Abstract
Background: The impact of subclinical hypothyroidism (SCH) and of levothyroxine replacement in pregnant women with SCH is unclear. The aims of this study were to assess (i) the impact of SCH during pregnancy on maternal and neonatal outcomes, and (ii) the effect of levothyroxine replacement therapy in these patients.
Methods: Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, the Cochrane Controlled Trials Register, Ovid EMBASE, Web of Science, and Scopus were searched from inception to January 2015. Randomized trials and cohort studies of pregnant women with SCH that examined adverse pregnancy and neonatal outcomes were included. Reviewers extracted data and assessed methodological quality in duplicate. Eighteen cohort studies at low-to-moderate risk of bias were included. Compared with euthyroid pregnant women, pregnant women with SCH were at higher risk for pregnancy loss (relative risk [RR] 2.01 [confidence interval (CI) 1.66–2.44]), placental abruption (RR 2.14 [CI 1.23–3.70]), premature rupture of membranes (RR 1.43 [CI 1.04–1.95]), and neonatal death (RR 2.58 [CI 1.41–4.73]). One study at high risk of bias compared pregnant women with SCH who received levothyroxine to those who did not and found no significant decrease in the rate of pregnancy loss, preterm delivery, gestational hypertension, low birth weight, or low Apgar score.
Conclusions: SCH during pregnancy is associated with multiple adverse maternal and neonatal outcomes. The value of levothyroxine therapy in preventing these adverse outcomes remains uncertain.
Introduction
Subclinical hypothyroidism (SCH) is defined as an elevated thyrotropin (TSH) concentration with normal serum levels of thyroxine (T4). Historically, the prevalence of SCH during pregnancy in the United States ranged from 2% to 2.5%. In contrast, overt hypothyroidism (OH; elevated TSH, low T4) has a prevalence of 0.2–0.5% (1). Recently, the normal range of TSH during pregnancy was redefined to an upper limit of 2.5 mIU/L during the first trimester and 3.0 mIU/L during the second and third trimesters (2). Applying the current diagnostic criteria, 15% of pregnant women in the United States have SCH, a fivefold increase in the prevalence of SCH (3).
In comparison with OH where there is clear evidence for adverse events, the impact of SCH on pregnancy is unclear (4). Multiple studies have reported an association of SCH with an increase in the risk of adverse pregnancy and neonatal outcomes, including pregnancy loss, preterm delivery, gestational diabetes, gestational hypertension, preeclampsia, placental abruption, premature rupture of membranes, intrauterine growth restriction, low birth weight, small for gestational age, low Apgar score, and neonatal death (5–16). Furthermore, high TSH levels in women during pregnancy have been associated with an increased risk of neurocognitive deficits in the offspring (17). Other studies, however, have not found any adverse outcomes associated with SCH (18–21). Moreover, there is uncertainty regarding the impact of levothyroxine replacement on improving outcomes in pregnant women with SCH (4). A previous systematic review in 2011 included five articles reporting on the adverse outcomes associated with SCH, and the meta-analysis included a maximum of three studies for each of the evaluated outcomes (22). In 2013, a Cochrane review on interventions for SCH during pregnancy did not identify any studies evaluating the effectiveness of levothyroxine therapy on maternal and neonatal outcomes (23). Since the publication of those two reviews, more studies have become available, which justifies a new quantitative synthesis of the available evidence.
A systematic review was therefore conducted, summarizing the evidence for the adverse clinical impact of SCH during pregnancy and for the value of levothyroxine therapy in mitigating that impact.
Methods
A systematic review and meta-analyses were performed to estimate (i) the impact of SCH compared to euthyroidism on maternal and neonatal outcomes in pregnant women, and (ii) the efficacy of levothyroxine therapy in preventing adverse maternal and neonatal events in pregnant women with SCH. This report follows a review protocol adhering to current standards for reporting of systematic reviews (24).
Eligibility criteria
To assess the impact of SCH on maternal and neonatal outcomes, randomized trials and cohort studies were sought that compared pregnant women with SCH to euthyroid pregnant women. Participants were pregnant women who had thyroid function tests during pregnancy to determine their thyroid status. SCH was defined as an elevated TSH concentration with normal serum T4 level (either total of free) or as an elevated TSH concentration between 2.5 and 5 mIU/L. To determine the impact of levothyroxine therapy, randomized trials and cohort studies were sought that compared pregnant women with SCH who received levothyroxine replacement therapy to those who did not. Studies in which the required information to determine eligibility was not available in the manuscript and where no response from the authors seeking that information was obtained were excluded. Studies that reported on a mixed population of SCH and OH during pregnancy were also excluded.
The main outcome measure was pregnancy loss (miscarriage, intrauterine death, fetal loss). Other outcomes included: preterm labor (onset of labor ≤37 weeks' gestation), preterm delivery (delivery ≤37 weeks' gestation), gestational hypertension (variously defined), preeclampsia (variously defined), eclampsia (variously defined), gestational diabetes (variously defined), placental abruption (premature separation of a normally implanted placenta), placenta previa (placental completely or partially covering the internal cervical os), premature rupture of membranes (PROM; variously defined), cesarean delivery, intrauterine growth restriction (IUGR; variously defined), low birth weight (≤2500 g), low Apgar score (≤7 at 5 min), small for gestational age (variously defined), and neonatal death (variously defined). Pregnancy loss was chosen as the main outcome because it is an outcome important to patients (25) that has significant consequences for pregnant women.
Study identification
A comprehensive search from each database's inception to January 2015 was conducted with no language restrictions. The databases included Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, the Cochrane Controlled Trials Register, Ovid EMBASE, Web of Science, and Scopus. An experienced librarian (P.J.E.) designed the search strategy with input from the study's principal investigator (S.M.). Controlled vocabulary supplemented with keywords was used to search for studies of SCH during pregnancy. The search strategy is available in Appendix 1. The reference list of narrative reviews was reviewed, and experts were consulted to identify additional references.
