Abstract
Background: The global epidemic of diabetes calls for innovative interventions. This study evaluated the effectiveness of the Project Dulce model, with and without wireless technology, on glycemic control and other clinical and self-reported outcomes in patients with poorly controlled type 2 diabetes in Mexico.
Subjects and Methods: Adults with type 2 diabetes and a glycated hemoglobin A1c (HbA1c) level of ≥8% were recruited from Family Medical Unit #27 of the Instituto Mexicano del Seguro Social (IMSS) in Tijuana, México, and randomly assigned to one of three groups: Project Dulce–only (PD); Project Dulce technology-enhanced with mobile tools (PD-TE); or IMSS standard of care/control group (CG). Clinical and self-reported outcomes were assessed at baseline, Month 4, and Month 10. Time-by-group interactions and within-group changes were analyzed.
Results: HbA1c reductions from baseline to Month 10 were significantly greater in PD-TE (−3.0% [−33 mmol/mol]) and PD (−2.6% [−28.7 mmol/mol]) compared with CG (−1.3% [−14.2 mmol/mol]) (P = 0.009 and 0.001, respectively). PD-TE and PD also exhibited significant improvement in diabetes knowledge when compared with CG (P < 0.05 for both). No statistically significant differences were detected between PD and PD-TE on these indicators (P = 0.54 and 0.86, respectively). Several within-group improvements were observed on other clinical and self-report indicators but did not vary significantly across groups.
Conclusions: Project Dulce with and without wireless technology substantially improved glycemic control and diabetes knowledge in high-risk patients with type 2 diabetes in a Mexican family medical unit, suggesting that integrating peer-led education, nurse coordination, and 3G wireless technology is an effective approach for improving diabetes outcomes in high-risk populations.
Introduction
In recent decades, the incidence and prevalence of diabetes have increased worldwide.1 The United States–Mexico border region is highly affected, with a 15.4% prevalence of type 2 diabetes mellitus in adults,2 exceeding the overall prevalence described for the Hispanic population in the United States (11.4%)3 or for the general population in Mexico (11.9%).4 Diabetes represents one of the greatest burdens for the Mexican health and social insurance provider, the Mexican Institute of Social Security (Instituto Mexicano del Seguro Social [IMSS]).5,6
Glycemic control in type 2 diabetes continues to be a major challenge.1,7 In Mexico, approximately 75% of individuals with diabetes have not reached adequate glycemic control.8 Systematic reviews have reported that diabetes self-management education programs can help patients with poor blood glucose control achieve glycemic targets.9–11 Although most of these studies have been conducted in high-income countries, 80% of the population affected with diabetes lives in low- and middle-income countries.1 Thus it is important to identify effective interventions for underserved, high-need populations in low-resource settings.
The International Diabetes Federation affirms that culturally appropriate and well-tested diabetes education models remain severely limited in low- and middle-income populations.1 One program that has demonstrated efficacy in improving glycemic control, behavioral indicators, and long-term cost reductions for high-risk (patients with poor glycemic control) Mexican-American patients diagnosed with type 2 diabetes mellitus in the United States is Project Dulce.12–14 The Project Dulce model uses nurse care support, peer-led diabetes self-management education, and a basic registry. Project Dulce uses trained diabetes nurses to assist physicians by collecting and reviewing patient data and taking anthropometric and sensory measures. Nurses also educate patients and promote adherence to treatment following evidence-based guidelines. Peer educators (individuals from the patients' communities with personal experience with diabetes) provide eight weekly educational sessions of 2 h each, using the Diabetes Among Friends curriculum13 followed by monthly support groups.
Several studies and systematic reviews of information and mobile technology have reported small to moderate glycated hemoglobin A1c (HbA1c) reduction and benefit in behavioral outcomes.15–23 Some of the largest reductions in HbA1c were reported by Quinn et al.,24 with differences in HbA1c between a technology intervention and standard care of 1.2% (13.1 mmol/mol) in a population of commercially insured patients in the United States. Systematic reviews emphasize the need for more randomized, controlled clinical trials that assess the value of wireless technology in diabetes management added to comprehensive, culturally sensitive, and innovative clinical and health education interventions for ethnic minorities and low- or middle-income countries.17,19 Given that Mexico is a country with high diabetes prevalence and high mobile technology penetration, where at least 85% of adults use cell phones,25 this study proposed to adapt and evaluate the Project Dulce program, with and without mobile technology, for patients with type 2 diabetes mellitus from a medical familiy unit in the United States–Mexican border region of Tijuana, Mexico. This study, known as Dulce Wireless Tijuana (DWT), evaluated if the adapted Project Dulce model for the Mexican population, with and without mobile technology, was effective in a “real-world” environment (i.e., under routine practice conditions with evolving management approaches), compared with usual clinical care, at improving clinical and self-report outcomes in patients with type 2 diabetes in Mexico.
Research Design and Methods
Study design
The DWT study is an open-label randomized controlled trial conducted from November 2011 to April 2014 at the Family Medical Unit #27 (UMF #27) of the IMSS in the city of Tijuana. Patients were identified as potential candidates by medical staff of the 81 medical offices in this health facility and recruited through direct patient evaluation and review of the patient's medical records by trained nurses. Participant inclusion criteria were as follows: 18–75 years of age, diagnosis of type 2 diabetes, HbA1c ≥8% (≥64 mmol/mol), no current insulin use, active IMSS health coverage, and able to read. Patients with severe medical or psychiatric conditions and who were unable to visit the clinic were excluded from the study.
The protocol of this study protocol was approved by the Universidad Autónoma de Baja California and the Bioethical Committee of the IMSS National Research Commission (protocol CNIC-R-2011-785-019).
Medical and laboratory records were reviewed to confirm eligibility. Insulin-naive patients were selected because they provided a participant group with more uniform clinical features by which to study the interventions. Patients are eligible to obtain IMSS health and social services if they are either dependents of or themselves active employers or employees registered in the IMSS national labor registry. This includes provision of laboratory services, medications, hospital services, and disability coverage. Alternatively, most uninsured have to pay out-of-pocket medications, laboratories, or disability coverage. Similar to designs in pragmatic studies, this study was conducted in a real-world environment with evolving management approaches, but the interventions for Project Dulce diabetes self-management education and technology remained consistent for the duration of the study.
