Abstract
We used magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) to evaluate the effects of boxing on brain structure and cognition in 10 boxers (8 retired, 2 active; mean age = 45.7 years; standard deviation [SD] = 9.71) and 9 participants (mean age = 43.44; SD = 9.11) in noncombative sports. Evans Index (maximum width of the anterior horns of the lateral ventricles/maximal width of the internal diameter of the skull) was significantly larger in the boxers (F = 4.52; p = 0.050; Cohen's f = 0.531). Word list recall was impaired in the boxers (F(1,14) = 10.70; p = 0.006; f = 0.84), whereas implicit memory measured by faster reaction time (RT) to a repeating sequence of numbers than to a random sequence was preserved (t = 2.52; p < 0.04). Fractional anisotropy (FA) and the apparent diffusion coefficient (ADC) measured by tractography did not significantly differ between groups. However, DTI metrics were significantly correlated with declarative memory (e.g., left ventral striatum ADC with delayed recall, r = −0.74; p = 0.02) and with RT to the repeating number sequence (r = 0.70; p = 0.04) in the boxers. Years of boxing had the most consistent, negative correlations with FA, ranging from −0.65 for the right ventral striatum to −0.92 for the right cerebral peduncle. Years of boxing was negatively related to the number of words consistently recalled over trials (r = −0.74; p = 0.02), delayed recall (r = −0.83; p = 0.003), and serial RT (r = 0.66; p = 0.05). We conclude that microstructural integrity of white matter tracts is related to declarative memory and response speed in boxers and to the extent of boxing exposure. Implications for chronic traumatic encephalopathy are discussed.
Key words: : boxing, chronic effects, cognition, imaging
Introduction
With recent neuropathology reports of chronic traumatic encephalopathy (CTE) in athletes exposed to repetitive head trauma,1 there is renewed interest in the chronic effects of boxing and other contact sports.2 The term chronic traumatic brain injury (CTBI) was proposed to describe the spectrum of neurological conditions that are the long-term consequences of repetitive head trauma.2 Subsequent to Martland's early description of “punch drunk syndrome” in boxers who developed motor and cognitive deficits after many bouts and extensive sparring,3,4 later neuropathology studies5 showed that CTE is characterized primarily by proliferation of phosphorylated tau (p-tau) deposits.
The phenotype of CTE includes a latent period of 8–10 years followed by onset of behavioral disturbance, characterized by impulsivity and depression.1 Cognitive features include disturbance of attention and memory.1 In a seminal follow-up study of former professional boxers, Roberts6 reported that 17% had clinical features that were consistent with CTE. Other neurodegenerative conditions related to extensive boxing exposure include post-traumatic parkinsonism.2 Exposure variables purportedly related to CTE and associated conditions include the length of boxing career, number of bouts, training schedule, associated subconcussive head impacts, knockouts, and the duration of retirement from boxing (i.e., postexposure interval).1,2
Recent advances in brain imaging provide an opportunity to characterize in vivo neurodegeneration in athletes at risk for CTE. Early identification of neurodegenerative changes may also facilitate treatment during a pre-clinical phase of CTE once an effective intervention is available. Frequent magnetic resonance imaging (MRI) findings reported in professional boxers include hippocampal atrophy, cavum septum pellucidum, dilated perivascular spaces, indications of diffuse axonal injury (DAI), pituitary gland atrophy, and ventricular enlargement.7 Diffusion tensor imaging (DTI) is a more advanced MRI technique that measures the microstructural integrity of brain tissue using metrics such as fractional anisotropy (FA) and apparent diffusion coefficient (ADC). FA indirectly measures white matter microstructure by assessing the tendency of water molecules to move parallel to structural components of axons (anisotropic diffusion) that act as barriers to diffusion rather than across them. Higher FA is generally associated with a structural environment characterized by higher fiber density and organization, homogeneity of the direction of the fibers, axonal diameter, and degree of myelination. ADC assesses how freely water moves inside the brain tissue (isotropic diffusion or diffusivity) and is generally negatively correlated with the components of the structural environment described above. Moderate-to-severe traumatic brain injury (TBI) generally results in decreased FA and increased ADC in the chronic post-injury interval.8–10 In contrast, the directionality of FA changes in acute mild TBI has been reported as elevated in some studies and reduced in others; the basis for this inconsistency in FA findings is poorly understood.11 However, there is agreement that DTI can detect changes in white matter tracts that are not observed on conventional MRI, including potential DAI associated with CTE.1
Previous DTI studies concerning the chronic effects of boxing have reported significantly lower FA and higher diffusivity in professional boxers than in a control group.12–14 Chappell and colleagues12 used a voxel-based analysis to compare DTI in 81 professional boxers with 12 control subjects and found white matter regions that had both lower FA and increased ADC, including the midbrain, medial temporal lobe, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and cerebral peduncles. Using a region of interest (ROI) analysis of DTI in 49 professional boxers and 19 comparison subjects, Zhang and colleagues14 found lower FA in the genu, splenium, and posterior internal capsule of the boxers who also had higher diffusivity in the anterior and posterior limbs of the internal capsule. However, the comparison groups in these studies were comprised of healthy subjects and did not control for participation in sports.
