domain, C2A, mediates Ca2+-sensitive lipid binding, and this topology positions the C2A domain to mediate membrane fusion within the cell (Davis et al., 2002; Therrien et al., 2009). In addition to the C2 domains, ferlins contain Ferlin-motifs, FerI, FerA and FerB, although these 60 amino acid structures while conserved have no yet defined function. A DysF domain, situated as a nested repeat, is also present. The DysF domain also has no known function, but a similar domain is found in the peroxisomal proteins (Lek et al., 2012).
Dysferlin is highly expressed in skeletal and cardiac tissue, despite the lack of cardiac involvement in the majority of patients and mouse models (Bashir et al., 1998). Dysferlin expression is comparatively low during muscle development with progressive upregulation as muscle differentiates into mature myotubes (Davis et al., 2002). Previously, dysferlin was thought to localize primarily to the sarcolemma in muscle cells (Bansal et al., 2003; Piccolo et al., 2000). However, using a pH-sensitive tag fused to the carboxy-terminus of dysferlin, dysferlin was found to enrich in the transverse (T)-tubule structure of mature muscle fibers. T-tubules are specialized membranous invagination, continuous with the sarcolemma, and these invaginations are responsible for promoting Ca2+ propagation throughout the myofiber (Flucher, 1992; Porter and Palade, 1957). The localization of dysferlin at the T-tubule correlates developmentally with the onset of dysferlin expression, as T-tubules form as muscle differentiation proceeds (Klinge et al., 2010; Lee et al., 2002). Additionally, dysferlin has been found in tubular aggregates derived from sarcoplasmic reticular (SR), another membranous compartment critically important in the regulation of calcium (Ikezoe et al., 2003). Dysferlin is expressed in many other tissue types including, monocytes, lung, skin, brain, testis, and placenta (Bashir et al., 1998). The role of dysferlin in these tissues is not known.
Due to the high homology of dysferlin to synaptotagmin and the muscle pathology found in dysferlinopathy patients it was hypothesized that dysferlin may play a role in vesicle fusion induced repair within skeletal muscle. Bansal et al. first described dysferlin’s role in repair by generating mice lacking dysferlin and using laser-induced injury on isolated myofibers (Bansal et al., 2003). Laser injury was conducted in the presence of FM1-43, a lipophilic dye that normally is nonfluorescent but upon binding negatively charged phospholipids exhibits increased fluorescence intensity. Dysferlin-null myofibers had much slower membrane resealing than control myofibers (Bansal et al., 2003). Control myofibers damaged in the absence of Ca2+ displayed repair defects similar to that of dysferlin-null muscle, demonstrating the requirement of extracellular Ca2+ for membrane repair. Dysferlin has roles beyond membrane repair, extending to general trafficking. For example, dysferlin-regulated vesicle trafficking in both fibroblasts and myoblasts was shown using pulse chase experiments with Alexa-488 labeled transferrin (Demonbreun et al., 2010a). In dysferlin-null cells, labeled transferrin accumulated within cells indicative of delayed recycling. The accumulation of transferrin in dysferlin-null cells is consistent with the role of dysferlin as a participant in the endocytic pathway regulating membrane fusion. Dysferlin-null mice exhibit muscle wasting and weakness, similar to humans with LGMD2B. To determine the muscle specific role of dysferlin in muscle disease, dysferlin was overexpressed in the skeletal muscle of dysferlin-null mice. Overexpression rescued the pathological phenotype and functional deficits. These data suggest that functional deficits due to dysferlin are muscle-intrinsic and not caused by a more general role dysferlin may play in recycling (Lostal et al., 2010; Millay et al., 2009).
Myoferlin, another ferlin family member, is highly homologous to dysferlin, 57% at the amino acid level. Like dysferlin, myoferlin contains seven C2 domains, and like dysferlin, myoferlin’s C2A domain binds phospholipids in a Ca2+-sensitive manner (Davis et al., 2002). Unlike dysferlin, myoferlin is highly expressed during early muscle development in the skeletal muscle precursor cell, the myoblast, and is downregulated as the muscle fuses into mature myotubes (Doherty et al., 2005). During the repair process when the embryonic programs are reinitiated, myoferlin expression is upregulated (Demonbreun et al., 2010b). Upregulation of myoferlin is thought to promote myoblast fusion enhancing regeneration during repair. Myoblasts from a mouse model lacking myoferlin display delayed myoblast fusion resulting in smaller myotubes in vitro. Concomitantly, myoferlin-null mice have a mild dystrophic phenotype characterized by smaller myofibers and delayed regeneration, characteristic of fusion defects (Doherty et al., 2005). Like dysferlin, myoferlin regulates endocytic recycling and receptor trafficking, and these effects were seen in both fibroblasts and myoblasts (Demonbreun et al., 2010c; Doherty et al., 2008). Mice lacking myoferlin not only have delayed trafficking of transferrin but also of receptors that are translocated like the IGF1-receptor. Delayed recycling of the IGF1-receptor was also correlated with decreased downstream signaling cascades. Vesicle trafficking and signaling are critical components required for proper muscle growth and membrane repair (Demonbreun et al., 2010c; Doherty et al., 2008). This role for myoferlin in regulating vesicle trafficking explains both growth and repair defects observed in myoferlin-null muscle.
Myoferlin-null mice are born in Mendelian ratios and live a normal life span. Human diseases linked to mutations in the gene encoding myoferlin have not been reported. However, recent reports have linked decreased levels of myoferlin to modulating the invasive potential of cancerous cells, where decreased proliferation and migration of myoferlin-depleted cells was seen in vitro. Additionally, myoferlin reduction through siRNA of Lewis Lung Carcinoma (LLC) cells, resulted in reduced membrane repair measured through dye uptake after laser damage (Leung et al., 2013). This data provides evidence myoferlin directs membrane repair and plays a role in efficient proliferation and growth in tumor cells. Overall, the roles of dysferlin and myoferlin in nonmuscle cells are expected to be similar to what has been seen in muscle cells.
In silico prediction algorithms have identified three other potential ferlin-like proteins Fer1L4, Fer1L5, and Fer1L6. Fer1L5 is most similar to myoferlin and has been studied in vitro. Similar to myoferlin, Fer1L5 is expressed during skeletal muscle differentiation and has been shown to interact with the Eps15 homology domain containing protein EHD1 and EHD2 through the interaction of the C2 domains (Posey et al., 2011). The interaction with the EHD proteins is discussed below. In total, in humans there are six ferlin family members, including otoferlin, which has been linked to genetically mediated deafness (Roux et al., 2006). The six ferlin proteins all share their multi-C2 domain nature and the presence of additional conserved regions (FIGURE 1).
Figure 1. Schematic of ferlin family members.
The ferlins share similar structure. Each contains multiple C2 domains (circles), which bind phospholipids in a calcium dependent manner. Fer domains (dark ovals) and DysF domains (light ovals) have unknown function and are present within only some ferlin family members. A carboxy-terminal transmembrane domain (rectangle) anchors the protein.
Anoctomin 5
Anoctamin 5 (ANO5/TMEM16E) belongs to a large family of transmembrane (TMEM) proteins. ANO5, specifically belongs to the TMEM16 family, consisting of 10 highly homologous members. TMEM16 proteins contain eight hydrophobic helices predicted to be transmembrane domains and are described as Ca2+-activated chloride channels (CaCCs), phospholipid scramblases or important for protein-protein interactions (Pedemonte and Galietta, 2014). ANO1 and ANO2 were shown to function as CaCCs, and the remaining family members are thought to have this role by analogy (Ousingsawat et al., 2009; Stephan et al., 2009). Loss of function mutations ANO5 result in Limb Girdle Muscular Dystrophy 2L (LGMD2L) and Miyoshi Myopathy Dystrophy-3 (MMD-3). The muscle manifestations in LGMD2L and MMD-3 resemble the clinical presentation of dysferlinopathies, LGMD2B and MM (Bolduc et al., 2010). Specifically, individuals with ANO5-mutations have lower limb weakness and progressive muscle atrophy. Like dysferlin gene mutations, ANO5 mutations are associated with very elevated serum CK levels and a similar phenotypic range of muscle disease that typically does not associate with cardiac defects (Mahjneh et al., 2010). The ANO5 protein is localized to the endoplasmic reticulum and intracellular vesicles, and ANO5 is upregulated during muscle differentiation similar to dysferlin, suggesting an equivalent role in muscle (Mizuta et al., 2007; Tsutsumi et al., 2005). Although not specifically localized to the sarcolemma, ANO5 was analyzed for a role in membrane repair. In HEK293 cells ANO5 was seen to translocate to the cell periphery after saponin treatment suggesting ANO5 is participating in membrane repair (Tian Y, 2015). Using adeno-associated virus (AAV), ANO5 was introduced into dysferlin-null mice as a potential membrane repair therapy (Monjaret et al., 2013). Laser injury was used to assess damage, and dysferlin-null myofibers with and without ANO5 expression were damaged (Monjaret et al., 2013). The presence of ANO5 did not significantly improve the membrane repair capacities of dysferlin-null myofibers, nor did ANO5 improve the histological pathology of skeletal muscle in mice, suggesting distinct roles for ANO5 and dysferlin in membrane repair (Monjaret et al., 2013). Members of the TMEM16 family have also been implicated in distributing phospholipids within membranes, referred to as phospholipid scramblase activity (Suzuki et al., 2013). Further studies are required to elucidate the role of ANO5 in membrane repair and determine how ANO5 functions within the membrane repair complex. Dominant mutations in ANO5 lead to gnathodiaphyseal dysplasia (GGD), a disorder of bone dystrophy that results in bone thinning, bowing, and occasionally bony necrosis (Marconi et al., 2013). The role of ANO5 gene mutations in both bone and muscle disease suggests that these pathways are used by multiple tissues and cell types.