The search results were uploaded into a systematic review software (DistillerSR, Ottawa, Canada). Reviewers working independently and in duplicate reviewed all abstracts and titles for inclusion. After abstract screening and retrieval of potentially eligible studies, the full-text publications were assessed for eligibility with excellent chance-adjusted inter-reviewer agreement (κ statistic = 0.87). Duplicate studies were excluded. Disagreements were resolved by consensus.
Data collection and management
Reviewers working independently and in duplicate using a standardized web-based form collected the following information from each eligible study: (i) baseline clinical features: gestational age at screening, race/ethnicity, body mass index (BMI), history of smoking, previous pregnancy, pregnancy loss, and preterm delivery, family history of thyroid disease, use of in vitro fertilization/assisted reproduction to achieve the index pregnancy, and educational level; (ii) TSH, T4, and thyroid peroxidase (TPO) antibody levels; (iii) main and other outcomes. The definition of SCH used in each study was also extracted. Disagreements were resolved by discussion and consensus. Unclear data were confirmed with the study author when possible.
Risk of bias assessment
The Newcastle-Ottawa risk of bias tool for observational studies was used to evaluate the methodological quality of included studies (26). This tool determines the comparability of the cohorts, their representativeness, and the ascertainment of exposure and outcomes. For the study assessing the impact of levothyroxine on SCH-related pregnancy and neonatal outcomes, risk-of-bias criteria for causal inferences about therapy were adapted (27). Reviewers working independently assessed the risk of bias of included studies in duplicate. Any disagreements were resolved by consensus.
Author contact
To reduce reporting bias, the authors of studies in which clarification or more information was needed to determine eligibility or to complete analyses were contacted. If no response was received from an initial e-mail contact, authors were contacted again after a four-week period by e-mail.
Meta-analysis
Random-effects meta-analyses were conducted using the DerSimonian and Laird method (28) to pool relative risk (RR) and estimate 95% confidence intervals (CI) for each of the outcome measures. Inconsistency was assessed using the I2 statistic, with values <25% indicative of low and >75% indicative of high inconsistency not due to chance (29). Review Manager v5.2 was used for statistical analyses (30).
Subgroup and sensitivity analyses
Sensitivity analyses were conducted to explain possible inconsistencies across study results on the main outcome of pregnancy loss. To understand the effect of gestational age at screening for thyroid dysfunction, only studies that included women screened between 0 and 12 weeks of pregnancy (first trimester) and then studies that included women screened at early pregnancy (20th week of gestation or earlier) were analyzed. A sensitivity analysis omitting studies at high risk of bias was planned. Finally, a subgroup analysis was also planned based on the TPO antibody status of the study population.
Results
Study identification
The search identified 1108 potentially eligible studies, of which 18 cohort studies studying 3995 pregnant women with SCH were eligible (5–8,10–14,18–21,31–35) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/thy). One study by Casey et al. from 2005 (5) reported the same outcomes in an overlapping population with Casey et al. from 2007 (12) and was used for sensitivity analysis. Only one eligible observational study assessing the effect of levothyroxine therapy in pregnant women with SCH was found (11).
Study characteristics
Table 1 summarizes study characteristics. Due to inconsistent reporting, it was not possible to present data regarding BMI, history of smoking, history of previous pregnancy, pregnancy loss, and preterm delivery, family history of thyroid disease, use of in vitro fertilization/assisted reproduction to achieve the index pregnancy, TSH, T4, and TPO antibody levels. Five studies screened women for thyroid dysfunction during the first trimester of pregnancy, and 14 studies screened women during early pregnancy (20th week of gestation or earlier). There was no eligible study reporting on eclampsia.
Table 1.
Study Characteristics
| Author | Year | Country | SCH definition | SCH (N) | SCH-age (years) | Gestational week (weeks) | SCH main race/ethnicity |
|---|---|---|---|---|---|---|---|
| Negro | 2010 | Italy | TSH 2.5–5 mIU/L | 642 | 29.2 (5.0) | ≤11 | NA |
| Kumar | 2009 | India | TSH >5 mIU/L, fT4 normal | 13 | 25 (20–32)* | All trimesters | NA |
| Casey | 2007 | United States | TSH ≥3.0 mIU/L, fT4 normal | 598 | 26.6 (6.0) | ≤20 | 86% Hispanic |
| Caseya | 2005 | United States | TSH ≥97.5th percentile (>2.74–5.09 mIU/L) and fT4 >2nd percentile | 404 | 26.9 (5.9) | ≤20 | 84% Hispanic |
| Sahu | 2010 | India | TSH >5.5 mIU/L, fT4 normal | 41 | 27.2 (4.1) | 13–26 | NA |
| Cleary-Goldman | 2008 | United States | TSH >97.5th percentile (TSH >4.29 mIU/L), fT4 2.5th–97.5th percentile | 240 | 29.8 (5.7) | 10–13 | 91% white |
| Breathnach | 2013 | Ireland | TSH >98th percentile (4.1 mIU/L), fT4 normal | 16 | 27 (5) | Early 2nd trimester | 100% white |
| Wang | 2012 | China | TSH ≥2.5 mIU/L, fT4 normal | 196 | Tx 27.8 (0.3); Non-Tx 30.4 (0.9) | ≤12 | NA |
| Mannisto | 2009 | Finland | TSH >95th percentile (>3.6 mIU/L), fT4 5th–95th percentiles | 224 | 28.6 (5.8) | ≤20 | NA |
| Mannistob | 2010 | Finland | TSH >95th percentile (>3.6 mIU/L), fT4 5th–95th percentiles | 224 | 28.6 (5.8) | ≤20 | NA |
| Su | 2011 | China | TSH >95th percentile (>3.6 mIU/L), fT4 5th–95th percentiles | 41 | NA | ≤20 | NA |
| Yuan | 2013 | China | TSH elevated and fT4 normal based on self-sequential longitudinal reference interval | 67 | NA | All trimesters | NA |
| Korevaar | 2013 | Netherlands | TSH >97.5th percentile (TSH >4.04 mIU/L), fT4 normal | 188 | NA | Early pregnancy | NA |
| Feldthusen | 2014 | Denmark | TSH ≥3.4 mIU/L, fT4 normal | 19 | 30 (range 21–41) | 3rd trimester | 100% white |
| Chen | 2014 | China | TSH >97.5th percentile (1st trimester >3.47, 2nd trimester >3.81, 3rd trimester >4.99 mIU/L), fT4 2.5th–97.5th percentile | 371 | 26.3 (0.2) | All trimesters | NA |
| Ong | 2014 | Australia | TSH >2.15 mIU/L, fT4 normal | 117 | 32.5 (4.6) | 9–14 | NA |
| Liu | 2014 | China | TSH 2.5–10 mIU/L, fT4 normal | 959 | 29.5 (3.4) 29.9 (3.5) | 4–8 | NA |
| Jacob | 2012 | India | TSH >2.5 but ≤4 mIU/L | 263 | 26.2 (3.5) | 1st trimester | 100% Asian |
Data presented as mean (standard deviation).