Investigators have also examined subject variables and boxing history in relation to DTI. Chappell and colleagues12 found that diffusivity in subcortical structures increased with age in both boxers and a comparison group, but the correlation between diffusion parameters, such as ADC or mean diffusivity and age in boxers (mean age of 28 years), was significantly higher than in the comparison group. This suggests that although diffusion parameters generally increase with age, they do so at a higher rate in boxers, possibly indicative of a neurodegenerative process. Exposure to boxing is often estimated by the number of bouts and years of boxing. Shin and colleagues15 studied the relationship between FA, measures of diffusion, and knockouts (KOs) in boxers. These investigators found a positive correlation between KOs and diffusion and a negative correlation between KOs and FA in the corpus callosum, isthmus of the cingulum bundle, pericalcarine region, precuneus, and amygdala.15
To date, most DTI studies have imaged professional boxers.12–14 Although several studies using conventional MRI have suggested that amateur boxers also exhibit chronic, neurobehavioral effects of repetitive head trauma,16,17 an investigation of amateur boxers and boxers who had recently become professionals reported negative MRI scans and neurobehavioral findings that did not differ from a comparison group of athletes engaged in noncontact sports.18 Moreover, the rate of CTBI among amateur boxers has been viewed as insignificant.2 Gaps in the boxing literature include the sparse data relating DTI and MRI findings to both cognitive performance and exposure history. Moreover, not all studies have included a comparison group with similar demographic features and participation in noncontact sports.
We conducted a pilot study to evaluate imaging and cognitive indications of neurodegeneration after repetitive head trauma and mild TBI associated with extended exposure to boxing. The goals of the study were to: 1) compare DTI and structural MRI findings in boxers and a comparison group who had played noncontact sports; 2) evaluate declarative memory and implicit memory in boxers and a comparison group; 3) study the relation of brain imaging findings to cognitive performance; and 4) analyze the relation of boxing history to brain imaging and cognitive performance. We hypothesized that MRI would disclose cerebral atrophy in boxers relative to a nonboxer comparison group and that DTI would show reduced microstructural integrity of white matter tracts in the boxers. With regard to the cognitive testing, we hypothesized that boxers would exhibit impaired declarative memory on a verbal list learning test. Although we predicted that boxers would show response slowing on a motor sequence learning task, we hypothesized that their implicit learning would be preserved, as reflected by shorter reaction time (RT), to a repeating sequence than to a random sequence of numbers. In addition, we hypothesized that the microstructural integrity of the white matter tracts in boxers would be related to declarative memory and performance on the motor sequence learning task. We also hypothesized that exposure to boxing indexed by the duration of participation, number of bouts, and number of KOs would be related to the brain imaging and cognitive findings. Last, we tested the hypothesis that the interval since the boxer's most recent bout (and, in most cases, final bout) would be negatively related to microstructural integrity of white matter and to cognitive performance.
Methods
Participants
We studied 10 male athletes with a history of amateur and/or professional boxing who ranged in age from 27 to 59 years (Table 1). We recruited a comparison group through community advertising including 9 males who had a history of participating in predominantly noncontact sports and were 26–57 years old. A research coordinator performed a structured boxing history interview with the boxers, including amateur and professional experience and TBI events (Table 1). A sports history interview covering sports participation and injuries was done with comparison group participants. In addition to diverse noncontact sports, 2 participants in the comparison group had played football and 2 had exposure to soccer. However, no participant in the comparison group had sustained a concussion or other TBI; no events associated with loss or alteration of consciousness or post-traumatic amnesia were reported. The duration of sports participation in the comparison group (mean = 30.89 years; median = 31.0; range = 3–50 years) approximated the duration of participation in the boxers (Table 1), but included more than a single sport. Although all participants underwent MRI, useable DTI data were acquired from 7 of the 9 comparison group participants. Nine boxers fought amateur and professional bouts, and a single boxer had only amateur bouts. Two of the boxers were still active and 8 had retired from the ring.
Table 1.
Demographic Features for Boxers and Comparison Group and Exposure Variables in Boxers
| Group | N | Variable | N | Mean | Median | SD | Min | Max |
|---|---|---|---|---|---|---|---|---|
| Boxer | 10 | Age | 10 | 45.70 | 47.00 | 9.71 | 27.00 | 59.00 |
| Education (years) | 10 | 13.00 | 12.50 | 1.49 | 11.00 | 16.00 | ||
| Years boxing | 10 | 35.70 | 37.00 | 9.15 | 16.00 | 51.00 | ||
| KOs (N) | 9 | 0.89 | 1.00 | 0.60 | 0.00 | 2.00 | ||
| KOs with LOC (N) | 9 | 0.67 | 1.00 | 0.71 | 0.00 | 2.00 | ||
| Bouts (N) | 10 | 164.00 | 132.50 | 116.80 | 40.00 | 386.00 | ||
| Comparison | 9 | Age | 9 | 43.44 | 44 | 9.11 | 26 | 57 |
| Education (years) | 9 | 13.78 | 14 | 1.92 | 10 | 16 |
KOs, knockouts; LOC, loss of consciousness; SD, standard deviation; Min, minimum; Max, maximum.