Regulators of membrane repair
Annexins
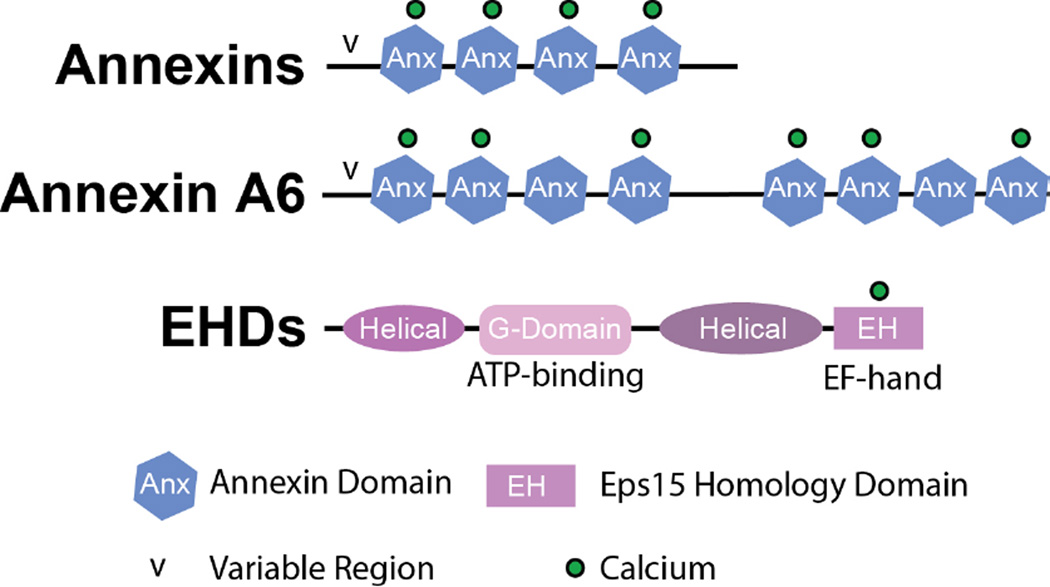
The annexin protein family characterized by the ability to bind phospholipids and actin in a Ca2+-dependent manner. Annexins preferentially bind phosphatidylserine, phosphatidylinositols, and cholesterol (Gerke et al., 2005). Dominant or recessive mutations in annexin genes have not been associated with muscle disease, however annexin A5 genetic variants associate with pregnancy loss (de Laat et al., 2006). The annexin family is known to comprise over 160 distinct proteins that are present in more than 65 unique species (Gerke and Moss, 2002). Humans have 12 different annexin genes, characterized by distinct tissue expression and localization. Annexins are involved in a variety of cellular processes including membrane permeability, mobility, and vesicle fusion. These properties are Ca2+-dependent. Although annexins do not contain EF hand domains, calcium ions bind to the individual annexin repeat domains (FIGURE 2). Differential Ca2+ affinity allows each annexin protein to respond to changes in intracellular calcium levels under unique spatiotemporal conditions (Blackwood and Ernst, 1990).
Figure 2. Schematic of Annexin and EHD proteins.
The annexin family contains 12 mammalian proteins each containing 4 annexin repeats (hexagon) and an amino-terminal variable (v) region. Annexin A6 is an atypical annexin containing eight annexin repeats and variable (v) amino-terminal region. The annexin repeats coordinate calcium binding (*) despite lacking EF-hands. The Eps 15 Homology Domain containing (EHD) family of proteins contains four highly homologous family members, EHD1-4. Each members is composed of an amino-terminal helical region, an ATP-binding G-domain, a helical region and an EH domain containing a calcium binding EF hand.
Structurally, the annexin family of proteins contains a conserved carboxy-terminal core domain composed of multiple annexin repeats and a variable amino-terminal head. The amino-terminus differs in length and amino acid sequence amongst the annexin family members. Additionally, post-translational modifications alter protein function and protein localization (Goulet et al., 1992; Kaetzel et al., 2001). Annexin proteins have the potential to self-oligomerize and interact with membrane surfaces in the presence of Ca2+ (Zaks and Creutz, 1991). The amino-terminal region is thought to bind one lipid membrane in a Ca2+-dependent manner, while the annexin core region binds an additional lipid membrane. In vitro studies have shown annexin domains lacking the amino-terminus can aggregate membrane through dimerization and annexin complex formation (Wang and Creutz, 1994).
Because of this plasma membrane binding capacity, annexins have broad membrane trafficking and actin organization roles. Specifically, annexins have been shown to interact with actin in a Ca2+-dependent manner coordinating the assembly and organization of the cytoskeleton, another critical component required during the repair process. Annexin A2 is linked to the secretory protein transport pathway and facilitates formation of filamentous (F)-actin (Hayes et al., 2006). The loss of annexin A2 decreases actin formation and vesicle trafficking. Additionally, annexin A2 binds phosphatidylinositol 4,5 bisphosphate (PIP2) and cholesterol with high affinity, making the lipid bilayer a target for annexin A2 binding (Hayes et al., 2004). The binding of annexin A2 to both actin and lipids is Ca2+-dependent allowing for tight control of the repair process.
Jaiswal and colleagues showed that the influx of Ca2+ upon membrane damage triggers annexin A2 to the site of membrane injury, facilitating actin reorganization near the injury lesion (Jaiswal et al., 2014). It is hypothesized that the reorganization of actin at the site of damage facilitates aggregation of vesicles and phospholipids at the site of damage as a well as providing changes in membrane tension required that aid repair. Notably, these studies were performed in MCF7 and HeLa cancer cells, although similar mechanisms may occur in other cells types, as actin reorganization at membrane lesions is observed in both Xenopus and Drosophila (Bement et al., 1999; Clark et al., 2009).
Annexins do not contain a predicted hydrophobic signal sequence targeting the annexins for classical secretion through the endoplasmic reticulum, yet annexins are found both on the interior and exterior of the cell (Christmas et al., 1991; Deora et al., 2004; Wallner et al., 1986). The process by which the annexins are externalized remains unknown. It is hypothesized that annexins may be released through granular exocytosis or cell lysis, however the method of externalization may also vary by cell type. Functionally, localization both inside and outside the cell adds to the complexity of the roles annexins play within tissues and cell types. Annexins have been shown to have anti-inflammatory, pro-fibrinolytic, and anti-thrombotic effects. The annexin A1-deleted mouse model exhibits an exacerbated inflammatory response when challenged and is resistant to the anti-inflammatory effects of glucocorticoids (Hannon et al., 2003). The annexin A2 null-mouse develops fibrin accumulation in the microvasculature and is defective in clearance of arterial thrombi (Ling et al., 2004).
Annexins have been shown to directly regulate membrane repair (Babbin et al., 2008; Lennon et al., 2003; McNeil et al., 2006). Annexins A1, A2, A5, and A6 localize to the site of muscle membrane repair in zebrafish muscle (Roostalu and Strahle, 2012). This localization at the sarcolemma occurs in a sequential manner with annexin A6 arriving at the site first (Roostalu and Strahle, 2012). Marg et al. also identified annexin A1 at the site of sarcolemmal damage in cultured human muscle cells (Marg et al., 2012). Jaiswal et al. found annexin A1 and annexin A2 at the site of membrane injury in two human cancer cell lines, MCF and HeLa cells (Jaiswal et al., 2014). Although little is known about the precise function of annexin A1 and annexin A2 in membrane repair, the expression level of both proteins may function as a diagnostic marker for a number of muscle diseases due to the strong correlation between high expression levels of annexin A1 and A2 and the clinical severity of such forms of muscular dystrophies (Cagliani et al., 2005). Annexins A1 and A2 directly bind dysferlin and are hypothesized to play a role in a larger membrane repair complex (Lennon et al., 2003; Roostalu and Strahle, 2012).
