Abstract
Less than half of the 1.2 million HIV-infected individuals in the United States are in consistent medical care, with only a third receiving treatment resulting in viral suppression. Novel interventions to improve engagement are necessary to ensure medical adherence, improve long-term outcomes, and reduce HIV transmission. Mobile health (mHealth) strategies including cell phone and text messaging have shown success in the developing world for medical adherence, yet mHealth interventions have not been developed and evaluated to improve retention in HIV care in the United States. We conducted a 6-month pilot study investigating the use of a clinic-based bi-directional texting intervention to enhance engagement in HIV care among those with higher risk of loss to follow up, including those with a recent HIV diagnosis or those re-engaging in HIV care at a large urban clinic in New England.
Introduction
National estimates indicate that less than 30% of HIV-infected individuals in the United States receive the maximal benefit of HIV care and treatment with virologic suppression.1 HIV virologic suppression is a critical component of the “Treatment as Prevention” strategy to control the HIV epidemic.2–4 Retaining HIV-infected individuals in care is a significant hurdle to overcome: one in five HIV-infected patients will not establish care after their initial visit,5 and those returning to care have higher rates of morbidity and mortality compared to those with consistent medical care.6
Mobile technology-based methods (mHealth) may play a supportive role in improving self-care of HIV-infected individuals, particularly those at risk for disengaging with care, including newly diagnosed or those with a history of non-adherence. Prior research in the US has shown that technology-based HIV medication adherence interventions are promising, including those using stand-alone technology-based interventions7,8 and those with multi-component interventions including: a technology-based method, such as telephone support9,10 or text messages11,12 combined with individualized counseling appointments12 or group sessions,13,14 or a combination of the two.14 While the outcomes of these studies focused primarily on either prevention or adherence to medications, none of these studies examined the use of mHealth for improving consistent engagement with medical care among HIV-infected individuals.
We conducted a 6-month pilot study at a large urban health center in New England to investigate the use of a clinic-based bi-directional texting intervention with appointment and medication adherence reminders, additional supportive messages, and assistance with problem-solving to improve engagement in HIV care among patients who were newly diagnosed with HIV, re-engaging with HIV medical care, or those considered by their medical providers to be at risk for non-adherence to medications or appointments.
Methods
Settings and participants
The Miriam Hospital Immunology Center (TMH IC) is the largest provider of HIV primary care services in Rhode Island and the bordering regions of Connecticut and Massachusetts. The clinic provides care for 1600 active clients, with 80–100 newly diagnosed patients entering care annually, and 30–50 patients re-entering clinical care after a gap of greater than one year; the clinic population is 70% male, 61% white, 25% Hispanic, with the most common HIV risk factors as men who have sex with men (42%), heterosexual sex (36%), and injection drug use (16%).15
Recruitment
HIV-infected English-speaking patients age 18 and older with a cell phone capable of sending/receiving text messages who were either: newly entering into care within one year of diagnosis, re-engaging with medical care after a lapse of ≥1 year, or, in the opinion of their medical providers, were at risk for antiretroviral therapy (ART) or appointment non-adherence, were eligible to participate. Participants were recruited using two methods. First, patients newly entering into care or re-entering into medical care were approached at the time of their clinic intake visit with a social worker, which is routinely completed prior to the first visit with the medical provider. During the intake visit, eligible participants were asked if they would like to participate in this study. Study staff contacted eligible participants who expressed interest either on the same day of their intake visit or at a mutually convenient time. Additionally, participants noted to have difficulty engaging in longitudinal care in the opinion of their primary HIV providers were referred to the study team and were contacted by phone.
Study design
Individuals who met eligibility criteria were offered enrollment in a 6-month pilot study of a bi-directional texting intervention, which included adherence reminders and appointment reminders with optional additional supportive messaging. Study assessments were completed at baseline, month 3, and month 6; and monthly queries by study staff were conducted to determine if changes in the frequency and content of messages were desired. At the end of study, participants were asked to complete an individual in-depth interview about their experiences with participating in the intervention.
