Abstract
Background: Insulin glargine 300 U/mL (Gla-300) has a more constant and prolonged action profile than insulin glargine 100 U/mL and in clinical studies is associated with similar glycemic control but less hypoglycemia. Whether its effects are altered by variability of injection time was examined in two 3-month substudies.
Materials and Methods: Eligible participants completing 6 months of optimized treatment with Gla-300 in EDITION 1 (n = 109) and EDITION 2 (n = 89), having a mean hemoglobin A1c (HbA1c) level of 7.3 % (SD 1.0 %), were randomized (1:1) to groups advised to increase variability of between-injection intervals to 24 ± up to 3 h or to maintain fixed 24-h intervals for 3 months. Changes of HbA1c level and other efficacy and safety measures were assessed.
Results: In the fixed-dosing group, 64% of participants reported all intervals within the 23–25-h range, compared with 15% of those advised flexible dosing. In the fixed- and flexible-dosing groups, 12% and 41%, respectively, of between-injection intervals were outside the 23–25-h range, and 2% and 16%, respectively, were outside the 21–27-h range. Least squares mean between-group difference in HbA1c change from baseline was 0.05 % (95% confidence interval [CI], −0.13 to 0.23); for fasting plasma glucose, 2.7 mg/dL (95% CI, −9.0 to 14.4); and for daily basal insulin dose, 0.00 U/kg (95% CI, −0.02 to 0.03). Frequencies of hypoglycemia and adverse events did not differ between groups.
Conclusions: The efficacy and safety of Gla-300 demonstrated in EDITION 1 and EDITION 2 are maintained in substudies when the insulin was injected up to 3 h before or after the usual time of administration.
Introduction
New insulin glargine 300 U/mL (Gla-300) has been shown to display more constant and prolonged pharmacokinetic and pharmacodynamic profiles compared with insulin glargine 100 U/mL (Gla-100).1 Comparisons of the clinical outcomes with Gla-300 versus Gla-100 in the EDITION 1 and EDITION 2 studies (registered with clinical trial registration numbers NCT01499082 and NCT01499095, respectively, at ClinicalTrials.gov) in people with type 2 diabetes mellitus (T2DM) have demonstrated that Gla-300 provides equivalent glycemic control to Gla-100 but with a lower risk of hypoglycemia.2,3 However, results of structured clinical studies may not always predict the results of treatments when they are applied in usual medical practice, in part because adherence to the timing of insulin injections may be more rigorous in the research setting. The pharmacokinetic and pharmacodynamic profiles of Gla-300, which extend beyond 24 h,1 may additionally allow greater flexibility in basal insulin injection time, permitting people to adapt injection times to their daily lifestyle needs.
To investigate the efficacy and safety of Gla-300 when there is greater variability in the timing of injections, two predefined, 3-month substudies were embedded within EDITION 1 and EDITION 2. Here we describe analyses of data from these two substudies, both pooled and considered separately.
Materials and Methods
Study design and participants
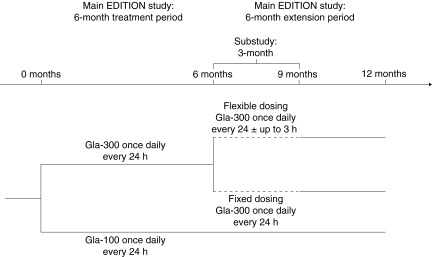
The EDITION 1 and EDITION 2 studies compared Gla-300 and Gla-100 in participants with T2DM using basal and mealtime insulin (EDITION 1) or basal insulin and oral antihyperglycemic drugs (EDITION 2).2,3 Following the main 6-month treatment period, 3-month substudies were conducted to examine the efficacy and safety of Gla-300 using flexible dosing (24 ± up to 3 h) or fixed dosing (24 h) intervals (Fig. 1). To enter the substudies the main study participants from the Gla-300 group had to agree to take part in the substudy, including the use of the flexible-dosing regimen, and to be judged by the investigator to be able to adhere to such a schedule.
FIG. 1.
EDITION 1 and EDITION 2 study design. Gla-100, insulin glargine 100 U/mL; Gla-300, insulin glargine 300 U/mL.