Median (range).
This study reported the same outcomes in an overlapping population with Casey 2007 and was used for sensitivity analysis.
This study has the same population with the study Mannisto 2009 but reports different outcomes.
fT4, free thyroxine; NA, not available; Tx, treated group; Non-Tx, non-treated group; SCH, subclinical hypothyroidism; TSH, thyrotropin.
Study quality
The risk of bias of included studies comparing pregnant women with SCH to euthyroid women was low to moderate, mainly due to limitations in the representativeness of study samples, lack of blinding when assessing the outcomes, and lack of adjustment for confounders (Table 2). The risk of bias of the study assessing the effect of levothyroxine therapy in pregnant women with SCH was high due to lack of randomization and blinding.
Table 2.
Risk of Bias Assessment
| Author | Representativeness of exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts |
|---|---|---|---|---|---|---|---|---|
| Negro, 2010 | Somewhat representative | Same community | Secure record | Yes | Study does not control for additional factors | Independent blind | Yes | Lost to follow-up unlikely to introduce bias |
| Kumar, 2009 | Selected group | Same community | Secure record | Yes | Study does not control for additional factors | Record linkage | Yes | Complete follow-up |
| Casey, 2007 | Somewhat representative | Same community | Secure record | Yes | Study controls for maternal age | Record linkage | Yes | Complete follow-up |
| Casey, 2005 | Somewhat representative | Same community | Secure record | Yes | Study controls for maternal age | Record linkage | Yes | Complete follow-up |
| Sahu, 2010 | Somewhat representative | Same community | Secure record | Yes | Study does not control for additional factors | Record linkage | Yes | Lost to follow-up rate >20%, and no description of those lost |
| Cleary-Goldman, 2008 | Somewhat representative | Same community | Secure record | Yes | Study controls for maternal age | Record linkage | Yes | Lost to follow-up unlikely to introduce bias |
| Breathnach, 2013 | Somewhat representative | Same community | Secure record | Yes | Study does not control for additional factors | Record linkage | Yes | Lost to follow-up unlikely to introduce bias |
| Wang, 2012 | Somewhat representative | Same community | Secure record | Yes | Study does not control for additional factors | Record linkage | Yes | Lost to follow-up unlikely to introduce bias |
| Mannisto, 2009 | Truly representative | Same community | Secure record | Yes | Study controls for TPO | Record linkage | Yes | Complete follow-up |
| Mannisto, 2010 | Truly representative | Same community | Secure record | Yes | Study controls for maternal age | Record linkage | Yes | Complete follow-up |
| Su, 2011 | Truly representative | Same community | Secure record | Yes | Study controls for maternal age | Record linkage | Yes | Complete follow-up |
| Yuan, 2013 | Somewhat representative | Same community | Secure record | Yes | Study does not control for additional factors | Record linkage | Yes | Complete follow-up |
| Korevaar, 2013 | Truly representative | Same community | Secure record | Yes | Study controls for maternal age | Record linkage | Yes | No statement |
| Feldthusen, 2014 | Somewhat representative | Same community | Secure record | Yes | Study controls for maternal age | Record linkage | Yes | Complete follow-up |
| Chen, 2014 | Somewhat representative | Same community | Secure record | Yes | Study controls for maternal age | Record linkage | Yes | Complete follow-up |
| Ong, 2014 | Somewhat representative | Same community | Secure record | Yes | Study controls for PAPP-A | Record linkage | Yes | Lost to follow-up unlikely to introduce bias |
| Liu, 2014 | Somewhat representative | Same community | Secure record | Yes | Study controls for confounders | Record linkage | Yes | Lost to follow-up unlikely to introduce bias |
| Jacob, 2012 | Somewhat representative | Same community | Secure record | Yes | Unknown | Record linkage + self-report | Yes | Lost to follow-up rate >20%, and no description of those lost |
Meta-analysis
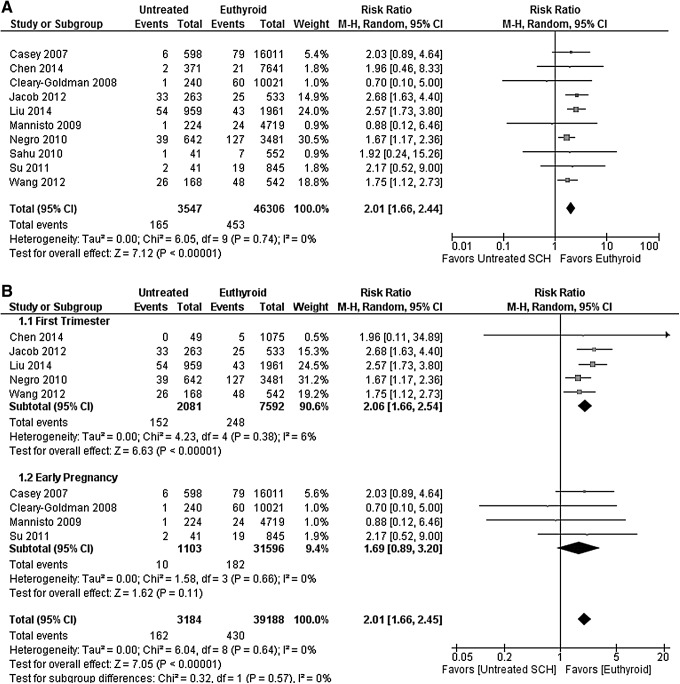
Table 3 presents the pooled estimates of association between SCH and pregnancy outcomes. Compared with euthyroid pregnant women, pregnant women with SCH had a higher risk of pregnancy loss (RR 2.01 [CI 1.66–2.44]; I2 = 0%; Fig. 1A), placental abruption (RR 2.14 [CI 1.23–3.70]; I2 = 0%), PROM (RR 1.43 [CI 1.04–1.95]; I2 = 9%), and neonatal death (RR 2.58 [CI 1.41–4.73]; I2 = 0%). There was no association found for gestational diabetes, preterm labor, preterm delivery, gestational hypertension, preeclampsia, placenta previa, cesarean delivery, IUGR, low birth weight, low Apgar score, and small for gestational age.