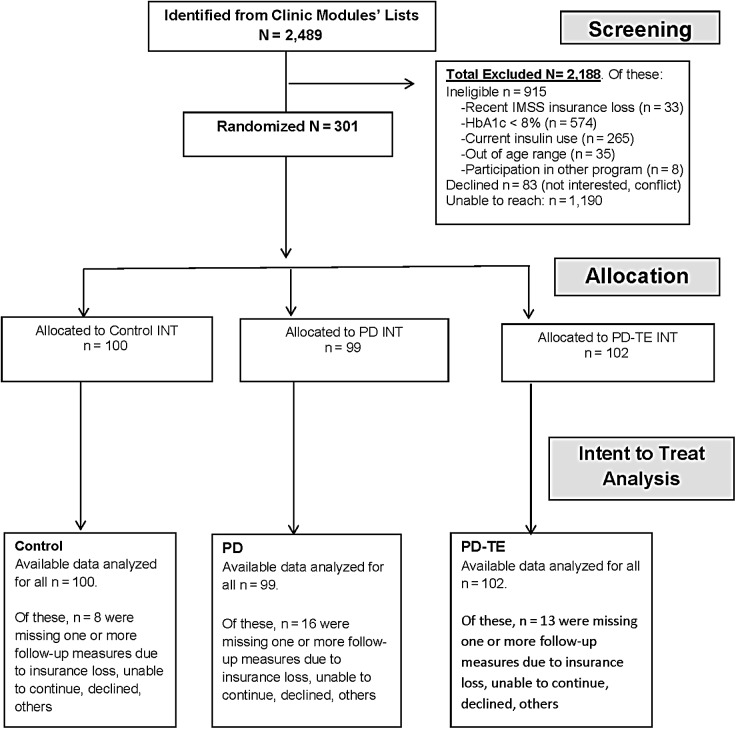
Patients who agreed to participate were randomly assigned to one of three groups: Project Dulce–only intervention (PD), Project Dulce technology-enhanced intervention (PD-TE), or control group (CG). A block randomization procedure26 was used to promote homogeneity among groups. Patients entered the study in successive cohorts as they were recruited, every 2 or 3 months. Figure 1 gives the Consolidated Standards of Reporting Trials diagram for the study.
FIG. 1.
Consolidated Standards of Reporting Trials (CONSORT) flowchart of enrollment and follow-up. HbA1c, glycated hemoglobin A1c; IMSS, Instituto Mexicano del Seguro Social; INT, intervention; PD, Project Dulce-only; PD-TE, Project Dulce technology-enhanced.
Assessments of clinical outcomes were conducted at baseline (Month 0), Month 4, and Month 10 by trained nurses. Self-reported oucomes were assessed at baseline and Month 10 only.
Interventions
PD
Consistent with the Project Dulce model,13 the PD group included a combination of care management by a multidisciplinary team led by trained clinicians and nurses, as well as a peer-led group education component. The flow of usual care at UMF #27 was modified to allow patients to be cared for by this multidisciplinary team.
Clinicians completed a 16-h training based on the American Diabetes Association guidelines27 that was delivered by the Scripps Whittier Diabetes Institute in San Diego, CA. They also participated in monthly multidisciplinary capacity-building case discussions and were responsible for prescribing and changing medications for patients as needed to meet glycemic control targets, adhering to current guidelines. These case discussions were added training components to meet the capacity-building needs of the local clinicians and the multidisciplinary team.
Nurses, also trained in diabetes management, played an important role in patient management and education. These diabetes-trained nurses collaborated with the attending physicians, providing personalized education to patients, reviewing their clinical history, and monitoring progress toward specific clinical or behavioral outcomes, all according to current clinical guidelines. Nurses also acted as liaisons with the community peer educators, referring patients to educational classes and support groups.
Peer educators, locally known as “promotoras,” who either had diabetes themselves or had lived with relatives or closely worked with people with diabetes, were trained in the “Diabetes Among Friends” curriculum of Project Dulce using an established training protocol, and competencies were met by the trainees.12,28 The order of the educational classes was modified to address the fears, myths, and barriers of the Mexican population. Peer educators were recruited from the same communities where the patients lived. Peer educators were supervised by staff from Fronteras Unidas Pro Salud, a community organization in Tijuana, as well as the IMSS. Promoting clinicians' adherence to guidelines and overcoming patients' and providers' fears and misconceptions of insulin use were part of the DWT training and education program. Observational data showed that providers in the clinic were hesitant to initiate and titrate insulin because of fear of hypoglycemia and the limited glucose monitoring resources available to patients. Patients had a strong fear of injections and misconceptions about the use of insulin.
The educational sessions were aimed at achieving effective diabetes self-management and were offered to patients and relatives on flexible schudules and locations. Patients received a total of eight 2-h, weekly sessions of peer-led diabetes self-management education during the first 2 months. Patient sessions were interactive and offered at the clinic and convenient community sites (public libraries, gymnasiums, and community centers). At the conclusion of the eight weekly sessions, patients were encouraged to attend monthly support groups through the 10th month of follow-up.
PD-TE
In addition to the PD intervention described above, PD-TE participants received a MyGlucoHealth glucose meter (Entra Health Systems, San Diego) with USB connection, 80 glucose test strips, and a 3G-enabled cell phone (Iusacell Mexico, Mexico City, Mexico). Patients received a 2-h orientation on how to use the glucose meter and the cell phone. IMSS usually does not provide glucose meters, test strips, or cell phones to patients, so the combination of technology tools used in this study for the PD-TE group can be considered a novel therapeutic approach in this institution.
Patients were asked to check their glucose level two times a day (fasting and postprandial), every day, during the first month and 2 days per week during the second month. Patients checked their glucose levels with the glucose meters and test strips provided. The glucose meter data were uploaded to the project's diabetes registry system. The system identified glucose readings considered too low or too high. The medical staff had access to the diabetes registry system developed to securely receive glucose meter readings and track patient information during visits and classes.
Patients were also encouraged to take interactive surveys, read text messages, watch short educational videos, and read brochures available through a Brew™ application developed by Iusacell (Geocontrol®) and installed on their cell phone. The interactive survey was sent from the patients' cell phone once a day during the first month and twice a week during the second month. The interactive survey was designed to promote tracking and accountability. It contained five simple questions related to their glucose readings, carbohydrate intake, physical activity, and medication adherence. The interactive survey had the capacity to relay feedback data to providers in real time via the Brew application. The system sent automatic reminders to complete the survey via short message service (text messaging). Alert messages were also sent to providers when patients reported out-of-range glucose levels or missed appointments. Lastly, PD-TE patients had access to diabetes care information through short, culturally appropriate videos and educational materials available in their cell phones. Videos followed the soap-opera style, known as “novela” in Spanish. The survey and videos were developed for and evaluated with Mexican patients with diabetes in a qualitative pilot test at UMF #27.
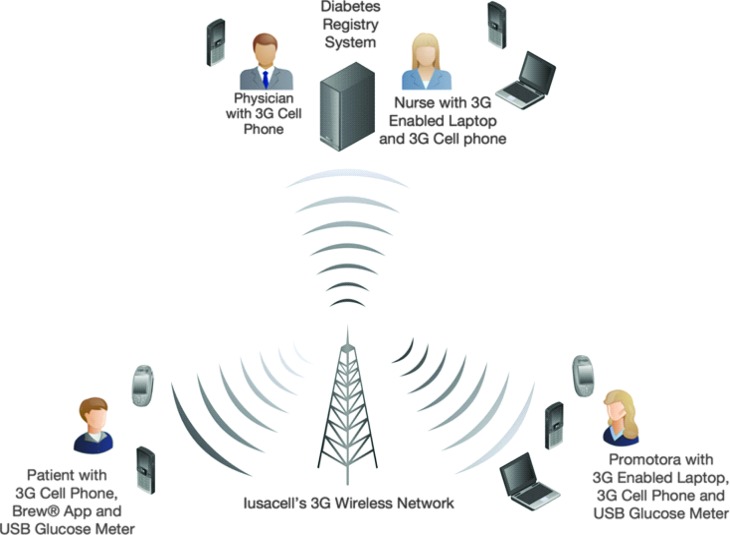
Patient data were protected using standard security protocols to guarantee integrity and confidentiality (Fig. 2).