Annexin A6 is involved in the membrane repair process in zebrafish and mouse skeletal muscle. During muscle membrane damage, annexin A6 translocates to the site of injury (Roostalu and Strahle, 2012; Swaggart et al., 2014). Annexin A6 is unique among the annexin family members, as it is the only annexin protein that contains two core domains and eight annexin repeat domains; all other annexin family members contain one core domain and four annexin repeats (Benz et al., 1996) (FIGURE 2). In vitro studies have shown that annexin A6 is capable of membrane binding through both the amino- and carboxy-terminal annexin core domains facilitating membrane coalescence of two opposing membranes, a requirement when coordinating membrane repair (Buzhynskyy et al., 2009). The annexin A6 null-mouse does not display any overt phenotype (Hawkins et al., 1999). However, annexin A6 was found to modify a mouse model of muscular dystrophy, the Sgcg mouse that lacks γ-sarcoglycan, a dystrophin associated protein (Swaggart et al., 2014). The Sgcg mouse was bred onto a two murine genetic backgrounds, DBA/2J (D2) and 129T2/SvEmsJ (129), and F4 progeny analyzed. These two models display distinct differences in the severity of muscle disease (Heydemann et al., 2005) and the DBA/2J strain has similarly been shown to enhance the mdx model of muscular dystrophy (Fukada et al., 2010). Quantitative trait loci mapping of membrane leak combined with RNAseq pointed identified Anxa6, the gene encoding annexin A6, as a modifier of membrane fragility. In the severe strain, a truncated form of the annexin A6 protein, representing the first 32 KDa of annexin A6, was expressed at low levels. This truncated annexin protein lacks the last four carboxy-terminal annexin repeats and is the result of a splice site variant located in the middle of exon 11 causing a premature stop codon in exon 16. This alternate splice form of annexin A6 was also found in the C57BL/6 (B6) mouse strain. The “N32” annexin A6 truncated protein was found to impair trafficking of full length annexin A6 to the sarcolemma after injury (Swaggart et al., 2014). Evidence for the dominant negative effect annexin A6’s amino-terminus was also seen in zebrafish and in human fibroblasts (Kamal et al., 1998; Roostalu and Strahle, 2012). Taken together, these data confirm a critical role for annexins in regulating membrane repair.
Although loss of annexin A6 in the mouse does not yield an overt phenotype, overexpression of annexin A6 is associated with a pathogenic phenotype (Gunteski-Hamblin et al., 1996). Using the α myosin heavy chain promoter, annexin A6 was overexpressed more than 10 fold in the heart. Annexin A6 overexpression resulted in dilated hearts with increased fibrosis. Cardiomyocytes from transgenic positive mice overexpressing A6 had reduced function resulting from altered levels of basal and free Ca2+. These data suggest that although annexin A6 is upregulated during muscle differentiation, coordinated overexpression is required to participate in efficient membrane repair and regeneration.
Annexin A5 is the smallest annexin and one of the most studied of the annexin family. A5 is a Ca2+-responsive protein that oligomerizes within membranes, and annexin A5 assembly is dependent on Ca2+ and phosphatidylserine (PS) concentrations (Mosser et al., 1991). Annexin A5 is also used commonly as a marker for apoptosis due to its binding with PS as PS is known to reverse membrane orientation during cell death from the inner to outer leaflet. Annexin A5 also oligomerizes and binds PS at the site of damage. Annexin A5 may provide stability to the site of injury by reducing the movement of PS containing membrane, and thereby preventing the torn membrane from extending (Saurel et al., 1998). Annexin A5-null perivascular cells were assessed for membrane repair capacity using laser-induced injury (Bouter et al., 2011). Lipophilic dye influx was increased in annexin A5 null cells after injury, compared to wildtype controls, suggesting that annexin A5 regulates the membrane resealing process. The introduction of extracellular recombinant annexin A5 prior to laser-induced damage was sufficient to improve the membrane repair capacity of annexin A5-null cells to near wildtype levels upon injury, suggesting that annexin A5 can act from the exterior of the cell (Bouter et al., 2011). This same process was also seen in human placental trophoblasts, which express both high levels of dysferlin and annexin A5 (Carmeille et al., 2015). In neuroblastoma cells, annexin A5 was shown to assemble into complexes that also contained annexins A1 and A2 in a time-dependent manner at the plasma membrane upon increased Ca2+ levels (Skrahina et al., 2008). Bouter et al. showed that preventing annexin A5 from forming two dimensional membrane-associated arrays resulted in defective repair (Bouter et al., 2011). These studies support a role for annexin A5 in membrane repair and suggest that annexin A5 functions as a molecular repair protein from both the interior and exterior of the cell.
Trafficking and membrane repair
The Eps15 Homology Domain (EHD) containing family of proteins is comprised of four family members, EHD1-4, and this family has been implicated in membrane trafficking and repair (Grant and Caplan, 2008; Naslavsky and Caplan, 2010; Posey et al., 2014; Rapaport et al., 2006). EHD proteins regulate cytoskeletal rearrangements, specifically actin, and also have been shown to interact with the ferlin family of proteins (Doherty et al., 2008; Posey et al., 2011; Posey et al., 2014). EHD proteins have an amino-terminal nucleotide binding domain, a central coiled-coiled domain, and a carboxy-terminal Epsin Homology (EH) domain, that harbor a Ca2+ binding EF hand domain. The EH domain is known to coordinate binding proteins that contain an asparagine-proline-phenylalanine (NPF) motif (Paoluzi et al., 1998; Salcini et al., 1997). EHDs are evolutionarily conserved and have been implicated in vesicle trafficking and recycling of many signaling molecules including IGF1, GLUT4, EGFR, and the transferrin receptor, in muscle, fibroblasts and HeLa cells (Naslavsky and Caplan, 2010; Posey et al., 2014; Rapaport et al., 2006). While EHDs family members within a species are highly homologous, their roles are non-redundant (George et al., 2007).
EHD1 is the best characterized of the EHD family of proteins and bares the highest homology to the C.elegans RME-1. Ehd1-null mice have features of muscular dystrophy and also developmental abnormalities (Posey et al., 2014; Rainey et al., 2010). Ehd1-null muscle has elongated T-tubules similar to what is seen in dysferlin null muscle, and this observation is consistent with the role of EHD1 regulating endocytic recycling to the plasma membrane (Posey et al., 2014; Rapaport et al., 2006). Similar to other membrane-affiliated proteins, EHD1 binds to phosphatidylinositol (PtdIns), a primarily membrane-associated lipid. EHD1 does not bind sphingolipids, cholesterol, ceramides, phosphatidylcholine (PC) or PS, suggesting a preferential interaction with and localization to the membrane (Naslavsky et al., 2007). The preferred binding of EHD1 with PtdIns was shown to be Ca2+-dependent in vitro and was mediated by the second half of the EH domain (Naslavsky et al., 2007). Additional data suggests that the orientation of the EHD protein allows for simultaneous binding of both NPF motif-binding partners and PtdIns lipids. This dual binding would facilitate the involvement of EHDs in both endocytic recycling of membrane and membrane repair.
To investigate EHD1’s role in membrane repair, Marg et al. analyzed EHD1 localization after laser damage in cultured human myotubes (Marg et al., 2012). EHD1 did not traffic to the site of damage and instead remained in the cytoplasm. However, these data do not negate a role for EHD1 in repair. EHD1 is expressed in muscle during differentiation and localizes to the T-tubule in skeletal muscle similar to dysferlin (Posey et al., 2011). Cultured human myotubes do not form mature T-tubule structures and therefore the lack of EHD1 translocation in cultured myotubes may indicate that EHD1 requires its T-tubule location in order to participate in membrane repair. Unlike EHD1, EHD2 is expressed early in development, potentially before the formation of T-tubules and is downregulated as differentiation proceeds in a pattern similar to myoferlin. In cultured human cultured myotubes EHD2 was observed to translocate to the site of damage, indicating that EHD2 is not dependent on a T-tubule-dependent trafficking mechanism (Marg et al., 2012).
EHD3 and EHD4
EHD3 and EHD4 are highly homologous to EHD1 and EHD2, although their roles in skeletal muscle membrane repair have not been extensively tested. EHD4 expression during muscle differentiation shares the same pattern as dysferlin with low-level expression in the singly-nucleated myoblast and increasing levels of expression as myoblast differentiate into multinucleated myotubes (Posey et al., 2011). Gudmundsson et al. showed that in rat and mouse models of heart failure EHD3 expression levels were upregulated in the left ventricle compared to non-ischemic controls suggesting a role for EHD3 in cardiac repair (Gudmundsson et al., 2012). Additionally, upregulation of EHD3 was shown in human heart failure samples validating these studies. Due to the high homology of EHD3 and EHD4 to other EHD proteins further analysis of these proteins during membrane repair is needed.