Table 3.
Pooled Relative Risk with 95% Confidence Interval Comparing Pregnant Women with SCH to Pregnant Euthyroid Women for All Pregnancy Outcomes
| Pregnancy outcome | Pooled RR [95% CI] | I2(%) | Studies used for meta-analysis |
|---|---|---|---|
| Pregnancy loss | 2.01 [1.66–2.44] | 0 | (6,7,10–12,14,18–20,35) |
| Preterm labor | 0.93 [0.58–1.51] | 0 | (18,32,34) |
| Preterm delivery | 1.20 [0.97–1.50] | 39 | (6–8,11–14,18–20,31,33–35) |
| Gestational hypertension | 1.22 [0.84–1.78] | 52 | (11,12,14,18,20,21,32,33) |
| Preeclampsia | 1.30 [1.00–1.68] | 0 | (12,13,18,21,33,34) |
| Gestational diabetes | 1.28 [0.90–1.81] | 44 | (12,14,18,20,21,32–35) |
| Placental abruption | 2.14 [1.23–3.70] | 0 | (12–14,18,21,32,34) |
| Placenta previa | 0.78 [0.19–3.18] | 0 | (14,18,34) |
| PROM | 1.43 [1.04–1.95] | 9 | (8,14,18,32,34,35) |
| Caesarean delivery | 1.06 [0.94–1.19] | 0 | (12,13,19,20,31,32) |
| IUGR | 1.70 [0.83–3.50] | 47 | (14,20,32,35) |
| Low birth weight | 1.34 [0.98–1.82] | 52 | (7,11,12,14,18,19,35) |
| Low Apgar score | 1.08 [0.71–1.65] | 0 | (11,19,34) |
| Small for gestational age | 1.17 [0.65–2.09] | 43 | (7,19,34,35) |
| Neonatal death | 2.58 [1.41–4.73] | 0 | (7,12,18,19,34,35) |
RR, relative risk; CI, 95% confidence interval; PROM, premature rupture of membranes; IUGR, intrauterine growth restriction.
FIG. 1.
(A) Forest plot of relative risk and 95% confidence interval (CI) of pooled studies comparing pregnant women with subclinical hypothyroidism to euthyroid pregnant women for risk of pregnancy loss. (B) Forest plot of relative risk and 95% CI of pooled studies comparing pregnant women with subclinical hypothyroidism to euthyroid pregnant women for risk of pregnancy loss (i) gestational age at screening at 0–12 weeks (first trimester) and (ii) gestational age at screening including 13–20 weeks (early pregnancy).
Wang et al. (11) screened pregnant women in the first trimester (≤12 weeks) for thyroid dysfunction. Women with SCH were recommended to start on levothyroxine, but only 14% received therapy. The study found an increased risk of pregnancy loss in pregnant women with SCH compared with euthyroid pregnant women (RR 1.75 [CI 1.12–2.73]). However, comparing 28 pregnant women with SCH who received levothyroxine replacement therapy to 168 women who did not receive levothyroxine, the study did not find a statistically significant decrease in the rate of pregnancy loss (RR 0.46 [CI 0.12–1.84]), preterm delivery (RR 0.31 [CI 0.02–5.13]), gestational hypertension (RR 3.00 [CI 0.28–31.99]), low birth weight (RR 0.65 [CI 0.04–11.71]), and low Apgar score (RR 0.65 [CI 0.04–11.71]) with levothyroxine therapy. The confidence in these results is limited due to the small sample size and number of events (imprecision), as well as the high risk of bias (most importantly selection bias).
Subgroup and sensitivity analyses
Figure 1B shows the result of the pre-planned sensitivity analysis on gestational age: including only studies in which women were screened for thyroid dysfunction between 0 and 12 weeks of pregnancy (first trimester) resulted in a RR for pregnancy loss of 2.06 [CI 1.66–2.54], while including studies in which women were screened throughout early pregnancy (20th week of gestation or earlier) resulted in an RR of 1.69 ([CI 0.89–3.20]; p-value for difference = 0.57). We did not find any studies at high risk of bias for the comparison between pregnant women with SCH and euthyroid pregnant women. Therefore, no sensitivity analysis was conducted to explore the effects of risk of bias.