FIG. 2.
Project Dulce technology-enhanced topology. Promotoras, patients, physicians, and nurses use their 3G cell phones, laptops, and USB glucose meters to exchange clinical and educational information through an EV-DO Revision A wireless network.
CG
Participants in the CG received standard care outlined by IMSS guidelines29,30 via recently introduced DiabetIMSS group medical visits or one-on-one visits with a family physician. Medical group visits were provided according to the IMSS Institutional Program for Prevention and Treatment of Diabetes, called the DiabetIMSS technical guide.29 Physicians, nurses, and social workers are trained according to these diabetes guidelines. Clinicians prescribed and made changes to medications following these guidelines. The DiabetIMSS program encouraged patients to participate in monthly visits of approximately 3 h, where they received educational classes and were evaluated by a nurse and a physician.30,31 Thus, patients in the DiabetIMSS group had access to 10 monthly medical group visits during the study.
In order to prevent contamination among the three groups, different physicians and peer educators provided care for each of the study groups. Also, the classes and visits were offered separately and at different times to prevent contamination across conditions.
Measures
Clinical outcomes
The primary outcome of this study was HbA1c (expressed in % [mmol/mol]). Secondary clinical outcomes were total cholesterol, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), triglycerides, systolic blood pressure (SBP), diastolic blood pressure (DBP), and body mass index (BMI). Laboratory assays were processed by the regional laboratory of the IMSS at Hospital #1 in Tijuana, according to standardized procedures. HbA1c concentration was measured by a turbidimetric immunoinhibition method, using the UniCel® DxC Synchron® (Beckman Coulter, Inc., Brea, CA) systems hemoglobin (HbA1c–) assay, certified by the NGSP.
Self-reported outcomes
Self-efficacy, depression, lifestyle, quality of life, and diabetes knowledge were measured at baseline and Month 10 using the respective Spanish-validated survey instruments: the Spanish Diabetes Self-Efficacy,32 the Patient Health Questionnaire (PHQ-9),33 the Instrument to Measure Lifestyle of Type 2 Diabetes Mellitus Patients (IMEVID),34 Diabetes 39,35 and the Diabetes Knowledge Questionnaire 24 (DKQ24).36 These instruments were validated in Spanish-speaking populations in the United States or in Mexico.
The Spanish Diabetes Self-Efficacy is an eight-item instrument that uses a 1 (not at all confident) to 10 (totally confident) response scale to assess patients' level of confidence conducting basic diabetes management activities.32
The PHQ-9 is a questionnaire validated for use in primary care to assess the nine diagnostic criteria for major depressive disorder.33
The IMEVID contains 25 items that measure self-reported health behaviors across seven domains: nutrition, physical activity, tobacco/alcohol consumption, information on diabetes, emotions, and therapeutic adherence.34
The Diabetes 39 is a 39-item questionnaire that uses a 1 (not affected at all) to 7 (extremely affected) response scale to gauge psychological well-being and social functioning.35
The DKQ24 instrument is a 24-item questionnaire that uses a true–false response scale to gauge patients' knowledge of diabetes.36
The reported internal consistency reliability (α) of each of these Spanish-validated instruments was 0.85, 0.84, 0.81, 0.95, and 0.78, respectively.32–36
Sociodemographic and medical history
Information regarding age, gender, education, marital status, hypertension diagnosis, and previous hospitalizations were collected during the baseline assessment.
Statistical analysis
A sample size of 99 patients per group (total n = 297) was calculated based on differences of the primary outcome (changes in HbA1c) with a 97.5% power to detect an effect of 0.9% difference as reported in a mobile diabetes intervention study.24 A 25% attrition rate was considered realistic given the high mobility of this population.
Descriptive and graphical analyses were used to examine outcome distributions, as well as to calculate means and SDs. Multilevel modeling analyses examined differential changes across time among the three groups on clinical and self-reported outcomes. Multilevel analysis was specifically used to correct for any potential nonindependence among the subjects and their groups. These analyses were conducted with the entire cohort (i.e., participants with at least one data point) in an intent-to-treat approach. Subsequent analyses examined within-group change on clinical and self-reported outcomes using analyses of covariance and controlling for age and sex. Medication adjustments were not included as a covariate because they were part of the DWT comprehensive interventions.
Furthermore, combined and individual associations of class and medical visit attendance with HbA1c changes were analyzed in dosage analyses. In an attempt to assess if interactive surveys in the PD-TE group had a predictive value by itself on the primary HbA1c outcome, an analysis of doses via linear regression adjusted for age and gender was conducted.
IBM (Armonk, NY) Statistical Package for the Social Sciences (version SPSS-21) and Scientific Software International (Skokie, IL) hierarchical linear modeling (version HLM-7) for Windows software were used to perform all analyses.
Results
In total, 2,489 potential study participants were identified and referred to staff for screening evaluation. Of those, 915 (37%) were considered ineligible, 83 declined (3%), and 1190 (48%) could not be reached after several contact attempts. The remaining 301 participants were enrolled in the study and were allocated randomly: 99 to PD, 102 to PD-TE, and 100 to CG (see the CONSORT diagram in Fig. 1).
The majority of patients were female, married, and middle-aged and reported middle school or lower educational attainment (Table 1). Average duration of diabetes since diagnosis was 8.3 years. All patients enrolled were taking oral hypoglycemic therapy, mainly metformin. The three study groups were similar at baseline, except for a larger proportion of women in the PD group (76.8%) compared with the CG (62.0%) and PD-TE (61.8%) groups (P < 0.05). There were no statistically significant differences of baseline HbA1c levels among the groups.
Table 1.
Demographic and General Characteristics of Groups at Baseline
| CG (n = 100) | PD (n = 99) | PD-TE (n = 102) | P | |
|---|---|---|---|---|
| Sex | 0.036a | |||
| Men | 38 (38.0) | 23 (23.2) | 39 (38.2) | |
| Women | 62 (62.0) | 76 (76.8) | 63 (61.8) | |
| Education | 0.757 | |||
| Basic (K–6th grade) | 51 (51.0) | 48 (48.5) | 58 (56.9) | |
| Middle (7th–12th grade) | 44 (44.0) | 46 (46.5) | 38 (37.3) | |
| College and above (13th + grade) | 5 (5.0) | 5 (5.1) | 6 (5.9) | |
| Marital status | 0.208 | |||
| Married or domestic partnership | 71 (71.0) | 60 (60.6) | 72 (70.6) | |
| Single, divorced, or widow | 29 (29.0) | 39 (39.4) | 30 (29.4) | |
| Hypertensionb | 0.725 | |||
| Yes | 47 (47.0) | 43 (43.4) | 50 (49.0) | |
| No | 53 (53.0) | 56 (56.6) | 52 (51.0) | |
| Hospitalizations | 0.844 | |||
| Yes | 7 (7.2) | 5 (5.3) | 6 (6.0) | |
| No | 93 (93.0) | 94 (94.9) | 94 (94.1) | |
| Age (years) [mean (SD)] | 52.5 (9.7) | 50.6 (10.7) | 51.5 (11.4) | 0.456 |
Data are n (%) unless indicated otherwise. Statistical tests used to assess differences were χ2 tests or one-way analysis of variance.