GRAF1
GTPase Regulator Associated with Focal adhesion kinase-1 (GRAF1) is a protein that contains an amino-terminal BAR domain; BAR domains (named for their presence in Bin1, amphyphisin and Rvs) are implicated in membrane bending (Lundmark et al., 2008). In addition, Graf1 contains a carboxy-terminal pleckstrin homology (PH)-domain that binds PIP2 and PS, and a carboxy-terminal src homology (SH3) domain. GRAF1 interacts with dynamin suggesting a role for GRAF1 in membrane remodeling and scission, similar to EHD1’s interaction with the BAR domain containing protein amphyphisin 2/Bin1 protein (Posey et al., 2014). Graf1 is expressed in punctate structures in fibroblasts, and it has been shown that the presence of GRAF1 can induce the formation of small intracellular tubules in vitro, a process referred to as tubulation. Unlike many other repair proteins, GRAF1 does not bind actin directly but can associate with actin through Cdc42. GRAF1 expression recapitulates that of myoferlin and EHD2, in that expression decreases with differentiation and is upregulated during muscle regeneration (Doherty et al., 2005; Lenhart et al., 2014). A GRAF1 null mouse was found to have defects in myoblast fusion and decreased myofiber size, similar to the phenotype observed in myoferlin deficient mice (Doherty et al., 2005; Lenhart et al., 2014). Consistent with this co-expression, Lenhart showed GRAF1 associated with the ferlin proteins myoferlin and Fer1L5 during muscle differentiation at pre-fusion complexes along with the EHD1 and EHD2. The interaction of GRAF1 with ferlin and EHD members suggests that GRAF1 is a member of the repair complex.
MG53 (Trim72)
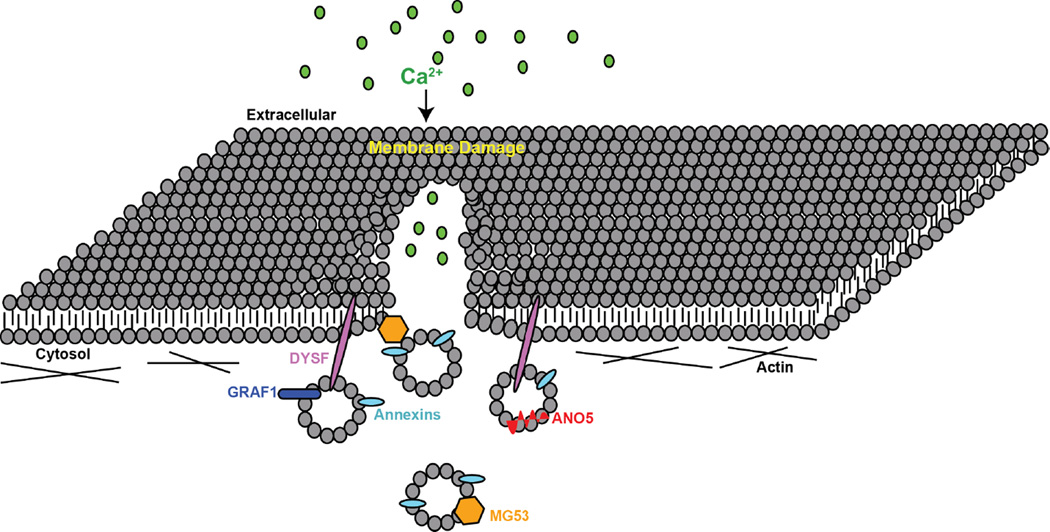
Mitsugumin 53, MG53, also known as Trim72 is part of the larger family of E3 ubiquitin ligases, Tripartite Motif (TRIM) proteins (Meroni and Diez-Roux, 2005). MG53 consists of an amino-terminal TRIM domain and a carboxy-terminal SPRY domain, a common structure of most TRIM family proteins. RNA analysis revealed MG53 is highly expressed in striated muscle and to a lower extent in lung and kidney epithelia (Cai et al., 2009a; Duann et al., 2015; Jia et al., 2014). Because of its high expression in skeletal muscle, MG53 was investigated as a potential mediator of membrane repair at the site of injury (Cai et al., 2009a). MG53-null mice develop progressive myopathy consistent with decreased fiber repair. MG53-defiecient muscle fibers exhibit decreased repair after laser-induced damage (Cai et al., 2009a). Mechanical electrode studies in both lung and kidney epithelia were used to show that MG53 translocates to the site of membrane disruption, indicative of a role in membrane repair in multiple cell types (Duann et al., 2015; Jia et al., 2014). MG53 preferentially binds phosphatidylserine (PS) at the site of damage, similar to the annexins (Cai et al., 2009a). Mechanical damage of the muscle cell line C2C12 resulted in MG53 translocation to the site of damage. MG53 was also found to colocalize with annexin A5, a surrogate marker of PS and another protein involved in membrane repair (Cai et al., 2009b). MG53 also interacts with dysferlin through its the C2A domain in a Ca2+-sensitive manner (Flix et al., 2013; Matsuda et al., 2012). Upon membrane damage, dysferlin was found to travel to the site of injury, followed by MG53. MG53 contains several cysteine residues that upon reduction, prevent MG53 oligomerization and the capacity to facilitate membrane repair (Cai et al., 2009a). Mutations in MG53 that inhibit MG53-oligimerization inhibited dysferlin localization at the sarcolemma (Matsuda et al., 2012). This suggests that MG53 acts in conjunction with other proteins, including dysferlin and annexins, in a larger repair complex to facilitate membrane repair (FIGURE 3).
Figure 3. Model for plasma membrane repair.
A membrane lesion is formed within the sarcolemma creating a micro-hole within the membrane. The influx of calcium triggers annexin A6 (A6) localization to the repair cap. Other repair complex proteins including annexin A1, A2, A5, dysferlin (DYSF) and MG53 are recruited to the lesion aiding in the re-sealing of the sarcolemma.
Therapies used to promote repair
Maintaining the integrity of membranes is critical in the prevention of disease. The sarcolemma membrane of muscle is a unique model to understand membrane repair processes. The sarcolemma is well-organized, and its primary components are well-studied. The sarcolemma is also under heavy mechanical load compared to other tissues providing a “testing ground” to better understand the repair process as muscle membranes are continually damaged and repaired in the course of normal activity. In the muscular dystrophies and myopathies, this process is often perturbed. In some forms of muscle disease, damage is accelerated, outstripping repair. In other forms of disease, repair is defective. Cells and animal models of these genetic defects provide unique avenues to study the repair process and, in turn, membrane repair treatments. Many other tissue types including heart and lung, also rely on intact membranes to prevent disease. Thus, methods and or reagents that increase the efficiency of membrane repair across multiple tissue types could potential impact multiple disease processes.
MG53
Extensive work by Weisleder and colleagues has shown the TRIM family member, MG53 is useful after multiple forms of injury to many different cell types. In muscle fibers, addition of recombinant MG53, rhMG53, to the exterior of the cell localizes to the site of injury, preventing the influx of impermeable dye normally seen in damaged fibers (Weisleder et al., 2012). It is hypothesized that MG53 acts as a molecular “band-aid” on injured cells allowing for the recovery of a membrane that otherwise is unable to repair. These findings are reminiscent was of what was seen when purified annexin A5 was introduced into cellular models of injury (Bouter et al., 2011). The observation that MG53 co-localizes with annexin A5 supports that this complex can perhaps be assembled by adding a number of its protein components.
Recombinant MG53 was also tested in vivo in the mdx animal model (Weisleder et al., 2012). Initial short-term in vivo studies showed that the addition of rhMG53 improved the membrane integrity of the myofibers preventing dye uptake into the muscle after eccentric running in the mdx mouse model. rhMG53 also reduced areas of myonecrosis within these animals suggesting rhMG53 stabilizes the membrane in vivo (Weisleder et al., 2012). Similar protective effects are seen in heart, lung, and kidney cells treated with rhMG53 or adeno-associated virus AAV-MG53 (Duann et al., 2015; He et al., 2012; Jia et al., 2014; Liu et al., 2015). These data are consistent with the idea that MG53 contributes to normal membrane repair and improves the reparative capacity in multiple organs in vitro and in vivo The results from long-term rhMG53 studies will prove useful to determine if MG53 repair can maintain improved membrane integrity after cyclic rounds of cellular damage.
Poloxamer 188 (P188)
Poloxamers are non-ionic copolymers composed of a hydrophilic poly (ethylene oxide) (PEO) block and a hydrophobic poly (propylene oxide) (PPO) block (Marks et al., 2001). Poloxamer 188 also known as pluronic F68, flocor, or rheothRx and one of the most commonly used copolymers. P188 is used in surfactants and emulsifying agents, and has been supported as an in vivo for therapies improving the membrane instability that occurs in the absence of dystrophin (Yasuda et al., 2005). In humans, P188 has a half-life of 18 hours and is nontoxic. Using X-ray diffraction of a lipid model in vitro, it was shown P188 likely forces lipid compaction restoring the membrane barrier through insertion into the lipid bilayer (Maskarinec et al., 2002). It has been shown that P188 can induce sealing of damaged membranes in vivo following electrical injury, which creates micro-holes within the membrane, a process termed electroporation now utilized in experimentation for plasmid DNA uptake (Weaver, 1995). Electrical burns produce extensive local trauma to the skin and skeletal muscle, and although this is not a disease in itself, produces injury similar to epidermal and dystrophic diseases. Addition of P188 prevented fluorescent dextran from entering damaged muscle fibers as well as reduced post-trauma inflammatory infiltration (Lee et al., 1992).