Interventions
Immediately after the main 6-month treatment period of EDITION 1 and EDITION 2, eligible participants previously using Gla-300 were randomized (1:1) using a remote telephone (interactive voice response system) or online system (interactive Web response system) to continue with fixed dosing or to start using a flexible-dosing regimen. Gla-300 was administered each evening using a modified SoloSTAR® (Sanofi, Paris, France) pen injector, with its dose usually adjusted weekly (and no more often than every 3 days) seeking a fasting self-measured plasma glucose of 80–100 mg/dL (4.4–5.6 mmol/L) prior to breakfast, as in the main studies.2,3 Participants documented their daily basal insulin dosage and time of administration. The reference injection times for all individuals were established at the start of the main studies. Participants in the flexible-dosing groups were instructed to inject insulin doses within 3 h earlier or later than their injection reference time (24 ± up to 3 h) and at the maximum interval (i.e., 3 h earlier or later than the reference injection time in the evening) on at least 2 days in each week. Participants in the fixed-dosing groups were instructed to continue with the injection of basal insulin doses at their reference injection time each evening, with 24 h between injections.
In the EDITION 1 substudy, mealtime insulin doses were adjusted at the discretion of the investigator. In the EDITION 2 substudy, if hemoglobin A1c (HbA1c) or fasting self-measured plasma glucose measurements were above target values and there was no reasonable explanation for inadequate glucose control, or if appropriate action failed to decrease the levels to below threshold values, rescue therapy could be considered by the investigator, based on a thorough evaluation of the participant's glycemic control. The choice of rescue medication was based on the investigator's decision and local approved guidelines. Assessment visits occurred at the baseline of each substudy (Month 6 of the main EDITION studies) and at the end of each 3-month substudy (Month 9 of the main EDITION studies). Telephone interviews were conducted at Week 1, Week 3, and Month 1.5 during each substudy.
Outcomes
The time intervals between two consecutive injections in the last 7 days before the assessments at Month 1.5 and Month 3 (Month 7.5 and Month 9, respectively, of the main EDITION studies) were analyzed. Thus, for each participant two 7-day sequences of injections were available for analysis. For purposes of analysis, dosing intervals were assigned to the following categories: 24 ± up to 1 h (within a 23–25-h interval), 24 ± 1–3 h (within a 21–23-h or within a 25–27-h interval), and 24 ± more than 3 h (beyond a 21–27-h interval). The percentage of all between-injection intervals within or outside each of these ranges was calculated. In addition, the percentage of participants with all injection intervals in the 23–25-h range was determined.
The primary efficacy end point was the change in HbA1c from substudy baseline to the end of the 3-month substudy. Secondary end points included the change in laboratory-measured (clinic-collected) fasting plasma glucose (FPG) and daily basal insulin doses. Hypoglycemia occurring between the substudy baseline and the end of the substudy was analyzed by categories defined by the American Diabetes Association4 and was classified as occurring during the night (“nocturnal”; 00:00–05:59 h) and at any time (24 h). Both percentages of participants with one or more hypoglycemic event and annualized rates (events per participant-year) were calculated. Adverse events (AEs) were recorded.
Data analysis and statistics
Outcomes were assessed separately for each substudy, as well as a pooled analysis of both. The individual substudy data were analyzed using analysis of covariance with treatment regimen and country as fixed effects and using the corresponding baseline value (at 6 months of the main study) as a covariate. A fixed-effect analysis based on the pooled data from EDITION 1 and EDITION 2 was performed for the modified intent-to-treat population (all participants who were randomized in the substudies and who, in the substudies, received at least one dose of Gla-300 and had an initial assessment and one or more subsequent assessment). For the change in HbA1c, FPG, and daily basal insulin dose from the substudy baseline to the end of the 3-month substudy, analysis of covariance was performed with fixed categorical effects of treatment regimen and substudy, as well as the continuous fixed covariates of corresponding baseline value. Safety end points were analyzed descriptively using the safety population, defined as all participants randomized within the substudies and exposed to at least one dose of Gla-300, regardless of dosage or duration.
Results
Study population
In total, 109 participants from EDITION 1 and 89 from EDITION 2 were enrolled in the substudies. These 198 participants were randomized to either the flexible-dosing (n = 101) or fixed-dosing (n = 97) regimens. The safety population comprised 100 and 96 participants for the respective regimens, and the modified intent-to-treat population included 99 and 95 participants, respectively. Baseline (Month 6 in the main studies) age, gender distribution, body mass index, and HbA1c for the randomized population were similar between the flexible- and fixed-dosing groups within each substudy and between the pooled flexible- and fixed-dosing groups (Supplementary Table S1; Supplementary Data are available online at www.liebertonline.com/dia).