In addition to the pre-planned sensitivity analysis, sensitivity analyses analyzing the impact of certain decisions made during the conduct of the study were performed. First, a sensitivity analysis was conducted in which the study from Casey et al. from 2007 (12) was replaced with the overlapping study from Casey et al. from 2005 (5). The analysis revealed similar pooled estimate results (data not shown). Second, a sensitivity analysis in which the unpublished study from Jacob et al. from 2012 (35) was removed resulted in similar pooled estimates (data not shown). Finally, a sensitivity analysis was performed in which both the study by Negro et al. from 2010 (6) and Jacob et al. from 2012 (35) were removed on the basis of their different SCH definitions with no significant change in the results (Supplementary Table S1).
Finally, due to insufficient data, it was not possible to perform a subgroup analysis based on the TPO antibody status of the study population.
Discussion
Summary of evidence
In this systematic review of 18 studies at low-to-moderate risk of bias including 3995 pregnant women with SCH, it was found that pregnant women with SCH were at higher risk for pregnancy loss, placental abruption, PROM, and neonatal death compared with euthyroid pregnant women. Only one observational study on the effect of levothyroxine in pregnant women with SCH was identified, but this study was at high risk of bias and yielded imprecise results.
Limitations and strengths
Incomplete searching and arbitrary study selection represent potential limitations of systematic reviews. However, the rigorous and comprehensive nature of the overlapping search strategies should have minimized the possibility that studies were missed that could substantially change the inferences drawn from the study. The risk of publication bias is high, particularly when the body of evidence is based on small observational studies. The results of this meta-analysis are driven by 10 larger studies that included >100 pregnant women with SCH. Despite the inconsistency in the gestational age at screening for thyroid function and in the definitions of SCH used, this seemed to contribute little to the variability in the estimates of association, which was moderate at worst. Although it would have been clinically meaningful, due to insufficient data, it was not possible to stratify the results by TPO antibody status. Another limitation is the paucity of evidence regarding the effect of levothyroxine replacement therapy in pregnant women with SCH. Although these limitations cannot be overcome methodologically, this review exhibited important strengths, as the study sought to summarize the totality of the available evidence following a predesigned protocol, reproducible judgments about study selection and quality, author contact, and focused analyses including an assessment of the effect of gestational age at screening for thyroid dysfunction that has not been performed previously (36).
Implications for practice and research
The present results support an association of SCH during pregnancy with adverse maternal and neonatal outcomes. This association may not be causal, and its magnitude may be overestimated by publication bias. Limitations and variability in study design reduce confidence in the results, and direct reliable evidence of the extent to which levothyroxine treatment in these women could improve pregnancy and neonatal outcomes is not available.
The study shows that there is inconsistency in the definitions of SCH used in the included studies, underscoring the lack of consensus among clinicians and researchers. Currently, in the absence of a laboratory trimester-specific reference range for TSH, a fixed cutoff of 2.5 mIU/L for the first trimester and 3 mIU/L for the second and third trimester is used (2). However, the reference range for TSH and free T4 can vary with geographic area and race/ethnicity (4,37). Indeed, 12/14 recent studies on reference range found that the upper trimester-specific TSH limit was >2.5 or 3 mIU/L, suggesting that a large number of pregnant women could be overdiagnosed with SCH and subsequently be overtreated when using a fixed TSH cutoff (37). Therefore, adapting a population-based reference range could lead to a more accurate diagnosis of SCH. The real challenge is to establish the TSH level above which women experience adverse pregnancy outcomes and levothyroxine therapy prevents these outcomes.
Indirect evidence for the effectiveness of levothyroxine in preventing pregnancy and neonatal complications is provided by one large randomized study designed to compare “universal screening” versus “case finding” in detecting thyroid dysfunction (38). Tests for thyroid function were performed immediately in women who were assigned in the “universal screening” group and in women at the “case finding” group only if there were deemed to be at high risk for thyroid dysfunction. In contrast, the serum samples were frozen and assayed after delivery for women at low risk for thyroid dysfunction in the “case finding” group. Hypothyroid pregnant women who were found to have a TSH >2.5 mIU/L and positive TPO antibody levels were started on levothyroxine. The study found that the proportion of hypothyroid women with at least one adverse obstetrical or neonatal outcome was significantly higher in the low-risk “case finding” group (not diagnosed and thus untreated, 91%) compared with the low-risk “universal screening” group (diagnosed and treated, 34%), suggesting a benefit from levothyroxine replacement. Moreover, adverse outcomes were less likely to occur among women in the “universal screening” group than among women in the “case finding” group (OR 0.43 [CI 0.26– 0.70]). This effect was driven primarily by adverse outcomes experienced by low-risk hypothyroid women (p = 0.005) who received treatment in the “universal screening” group but not in the “case finding” group. Given that there was no cutoff for T4 level, this study by design could have included pregnant women with OH who have more severe thyroid dysfunction compared with those with SCH and who are therefore at higher risk for adverse pregnancy and neonatal outcomes. Finally, the most commonly reported adverse outcome in this study was cesarean delivery with no further clarification as to the indication.
The same group conducted a prospective study where euthyroid TPOAb+ pregnant women were randomly assigned to levothyroxine therapy or no treatment, while TPOAb– pregnant women served as controls (39). The study found that euthyroid pregnant women who were TPOAb+ had an increased risk for pregnancy loss, which was mitigated with levothyroxine replacement.
A multicenter randomized trial assessed the impact of levothyroxine on the cognitive function among children of women who had TSH >97.5th percentile or free T4 <2.5th percentile, or both, during pregnancy (40). The treatment had no effect on the mean offspring IQ at three years or the proportion of children with IQ <85. A post hoc analysis for the subgroup of pregnant women who met the criteria for SCH had the same non-significant results.