Most notably, P188 stabilizes membrane in animal models with Duchene Muscular Dystrophy (DMD) related cardiomyopathy (Townsend et al., 2010; Yasuda et al., 2005). Addition of P188 was able to stabilize these membranes preventing the continual rounds of degeneration from occurring (Spurney et al., 2011). However, the effects of P188 on skeletal muscle have not been studied as intensely. Terry and colleagues found the administration of P188 through intraperitoneal injection in mdx mice resulted improved histopathology; however P188 was not able to prevent damage in response to eccentric contraction suggesting that P188 may be able to only partially protect against membrane damage (Terry et al., 2014). Evaluating how P188 induces protein relocalization may provide more insight into the mechanism by which P188 exerts its effect. Understanding if P188 only improves membrane stability or whether it also recruits repair proteins or alters phospholipid composition may improve the therapeutic indications for this and related compounds.
Myoferlin
Due to the high level of expression of myoferlin during growth and regeneration, myoferlin is a prime candidate for improving defects in membrane repair. Transgenic mice overexpressing myoferlin under the broadly expressed chicken beta-actin (CAG) promoter were used to examine the effect of myoferlin (Lostal et al., 2012). These mice express myoferlin mRNA at 200-fold greater levels than wildtype controls at 6-months, but only express myoferlin protein 4-fold higher at 3-weeks of age when myoferlin is expressed during the growth phase and 100-fold higher than controls at 4-months when myoferlin is downregulated in adulthood (Lostal et al., 2012). Myoferlin transgenic mice were crossed to dysferlin-null mice to determine if myoferlin overexpression could rescue in this model. Laser repair assays on myoferlin-TG+ myofibers showed that the increased expression of myoferlin in dysferlin-null muscle was sufficient to improve repair to wildtype levels as measured by FM dye influx. Despite a correction in the membrane repair assay, a lack of histological improvement was noted, and the authors suggested that myoferlin and dysferlin have different role during the process of muscle membrane repair. However because overexpression of membrane-associated proteins can exert stress on many cell types, including muscle, the presence of histopathology from myoferlin overexpression should be cautiously interpreted. Mice lacking both myoferlin and dysferlin do display a significantly enhanced muscular dystrophy phenotype suggesting that both these proteins are implicated in pathogenesis (Demonbreun et al., 2014). The dysferlin/myoferlin double null model developed a more progressive dystrophy with more severe T-tubule defects than that of either single mutant. Given that ferlin proteins regulate membrane trafficking beyond membrane repair with complex roles at the T-tubule, further studies are required to better understand the role of myoferlin in muscle repair.
Conclusions
The available genetic models in humans, mice and other genetic systems have produced a better understanding of the membrane repair complex. Notably, many of these proteins are important for muscle membrane repair, and this likely relates to the physiological roles of skeletal muscle in producing contraction and the manner under which muscle faces stress and strain. The patterns of membrane repair are also seen in nonmuscle cell types, suggesting that the basic molecular machinery is shared in many different types of cells and organisms. The process of membrane repair is critical to cell and organism survival and requires a complex orchestration of Ca2+ and phospholipid signaling, as well as the aggregation of protein-repair complexes. A better understanding of this process, including approaches to improving repair, remains central to health and well-being.
Acknowledgments
This work was supported by National Institutes of Health NS047726, NS072027, AR052646.
Abbreviations
- EHD
Eps15 Homology Domain
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- CK
Creatine Kinase
REFERENCES
- Achanzar WE, Ward S. A nematode gene required for sperm vesicle fusion. Journal of cell science. 1997;110(Pt 9):1073–1081. doi: 10.1242/jcs.110.9.1073. [DOI] [PubMed] [Google Scholar]
- Babbin BA, Laukoetter MG, Nava P, Koch S, Lee WY, Capaldo CT, Peatman E, Severson EA, Flower RJ, Perretti M, Parkos CA, Nusrat A. J Immunol. 2008;181:5035–5044. doi: 10.4049/jimmunol.181.7.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiychuk EB, Monastyrskaya K, Potez S, Draeger A. Blebbing confers resistance against cell lysis. Cell Death Differ. 2011;18:80–89. doi: 10.1038/cdd.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal D, Campbell KP. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 2004;14:206–213. doi: 10.1016/j.tcb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno MR, Moreira Ede S, Zatz M, Beckmann JS, Bushby K. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20:37–42. doi: 10.1038/1689. [DOI] [PubMed] [Google Scholar]
- Bement WM, Mandato CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Current biology : CB. 1999;9:579–587. doi: 10.1016/s0960-9822(99)80261-9. [DOI] [PubMed] [Google Scholar]
- Benz J, Bergner A, Hofmann A, Demange P, Gottig P, Liemann S, Huber R, Voges D. The structure of recombinant human annexin VI in crystals and membrane-bound. Journal of molecular biology. 1996;260:638–643. doi: 10.1006/jmbi.1996.0426. [DOI] [PubMed] [Google Scholar]
- Bittner RE, Anderson LV, Burkhardt E, Bashir R, Vafiadaki E, Ivanova S, Raffelsberger T, Maerk I, Hoger H, Jung M, Karbasiyan M, Storch M, Lassmann H, Moss JA, Davison K, Harrison R, Bushby KM, Reis A. Dysferlin deletion in SJL mice (SJL-Dysf) defines a natural model for limb girdle muscular dystrophy 2B. Nat Genet. 1999;23:141–142. doi: 10.1038/13770. [DOI] [PubMed] [Google Scholar]
- Blackwood RA, Ernst JD. Characterization of Ca2(+)-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. The Biochemical journal. 1990;266:195–200. doi: 10.1042/bj2660195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc V, Marlow G, Boycott KM, Saleki K, Inoue H, Kroon J, Itakura M, Robitaille Y, Parent L, Baas F, Mizuta K, Kamata N, Richard I, Linssen WH, Mahjneh I, de Visser M, Bashir R, Brais B. Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am J Hum Genet. 2010;86:213–221. doi: 10.1016/j.ajhg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouter A, Gounou C, Berat R, Tan S, Gallois B, Granier T, d'Estaintot BL, Poschl E, Brachvogel B, Brisson AR. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nature communications. 2011;2:270. doi: 10.1038/ncomms1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhynskyy N, Golczak M, Lai-Kee-Him J, Lambert O, Tessier B, Gounou C, Berat R, Simon A, Granier T, Chevalier JM, Mazeres S, Bandorowicz-Pikula J, Pikula S, Brisson AR. Annexin-A6 presents two modes of association with phospholipid membranes. A combined QCM-D, AFM and cryo-TEM study. Journal of structural biology. 2009;168:107–116. doi: 10.1016/j.jsb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Cagliani R, Magri F, Toscano A, Merlini L, Fortunato F, Lamperti C, Rodolico C, Prelle A, Sironi M, Aguennouz M, Ciscato P, Uncini A, Moggio M, Bresolin N, Comi GP. Mutation finding in patients with dysferlin deficiency and role of the dysferlin interacting proteins annexin A1 and A2 in muscular dystrophies. Hum Mutat. 2005;26:283. doi: 10.1002/humu.9364. [DOI] [PubMed] [Google Scholar]
- Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009a;11:56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. The Journal of biological chemistry. 2009b;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeille R, Degrelle SA, Plawinski L, Bouvet F, Gounou C, Evain-Brion D, Brisson AR, Bouter A. Annexin-A5 promotes membrane resealing in human trophoblasts. Biochimica et biophysica acta. 2015 doi: 10.1016/j.bbamcr.2014.12.038. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. The Journal of cell biology. 2003;162:543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christmas P, Callaway J, Fallon J, Jones J, Haigler HT. Selective secretion of annexin 1, a protein without a signal sequence, by the human prostate gland. The Journal of biological chemistry. 1991;266:2499–2507. [PubMed] [Google Scholar]