Baseline assessment
All participants completed a demographic questionnaire including age, race/ethnicity, marital status, education level, income, insurance, employment, health care utilization, and distance traveled for HIV care. Medical information was abstracted from their clinic charts including: HIV testing history, length of diagnosis, most recent immunologic and virologic lab results within 60 days of study entry, and ART history. Participants completed study assessments investigating stigma, substance use, depression, and perceived barriers and facilitators of HIV care. Stigma was assessed using the 28-item internalized HIV stigma measure.15 Substance use was measured with the 11-item Drug Use Disorders Identification Test and the 3-item Alcohol Use Disorders Identification Test.16,17 Depression was assessed using the 9-item Patient Health Questionnaire.18 Each of these measures are valid and reliable and have been used with HIV patient populations in prior research.
Perceived barriers and facilitators to HIV care
Assessments on perceived barriers and facilitators to HIV care were adapted from a measure developed by the Centers for Disease Control and Prevention Medical Monitoring Project, a surveillance study designed to learn more about the experiences and needs of people who are receiving care for HIV.16 Participants were asked to identify their own barriers and facilitators to HIV care; if they could not identify at least three from each category on their own, they were offered a list of barriers and facilitators to select from in order to help facilitate their responses. They were then asked to rank in order of impact up to three responses for both barriers and facilitators.
Text messaging
All participants received self-selected, text messages targeted towards: (1) daily medication adherence, (2) appointment adherence, and (3) addressing barriers to retention in care. Participants could create their own messaging or select from a table of pre-scripted messages. Participants were instructed that they could text back any questions or comments that they may have to the interventionist (a BA-level trained research assistant) with an expected response of 24 h during weekdays and 48 h during weekend. If the participant requested assistance, the interventionist provided assistance via cell phone for issues such as transportation or appointment scheduling. The content of the messages and frequency of messaging was determined between the interventionist and participants at the baseline visit (Table 1).
Table 1.
Sample Text Messaging
| Appointment reminder messages | Pre-selected: |
| • You're worth it – remember your clinic appointment | |
| • Call your case manager – he/she can help you get to clinic | |
| • Can't remember when your next appointment is? Call the clinic to find out. | |
| Participant-created: | |
| • Don't forget about your doctor's appointment…love, Godzilla | |
| • Your doctor wants you to come to your appointment | |
| Medication adherence reminder sent to participant on a selected schedule. | Pre-selected |
| • Meds keep your body strong and healthy. | |
| • Give meaning to your life … Now! | |
| • Hey, take your vitamins! | |
| Participant-created message | |
| • Take at 5 | |
| • [daughter's name] said to take your medicine | |
| A barrier to care reminder message is sent to participant between registration and first check-in appointment | Pre-selected: |
| • Need to go to my XX meeting. | |
| • Can't get your prescriptions? Call your clinic or | |
| • case manager. | |
| Participant-created message | |
| • Don't forget about God! | |
| • Recovery is important | |
| • Don't forget about your meeting! | |
| • Stop your smoking! |
Participants received monthly calls from the interventionist to inquire whether changes in messaging content or frequency were desired. All participants received an administrative message to confirm receipt of the initial text message. To maintain confidentiality with either pre-scripted or customized messages, messaging could not include participant names, reference to the Immunology Center, HIV infection, or HIV medications.
Retention in care content
Text messages were sent to remind the participant about his/her upcoming clinic appointment. This was a short message that included the date and time of the appointment and a request to contact the clinic if the participant anticipated any difficulties in attending the scheduled appointment. Appointment reminders were sent at the following frequency: once weekly reminder sent 3 weeks, 2 weeks, and 1 week before the scheduled clinic appointment; and a once daily reminder sent 2 days, and 1 day before clinic appointment.
Adherence to ART content
For participants prescribed ART (n = 30, 94%), a daily customizable text message was sent to remind the participant to take his/her ART. Given the potential for participants to find daily reminders intrusive, participants had the option of changing the frequency of ART and appointment reminders every month.
Barriers to care content
Finally, given evidence that counseling techniques are effective in enhancing ART adherence17 and reducing HIV-risk behaviors18–20 that also impact adherence to HIV care, problem-solving communication messages were provided in order to further promote retention to care and medication adherence by addressing potential barriers to treatment adherence. Participants had the option to choose a pre-scripted message or to create a customized message addressing potential barriers to care that were assessed at the baseline visit, such as transportation arrangements or attending substance use support groups.