P < 0.05, significant difference.
According to Instituto Mexicano del Seguro Social national guidelines, hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≤90 mm Hg, or normal ranges in people receiving antihypertensive therapy.
CG, control group; PD, Project Dulce-only; PD-TE, Project Dulce technology-enhanced.
Unadjusted means, SDs, and differences between baseline and Month 4 and between baseline and Month 10 are presented in Table 2 for all outcomes.
Table 2.
Group Means for Clinical and Self-Report Outcomes at 0, 4, and 10 Months Among the Three Groups
| CG | PD | PD-TE | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| HbA1c (% unit) | ||||||
| Baseline | 100 | 10.90 (2.01) | 99 | 11.39 (2.52) | 102 | 11.19 (2.03) |
| Month 4 | 84 | 9.66 (2.71) | 77 | 8.27 (2.13) | 85 | 7.68 (2.13) |
| Month 10 | 92 | 9.56 (2.79) | 82 | 8.68 (2.76) | 89 | 8.19 (2.17) |
| Change 0–4 monthsa | −1.20 (2.89) | −3.11 (2.86) | −3.37 (2.80) | |||
| Change 0–10 monthsa | −1.30 (3.29) | −2.63 (3.73) | −3.02 (2.83) | |||
| HbA1c (mmol/mol) | ||||||
| Baseline | 100 | 96 (22.0) | 99 | 101 (27.5) | 102 | 99 (22.2) |
| Month 4 | 84 | 83 (29.6) | 77 | 67 (23.3) | 85 | 61 (23.3) |
| Month 10 | 92 | 81 (30.5) | 82 | 72 (30.2) | 89 | 66 (23.7) |
| Change 0–4 monthsa | −13.1 (31.6) | −34.0 (31.3) | −36.8 (30.6) | |||
| Change 0–10 monthsa | −14.2 (36.0) | −28.7 (40.8) | −33.0 (30.9) | |||
| Total cholesterol (mg/dL) | ||||||
| Baseline | 89 | 209.92 (38.99) | 94 | 206.46 (39.79) | 100 | 206.02 (37.14) |
| Month 4 | 79 | 201.00 (36.73) | 74 | 189.47 (35.98) | 83 | 188.06 (31.71) |
| Month 10 | 90 | 205.13 (38.84) | 79 | 195.80 (40.17) | 85 | 191.12 (39.04) |
| Change 0–4 months | −10.91 (34.77) | −14.07 (36.89) | −14.08 (35.82) | |||
| Change 0–10 months | −6.96 (31.18) | −7.40 (33.19) | −13.75 (36.94) | |||
| Triglycerides (mg/dL) | ||||||
| Baseline | 87 | 249.09 (141.48) | 92 | 231.46 (169.83) | 99 | 231.20 (148.93) |
| Month 4 | 78 | 203.17 (115.00) | 74 | 168.22 (90.96) | 83 | 173.48 (90.67) |
| Month 10 | 86 | 210.57 (122.79) | 75 | 173.97 (81.61) | 86 | 180.16 (96.39) |
| Change 0–4 months | −49.05 (131.69) | −41.25 (113.65) | −49.74 (125.69) | |||
| Change 0–10 months | −25.30 (128.11) | −29.85 (106.45) | −46.76 (120.4) | |||
| LDL-c (mg/dL) | ||||||
| Baseline | 52 | 118.64 (38.05) | 67 | 114.91 (29.87) | 75 | 109.74 (35.59) |
| Month 4 | 68 | 117.83 (31.91) | 63 | 107.71 (24.83) | 78 | 108.20 (27.52) |
| Month 10 | 75 | 117.45 (34.92) | 63 | 112.00 (28.41) | 77 | 111.54 (26.10) |
| Change 0–4 months | +4.31 (31.59) | −4.11 (28.93) | −1.18 (35.70) | |||
| Change 0–10 months | +0.45 (35.22) | −0.40 (24.91) | −0.26 (33.52) | |||
| HDL-c (mg/dL) | ||||||
| Baseline | 51 | 40.08 (9.50) | 67 | 41.56 (8.42) | 75 | 40.83 (7.96) |
| Month 4 | 69 | 41.00 (10.17) | 66 | 43.28 (10.49) | 77 | 43.69 (10.72) |
| Month 10 | 77 | 44.75 (12.07) | 64 | 42.72 (10.61) | 77 | 43.38 (11.71) |
| Change 0–4 months | +1.67 (9.11) | +2.99 (11.97) | +2.35 (10.24) | |||
| Change 0–10 monthsa | +6.05 (13.47) | +1.57 (8.78) | +1.53 (9.30) | |||
| BMI (kg/m2) | ||||||
| Baseline | 100 | 30.79 (5.10) | 97 | 31.15 (5.41) | 100 | 30.74 (5.29) |
| Month 4 | 84 | 31.11 (5.32) | 75 | 31.55 (5.28) | 85 | 31.20 (5.19) |
| Month 10 | 91 | 30.79 (4.98) | 74 | 32.08 (5.01) | 88 | 31.23 (5.27) |
| Change 0–4 months | +0.07 (1.32) | +0.08 (1.58) | +0.01 (1.69) | |||
| Change 0–10 months | −0.08 (1.67) | +0.25 (2.06) | +0.23 (2.27) | |||
| SBP (mm Hg) | ||||||
| Baseline | 100 | 122.70 (15.65) | 97 | 120.05 (11.71) | 100 | 123.78 (15.35) |
| Month 4 | 84 | 121.26 (12.66) | 76 | 119.59 (13.51) | 85 | 120.00 (13.34) |
| Month 10 | 90 | 123.02 (12.58) | 77 | 119.69 (14.24) | 87 | 120.13 (14.26) |
| Change 0–4 monthsb | −2.43 (15.7) | −0.08 (15.85) | −4.47 (15.15) | |||
| Change 0–10 months | −0.64 (16.0) | +0.08 (15.72) | −4.05 (16.86) | |||
| DBP (mm Hg) | ||||||
| Baseline | 100 | 78.09 (9.73) | 97 | 75.90 (8.18) | 100 | 78.88 (8.54) |
| Month 4 | 84 | 75.98 (8.02) | 76 | 74.12 (8.11) | 85 | 74.78 (11.71) |
| Month 10 | 91 | 76.85 (6.82) | 77 | 75.60 (9.13) | 87 | 75.22 (8.55) |
| Change 0–4 months | −2.34 (10.85) | −0.68 (10.01) | −4.28 (13.13) | |||
| Change 0–10 months | −1.82 (10.77) | +0.14 (11.48) | −3.74 (10.32) | |||
| Self-efficacy | ||||||
| Baseline | 97 | 46.81 (16.37) | 88 | 48.82 (18.10) | 92 | 54.75 (16.02) |
| Month 10 | 81 | 57.37 (17.04) | 62 | 61.77 (18.93) | 77 | 63.29 (17.26) |
| Change 0–10 months | +9.66 (22.02) | +12.08 (21.63) | +9.12 (19.29) | |||
| Depression | ||||||
| Baseline | 97 | 6.52 (4.99) | 89 | 7.48 (5.29) | 93 | 7.73 (5.77) |
| Month 10 | 81 | 5.56 (4.79) | 62 | 4.81 (4.26) | 77 | 5.13 (4.48) |
| Change 0–10 months | −0.83 (5.44) | −2.35 (4.83) | −2.36 (5.07) | |||
| Lifestyle | ||||||
| Baseline | 95 | 58.26 (12.52) | 89 | 61.79 (13.36) | 93 | 62.57 (13.13) |
| Month 10 | 81 | 69.68 (11.93) | 62 | 77.60 (9.20) | 77 | 76.90 (11.80) |
| Change 0–10 months | +11.78 (13.99) | +14.08 (11.00) | +14.44 (13.95) | |||
| Quality of lifec | ||||||
| Baseline | 97 | 31.11 (17.60) | 88 | 31.40 (20.99) | 93 | 30.09 (18.10) |