Variability in dosing intervals
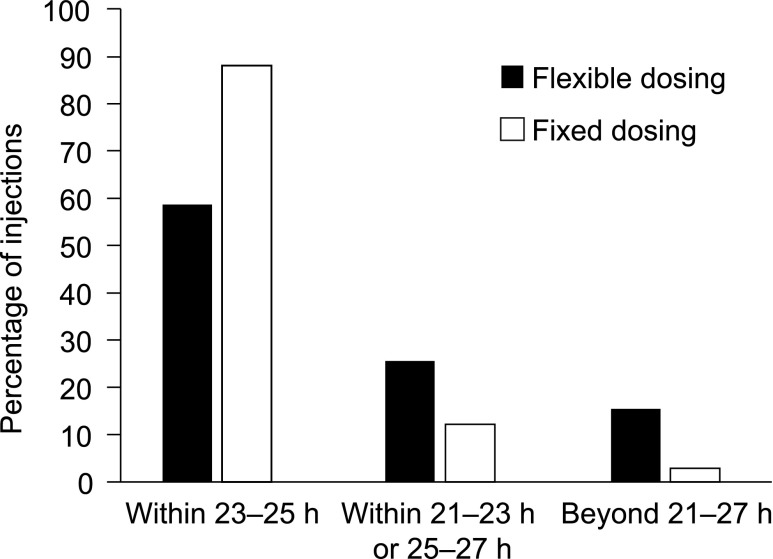
Data were available for 1,003 dosing intervals for 96 participants in the fixed-dosing group and 1,092 dosing intervals for 100 individuals in the flexible-dosing group. In the pooled analysis, 64% of participants in the fixed-dosing group maintained all observed intervals in the 23–25-h range, compared with just 15% of those in the flexible-dosing group. Of all intervals observed in the fixed-dosing group, 88% were in the 23–25-h range (and thus 12% were outside that range), whereas in comparison 59% of intervals in the flexible-dosing group were in the 23–25-h range (41% outside the range). Between-injection intervals outside the 21–27-h range were uncommon for the fixed-dosing group (2%), but 16% of intervals in the flexible-dosing group were outside this wider range (Fig. 2). Within the individual substudies (Supplementary Fig. S1), the pattern of injection intervals was similar to that observed in the pooled data.
FIG. 2.
Percentage of injections by the time interval between two consecutive insulin glargine 300 U/mL injections in EDITION 1 and EDITION 2 substudies (safety population).
Glycemic responses and insulin dosage
In the pooled analysis, HbA1c for both the flexible- and fixed-dosing groups after 3 months was similar to that observed at the substudy baseline (Table 1). The least squares (LS) mean difference between regimens in the mean change in HbA1c was 0.05 % (95% confidence interval [CI], −0.13 to 0.23) (0.5 [−1.4 to 2.5] mmol/mol). Laboratory-measured (clinic-collected) FPG levels were also similar at substudy baseline and after 3 months for both treatment regimens (Table 1). The LS mean difference between the flexible- and fixed-dosing regimens in mean FPG change was 2.7 (95% CI, −9.0 to 14.4) mg/dL (0.15 [–0.50 to 0.80] mmol/L). The change in mean daily basal insulin dose was identical, with the LS mean difference being 0.00 (95% CI, −0.02 to 0.03) U/kg between the treatment regimens (Table 1). The individual data for the EDITION 1 and EDITION two substudies are shown in Supplementary Table S2.
Table 1.