- Clark AG, Miller AL, Vaughan E, Yu HY, Penkert R, Bement WM. Integration of single and multicellular wound responses. Current biology : CB. 2009;19:1389–1395. doi: 10.1016/j.cub.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin B. Dystrophin complex functions as a scaffold for signalling proteins. Biochimica et biophysica acta. 2014;1838:635–642. doi: 10.1016/j.bbamem.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Corrotte M, Almeida PE, Tam C, Castro-Gomes T, Fernandes MC, Millis BA, Cortez M, Miller H, Song W, Maugel TK, Andrews NW. Caveolae internalization repairs wounded cells and muscle fibers. eLife. 2013;2:e00926. doi: 10.7554/eLife.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DB, Doherty KR, Delmonte AJ, McNally EM. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. The Journal of biological chemistry. 2002;277:22883–22888. doi: 10.1074/jbc.M201858200. [DOI] [PubMed] [Google Scholar]
- de Laat B, Derksen RH, Mackie IJ, Roest M, Schoormans S, Woodhams BJ, de Groot PG, van Heerde WL. Annexin A5 polymorphism (-1C-->T) and the presence of anti-annexin A5 antibodies in the antiphospholipid syndrome. Annals of the rheumatic diseases. 2006;65:1468–1472. doi: 10.1136/ard.2005.045237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonbreun AR, Fahrenbach JP, Deveaux K, Earley JU, Pytel P, McNally EM. Impaired muscle growth and response to insulin-like growth factor 1 in dysferlin-mediated muscular dystrophy. Hum Mol Genet. 2010a doi: 10.1093/hmg/ddq522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonbreun AR, Lapidos KA, Heretis K, Levin S, Dale R, Pytel P, Svensson EC, McNally EM. Myoferlin regulation by NFAT in muscle injury, regeneration and repair. Journal of cell science. 2010b;123:2413–2422. doi: 10.1242/jcs.065375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonbreun AR, Posey AD, Heretis K, Swaggart KA, Earley JU, Pytel P, McNally EM. Myoferlin is required for insulin-like growth factor response and muscle growth. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010c;24:1284–1295. doi: 10.1096/fj.09-136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonbreun AR, Rossi AE, Alvarez MG, Swanson KE, Deveaux HK, Earley JU, Hadhazy M, Vohra R, Walter GA, Pytel P, McNally EM. Dysferlin and myoferlin regulate transverse tubule formation and glycerol sensitivity. The American journal of pathology. 2014;184:248–259. doi: 10.1016/j.ajpath.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. The Journal of biological chemistry. 2004;279:43411–43418. doi: 10.1074/jbc.M408078200. [DOI] [PubMed] [Google Scholar]
- DiFranco M, Quinonez M, Capote J, Vergara J. DNA transfection of mammalian skeletal muscles using in vivo electroporation. J Vis Exp: (JoVE) 2009 doi: 10.3791/1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JT, Lenhart KC, Cameron MV, Mack CP, Conlon FL, Taylor JM. Skeletal muscle differentiation and fusion are regulated by the BAR-containing Rho-GTPase-activating protein (Rho-GAP), GRAF1. The Journal of biological chemistry. 2011;286:25903–25921. doi: 10.1074/jbc.M111.243030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty KR, Cave A, Davis DB, Delmonte AJ, Posey A, Earley JU, Hadhazy M, McNally EM. Normal myoblast fusion requires myoferlin. Development. 2005;132:5565–5575. doi: 10.1242/dev.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty KR, Demonbreun AR, Wallace GQ, Cave A, Posey AD, Heretis K, Pytel P, McNally EM. The Endocytic Recycling Protein EHD2 Interacts with Myoferlin to Regulate Myoblast Fusion. The Journal of biological chemistry. 2008;283:20252–20260. doi: 10.1074/jbc.M802306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann P, Li H, Lin P, Tan T, Wang Z, Chen K, Zhou X, Gumpper K, Zhu H, Ludwig T, Mohler PJ, Rovin B, Abraham WT, Zeng C, Ma J. MG53-mediated cell membrane repair protects against acute kidney injury. Science translational medicine. 2015;7:279–236. doi: 10.1126/scitranslmed.3010755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flix B, de la Torre C, Castillo J, Casal C, Illa I, Gallardo E. Dysferlin interacts with calsequestrin-1, myomesin-2 and dynein in human skeletal muscle. The international journal of biochemistry & cell biology. 2013;45:1927–1938. doi: 10.1016/j.biocel.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Flucher BE. Structural analysis of muscle development: transverse tubules, sarcoplasmic reticulum, and the triad. Developmental biology. 1992;154:245–260. doi: 10.1016/0012-1606(92)90065-o. [DOI] [PubMed] [Google Scholar]
- Fukada S, Morikawa D, Yamamoto Y, Yoshida T, Sumie N, Yamaguchi M, Ito T, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Yamamoto H. Genetic background affects properties of satellite cells and mdx phenotypes. The American journal of pathology. 2010;176:2414–2424. doi: 10.2353/ajpath.2010.090887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M, Ying G, Rainey MA, Solomon A, Parikh PT, Gao Q, Band V, Band H. Shared as well as distinct roles of EHD proteins revealed by biochemical and functional comparisons in mammalian cells and C. elegans. BMC Cell Biol. 2007;8:3. doi: 10.1186/1471-2121-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins: from structure to function. Physiological reviews. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Goulet F, Moore KG, Sartorelli AC. Glycosylation of annexin I and annexin II. Biochemical and biophysical research communications. 1992;188:554–558. doi: 10.1016/0006-291x(92)91091-4. [DOI] [PubMed] [Google Scholar]
- Grant BD, Caplan S. Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic. 2008;9:2043–2052. doi: 10.1111/j.1600-0854.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson H, Curran J, Kashef F, Snyder JS, Smith SA, Vargas-Pinto P, Bonilla IM, Weiss RM, Anderson ME, Binkley P, Felder RB, Carnes CA, Band H, Hund TJ, Mohler PJ. Differential regulation of EHD3 in human and mammalian heart failure. Journal of molecular and cellular cardiology. 2012;52:1183–1190. doi: 10.1016/j.yjmcc.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunteski-Hamblin AM, Song G, Walsh RA, Frenzke M, Boivin GP, Dorn GW, 2nd, Kaetzel MA, Horseman ND, Dedman JR. Annexin VI overexpression targeted to heart alters cardiomyocyte function in transgenic mice. The American journal of physiology. 1996;270:H1091–H1100. doi: 10.1152/ajpheart.1996.270.3.H1091. [DOI] [PubMed] [Google Scholar]
- Hack AA, Lam MY, Cordier L, Shoturma DI, Ly CT, Hadhazy MA, Hadhazy MR, Sweeney HL, McNally EM. Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex. Journal of cell science. 2000;113(Pt 14):2535–2544. doi: 10.1242/jcs.113.14.2535. [DOI] [PubMed] [Google Scholar]
- Hannon R, Croxtall JD, Getting SJ, Roviezzo F, Yona S, Paul-Clark MJ, Gavins FN, Perretti M, Morris JF, Buckingham JC, Flower RJ. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- Hawkins TE, Roes J, Rees D, Monkhouse J, Moss SE. Immunological development and cardiovascular function are normal in annexin VI null mutant mice. Molecular and cellular biology. 1999;19:8028–8032. doi: 10.1128/mcb.19.12.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MJ, Merrifield CJ, Shao D, Ayala-Sanmartin J, Schorey CD, Levine TP, Proust J, Curran J, Bailly M, Moss SE. Annexin 2 binding to phosphatidylinositol 4,5-bisphosphate on endocytic vesicles is regulated by the stress response pathway. The Journal of biological chemistry. 2004;279:14157–14164. doi: 10.1074/jbc.M313025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MJ, Shao D, Bailly M, Moss SE. Regulation of actin dynamics by annexin 2. The EMBO journal. 2006;25:1816–1826. doi: 10.1038/sj.emboj.7601078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Tang RH, Weisleder N, Xiao B, Yuan Z, Cai C, Zhu H, Lin P, Qiao C, Li J, Mayer C, Li J, Ma J, Xiao X. Enhancing muscle membrane repair by gene delivery of MG53 ameliorates muscular dystrophy and heart failure in delta-Sarcoglycan-deficient hamsters. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:727–735. doi: 10.1038/mt.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydemann A, Huber JM, Demonbreun A, Hadhazy M, McNally EM. Genetic background influences muscular dystrophy. Neuromuscular disorders : NMD. 2005;15:601–609. doi: 10.1016/j.nmd.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Ho M, Post CM, Donahue LR, Lidov HG, Bronson RT, Goolsby H, Watkins SC, Cox GA, Brown RH., Jr Disruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum Mol Genet. 2004;13:1999–2010. doi: 10.1093/hmg/ddh212. [DOI] [PubMed] [Google Scholar]