Quantitative analysis
Patient demographics, baseline CD4 and HIV PVL, and measurement responses (stigma, substance use, and depression) were summarized using means, medians, and ranges for the continuous measures and proportions for categorical and ordinal measures. Barriers and facilitators to care were summarized as primary, secondary and tertiary at each study visit. Data on the frequency and content of the text messaging were collected.
To measure adherence to medical care for the 6-month period of this study, we tracked both the number of medical visits attended by each participant after study entry and medical appointment adherence: the proportion of scheduled visits attended by each patient over the 6-month study observational period.21 As an exploratory analysis, we compared differences in the proportion of patients with virologic suppression from the beginning and end of the study using sample t-tests with a p value less than 0.05 considered statistically significant.
Qualitative data and analysis
All participants who completed the intervention were asked to complete an individual in-depth interview regarding the intervention including acceptability of the content, delivery method, and frequency of appointment reminders and ART reminders, as well as the value of the intervention perceived by the participant. Interviews were conducted in a private setting and were conducted by a doctoral-level investigator experienced in qualitative methods and HIV treatment adherence research. A semi-structured interview guide was used which allowed the interviewer and participant to discuss topics freely as appropriate. Interviews were digitally recorded and transcribed verbatim. Transcripts were coded initially individually by three members of the study team trained in qualitative interviewing. After the interviews were independently coded, the three coders convened to discuss and critically describe, analyze, and justify identified themes. A subsequent consensus meeting was held to resolve any coding discrepancies. Myriad themes related to the outcomes of interest were organized by topic, theme, and excerpt in the qualitative data management program, NVivo (Version 10, Los Angeles, CA).
Informed consent and reimbursement
All study procedures were reviewed and approved by The Miriam Hospital Institutional Review Board. All participants were compensated $20 after completion of the baseline study visit, $20 for a 3-month interim visit, $20 for completion of the final 6-month visit, and an additional $35 for completion of an individual in-depth interview during the final visit. Participants were also given $20 at the baseline visit, and an additional $20 at the 3-month visit to compensate for text messaging services delivered via their personal phones.
Results
From January to July 2013, 58 patients were screened for the study, 32 were enrolled (Fig. 1), and 20 completed all study assessments. Of note, only two individuals were not eligible due to lack of a cell phone that sends/receives text messages. The participants were 69% male, 66% white, 19% Hispanic, 81% with high school education or higher, with the majority low-income (72% reporting <$1250/month; Table 2). The median age was 36. Approximately half (n = 13, 43%) of them reported HIV risk as men who have sex with men, 35% reported heterosexual intercourse, and three (9%) reported sharing IV drug needles as their primary risk. Almost all subjects (94%) reported being prescribed ART, though 44% had a baseline HIV PVL >200 copies/mL and 16% had a baseline CD4 +T cell <200/μL (median 509/ μL).
FIG. 1.
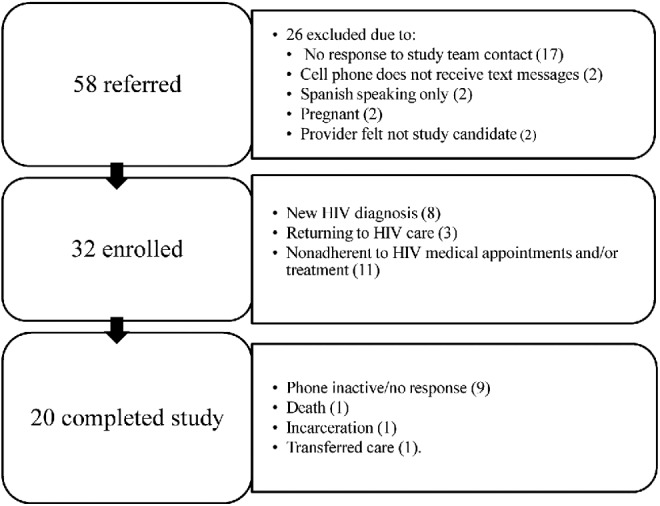
Study enrollment.