Based on the analysis of the concurrent evidence, the American Thyroid Association (ATA) released their guidelines in 2011; the strength of each recommendation was graded according to the United States Preventive Services Task Force (USPSTF) system. In the USPSTF system, the strength of a recommendation is graded A, B, C, D, or I (if insufficient) based on the quality of the evidence (good, fair, poor, insufficient). The ATA guidelines recommend treatment of pregnant women with SCH and positive TPO antibodies (Level B, fair evidence—USPSTF), but found insufficient evidence to recommend for or against universal levothyroxine treatment in pregnant women with SCH and negative TPO antibodies (Level I—USPSTF) (2). The Endocrine Society, in addition to the USPSTF system, followed the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system to evaluate the strength of each recommendation and the quality of the evidence. The Endocrine Society panel recommends levothyroxine replacement in all pregnant women with SCH (weak recommendation, low-quality evidence) (41). Implementation of these guidelines will result in diagnosis with SCH and treatment with levothyroxine of up to 15% of pregnant women. The guideline panel acknowledges that the evidence is not sufficient, but focuses on accruing any potential benefit from levothyroxine replacement therapy, noting that any adverse effects such as iatrogenic thyrotoxicosis are rare. However, clinicians and their patients need reliable evidence to decide whether pregnant women with SCH will have better outcomes with levothyroxine replacement therapy.
The uncertainty documented in this review, the variability in expert recommendations, and the impact on the pregnancy experience of a large proportion of pregnant women underscore the need for randomized trials to estimate the effectiveness of levothyroxine therapy in this population, a point that has been emphasized by others (2,22,41,42). An ongoing randomized trial will hopefully offer some answers in the near future (clinicaltrials.gov/NCT00388297). However, high-risk pregnant women, such as women with multiple gestation pregnancy and with medical comorbidities have been excluded from this trial. Additional trials including high-risk pregnant women as well as women from iodine-deficient areas or with positive TPO antibodies may identify subgroups of patients in whom levothyroxine replacement therapy may be more likely to be beneficial. Moreover, within these trials, different TSH strata should be explored to identify the optimal treatment threshold where the benefits of levothyroxine use outweigh the risks (43). In the meantime, the extent to which 600,000 otherwise normal pregnant women in the United States with laboratory parameters consistent with the current definition of SCH are benefiting from taking levothyroxine therapy is unknown (42). Clinicians and pregnant women with SCH will need to discuss the potential benefits of levothyroxine therapy while taking into consideration the burden of treatment (i.e., daily pills, frequent tests, healthcare visits) and each woman's preferences and context.
Conclusions
The extant body of evidence supports an association of SCH during pregnancy with multiple adverse maternal and neonatal outcomes, but there is paucity of evidence for the value of levothyroxine therapy to mitigate this association. Clinicians and patients must engage in frank and shared decision making while awaiting the results of ongoing efficacy trials and the conduct of larger trials of levothyroxine therapy in high-risk women with SCH during pregnancy.
Supplementary Material
Appendix 1.
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present
| # | Searches | Results | Search type |
|---|---|---|---|
| 1 | Hypothyroidism/or hypothyroidism.mp. | 34,300 | Advanced |
| 2 | Thyroxine/ad, tu [Administration & Dosage, Therapeutic Use] | 6409 | Advanced |
| 3 | ((l adj thyroxin*) or levothyroxine).mp. or 2 [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 9424 | Advanced |
| 4 | thyroxine.mp. and hormone replacement therapy/ [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 516 | Advanced |
| 5 | or/2–3 | 9424 | Advanced |
| 6 | 1 and 5 | 4670 | Advanced |
| 7 | exp Pregnancy Complications/ or Pregnancy Trimesters/ or pregnancy.mp. or Pregnancy Maintenance/ or exp Pregnancy/ or Pregnancy Rate/ or Pregnancy Trimester, Second/ or Pregnancy Outcome/ or Pregnancy Trimester, Third/ or Pregnancy Trimester, First/ | 804,495 | Advanced |
| 8 | (pregnancy or pregnant or preterm or gestational or apgar or intrauterine or birth or prematur*).mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 1,046,192 | Advanced |
| 9 | abortion, spontaneous/ or premature birth/ or obstetric labor, premature/ or diabetes, gestational/ or stillbirth.mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 42,777 | Advanced |
| 10 | fetal growth retardation/ or infant, low birth weight/ or infant, very low birth weight/ or infant, extremely low birth weight/ or infant, small for gestational age/ | 37,380 | Advanced |
| 11 | hypertension, pregnancy induced/ or hellp syndrome/ or eclampsia/ or abruptio placentiae/ or pre-eclampsia/ or apgar score/ | 34,310 | Advanced |
| 12 | pregnancy outcome/ or miscarriage/ or fetal membranes, premature rupture/ or placenta previa/ | 55,760 | Advanced |
| 13 | Cesarean Section/ | 35,016 | Advanced |
| 14 | cesarean*.mp. or 9 or 10 or 11 or 12 or 13 [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 172,657 | Advanced |
| 15 | or/7–8,14 | 1,082,749 | Advanced |
| 16 | 6 and 15 | 701 | Advanced |
| 17 | limit 16 to (clinical trial, all or clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or clinical trial or comparative study or controlled clinical trial or evaluation studies or meta analysis or multicenter study or observational study or randomized controlled trial) | 73 | Advanced |