| Month 10 | 81 | 24.51 (18.12) | 62 | 16.68 (14.81) | 77 | 19.28 (16.88) |
| Change 0–10 months | −6.53 (20.57) | −15.41 (21.66) | −11.40 (18.65) | |||
| Diabetes knowledge | ||||||
| Baseline | 51 | 13.69 (4.22) | 49 | 14.12 (3.45) | 48 | 14.21 (3.91) |
| Month 10 | 81 | 14.83 (3.16) | 62 | 16.76 (2.98) | 77 | 17.30 (3.56) |
| Change 0–10 months | +1.15 (4.05) | +3.20 (3.28) | +3.24 (4.15) | |||
The following Spanish-validated survey instruments were used to measure self-efficacy, depression, lifestyle, quality of life, and diabetes knowledge, respectively: the Spanish Diabetes Self-Efficacy,32 the Patient Health Questionnaire,33 the Instrument to Measure Lifestyle of Type 2 Diabetes Mellitus Patients,34 Diabetes 39,35 and the Diabetes Knowledge Questionnaire 24.36 Unadjusted means are presented for 0, 4, and 10 months for descriptive purposes. Results for within-group changes were examined using analyses of covariance and controlling for age and sex. Sample sizes vary for each analysis because of missing values.
Levels of significance are indicated: aP < 0.01, bP < 0.05.
Higher scores mean quality of life is adversely affected. Thus reductions in quality of life scores represent positive gains in quality of life.
BMI, body mass index; CG, control group; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin A1c; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; PD, Project Dulce-only; PD-TE, Project Dulce technology-enhanced; SBP, systolic blood pressure.
Differential change over time analyses
Clinical outcomes
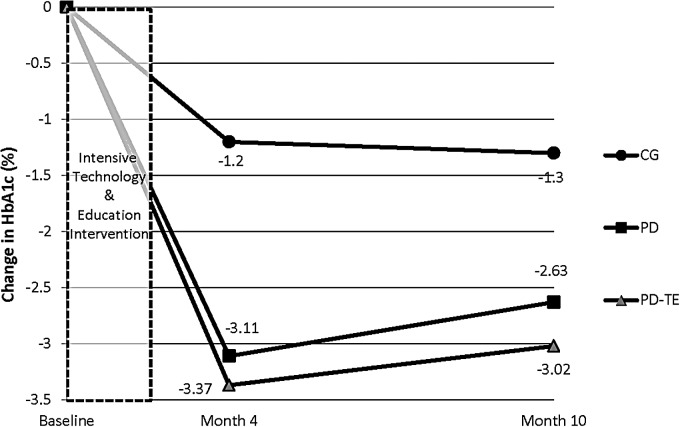
A significant time-by-group interaction effect was observed for HbA1c (P < 0.001) but not for any other clinical outcomes (all P values >0.05). Follow-up analyses were conducted to investigate differences among the three groups. Specifically, the PD-TE and PD groups exhibited significantly greater reductions in HbA1c over time compared with CG (P = 0.001 and P = 0.009, respectively). The degree of improvement in HbA1c did not vary significantly between PD and PD-TE over the 10-month follow-up (P = 0.86) (Fig. 3).
FIG. 3.
Changes in absolute levels of glycated hemoglobin A1c (HbA1c) (%) at baseline and at 4 and 10 months among Project Dulce-only (PD), Project Dulce technology-enhanced (PD-TE), and control group (CG) groups. PD-TE (P = 0.001) and PD (P = 0.009) groups exhibited significantly greater reductions in HbA1c over time compared with CG but did not vary significantly from each other (P = 0.86).
Self-reported outcomes
A significant time-by-group interaction effect was also observed for diabetes knowledge but not for any of the other self-reported outcomes: self-efficacy, depression, lifestyle, and quality of life (all P values >0.05). Follow-up analyses indicated that the PD-TE (P = 0.01) and PD (P = 0.03) groups exhibited significantly greater improvements in diabetes knowledge between baseline and Month 10 compared with CG. The degree of improvement in knowledge did not vary significantly between PD and PD-TE (P = 0.54).
Within-group changes analyses
Clinical outcomes
Unadjusted change scores are presented in Table 2 for descriptive purposes; however, P values are derived from age/gender-adjusted multivariable tests. An average within-group HbA1c decline between baseline and Month 10 of −3.02% (−33.0 mmol/mol) was observed for PD-TE with a decline of −2.63% (−28.7 mmol/mol) for PD (P values <0.01). CG showed a relatively smaller, but still significant, decline of −1.30% (−14.2 mmol/mol) in the same period (P < 0.01).
Significant within-group improvements in HDL-c were observed in the PD-TE (+1.53 mg/dL), PD (+1.57 mg/dL), and CG (+6.05 mg/dL) groups between baseline and Month 10 (P < 0.01). Also, statistically significant SBP reductions were observed in the PD-TE (−4.47 mm Hg), PD (−0.08 mm Hg), and CG (−2.43 mm Hg) groups between baseline and Month 4 (P values <0.05). No significant differences were observed for total cholesterol, LDL-c, triglycerides, DBP, and BMI in any of the three groups.
Self-reported outcomes
Within-group differences in quality of life and diabetes knowledge improvements approached statistical significance in the PD-TE and PD groups (P values <0.10).
Subanalyses
Changes in medication, from baseline to Month 10, showed a significant higher percentage of insulin use in patients of the PD and PD-TE groups (74% and 69%, respectively) compared with the CG (38%) (P < 0.001). Combined attendance to medical visits and peer-led education classes was a significant predictor of HbA1c changes for the PD and PD-TE groups (β = 0.2, P = 0.01). For every additional visit or class attended, a change in 0.2% (2.2 mmol/mol) of HbA1c was observed. In analyzing attendance to medical visits alone, medical visits were a significant predictor of HbA1c changes for the PD and PD-TE groups (β = 0.15, P = 0.02). Attendance to peer-led education alone did not reach a significant difference among groups. Patients in the PD-TE group participated on average in nine peer-led education classes, in the PD group in eight classes, and in the CG in seven classes (β = 0.16, P = 0.09).