Table 2.
Baseline Demographics
| n | %a | |
|---|---|---|
| Age: Median = 36 (range 19–64) | ||
| 18–25 | 5 | 16% |
| 25–34 | 9 | 28% |
| 35–44 | 6 | 19% |
| >45 | 12 | 38% |
| Gender | ||
| Male | 22 | 69% |
| Female | 10 | 31% |
| Ethnicity | ||
| Hispanic | 6 | 19% |
| Non Hispanic | 26 | 81% |
| Race | ||
| Native American | 1 | 3% |
| Black or African American | 7 | 22% |
| White | 20 | 66% |
| Multiracial | 2 | 6% |
| Education | ||
| <High school degree | 6 | 19% |
| High school degree | 12 | 38% |
| Some college or higher | 14 | 44% |
| Income | ||
| <$1250/month | 23 | 72% |
| $1250–3000/month | 5 | 16% |
| >$3000/month | 1 | 3% |
| Employment status | ||
| Not working | 15 | 47% |
| Working full time | 4 | 13% |
| Working part time | 3 | 9% |
| Other | 10 | 31% |
| Health insurance | ||
| Yes | 23 | 72% |
| No | 9 | 28% |
| Last HIV medical visit | ||
| Within 12 months | 8 | 25% |
| >12 months | 2 | 6% |
| >6 months | 1 | 3% |
| Regularly | 21 | 66% |
| Prescribed HIV medications | ||
| Yes | 30 | 94% |
| No | 2 | 6% |
| HIV risk factor (n = 20) | ||
| MSM | 10 | 50% |
| Heterosexual | 7 | 35% |
| Sharing IV needles | 3 | 15% |
| CD4b | ||
| <200 | 5 | 16% |
| HIV PVL >200 copies/mLb | 14 | 44% |
Percentages may not equal 100 due to rounding; bobtained within 60 days of study entry.
Psychosocial measures
Participants completed assessments of stigma, substance use, and depression at baseline (n = 32) and at final study visit (n = 20). At baseline, participants reported high levels of stigma (median score = 67, range = 45–93), high levels of substance use (male median score = 7.5, female median score = 4), moderate alcohol use (male median score = 4.5, female median score = 5), and mild to moderate levels of depression (median score = 9, range = 0–18). There were no significant changes in the psychosocial measures between the baseline and final study visits among those who completed all study visits (n = 20).
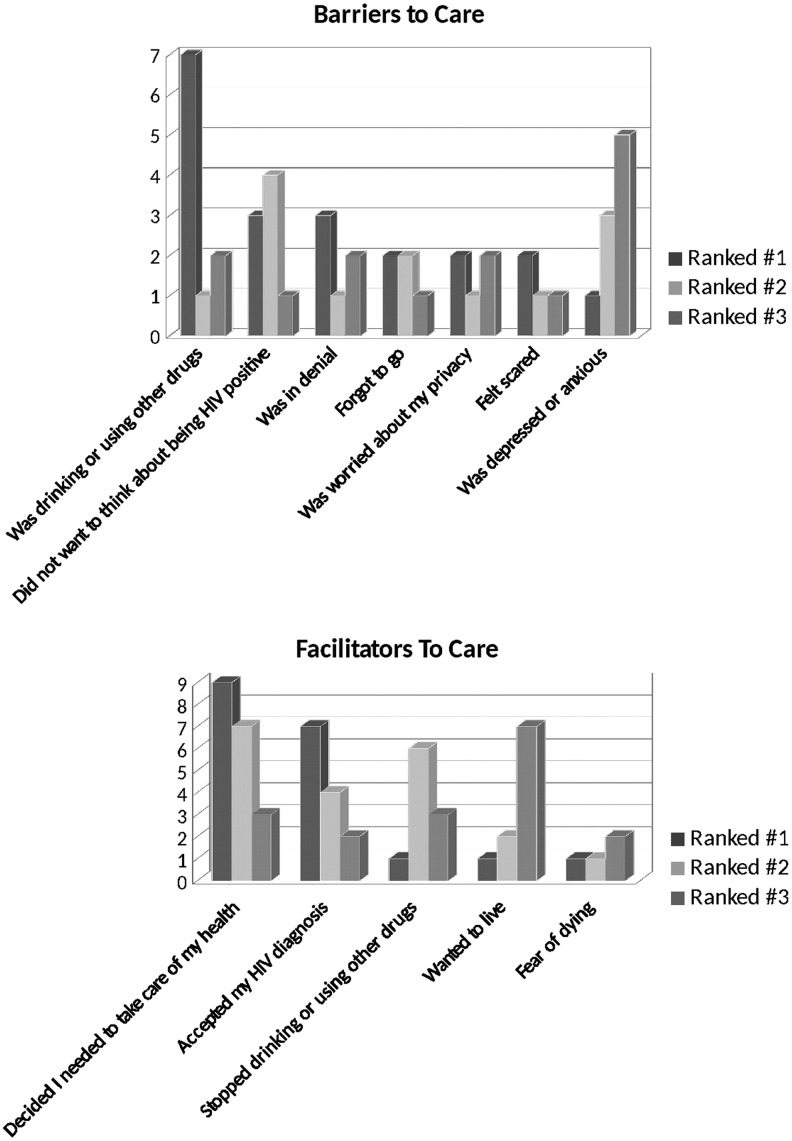
Barriers and facilitators
Substance use was reported as a barrier to care by 15 participants, with 7 (22%) reporting it as their primary barrier. Denial (n = 14, 48%) and “did not want to think about being HIV positive” (n = 19, 66%) were commonly reported as both primary and secondary barriers. Depression was reported as a barrier by most of the cohort (n = 20, 69%), though few listed it as a primary barrier (Fig. 2). Most patients ranked “deciding to take better care of my health” (n = 19, 59%) and “acceptance of HIV diagnosis” (n = 13, 41%) as either their main facilitators of medical care, followed by “cessation of substance use” (n = 10, 31%) and “the will to live” (n = 10, 31%).