| 18 | 16 and (followup.mp. or follow-up studies/ or cohort*.mp. or prospective*.mp. or retrospective*.mp. or “cross-section*”.mp. or trial*.mp. or meta-analysis.mp.) [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] | 180 | Advanced |
| 19 | 17 or 18 | 203 | |
| Cochrane—same strategy, 21 | |||
| Embase 1988 to 2015 Week 04 | |||
| 1 | hypothyroidism/ or subclinical hypothyroidism/ | 31,682 | Advanced |
| 2 | thyroxine/ct, ad, dt | 5402 | Advanced |
| 3 | (l adj thyroxine*).mp. [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] | 2127 | Advanced |
| 4 | levothyroxine sodium/ or levothyroxine/ | 12,441 | Advanced |
| 5 | or/2–4 | 18,035 | Advanced |
| 6 | 1 and 5 | 7932 | Advanced |
| 7 | exp pregnancy/ or exp pregnancy complications/ | 377,906 | Advanced |
| 8 | spontaneous abortion/ | 22,634 | Advanced |
| 9 | exp labor complication/ | 106,863 | Advanced |
| 10 | exp pregnancy disorder/ | 312,598 | Advanced |
| 11 | intrauterine growth retardation/ | 16,924 | Advanced |
| 12 | exp low birth weight/ | 37,013 | Advanced |
| 13 | exp “eclampsia and preeclampsia”/ | 33,153 | Advanced |
| 14 | exp “parameters concerning the fetus, newborn and pregnancy”/ | 213,446 | Advanced |
| 15 | or/7–14 | 598,381 | Advanced |
| 16 | 6 and 15 | 976 | Advanced |
| 17 | exp evidence-based medicine/ | 687,624 | Advanced |
| 18 | exp cross-sectional study/ or exp incidence/ or exp seasonal variation/ | 420,077 | Advanced |
| 19 | follow up/ | 829,505 | Advanced |
| 20 | exp cohort analysis/ or exp correlational study/ or exp cross-sectional study/ or exp double blind procedure/ | 428,223 | Advanced |
| 21 | prospective study/ or retrospective study/ | 618,004 | Advanced |
| 22 | exp case control study/ or exp case study/ or exp clinical trial/ or exp “clinical trial (topic)”/ or exp longitudinal study/ or exp major clinical study/ or exp prospective study/ or exp retrospective study/ | 2,996,565 | Advanced |
| 23 | or/17–22 | 3,909,344 | Advanced |
| 24 | 16 and 23 | 321 | Advanced |
| 25 | limit 24 to human | 316 | Advanced |
| 26 | 25 not case report/ | 265 | Advanced |
WoS
TOPIC: (hypothyroid* AND (pregnan* OR trimester* OR birth OR gestation* OR abortion OR obstetric* OR prematur* OR fetus OR fetal OR foetus OR foetal OR preeclampsia OR “pre-eclampsia” OR hellp)) AND TOPIC: (levothyroxin* OR lt4 OR “l t4” OR thyroxin*) AND TOPIC: (outcome* OR trial* OR study OR studies OR series OR follow* or cohort* or prospective* OR retrospective* OR meta-analysis) AND TOPIC: (subclinical* OR “sub clinical*” OR asymptomatic* OR silent OR covert OR screen*) 396
Scopus
( TITLE-ABS-KEY ( : ( hypothyroid* AND ( pregnan* OR trimester* OR birth OR gestation* OR abortion OR obstetric* OR prematur* OR fetus OR fetal OR foetus OR foetal OR preeclampsia OR ”pre-eclampsia” OR hellp ) ) ) AND TITLE-ABS-KEY( ( levothyroxin* OR lt4 OR ”l t4” OR thyroxin* ) ) AND TITLE-ABS-KEY ( ( outcome* OR trial* OR study OR studies OR series OR follow* OR cohort* OR prospective* OR retrospective* OR meta-analysis ) ) AND TITLE-ABS-KEY ( ( subclinical* OR ”sub clinical*” OR asymptomatic* OR silent OR covert OR screen* ) ) AND NOT TITLE-ABS-KEY ( rats OR rat OR mice OR mouse OR rodent* ) ) AND ( LIMIT-TO ( DOCTYPE, ”ar” ) OR LIMIT-TO ( DOCTYPE, ”re” ) OR LIMIT-TO ( DOCTYPE, ”cp” ) 799
Acknowledgments
We are grateful to the authors of primary studies who responded to our requests for data confirmation and missing data (C. Daumerie, J. Walsh, J. Jacob, R. Negro, M. Penin, M. Poulasouchidou, and S. Prema). We would like to thank Dr. Ana Castaneda-Guarderas for technical support. This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, Faix JD, Klein RZ. 2000. Maternal thyroid deficiency and pregnancy complications: Implications for population screening. J Med Screen 7:127–130 [DOI] [PubMed] [Google Scholar]
- 2.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W. 2011. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 21:1081–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatt AJ, Nakamoto JM, Kaufman HW. 2012. National status of testing for hypothyroidism during pregnancy and postpartum. J Clin Endocrinol Metab 97:777–784 [DOI] [PubMed] [Google Scholar]
- 4.Negro R, Stagnaro-Green A. 2014. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 349:g4929. [DOI] [PubMed] [Google Scholar]
- 5.Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG. 2005. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 105:239–245 [DOI] [PubMed] [Google Scholar]
- 6.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. 2010. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 95:E44–48 [DOI] [PubMed] [Google Scholar]
- 7.Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, Xu YH, Tao FB. 2011. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 96:3234–3241 [DOI] [PubMed] [Google Scholar]
- 8.Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Muinck Keizer-Schrama SM, Hofman A, Ross HA, Hooijkaas H, Tiemeier H, Bongers-Schokking JJ, Jaddoe VW, Visser TJ, Steegers EA, Medici M, Peeters RP. 2013. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab 98:4382–4390 [DOI] [PubMed] [Google Scholar]
- 9.Tudela CM, Casey BM, McIntire DD, Cunningham FG. 2012. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 119:983–988 [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Shan Z, Li C, Mao J, Xie X, Wang W, Fan C, Wang H, Zhang H, Han C, Wang X, Liu X, Fan Y, Bao S, Teng W. 2014. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid 24:1642–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Teng WP, Li JX, Wang WW, Shan ZY. 2012. Effects of maternal subclinical hypothyroidism on obstetrical outcomes during early pregnancy. J Endocrinol Invest 35:322–325 [DOI] [PubMed] [Google Scholar]
- 12.Casey BM, Dashe JS, Spong CY, McIntire DD, Leveno KJ, Cunningham GF. 2007. Perinatal significance of isolated maternal hypothyroxinemia identified in the first half of pregnancy. Obstet Gynecol 109:1129–1135 [DOI] [PubMed] [Google Scholar]