- Ikezoe K, Furuya H, Ohyagi Y, Osoegawa M, Nishino I, Nonaka I, Kira J. Dysferlin expression in tubular aggregates: their possible relationship to endoplasmic reticulum stress. Acta neuropathologica. 2003;105:603–609. doi: 10.1007/s00401-003-0686-1. [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Lauritzen SP, Scheffer L, Sakaguchi M, Bunkenborg J, Simon SM, Kallunki T, Jaattela M, Nylandsted J. S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nature communications. 2014;5:3795. doi: 10.1038/ncomms4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Chen K, Lin P, Lieber G, Nishi M, Yan R, Wang Z, Yao Y, Li Y, Whitson BA, Duann P, Li H, Zhou X, Zhu H, Takeshima H, Hunter JC, McLeod RL, Weisleder N, Zeng C, Ma J. Treatment of acute lung injury by targeting MG53-mediated cell membrane repair. Nature communications. 2014;5:4387. doi: 10.1038/ncomms5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel MA, Mo YD, Mealy TR, Campos B, Bergsma-Schutter W, Brisson A, Dedman JR, Seaton BA. Phosphorylation mutants elucidate the mechanism of annexin IV-mediated membrane aggregation. Biochemistry. 2001;40:4192–4199. doi: 10.1021/bi002507s. [DOI] [PubMed] [Google Scholar]
- Kamal A, Ying Y, Anderson RG. Annexin VI-mediated loss of spectrin during coated pit budding is coupled to delivery of LDL to lysosomes. The Journal of cell biology. 1998;142:937–947. doi: 10.1083/jcb.142.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JP, Ziman AP, Mueller AL, Muriel JM, Kleinhans-Welte E, Gumerson JD, Vogel SS, Ward CW, Roche JA, Bloch RJ. Dysferlin stabilizes stress-induced Ca2+ signaling in the transverse tubule membrane. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20831–20836. doi: 10.1073/pnas.1307960110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge L, Harris J, Sewry C, Charlton R, Anderson L, Laval S, Chiu YH, Hornsey M, Straub V, Barresi R, Lochmuller H, Bushby K. Dysferlin associates with the developing T-tubule system in rodent and human skeletal muscle. Muscle Nerve. 2010;41:166–173. doi: 10.1002/mus.21166. [DOI] [PubMed] [Google Scholar]
- Kuru S, Yasuma F, Wakayama T, Kimura S, Konagaya M, Aoki M, Tanabe M, Takahashi T. [A patient with limb girdle muscular dystrophy type 2B (LGMD2B) manifesting cardiomyopathy] Rinsho shinkeigaku = Clinical neurology. 2004;44:375–378. [PubMed] [Google Scholar]
- Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- Lee RC, River LP, Pan FS, Ji L, Wollmann RL. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4524–4528. doi: 10.1073/pnas.89.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek A, Evesson FJ, Sutton RB, North KN, Cooper ST. Ferlins: regulators of vesicle fusion for auditory neurotransmission, receptor trafficking and membrane repair. Traffic. 2012;13:185–194. doi: 10.1111/j.1600-0854.2011.01267.x. [DOI] [PubMed] [Google Scholar]
- Lenhart KC, Becherer AL, Li J, Xiao X, McNally EM, Mack CP, Taylor JM. GRAF1 promotes ferlin-dependent myoblast fusion. Developmental biology. 2014;393:298–311. doi: 10.1016/j.ydbio.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., Jr Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. The Journal of biological chemistry. 2003 doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- Leung C, Yu C, Lin MI, Tognon C, Bernatchez P. Expression of myoferlin in human and murine carcinoma tumors: role in membrane repair, cell proliferation, and tumorigenesis. The American journal of pathology. 2013;182:1900–1909. doi: 10.1016/j.ajpath.2013.01.041. [DOI] [PubMed] [Google Scholar]
- Ling Q, Jacovina AT, Deora A, Febbraio M, Simantov R, Silverstein RL, Hempstead B, Mark WH, Hajjar KA. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. The Journal of clinical investigation. 2004;113:38–48. doi: 10.1172/JCI200419684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, Serrano C, Urtizberea JA, Hentati F, Hamida MB, Bohlega S, Culper EJ, Amato AA, Bossie K, Oeltjen J, Bejaoui K, McKenna-Yasek D, Hosler BA, Schurr E, Arahata K, de Jong PJ, Brown RH., Jr Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhu H, Zheng Y, Xu Z, Li L, Tan T, Park KH, Hou J, Zhang C, Li D, Li R, Liu Z, Weisleder N, Zhu D, Lin P, Ma J. Cardioprotection of recombinant human MG53 protein in a porcine model of ischemia and reperfusion injury. Journal of molecular and cellular cardiology. 2015;80:10–19. doi: 10.1016/j.yjmcc.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lostal W, Bartoli M, Bourg N, Roudaut C, Bentaib A, Miyake K, Guerchet N, Fougerousse F, McNeil P, Richard I. Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer. Hum Mol Genet. 2010;19:1897–1907. doi: 10.1093/hmg/ddq065. [DOI] [PubMed] [Google Scholar]
- Lostal W, Bartoli M, Roudaut C, Bourg N, Krahn M, Pryadkina M, Borel P, Suel L, Roche JA, Stockholm D, Bloch RJ, Levy N, Bashir R, Richard I. Lack of correlation between outcomes of membrane repair assay and correction of dystrophic changes in experimental therapeutic strategy in dysferlinopathy. PloS one. 2012;7:e38036. doi: 10.1371/journal.pone.0038036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark R, Doherty GJ, Howes MT, Cortese K, Vallis Y, Parton RG, McMahon HT. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Current biology : CB. 2008;18:1802–1808. doi: 10.1016/j.cub.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjneh I, Jaiswal J, Lamminen A, Somer M, Marlow G, Kiuru-Enari S, Bashir R. A new distal myopathy with mutation in anoctamin 5. Neuromuscular disorders : NMD. 2010;20:791–795. doi: 10.1016/j.nmd.2010.07.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi C, Brunamonti Binello P, Badiali G, Caci E, Cusano R, Garibaldi J, Pippucci T, Merlini A, Marchetti C, Rhoden KJ, Galietta LJ, Lalatta F, Balbi P, Seri M. A novel missense mutation in ANO5/TMEM16E is causative for gnathodiaphyseal dyplasia in a large Italian pedigree. European journal of human genetics : EJHG. 2013;21:613–619. doi: 10.1038/ejhg.2012.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marg A, Schoewel V, Timmel T, Schulze A, Shah C, Daumke O, Spuler S. Sarcolemmal repair is a slow process and includes EHD2. Traffic. 2012;13:1286–1294. doi: 10.1111/j.1600-0854.2012.01386.x. [DOI] [PubMed] [Google Scholar]
- Marks JD, Pan CY, Bushell T, Cromie W, Lee RC. Amphiphilic, tri-block copolymers provide potent membrane-targeted neuroprotection. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:1107–1109. doi: 10.1096/fj.00-0547fje. [DOI] [PubMed] [Google Scholar]
- Maskarinec SA, Hannig J, Lee RC, Lee KY. Direct observation of poloxamer 188 insertion into lipid monolayers. Biophysical journal. 2002;82:1453–1459. doi: 10.1016/S0006-3495(02)75499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda C, Miyake K, Kameyama K, Keduka E, Takeshima H, Imamura T, Araki N, Nishino I, Hayashi Y. The C2A domain in dysferlin is important for association with MG53 (TRIM72) PLoS currents. 2012;4 doi: 10.1371/5035add8caff4. e5035add5038caff5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade JR, Archambeau A, Michele DE. Rapid actin-cytoskeleton-dependent recruitment of plasma membrane-derived dysferlin at wounds is critical for muscle membrane repair. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:3660–3670. doi: 10.1096/fj.14-250191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil AK, Rescher U, Gerke V, McNeil PL. J Biol Chem. 2006;281:35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. The American journal of pathology. 1992;140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA. Loss, restoration, and maintenance of plasma membrane integrity. The Journal of cell biology. 1997;137:1–4. doi: 10.1083/jcb.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of 'single protein RING finger' E3 ubiquitin ligases. BioEssays : news and reviews in molecular, cellular and developmental biology. 2005;27:1147–1157. doi: 10.1002/bies.20304. [DOI] [PubMed] [Google Scholar]
- Millay DP, Maillet M, Roche JA, Sargent MA, McNally EM, Bloch RJ, Molkentin JD. Genetic manipulation of dysferlin expression in skeletal muscle: novel insights into muscular dystrophy. The American journal of pathology. 2009;175:1817–1823. doi: 10.2353/ajpath.2009.090107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta K, Tsutsumi S, Inoue H, Sakamoto Y, Miyatake K, Miyawaki K, Noji S, Kamata N, Itakura M. Molecular characterization of GDD1/TMEM16E, the gene product responsible for autosomal dominant gnathodiaphyseal dysplasia. Biochemical and biophysical research communications. 2007;357:126–132. doi: 10.1016/j.bbrc.2007.03.108. [DOI] [PubMed] [Google Scholar]
- Monjaret F, Suel-Petat L, Bourg-Alibert N, Vihola A, Marchand S, Roudaut C, Gicquel E, Udd B, Richard I, Charton K. The phenotype of dysferlin-deficient mice is not rescued by adeno-associated virus-mediated transfer of anoctamin 5. Human gene therapy. Clinical development. 2013;24:65–76. doi: 10.1089/humc.2012.217. [DOI] [PubMed] [Google Scholar]