FIG. 2.
Barriers and facilitators to HIV care.
Messaging
Participants chose confidential pre-selected (69%) or self-created (31%) medication and appointment text-message reminders. Eight participants opted for a third text message that included reminders for substance use/abuse support meetings, smoking cessation, or supportive messaging. Participant-initiated messages included inquiries on clinic appointments (5), study visits (13), clinic contact information (2), and notifications of number change (4). None of the participants modified the frequency of their messaging, although three participants modified the content of their messaging.
Retention in care and virologic suppression
As a measure of engagement with care, two (6%) participants did not complete any medical visits during the 6-month period after study entry, 30 (94%) participants completed at least one visit, and 23 (72%) participants completed two visits. Appointment adherence, defined as the proportion of scheduled visits attended, in this cohort averaged 79.1% (range 0–100%) with two patients (6%) not keeping any scheduled visits, seven patients (22%) keeping only one-half of scheduled appointments, and fifteen patients ( 47%) with 100% appointment adherence. Eighteen of the 32 (56%) participants had an HIV PVL <20 copies/mL at baseline; by the end of the study, 25 (78%) participants were virologically suppressed (<20 copies/mL, p = 0.002).
Twenty participants (62.5%) completed all visits in the 6-month study: All 32 completed baseline assessments, 28 completed visit 2, and 20 completed visit 3. For the 12 who did not complete all study visits and were lost-to-follow-up, reasons included: phone inactive/no response (9), death (1), incarceration (1), and transfer of care (1). Of those 12, four completed only the baseline visit and eight completed visit 2. There were no statistically significant differences in baseline characteristics of patients (demographics or assessment measures including stigma, substance use, and depression) between those who did (n = 20) and did not (n = 12) complete all three study visits.
Qualitative results
All twenty participants who completed visit 3 (final visit) were invited to participate in an in-depth qualitative interview investigating their views on the acceptability and feasibility of the mHealth intervention, the content and types of text messages, and the impact of the intervention on their medical engagement; all 20 participants who completed visit 3 participated in the interview.