Clinical Measures for the Flexible-Dosing and Fixed-Dosing Insulin Glargine 300 U/mL Regimens (Pooled Data, EDITION 1 and EDITION 2 Substudies, Modified Intent-to-Treat Population)
| Flexible dosing (n = 99) | Fixed dosing (n = 95) | |
|---|---|---|
| Daily basal insulin dose (U/kg) | ||
| Baseline of substudy [mean (SD)]a | 1.00 (0.36) | 0.93 (0.33) |
| End of substudy (Month 3) [mean (SD)]b | 1.04 (0.39) | 0.96 (0.37) |
| Baseline of substudy to end of substudy (Month 3) [LS mean change (SE)] | 0.03 (0.01) | 0.03 (0.01) |
| LS difference [mean (95% CI)] | 0.00 (−0.02 to 0.03) | |
| HbA1c | ||
| Baseline of substudy [mean (SD)]a | ||
| % | 7.30 (0.93) | 7.30 (0.96) |
| mmol/mol | 56.3 (10.2) | 56.3 (10.5) |
| End of substudy (Month 3) [mean (SD)]b | ||
| % | 7.34 (0.92) | 7.29 (1.03) |
| mmol/mol | 56.7 (10.1) | 56.2 (11.3) |
| Baseline of substudy to end of substudy (Month 3) [LS mean change (SE)] | ||
| % | 0.05 (0.06) | 0.00 (0.07) |
| mmol/mol | 0.5 (0.7) | 0.0 (0.7) |
| LS difference [mean (95% CI)] | ||
| % | 0.05 (−0.13 to 0.23) | |
| mmol/mol | 0.5 (−1.4 to 2.5) | |
| FPG | ||
| Baseline of substudy [mean (SD)]a | ||
| mg/dL | 130.2 (35.6) | 124.2 (47.2) |
| mmol/L | 7.2 (2.0) | 6.9 (2.6) |
| End of substudy (Month 3) [mean (SD)]b | ||
| mg/dL | 135.4 (42.1) | 129.5 (48.6) |
| mmol/L | 7.5 (2.3) | 7.2 (2.7) |
| Baseline of substudy to end of substudy (Month 3) [LS mean change (SE)] | ||
| mg/dL | 6.6 (4.1) | 3.9 (4.3) |
| mmol/L | 0.37 (0.23) | 0.22 (0.24) |
| LS difference [mean (95% CI)] | ||
| mg/dL | 2.7 (−9.0 to 14.4) | |
| mmol/L | 0.15 (−0.50 to 0.80) | |
Month 6 of the main EDITION study.
Month 9 of the main EDITION study.
CI, confidence interval; FPG, fasting plasma glucose (central laboratory-measured); HbA1c, hemoglobin A1c; LS, least squares; mITT, modified intent-to-treat.
Hypoglycemia
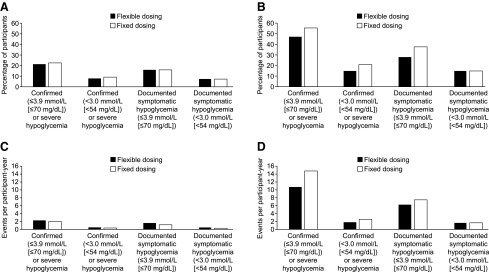
The percentage of participants experiencing one or more nocturnal confirmed (≤70 mg/dL [≤3.9 mmol/L]) or severe hypoglycemic events was similar in the pooled data analysis, at 21% and 23% in the flexible-dosing and fixed-dosing groups, respectively (Fig. 3A and Supplementary Table S3). This was also true for the events at any time (24 h) and for nocturnal and any-time events analyzed using other definitions of hypoglycemia (Fig. 3A and B and Supplementary Table S3). One severe hypoglycemic event was reported in the fixed-dosing group and none in the flexible-dosing group (Supplementary Table S3).
FIG. 3.
Hypoglycemic events in each category (pooled EDITION 1 and EDITION 2 substudies; safety population). (A) Percentage of participants experiencing one or more nocturnal (00:00–05:59 h) events in each category. (B) Percentage of participants experiencing one or more events at any time (24 h). (C) Annualized nocturnal (00:00–05:59 h) rates (events per participant-year). (D) Annualized rates (events per participant-year) at any time (24 h).
Likewise, the annualized rates of hypoglycemia compared using any definition, at any time (24 h) and during the night, were similar with the flexible-dosing and fixed-dosing regimens (Fig. 3C and D and Supplementary Table S3). The occurrence of hypoglycemia is shown separately for EDITION 1 and EDITION 2 in Supplementary Tables S4 and S5, respectively.
AEs
The number of participants experiencing any treatment-emergent AEs in the pooled data analysis was similar in both the flexible-dosing and fixed-dosing groups (24 [24%] and 26 [27%], respectively). No signal for differences in any category of event was detected. A comparably low number of participants experienced serious AEs in the flexible- and fixed-dosing groups (6 [6%] and 5 [5%], respectively). The serious AEs were not considered related to the study medication, and no deaths were reported. No differences between regimens within each substudy were detected.