Descriptive analysis showed that patients had high levels of response to the interactive surveys designed to promote tracking and accountability.On average, patients responded to 30 of the 40 interactive surveys they received via cell phone during the first 2 months of the study (75% adherence). Nevertheless, the results of the linear regression indicate that the number of interactive surveys by itself does not significantly predict the observed reduction in the primary outcome HbA1c (P = 0.152).
Discussion
Findings indicate that the PD-TE and PD groups exhibited significantly larger reductions in HbA1c levels at Month 10 (−3.02% [−33 mmol/mol] and −2.63% [−28.7 mmol/mol]) than CG (−1.30% [−14.2 mmol/mol]). However, no significant differences were observed between the PD-TE and PD groups. A similar pattern was observed for diabetes knowledge. Although significant within-group changes were observed on several of the remaining clinical and self-reported outcomes, these improvements did not vary significantly among the groups.
These results suggest that the adapted Project Dulce model for the Mexican population of IMSS in Tijuana, with and without technology, is feasible and effective in improving glycemic control in high-risk patients with type 2 diabetes mellitus attending IMSS clinic UMF #27 in Tijuana. Absolute reductions in HbA1c in the PD-TE and PD groups were larger in this study than the declines in HbA1c published in high-risk patients in the United States using the same peer education curriculum (−1.5% [−16.4 mmol/mol]).13
Although in this study no significant ifferences were found between PD-TE (technology-enhanced) and PD alone, this study did demonstrate acceptability in and engagement of a low-income patient population with the 3G technology used in this trial. This is consistent with the argument presented by the systematic review of Free et al.,16 which stated that mobile interventions are probably more relevant to providers in developing countries where mobile technology may offer valuable clinical support and guidance remotely. Free et al.16,17 further explained that most controlled trials have been conducted in high-income countries where the standard of care available “may be very different to the standard care in low- or middle-income countries.” It was promising to see that patients did not regress back to their initial high HbA1c levels (Fig. 2), as may be commonly reported soon after interventions.9 This indicates the potential to maintain glycemic control over a longer period of intervention.
Potential explanations for the within-group changes observed in the CG may include the facts that a new program (DiabetIMSS)30,31 was introduced as part of the standard of care at IMSS UMF #27, and a high influx of recently graduated and trained family physicians was experienced at the clinic during the implementation of the study. Because this was a real-world study it was possible that new interventions may be introduced during the conduct of the study. Nonetheless, the improvement observed in the CG was significantly smaller than those observed in the PD-TE and PD interventions, which supports the proposal that other elements in the PD-TE and PD further improved care.
As demonstrated in previous studies, improvements in glycemic control can be effectively achieved with the use of team-based care that includes nurse care managers and peer educators.12,13 In both the PD-TE and PD groups, adding a nurse care manager to the workflow and treatment algorithms allowed the family physician to conduct a no-rush, more complete review of the patient's clinical status and medication adjustments, as well as a more thorough conversation with the patient. The use of a multidisciplinary team, together with reinforced clinician education and the DWT program's emphasis on overcoming patients' and providers' fears and misconceptions on the use of insulin, may have facilitated the adherence to medication and explain the higher percentages of insulin use in the PD and PD-TE groups. However, because appropriate medication adjustments were part of the DWT comprehensive interventions, we are unable to isolate the specific effect of adherence to medications on glycemic outcomes. In the case of PD-TE, the short-term use of the glucose meters during the period of medication adjustment may have improved clinicians' confidence when prescribing or titrating insulin. Future studies should examine medication adjustment or adherence as a pathway to improved outcomes.
Community-based, peer diabetes education was also an important and culturally tailored strategy used in this setting where education is traditionally provided by healthcare professionals. This was the first time that diabetes educational sessions were provided outside the IMSS UMF #27 clinic at convenient locations and times by peer educators from a nonprofit organization. Patients expressed high levels of satisfaction with the peer and patient-centered approach to education as well as the use of technology tools, especially the glucose meter and the interactive survey (PD-TE group).
Although the declines in HbA1c level between the PD-TE and PD groups did not reach statistical significance, there is a modest difference of 0.4% (4.4 mmol/mol) between these two groups, favoring the PD-TE intervention. The magnitude of this decline is similar to those in several studies for different mobile technology interventions.15,21 Given the higher reductions in HbA1c trends observed at Month 4, right after the intensive phase of the technology, future research should examine if a longer period of mobile technology intervention leads to greater effects on glycemic control. This recommendation is consistent with the findings of the systematic review by Clark9 that consider duration of interventions as key to success. Also, studies with larger samples sizes may be needed to reach statistical significance when evaluating the mobile health impact alone.
The completion of the interactive surveys by the participants had a very high uptake (75%), demonstrating widespread willingness to respond via mobile technology. One possible explanation of the high survey response was the automatic alerts sent to the patients as reminders. Although we cannot conclude this for certain, we suspect that a high survey response may have indicated high adherence or use of self-management activities. A dosage effect of the mobile technology was not detected, but with such high, homogeneous dosage across groups (i.e., small effect size), it was more challenging to detect statistical significance. In terms of feasibility, the basic literacy levels and wide range in age of the patients did not seem to prevent the use of the technology adaptation. However, an initial orientation and ongoing instructions on the use of the cell phone interactive survey, videos, and glucose meters were required.
Preliminary qualitative reports reveal that participants particularly valued the ability to see how their glucose levels responded to the changes in medications, physical activity, and nutrition. The value of receiving timely feedback coincides with the Control Theory and the Goal Setting Theory, which explain how people can react or adapt to feedback and that actions or behaviors can be adjusted.37,38 Because the introduction of mobile technology was quite feasible in a population with high mobile penetration like Mexico, it merits a more in-depth analysis of the additional costs to the healthcare system. Additional qualitative and cost-effectiveness secondary data will be reported in future publications to further assess the sustainability of these interventions.
By design, this study purposefully incorporated the technology into the multidisciplinary team and peer education approaches to assess the combination of technology tools with promising self-management interventions, but future research may focus on studying the specific components to further evaluate the individual effects. Thus the current study provides insight on the effectiveness of a comprehensive educational approach, including mobile health tools, but insufficient information on the impact of mobile health tools alone.
Findings for total cholesterol, triglycerides, LDL-c, HDL-c, BMI, SBP, DBP, self-efficacy, depression, lifestyle, and quality of life did not show statistically significant differences among the study groups. The greater increase of HDL-c level in the control group needs to be further analyzed and may be due to the new 10-month group visit education model (DiabetIMSS). The small reductions in total cholesterol, LDL-c, SBP, and DBP may be due to the fact that patients were selected based on HbA1c, and thus baseline levels on the other indicators were largely near clinical targets at baseline for all three groups. These results are consistent with the mixed results in similar outcomes reported by the systematic review of Jackson et al.19 Future studies could focus on recruiting populations at higher risk of hypertension and dyslipidemia that have elevated blood pressure and lipid values in addition to poor glycemic control. Nevertheless, more emphasis on lipid management and access to more pharmacological options at the primary level are recommended for more significant reductions in these risk factors.