Acceptability and feasibility of the mHealth intervention
The majority of participants responded favorably to receiving the daily medication reminders, as well as the reminders of their scheduled medical appointments for HIV care. Many of them indicated that they liked how easy and convenient it was to receive the messages on their personal cell phones, such as noted by this one participant: “I think the texts are so modern. They're easy. They're quick. Everyone can receive them. Most people nowadays, they have unlimited text on their plan.” Several participants also reported that the texts assisted them with incorporating their HIV medication into their daily routine and that receiving the messages eventually became a conditioned response for them to take their medication, like the following participant who said: “By the end of the study, it was so in my head that I was taking it [HIV medication] sometimes before I would get the text.”
Another frequent theme was the perception of increased support through receipt of the messages regardless of their specific content as conveyed by this one participant: “I like it when it [the phone] goes beep-beep. I know who it is. I'll be cooking…I know who it is. It's just like my heart opens up. I don't feel alone.” Another participant felt the messaging conveyed a responsible connection that someone was keeping them accountable for their actions: “A text message reminder every day would be beneficial for people. It feels a little bit more accountable when someone's actually taking their time to text message you…” Many participants indicated that they appreciated knowing that there was an actual person on the other end sending the messages to them, which made it more personal than if it was just an automated system as this one participant conveys: “I was talking to a human being, so that's what makes it more worth it. If I'm talking to a recording and they're recording me, it's almost like that wasn't worth it at all.”
Content and types of text messages
Several participants remarked on how much they liked the customizability and personalized messaging, such as the following participant who said that it was important for her to hear her daughter's voice in the messages to inspire her to take her medication:“…I personalized my texts sayin' that my daughter's tellin' me to take my medicine. That helped me out a lot.” Similarly, many participants indicated that the personalized messaging itself facilitated ownership over their actions in order to continue to live, such as conveyed by this participant: “We came up with the one, ‘Give meaning to your life’ and I liked it. I guess it's kind of symbolic in a weird way. Taking a pill means I wanna live.” They also commented positively on the ability to change their messaging if they chose to do so as noted by this participant here: “That part of the study was good because you had the latitude of puttin’—changin’ it, addin’ it, or subtracting it, and making it into what you want.’
Most participants wanted to be able to personalize specific times of the messaging in accordance with their medication dosing times rather than the routine scheduled morning or afternoon texts sent by the study team. In addition, some participants stated that they did not understand that the communication between them and study staff was bi-directional (that they could respond back to the messages or initiate communication with clinical questions if they so desired), such as indicated by this one participant: “I wanted some affirmation or reassurance. Not that the texts were cold and impersonal because they're not, but there was a part of me that wanted to say, ‘hey, thanks.’ I guess that would be my biggest thing. I coulda used a little more coddling at that time.”
Impact of mHealth intervention on participant's medical engagement or self-care
A few participants also discussed how the messaging increased their engagement in medical care or self-care. One participant commented how the messaging can empower a patient to remain engaged with medical care: “…You just want to stay in bed. Then you get that text message, ‘Don't forget your health comes first. Don't forget your doctor's appointment.’ It's like, ‘Yeah, you know what? Let me get up and take this pill. Let me go see the doctor.” Another participant stated the following that it reminded them in the moment that they had a medical appointment: “It [the text] let me know, okay, this is what I gotta do today…I can't forget this appointment because this is what I got to do.” Some participants also felt empowered by the messaging to take additional steps to improve their self-care as noted by this participant: “Since I knew this study was coming to an end eventually…there are a number of apps out there, but I did settle on one and found one that it's like a virtual pill box. It gives you those text messages and things.”
Discussion
Our findings support the use of a clinic-based bi-directional mHealth intervention among patients at risk for disengaging with medical care given the prevalence of cell phone use, text messaging, and acceptability of the intervention among the study participants. This is particularly important since these participants were at an increased risk for loss to follow-up due to their status as being newly diagnosed, returning to care, or having a prior history of non-adherence to care and treatment. Additionally, participants identified multiple barriers to care including substance use, denial, and depression. While this intervention was staff-delivered with messaging sent at particular times of the day, participants preferred the ability to customize timing and frequency, which would require an automated system.