Discussion
In clinical research studies comparing insulin regimens, the timing of insulin injections is usually mandated by protocol to allow comparisons between the properties of the insulins tested. However, both people with diabetes and physicians agree that a daily fixed injection time is restrictive, and in real life individuals often deviate from a fixed injection time in order to adapt to daily changes of schedule.5,6 In one survey, only 19% of physicians reported that their patients were “very successful” at taking their insulin injection at a fixed time every day.5 In a survey of people with diabetes,7 24% of respondents reported they had mistimed their basal insulin injection (±2 h from the prescribed injection time) on one or more occasions in the previous 30 days, with a mean number of 4.2 mistimed doses in the previous 30 days. Some individuals (6.5%) had mistimed their injections on more than five occasions in the previous 30 days. In addition, 47% of people in this survey reported that a fixed-dosing schedule negatively affected many daily activities. Hence the results of clinical studies may not always be reflected in clinical practice, when people are less consistent in using insulin. New basal insulin analogs with longer and more stable profiles of action might be less affected by greater variation of injection time. Thus, finding no loss of efficacy or safety when intervals between injections of these agents are varied in research studies might allay concerns that such studies are not relevant to clinical practice.
These substudies of EDITION 1 and EDITION 2 were designed to address this issue, in the setting of glycemic control that had previously been optimized. The advice given to participants in the flexible-dosing arm of the study clearly increased the variability of injection timing compared with that observed in the fixed-dosing interval arm, both with regard to the number of participants with at least one interval outside the 23–25-h range (85% vs. 36%) and in the percentage of all observed intervals outside the range (41% vs. 12%). Similarly, more extreme deviations of injection intervals, greater than ±3 h and thus outside a 21–27-h range, were more frequent in the flexible-dosing group compared with the fixed-dosing group (16% vs. 2%). The degree of variability observed in the flexible-dosing group in this study appears similar to that commonly seen in daily life, as reported in the study described above,7 where the timing of injections may be influenced by variations in timing of the evening meal or bedtime, as required by work or family activities, or by travel.
Therefore the present findings with Gla-300 provide reassurance that, given this much variability of injection timing, Gla-300 may be expected to have similar efficacy and tolerability in routine clinical use as in EDITION 1 and EDITION 2. Specifically, no differences between the treatment regimens in glycemic control assessed by HbA1c or by clinic-collected and laboratory-measured FPG were found, and insulin doses were the same with each regimen. There was no evidence that the occurrence of nonsevere hypoglycemia, defined as confirmed by glucose measurement of ≤70 mg/dL or <54 mg/dL, was affected by greater flexibility of timing. Severe hypoglycemic events were uncommon with both regimens. Moreover, when data from each of the two substudies embedded within EDITION 1 and EDITION 2 were considered separately, no clear differences were apparent. Thus, increased flexibility of timing of Gla-300 injection led to no difficulties in the setting of either mealtime and basal insulin therapy or basal insulin without mealtime insulin.
Of interest in relation to these findings with Gla-300 is a previous report of use of insulin degludec, a basal insulin with an even longer action profile than Gla-300, for T2DM.8 Because insulin degludec has a duration of action approaching 2 days (with a half-life of approximately 25 h),9 its efficacy and safety were tested when it was administered with very widely variable (8–40-h) dosing intervals and compared with once-daily degludec and once-daily Gla-100 that were always to be injected at the same time of day. In addition to testing dosing intervals that are well beyond those usually recommended in clinical practice, the insulin degludec study differed from the present study by including insulin-naive participants, having a longer follow-up (26 weeks vs. 12 weeks), and enrolling more participants in the variable dosing arm (229 vs. 99). Under these conditions, variable dosing intervals with insulin degludec resulted in equivalent levels of glycemic control and similar rates of hypoglycemia to fixed dosing intervals with either Gla-100 or insulin degludec, providing reassurance that substantially altering injection timing altered neither the risks nor the efficacy of insulin degludec.