This study had several limitations. In this study, the mobile technology tools were actively only offered to the participants for the first 2 months of follow-up in order to coincide with the intensive phase of the self-management education. Based on the results at Month 4, a greater reduction among the groups might have been noted if the mobile technology was made available throughout the entire study period. Another limitation to this study included difficulties in obtaining a full dataset on all patients due to patients entering and leaving the IMSS healthcare system. This was especially due to temporarily or permanent changes in job or insurance coverage status. This challenge is not unusual for a study taking place in a setting like Eastern Tijuana with a highly mobile population and high employment rotation.39 This pattern is common and perhaps more pronounced in the uninsured population. Nevertheless, the intent-to-treat approach and multilevel analysis were used to include all the available measures of the patients enrolled in the study. In general, IMSS patients visit the clinic on a regular basis to receive their medications. This may be considered a potential bias for generalization to other uninsured patient populations. However, the fact that patients visited the clinic to receive their medications regularly was not enough to maintain adequate control. Additionally, temporary shortages of lipid-testing supplies reduced the number of laboratory studies conducted, especially at Month 4, resulting in a more limited analysis for lipid results.
In conclusion, the findings further add to the literature that the Project Dulce model with or without short-term mobile wireless technology offers an effective approach in low-income, publicly insured patients outside of the United States for improving glycemic control and disease knowledge in the management of patients with poorly controlled type 2 diabetes. In addition to technology, this study was able to incorporate and combine strategies known for their cost savings potential, including the use of nurses as chronic care coordinators, the use of outreach workers or peer educators in chronic disease management, the community-based and patient-centered approach, and the use of partnerships to maximize resources. Recent literature indicates that it is essential to evaluate the impact of combining self-management education approaches with innovative diverse technology tools versus evaluating individual component interventions.17 This study assessed the impact of combining these two methods and evaluating their effect on clinical and behavioral parameters. The observed declines in HbA1c levels of the PD-TE and PD groups indicate the feasibility and success of adapting the multidisciplinary care model of Project Dulce, with and without short-term mobile wireless technology tools, to the care of uncontrolled patients with type 2 diabetes in publicly insured populations of low- or middle-income countries. Future research should examine if a longer period of mobile technology intervention and larger sample-sized studies may lead to even greater and statistically significant effects on glycemic control.
Acknowledgments
Funding for this study was provided by Qualcomm Inc. and Iusacell, with the in-kind support of all partner organizations: the International Community Foundation, the Universidad Autónoma de Baja California, Fronteras Unidas Pro-Salud, Entra Health Systems, Scripps Whittier Diabetes Institute, and the Fundación Internacional de la Comunidad. National Center for Research Resources grant 1UL1 TR001114-01 supported the researchers at the Scripps Whittier Diabetes Institute. The International Community Foundation, a not-for-profit dedicated to expanding philanthropy in Mexico and Latin America, was responsible for managing the funds of this study. In particular, we recognize the vision of Richard Kiy and Dr. Manuel Acosta-Meza (deceased) for promoting this innovative collaboration. The authors especially recognize the dedication of IMSS UMF #27 nurses Clementina Mejia and Guadalupe Peña, ProSalud peer educators Karla Vasquez, Marisela Gonzalez, Enriqueta Dueñes, and Adriana Gutierrez, with Director Marcela Merino and Coordinator Dr. Veronica Avalos, IMSS UMF #27 family physicians Abraham Martinez, Orlando Salinas, Dulce Hernandez, Jair Montoya, Jesus Rivas, and Jessica Camarena, with IMSS social worker and administrators Eulalia Velasquez, Berenice Cota, and Dr. Clemente Martinez and engineers Daniel Gutierrez, Marco Bonilla, and Oscar Olivares, Adelaide Fortmann, Rachael Araujo, Aurelia Stephens, Norma Mendoza, and Chris Walker of the Scripps Whittier Diabetes Institute, Beatriz Alfaro of the Universidad Autónoma de Baja California faculty, students Illiana Ortiz, Ana Lucia Rivera, Erick Rivera, and Nohemi Gonzalez at the Universidad Autónoma de Baja California, IMSS volunteers Mario Espinoza, Jenny Gonzalez, and Isabel Gandarilla, and International Community Foundation staff Julieta Mendez, Courtney Corle, and Alicia Milla.
Author Disclosure Statement
No competing financial interests exist.
M.C.A.-C., S.C., R.M.-D., and A.V.-O. wrote the study protocol and design. M.C.A.-C. as Principal Investigator and S.C. as Co-Principal Investigator oversaw the implementation of the study. S.C. wrote the manuscript and researched literature. R.M.-D. conducted data analysis. A.F. helped with the hierarchical linear model software analysis. All authors contributed to the discussion, reviewed, and edited the manuscript. Dr. Manuel Acosta-Meza (deceased) contributed to the design and protocol development. Drs. Maria Luisa Zuñiga from San Diego State University and Evarista Arellano from the Universidad Autónoma de Baja California, Ensenada Campus, reviewed and provided feedback on one preliminary version of the manuscript. The sponsors of this study did not play a role in the study design, management, data collection, data analysis, writing, or reviewing of this manuscript. Only the authors contributed to the writing and reviewed this manuscript.