Even though only 20 of the 32 (62%) participants completed all study procedures through 6 months of follow-up, most participants' loss to follow-up was due to the inability of staff to reach participants to complete the study visits. This is likely reflective of the challenges often associated with this particular population of patients at increased risk for loss to follow-up. A recent study of a cell phone-based supportive intervention for HIV-infected youth with a history of adherence problems had a similar completion rate for the intervention.10 In the clinical setting, mHealth interventions could play a supportive role in conjunction with other interventions to retain the most challenging patients in care where primary clinic communication has halted, such as community-based case management or assistance from integrated public health programs to retain individuals in care.
In the current study, participants were provided with the opportunity to change medication message frequency after 1 month of daily messaging; no participants chose this option, though during the individual in-depth interview at the end of the study, several participants commented that this would have been a good option and denied text message fatigue. Prior studies investigating message fatigue suggest frequent messaging may decrease quality of life,8 and changing messaging content and frequency may serve to counteract this problem.22 Our study followed participants for 6 months. With a longer follow-up period, participants might have changed the frequency of messaging. Effectively communicating the ability to change the frequency of the text messages should be emphasized and reiterated multiple times with participants in future research.
Most participants (94%) engaged with their medical provider at least once during the study period, with the majority seeing their providers at least twice within 6 months, and almost half making it to every appointment scheduled by their HIV provider. Of the 20 participants who completed the individual in-depth interviews, the combination of the dedicated interventionist and messaging improved their perception of support. Intervention studies using mHealth that included a combination of individualized counseling sessions, as well as choices of adherence aids, seem to produce the most beneficial effects on medication adherence23 and could be expanded to improve overall engagement with medical care.
It is interesting to note that there was limited use of the bi-directionality option by participants in this study. During the individual in-depth interview, some participants noted that they did not understand they could initiate communication for clinical needs with the messaging, which may partly explain the lack of use. However, most participants indicated that they did not feel the need to text the interventionist back unless they had specific questions about the date and time of their medical appointments. Expansion of this intervention would need to emphasize the role of patient-initiated communication to support engagement with care.
While this study, and other research and policy recommendations point to acceptability and potential benefits of using a mHealth approach to improving adherence to treatment,7–9,11–14 implementation into routine medical care will require further development. In this study, messages were sent manually on a daily basis by a research interventionist using a platform that is separate from the clinical medical record. While we found acceptability and feasibility of this intervention format, this was a small pilot and a larger trial of bi-directional texting with enhanced features is necessary to comprehensively assess the effect of this intervention. In design, a clinic-wide integrated program would ideally be automated, easily customizable, and allow bi-directional communication with medical providers while still maintaining confidentiality.
Additionally, this communication would become part of the existing electronic health record infrastructure to minimize duplication of services as well as to allow access of these communications to all necessary medical staff. A cost analysis will need to be performed as well, taking into consideration need for updated software platforms and ongoing staff training. In this study, participants used their own personal phones which typically had free text messaging plans, with messages sent from the study team using a free web-based messaging service. This approach, while minimizing cost, is not necessarily scalable for integrated clinical use.
Conclusions
Keeping HIV-infected patients connected to care is a major health care and public health priority as improving patient retention will reduce mortality, co-morbidities, and transmission. This study contributes to the growing research supporting the use of mHealth to improve self-care of HIV-infected patients by incorporating appointment reminders, supportive messaging, and assistance to address barriers with a dedicated interventionist. Future studies should explore integration of mHealth strategies into the clinical setting. With the expansion of electronic medical records (EMR) in clinical practice, integration of mHealth within existing EMR should be a priority of future research.