- 13.Feldthusen AD, Larsen J, Pedersen PL, Kristensen TT, Kvetny J. 2014. Pregnancy-induced alterations in mitochondrial function in euthyroid pregnant women and pregnant women with subclinical hypothyroidism; relation to adverse outcome. J Clin Transl Endocrinol 1:e13–e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen LM, Du WJ, Dai J, Zhang Q, Si GX, Yang H, Ye EL, Chen QS, Yu LC, Zhang C, Lu XM. 2014. Effects of subclinical hypothyroidism on maternal and perinatal outcomes during pregnancy: a single-center cohort study of a chinese population. PLoS One 9:e109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. 2012. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol 119:315–320 [DOI] [PubMed] [Google Scholar]
- 16.Stagnaro-Green A, Chen X, Bogden JD, Davies TF, Scholl TO. 2005. The thyroid and pregnancy: a novel risk factor for very preterm delivery. Thyroid 15:351–357 [DOI] [PubMed] [Google Scholar]
- 17.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. New Engl J Med 341:549–555 [DOI] [PubMed] [Google Scholar]
- 18.Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF, Luthy D, Gross S, Bianchi DW, D'Alton ME. 2008. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 112:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Männistö T, Vääräsmäki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, Bloigu A, Järvelin MR, Suvanto-Luukkonen E. 2009. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J Clin Endocrinol Metab 94:772–779 [DOI] [PubMed] [Google Scholar]
- 20.Sahu MT, Das V, Mittal S, Agarwal A, Sahu M. 2010. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Gynecol Obstet 281:215–220 [DOI] [PubMed] [Google Scholar]
- 21.Männistö T, Vääräsmäki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, Bloigu A, Järvelin MR, Suvanto E. 2010. Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J Clin Endocrinol Metab 95:1084–1094 [DOI] [PubMed] [Google Scholar]
- 22.van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, Bisschop PH. 2011. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 17:605–619 [DOI] [PubMed] [Google Scholar]
- 23.Reid SM, Middleton P, Cossich MC, Crowther CA, Bain E. 2013. Interventions for clinical and subclinical hypothyroidism pre-pregnancy and during pregnancy. Cochrane Database Syst Rev 5:CD007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012 [DOI] [PubMed] [Google Scholar]
- 25.Guyatt G, Montori V, Devereaux PJ, Schunemann H, Bhandari M. 2004. Patients at the center: in our practice, and in our use of language. ACP J Club 140:A11–12 [PubMed] [Google Scholar]
- 26.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed June30, 2015)
- 27.Higgins JPT, Green S. (Eds) 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Available at: http://handbook.cochrane.org (accessed June30, 2015)
- 28.DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin Trials 7:177–188 [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. BMJ 327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014 [Google Scholar]
- 31.Kumar A, Agarwal K, Gupta RK, Kar P. 2009. Obstetric outcome in women with hepatitis C virus infection and thyroid dysfunction. Acta Obstet Gynecol Scand 88:1133–1137 [DOI] [PubMed] [Google Scholar]
- 32.Breathnach FM, Donnelly J, Cooley SM, Geary M, Malone FD. 2013. Subclinical hypothyroidism as a risk factor for placental abruption: evidence from a low-risk primigravid population. Aust N Z J Obstet Gynaecol 53:553–560 [DOI] [PubMed] [Google Scholar]
- 33.Yuan P, Wang Q, Huang R, Cao F, Zhu Z, Sun D, Zhou H, Yu B. 2013. Clinical evaluation with self-sequential longitudinal reference intervals: pregnancy outcome and neonatal thyroid stimulating hormone level associated with maternal thyroid diseases. West Indian Med J 62:28–34 [PubMed] [Google Scholar]
- 34.Ong GS, Hadlow NC, Brown SJ, Lim EM, Walsh JP. 2014. Does the thyroid-stimulating hormone measured concurrently with first trimester biochemical screening tests predict adverse pregnancy outcomes occurring after 20 weeks gestation? J Clin Endocrinol Metab 99:E2668–E2672 [DOI] [PubMed] [Google Scholar]
- 35.Jacob JJ, Aditya K, Achint S, Dhar T, Avasti K. 2012. Increased pregnancy losses and poor neonatal outcomes in women with first-trimester TSH levels between 2.5 and 4 mIU/L compared to euthyroid women with TSH less than or equal to 2.5. Endocr Rev 33:OR04-01 [Google Scholar]
- 36.Montori VM, Guyatt GH. 2003. Summarizing studies of diagnostic test performance. Clin Chem 49:1783–1784 [DOI] [PubMed] [Google Scholar]
- 37.Medici M, Korevaar TI, Visser WE, Visser TJ, Peeters RP. 2015. Thyroid function in pregnancy: what is normal? Clin Chem 61:704–713 [DOI] [PubMed] [Google Scholar]
- 38.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. 2010. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab 95:1699–1707 [DOI] [PubMed] [Google Scholar]
- 39.Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. 2006. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 91:2587–2591 [DOI] [PubMed] [Google Scholar]
- 40.Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, Chiusano E, John R, Guaraldo V, George LM, Perona M, Dall'Amico D, Parkes AB, Joomun M, Wald NJ. 2012. Antenatal thyroid screening and childhood cognitive function. New Engl J Med 366:493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. 2012. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97:2543–2565 [DOI] [PubMed] [Google Scholar]
- 42.Maraka S, O'Keeffe DT, Montori VM. 2015. Subclinical hypothyroidism during pregnancy—should you expect this when you are expecting?: A teachable moment. JAMA Intern Med 175:1088–1089 [DOI] [PubMed] [Google Scholar]
- 43.Maraka S, Ospina NS, Montori VM. 2015. Subclinical hypothyroidism overdiagnosis in pregnant women—reply. JAMA Intern Med. 175:1873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.