- Mosser G, Ravanat C, Freyssinet JM, Brisson A. Sub-domain structure of lipid-bound annexin-V resolved by electron image analysis. Journal of molecular biology. 1991;217:241–245. doi: 10.1016/0022-2836(91)90538-h. [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol. 2010 doi: 10.1016/j.tcb.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, Rahajeng J, Chenavas S, Sorgen PL, Caplan S. EHD1 and Eps15 interact with phosphatidylinositols via their Eps15 homology domains. The Journal of biological chemistry. 2007;282:16612–16622. doi: 10.1074/jbc.M609493200. [DOI] [PubMed] [Google Scholar]
- Nguyen K, Bassez G, Krahn M, Bernard R, Laforet P, Labelle V, Urtizberea JA, Figarella-Branger D, Romero N, Attarian S, Leturcq F, Pouget J, Levy N, Eymard B. Phenotypic study in 40 patients with dysferlin gene mutations: high frequency of atypical phenotypes. Arch Neurol. 2007;64:1176–1182. doi: 10.1001/archneur.64.8.1176. [DOI] [PubMed] [Google Scholar]
- Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. The Journal of biological chemistry. 2009;284:28698–28703. doi: 10.1074/jbc.M109.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoluzi S, Castagnoli L, Lauro I, Salcini AE, Coda L, Fre S, Confalonieri S, Pelicci PG, Di Fiore PP, Cesareni G. Recognition specificity of individual EH domains of mammals and yeast. The EMBO journal. 1998;17:6541–6550. doi: 10.1093/emboj/17.22.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins) Physiological reviews. 2014;94:419–459. doi: 10.1152/physrev.00039.2011. [DOI] [PubMed] [Google Scholar]
- Piccolo F, Moore SA, Ford GC, Campbell KP. Intracellular accumulation and reduced sarcolemmal expression of dysferlin in limb--girdle muscular dystrophies. Annals of neurology. 2000;48:902–912. [PubMed] [Google Scholar]
- Pohl U, Smith JS, Tachibana I, Ueki K, Lee HK, Ramaswamy S, Wu Q, Mohrenweiser HW, Jenkins RB, Louis DN. EHD2, EHD3, and EHD4 encode novel members of a highly conserved family of EH domain-containing proteins. Genomics. 2000;63:255–262. doi: 10.1006/geno.1999.6087. [DOI] [PubMed] [Google Scholar]
- Porter KR, Palade GE. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. The Journal of biophysical and biochemical cytology. 1957;3:269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey AD, Jr, Pytel P, Gardikiotes K, Demonbreun AR, Rainey M, George M, Band H, McNally EM. Endocytic recycling proteins EHD1 and EHD2 interact with fer-1-like-5 (Fer1L5) and mediate myoblast fusion. The Journal of biological chemistry. 2011;286:7379–7388. doi: 10.1074/jbc.M110.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey AD, Jr, Swanson KE, Alvarez MG, Krishnan S, Earley JU, Band H, Pytel P, McNally EM, Demonbreun AR. EHD1 mediates vesicle trafficking required for normal muscle growth and transverse tubule development. Developmental biology. 2014;387:179–190. doi: 10.1016/j.ydbio.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey MA, George M, Ying G, Akakura R, Burgess DJ, Siefker E, Bargar T, Doglio L, Crawford SE, Todd GL, Govindarajan V, Hess RA, Band V, Naramura M, Band H. The endocytic recycling regulator EHD1 is essential for spermatogenesis and male fertility in mice. BMC developmental biology. 2010;10:37. doi: 10.1186/1471-213X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D, Auerbach W, Naslavsky N, Pasmanik-Chor M, Galperin E, Fein A, Caplan S, Joyner AL, Horowitz M. Recycling to the plasma membrane is delayed in EHD1 knockout mice. Traffic. 2006;7:52–60. doi: 10.1111/j.1600-0854.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Roche JA, Ford-Speelman DL, Ru LW, Densmore AL, Roche R, Reed PW, Bloch RJ. Physiological and histological changes in skeletal muscle following in vivo gene transfer by electroporation. American journal of physiology. Cell physiology. 2011;301:C1239–C1250. doi: 10.1152/ajpcell.00431.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. The Journal of cell biology. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roostalu U, Strahle U. In vivo imaging of molecular interactions at damaged sarcolemma. Dev Cell. 2012;22:515–529. doi: 10.1016/j.devcel.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Salcini AE, Confalonieri S, Doria M, Santolini E, Tassi E, Minenkova O, Cesareni G, Pelicci PG, Di Fiore PP. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes & development. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurel O, Cezanne L, Milon A, Tocanne JF, Demange P. Influence of annexin V on the structure and dynamics of phosphatidylcholine/phosphatidylserine bilayers: a fluorescence and NMR study. Biochemistry. 1998;37:1403–1410. doi: 10.1021/bi971484n. [DOI] [PubMed] [Google Scholar]
- Skrahina T, Piljic A, Schultz C. Heterogeneity and timing of translocation and membrane-mediated assembly of different annexins. Experimental cell research. 2008;314:1039–1047. doi: 10.1016/j.yexcr.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Spurney CF, Guerron AD, Yu Q, Sali A, van der Meulen JH, Hoffman EP, Nagaraju K. Membrane sealant Poloxamer P188 protects against isoproterenol induced cardiomyopathy in dystrophin deficient mice. BMC cardiovascular disorders. 2011;11:20. doi: 10.1186/1471-2261-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AB, Shum EY, Hirsh S, Cygnar KD, Reisert J, Zhao H. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11776–11781. doi: 10.1073/pnas.0903304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. The Journal of cell biology. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Fujii T, Imao T, Ishihara K, Kuba H, Nagata S. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. The Journal of biological chemistry. 2013;288:13305–13316. doi: 10.1074/jbc.M113.457937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaggart KA, Demonbreun AR, Vo AH, Swanson KE, Kim EY, Fahrenbach JP, Holley-Cuthrell J, Eskin A, Chen Z, Squire K, Heydemann A, Palmer AA, Nelson SF, McNally EM. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6004–6009. doi: 10.1073/pnas.1324242111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RL, Kaneb HM, Wells DJ. Poloxomer 188 has a deleterious effect on dystrophic skeletal muscle function. PloS one. 2014;9:e91221. doi: 10.1371/journal.pone.0091221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrien C, Di Fulvio S, Pickles S, Sinnreich M. Characterization of lipid binding specificities of dysferlin C2 domains reveals novel interactions with phosphoinositides. Biochemistry. 2009;48:2377–2384. doi: 10.1021/bi802242r. [DOI] [PubMed] [Google Scholar]
- Tian YWJ, Cebotaru L, Wang H, Guggino WB. Anoctamin5 is Related to Plasma Membrane Repair. JSM Regen Med Bio Eng. 2015;3:6. [Google Scholar]
- Townsend D, Turner I, Yasuda S, Martindale J, Davis J, Shillingford M, Kornegay JN, Metzger JM. Chronic administration of membrane sealant prevents severe cardiac injury and ventricular dilatation in dystrophic dogs. The Journal of clinical investigation. 2010;120:1140–1150. doi: 10.1172/JCI41329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Inoue H, Sakamoto Y, Mizuta K, Kamata N, Itakura M. Molecular cloning and characterization of the murine gnathodiaphyseal dysplasia gene GDD1. Biochemical and biophysical research communications. 2005;331:1099–1106. doi: 10.1016/j.bbrc.2005.03.226. [DOI] [PubMed] [Google Scholar]
- Wallner BP, Mattaliano RJ, Hession C, Cate RL, Tizard R, Sinclair LK, Foeller C, Chow EP, Browing JL, Ramachandran KL, et al. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986;320:77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]
- Wang W, Creutz CE. Role of the amino-terminal domain in regulating interactions of annexin I with membranes: effects of amino-terminal truncation and mutagenesis of the phosphorylation sites. Biochemistry. 1994;33:275–282. doi: 10.1021/bi00167a036. [DOI] [PubMed] [Google Scholar]
- Weaver JC. Electroporation theory. Concepts and mechanisms. Methods in molecular biology. 1995;55:3–28. doi: 10.1385/0-89603-328-7:3. [DOI] [PubMed] [Google Scholar]
- Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen PJ, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao RP, Ma J. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Science translational medicine. 2012;4:139–185. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Townsend D, Michele DE, Favre EG, Day SM, Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–1029. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- Yu QC, McNeil PL. Transient disruptions of aortic endothelial cell plasma membranes. The American journal of pathology. 1992;141:1349–1360. [PMC free article] [PubMed] [Google Scholar]
- Zaks WJ, Creutz CE. Ca(2+)-dependent annexin self-association on membrane surfaces. Biochemistry. 1991;30:9607–9615. doi: 10.1021/bi00104a007. [DOI] [PubMed] [Google Scholar]