Acknowledgments
This research was supported by a Lifespan Developmental grant (AIR), the Lifespan/Tufts/Brown Center for AIDS Research (P30AI042853), and by supporting grants from the National Institute of Mental Health (AIR 1K23MH100955). The authors would also like to thank Liem Tran for his support on the study.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Bradley H, Hall HI, Wolitski RJ, et al. . Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR 2014;63:1113–1117 [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N Engl J Med 2011;364:1943–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. . Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011;52:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr 2012;60:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ndiaye B, Ould-Kaci K, Salleron J, et al. . Characteristics of and outcomes in HIV-infected patients who return to care after loss to follow-up. AIDS 2009;23:1786–1789 [DOI] [PubMed] [Google Scholar]
- 7.Andrade AS, McGruder HF, Wu AW, et al. . A programmable prompting device improves adherence to highly active antiretroviral therapy in HIV-infected subjects with memory impairment. Clin Infect Dis 2005;41:875–882 [DOI] [PubMed] [Google Scholar]
- 8.Safren SA, Hendriksen ES, Desousa N, Boswell SL, Mayer KH. Use of an on-line pager system to increase adherence to antiretroviral medications. AIDS Care 2003;15:787–793 [DOI] [PubMed] [Google Scholar]
- 9.Reynolds NR, Testa MA, Su M, et al. . Telephone support to improve antiretroviral medication adherence: A multisite, randomized controlled trial. J Acquir Immune Defic Syndr 2008;47:62–68 [DOI] [PubMed] [Google Scholar]
- 10.Belzer ME, Kolmodin MacDonell K, Clark LF, et al. . Acceptability and feasibility of a cell phone support intervention for youth living with HIV with nonadherence to antiretroviral therapy. AIDS Patient Care STDS 2015;29:338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairley CK, Levy R, Rayner CR, et al. . Randomized trial of an adherence programme for clients with HIV. Int J STD AIDS 2003;14:805–809 [DOI] [PubMed] [Google Scholar]
- 12.Simoni JM, Chen WT, Huh D, et al. . A preliminary randomized controlled trial of a nurse-delivered medication adherence intervention among HIV-positive outpatients initiating antiretroviral therapy in Beijing, China. AIDS Behav 2011;15:919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyon ME, Trexler C, Akpan-Townsend C, et al. . A family group approach to increasing adherence to therapy in HIV-infected youths: Results of a pilot project. AIDS Patient Care STDS 2003;17:299–308 [DOI] [PubMed] [Google Scholar]
- 14.Murphy DA, Lu MC, Martin D, Hoffman D, Marelich WD. Results of a pilot intervention trial to improve antiretroviral adherence among HIV-positive patients. J Assoc Nurses AIDS Care 2002;13:57–69 [DOI] [PubMed] [Google Scholar]
- 15.Gillani FS. The Miriam Hospital Immunology Center Database (ICDB) Annual Data Report. 2011; Available at: http://192.138.176.29/cfar/icdb%20annual%20report%202010_final.pdf (Last accessed May20, 2012)
- 16.HIV/AIDS Do. Medical Monitoring Project-Percieved Barriers and Facilitators to HIV Care. Atlanta: CDC, 2008 [Google Scholar]
- 17.Samet JH, Horton NJ, Meli S, et al. . A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther 2005;10:83–93 [DOI] [PubMed] [Google Scholar]
- 18.Carey MP, Lewis BP. Motivational strategies can augment HIV-risk reduction programs. AIDS Behav 1999;3:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carey MP, Braaten LS, Maisto SA, et al. . Using information, motivational enhancement, and skills training to reduce the risk of HIV infection for low-income urban women: A second randomized clinical trial. Health Psychol 2000;19:3–11 [DOI] [PubMed] [Google Scholar]
- 20.Van Kesteren NM, Kok G, Hospers HJ, Schippers J, De Wildt W. Systematic development of a self-help and motivational enhancement intervention to promote sexual health in HIV-positive men who have sex with men. AIDS Patient Care STDS 2006;20:858–875 [DOI] [PubMed] [Google Scholar]
- 21.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: Measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS 2010;24:607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris JL, Furberg R, Martin N, et al. . Implementing an SMS-based intervention for persons living with human immunodeficiency virus. J Public Health Manag Pract 2013;19:E9–E16 [DOI] [PubMed] [Google Scholar]
- 23.Saberi P, Johnson MO. Technology-based self-care methods of improving antiretroviral adherence: A systematic review. PLoS One 2011;6:e27533. [DOI] [PMC free article] [PubMed] [Google Scholar]