References
- 1.International Diabetes Federation: The IDF Diabetes Atlas. Sixth Edition. 2013. www.idf.org/diabetesatlas (accessed February23, 2014)
- 2.Díaz-Apodaca BA, Ebrahim S, McCormack V, de Cosío FG, Ruiz-Holguín R: Prevalence of type 2 diabetes and impaired fasting glucose: cross-sectional study of multiethnic adult population at the United States-Mexico border. Rev Panam Salud Publica 2010;28:174–181 [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation: North America and Caribbean, Mexico, 2014. www.idf.org/membership/nac/mexico (accessed February23, 2014) [Google Scholar]
- 4.International Diabetes Federation: North America and Caribbean, United States of America, 2014. www.idf.org/membership/nac/united-states (accessed February23, 2014) [Google Scholar]
- 5.Vázquez-Martínez JL, Gómez-Dantés H, Fernández-Cantón S: Diabetes mellitus in an adult population of the IMSS (Mexican Institute of Social Security). Results of the National Health Survey 2000 [in Spanish]. Rev Med Inst Mex Seguro Soc 2006;44:13–26 [PubMed] [Google Scholar]
- 6.Instituto Mexicano del Seguro Social: Programa Institucional del Instituto Mexicano del Seguro Social (PIIMSS) 2014–2018. Diario Oficial; 2014. www.imss.gob.mx/sites/all/statics/pdf/PIIMSS_2014-2018_FINAL_230414.pdf (accessed February8, 2016) [Google Scholar]
- 7.Keogh KM, Smith SM, White P, McGilloway S, Kelly A, Gibney J, O'Dowd T: Psychological family intervention for poorly controlled type 2 diabetes. Am J Manag Care 2011;17:105–113 [PubMed] [Google Scholar]
- 8.Instituto Nacional de Salud Pública: Encuesta Nacional de Salud y Nutrición 2012. Síntesis Ejecutiva. Subdirección de Comunicación Científica y Publicaciones, Instituto Nacional de Salud Pública, 2012. http://ensanut.insp.mx/doctos/ENSANUT2012_Sint_Ejec-24oct.pdf (accessed February8, 2016) [Google Scholar]
- 9.Clark M: Diabetes self-management education: a review of published studies. Primary Care Diabetes 2008;2:113–120 [DOI] [PubMed] [Google Scholar]
- 10.Duke SAS, Colagiuri S, Colagiuri R: Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2009;(1):CD005268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norris SL, Engelgau MM, Venkat Narayan KM: Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001;24:561–587 [DOI] [PubMed] [Google Scholar]
- 12.Philis-Tsimikas A, Gallo L, Brewer A, Dodgen-Bower P, Walker C: Peer-led diabetes education programs in high-risk Mexican-Americans improve glycemic control compared to standard approaches: a Project Dulce promotora randomized study. Diabetes Care 2011;34:1926–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philis-Tsimikas A, Gilmer TP, Schultz J, Walker C, Fortmann AL, Gallo LC: Community-created programs: can they be the basis of innovative transformations in our health care practice? Implications from 15 years of testing, translating, and implementing community-based, culturally tailored diabetes management programs. Clin Diabetes 2012;30:3–7 [Google Scholar]
- 14.Gilmer TP, Roze S, Valentine WJ, Emy-Albrecht K, Ray JA, Cobden D, Nicklasson L, Philis-Tsimikas A, Palmer AJ: Cost-effectiveness of diabetes case management for low-income populations. Health Serv Res 2007;42:1493–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal K, Eastwood SV, Farmer A, Barnard ML, Peacock R, Wood B, Edwards P, Murray E: Computed-based interventions to improve self-management in adults with type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2014;37:1759–1766 [DOI] [PubMed] [Google Scholar]
- 16.Free C, Phillips G, Watson L, Gali L, Felix L, Edwards P, Patel V, Haines A: The effectiveness of mobile-health technologies to improve health care service delivery processes: a systematic review and meta-analysis. PLoS Med 2013;10:e1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A: The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med 2013;10:e1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleveringa FGW, Gorter KJ, van den Donk M, van Gijsel J, Rutten GEHM: Computerized decision support systems in primary care for type 2 diabetes patients only improve patient's outcomes when combined with feedback on performance and care management: a systematic review. Diabetes Technol Ther 2013;15:180–192 [DOI] [PubMed] [Google Scholar]
- 19.Jackson CL, Bolen S, Bacanti FL, Batts-Turner ML, Gary TL: A systematic review of interactive computer-assisted technology in diabetes care. J Gen Intern Med 2006;21:105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franklin VL, Waller A, Pagliari C, Greene SA: A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet Med 2006;23:1332–1338 [DOI] [PubMed] [Google Scholar]
- 21.Liang X, Wang Q, Yang X, Cao J, Chen J, Mo X, Huang J, Wang L, Gu D: Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med 2011;28:455–463 [DOI] [PubMed] [Google Scholar]
- 22.Krishna S, Boren SA: Diabetes self management care via cell phone: a systematic review. J Diabetes Sci Technol 2008;2:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishna S, Austin Boren S, Balas EA: Healthcare via cell phones: a systematic review. Telemed J E Health 2009;15:231–240 [DOI] [PubMed] [Google Scholar]
- 24.Quinn CC, Barr EA, Shardell MD, Ballew SH, Terrin ML, Gruber-Baldani AL: Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011;34:1934–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Central Intelligence Agency: The World Factbook. Updated January 20, 2016. https://www.cia.gov/library/publications/the-world-factbook/geos/mx.html (accessed February8, 2016)
- 26.Efird J: Blocked randomization with randomly selected block sizes. Int J Environ Res Public Health 2011;8:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes Association: Standards of medical care in diabetes. Diabetes Care 2013;36(Suppl 1):S17–S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philis-Tsimikas A, Walker C, Rivard L, Talavera G, Reimann J, Salmon M, Araujo R; Project Dulce: Improvement in diabetes care of underinsured patients enrolled in Project Dulce. Diabetes Care 2004;27:110–115 [DOI] [PubMed] [Google Scholar]
- 29.Instituto Mexicano del Seguro Social: Tratamiento de la Diabetes Mellitus Tipo 2 en el Primer Nivel de Atención. Updated July 2014. www.imss.gob.mx/sites/all/statics/guiasclinicas/718GER.pdf (accessed February8, 2016)
- 30.Instituto Mexicano del Seguro Social: Guía técnica para otorgar atención médica en el módulo DiabetIMSS a derechohabientes con diagnósticos de diabetes mellitus, en Unidades de Medicina Familiar. Distrito Federal (México): IMSS, Dirección de Prestaciones Médicas, Unidad de Atención Médica, Coordinación de Áreas Médicas, 2009 [Google Scholar]
- 31.Instituto Mexicano del Seguro Social: Manual del Aplicador del Modulo Diabet IMSS. Distrito Federal (México): Dirección de Prestaciones Médicas, Unidad de Atención Médica, Coordinación de Áreas Médicas, 2009 [Google Scholar]
- 32.Lorig K, Stewart A, Ritter P, González V, Laurent D, Lynch J: Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks, CA: Sage Publications, 1996. pp. 24–25, 41–45 [Google Scholar]
- 33.Baader TM, Molina JL, Venezian S, Rojas C, Farías R, Fierro-Freixeneta C, Backenstras M, Mundt C: Validity and utility of PHQ9 (Patient Health Questionnaire) in the diagnosis of depression in user patients of primary care in Chile [in Spanish]. Rev Chil Neuropsiquiatr 2012;50:10–22 [Google Scholar]
- 34.López-Carmona JM, Ariza-Andraca CR, Rodríguez-Moctezuma JR, Munguía-Miranda C: Construcción y validación inicial de un instrumento para medir el estilo de vida en pacientes con diabetes mellitus tipo 2. Salud Publica Mex 2003;45:259–68 [PubMed] [Google Scholar]
- 35.López-Carmona JM, Rodríguez-Moctezuma R: Adaptación y validación del instrumento de calidad de vida Diabetes 39 en pacientes mexicanos con diabetes mellitus tipo 2. Salud Publica Mex 2006;48:200–211 [DOI] [PubMed] [Google Scholar]
- 36.García AA, Villagomez ET, Brown SA, Kouzekanani K, Hanis CL: The Starr County Diabetes Education Study. Development of the Spanish-language Diabetes Knowledge Questionnaire. Diabetes Care 2001;24:16–21 [DOI] [PubMed] [Google Scholar]
- 37.Ritter JB: World Campus. Lesson 9: Control Theory: How Do I Regulate My Behavior? Work Attitudes and Motivations. 2014. https://courses.worldcampus.psu.edu/sp14/psych484/001/content/lesson09/lesson09_01.html (accessed February23, 2014)
- 38.Erez M: Feedback: a necessary condition for the goal setting-performance relationship. J Appl Psychol 1977;62:624–627 [Google Scholar]
- 39.Carrillo J, Santibáñez Romellón J: Rotación de Personal en Las Maquiladoras Tijuana, 2nd ed. Mexico: Plaza y Valdez, SA, 2001 [Google Scholar]