The strengths of our substudies include the success of the intervention in reproducing a degree of variability of injection time that might be expected in usual daily life, as well as the lack of any trend toward undesirable effects of this variability. Limitations include the small numbers of participants enrolled and observation of participants for a period of just 3 months (resulting in relatively low statistical power), dependence on participant reports of the timing of dosing, and some uncertainty as to whether the findings can be generalized to longer-term periods of treatment or other populations. Also, the variability resulting from the advice given to the flexible-dosing group might be less than experienced by some individuals in daily life, and no comparison with an alternative insulin product was included in the substudies. Confirmation of the lack of untoward consequences of greater variability of injection timing will require larger and longer studies in people with T2DM that can provide greater statistical power. Studies of this issue in people with type 1 diabetes would also be of interest.
In conclusion, the results suggest that allowing greater variability of injection timing (24 ± up to 3 h) of Gla-300 in the setting of a clinical study did not compromise efficacy and safety profiles in people with T2DM. Therefore, Gla-300 may allow people with T2DM more freedom in timing their basal insulin injections to deal with the situational variability experienced in daily life.
Supplementary Material
Acknowledgments
This study was funded by Sanofi. The authors thank the study participants, trial staff, and investigators for their participation. The authors would also like to thank Cassandra Pessina (Sanofi) for critical review of the manuscript and for assistance with management of the manuscript development. Editorial assistance was provided by Don Smyth of Fishawack Communications, funded by Sanofi.
Author Disclosure Statement
M.C.R. receives research grant support from AstraZeneca, Eli Lilly, and Sanofi and honoraria for consulting and/or speaking from AstraZeneca, Elcelyx, Eli Lilly, Sanofi, and Valeritas. These dualities of interest have been reviewed and managed by Oregon Health & Science University. G.B.B. receives honoraria for advising and lecturing from Sanofi, Eli Lilly, and Novartis. P.D.H. or institutions with which he is connected receive funding from AntriaBio, Biocon, AstraZeneca, Eli Lilly, GlaxoSmithKline, Hanmi, Janssen/Johnson & Johnson, Merck (MSD), Novo Nordisk, Roche Diagnostics, Sanofi, and Skyepharma. R.M.B has received research support or funding for consultancy or serving on a scientific advisory board from Abbott Diabetes Care, Amylin, Bayer, Becton Dickinson, Boehringer Ingelheim, AstraZeneca/Bristol-Myers Squibb Alliance, Calibra, DexCom, Eli Lilly, Halozyme, Hygieia, Johnson & Johnson, Medtronic, Merck, Novo Nordisk, Roche, Sanofi, and Takeda. His employer, the nonprofit Park Nicollet Institute, contracts for his services, and no personal income goes to R.M.B. He has inherited Merck stock and has been a volunteer for the American Diabetes Association and JDRF. M.Z., I.M.-B., and M.W. are employees of Sanofi. L.V. is an employee of EXPERIS. N.J. receives honoraria for consulting and speaking from Novartis, Ipsen, Eli Lilly, Novo Nordisk, and Sanofi. H.Y-J. receives honoraria for consulting and speaking from Eli Lilly, Boehringer Ingelheim, Sanofi, and Merck (MSD). The authors had full access to all the data in the studies and had the final responsibility for the decision to submit this publication.
References
- 1.Becker RH, Dahmen R, Bergmann K, et al. : New insulin glargine 300 units·mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units·mL−1. Diabetes Care 2014;38:637–643 [DOI] [PubMed] [Google Scholar]
- 2.Riddle MC, Bolli GB, Ziemen M, et al. : New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care 2014;37:2755–2762 [DOI] [PubMed] [Google Scholar]
- 3.Yki-Järvinen H, Bergenstal R, Ziemen M, et al. : New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014;37:3235–3243 [DOI] [PubMed] [Google Scholar]
- 4.Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245–1249 [DOI] [PubMed] [Google Scholar]
- 5.Peyrot M, Barnett AH, Meneghini LF, et al. : Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012;29:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies MJ, Gagliardino JJ, Gray LJ, et al. : Real-world factors affecting adherence to insulin therapy in patients with Type 1 or Type 2 diabetes mellitus: a systematic review. Diabet Med 2013;30:512–524 [DOI] [PubMed] [Google Scholar]
- 7.Brod M, Rana A, Barnett AH: Adherence patterns in patients with type 2 diabetes on basal insulin analogues: missed, mistimed and reduced doses. Curr Med Res Opin 2012;28:1933–1946 [DOI] [PubMed] [Google Scholar]
- 8.Meneghini L, Atkin SL, Gough SC, et al. : The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care 2013;36:858–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heise T, Nosek L, Bøttcher SG, et al. : Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab 2012;14:944–950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.