Inclusion criteria for the boxers were: 1) 15 or more years of amateur and/or professional boxing, or at least 20 bouts and a regular schedule of sparring; 2) current age 25–60 years; 3) no medical history of a neurological or psychiatric disorder unrelated to boxing (e.g., brain tumor, multiple sclerosis, schizophrenia, or bipolar disorder); 4) male; and 5) fluency in English (not all of the neurocognitive measures have been appropriately normed and validated in other languages). Apart from boxing experience and no history of concussion or TBI, the inclusion and exclusion criteria for the comparison group who had engaged in noncontact and contact sports were similar. Other exclusion criteria for both groups included 1) contraindications to undergoing MRI and 2) cognitive deficits that precluded informed consent. Boxers were recruited from local boxing gyms, and the comparison group was recruited through the community by advertisement or referral. The research protocol was approved by the institutional review board of the Baylor College of Medicine (Houston, TX). Informed consent was obtained from each participant.
Imaging protocols
All participants underwent MRI without sedation on the same Siemens 3.0 Tesla Trio scanner (Siemens Healthcare, Erlangen, Germany). Regular quality assurance testing was performed, including American College of Radiology phantom testing, and no concerns with quality assurance were noted through the course of the study. Anatomical series to assess neuropathology included a three dimensional (3D) magnetization prepared rapid gradient echo sequence (2600 ms repetition time [TR], 3.02 ms echo time [TE], 900 ms inversion time [TI], 1.0-mm axial slices, 0-mm gap, 176 slices, 256-mm field of view [FOV], 8-degree flip angle, 130 Hz BW/pixel), a T2-weighted gradient echo sequence (668 ms TR, 20 ms TE, 4.0-mm axial slices, 1.2-mm gap, 27 slices, 256-mm FOV, 20-degree flip angle, 199 Hz BW/pixel), a T2-weighted fluid-attenuated inversion recovery sequence (10,000 ms TR, 105 ms TE, 2500 ms TI, echo train length [ETL] 21, 5-mm axial slices, 1-mm gap, 28 slices, 240-mm FOV, 120-degree flip angle, 191 Hz BW/pixel), and a T2-weighted turbo spin echo sequence (5770 ms TR, 80 ms TE, ETL 9, 5.0-mm axial slices, 0-mm gap, 46 slices, 256-mm FOV, 150-degree flip angle, 200 Hz BW/pixel).
Diffusion tensor imaging
Transverse multi-slice spin echo, single shot, echo planar imaging sequences were used (8000 ms TR, 92 ms TE, 2.0-mm axial slices, 0.6-mm gap). A 256-mm FOV was used with a measured voxel size of 2.67 × 2.67 × 2.6 mm. Diffusion was measured along 30 directions (number of b-value = 2; low b-value = 0; and high b-value = 1000 sec/mm2). To improve signal-to-noise ratio, high b-images were acquired twice and averaged in most cases. Each acquisition took approximately 4 min 44 sec, and 56 slices were acquired.
Image processing
Diffusion tensor imaging
A multiple ROI approach to quantitative DTI tractography was applied. After ROIs were selected, an automated Philips 3D fiber tracking tool was utilized to create fiber tracts passing through the ROIs. The algorithm for fiber tracking is based upon the fiber assignment by continuous tracking method.19 Tracking terminated if the FA in the voxels decreased below 0.2 or if the angle between adjacent voxels along the track was larger than 7 degrees. Mean FA and ADC for the white matter tracts described below were the DTI measures used to compare the groups.
We measured FA and ADC in the following right and left regions: uncinate fasciculus; inferior longitudinal fasciculus; ventral striatum; and cerebral peduncle. The rationale for this selection was: 1) Uncinate fasciculus connects orbitofrontal cortex with the temporal lobe and is implicated in declarative memory20; 2) inferior longitudinal fasciculus connects hippocampus with the occipital region and is implicated in visual memory,20 which is relevant to learning the serial, visual RT task; (3) ventral striatum has an interactive role with hippocampus in feedback-based learning over trials and is also involved in learning habits21; and 4) cerebral peduncle connects the cortex and the brain stem and is involved in the refined control of body movements. With a cortico-striato-cerebellar-thalamo-cortical loop implicated in motor sequence learning,22 we postulated that the cerebral peduncle is relevant to serial RT performance used to measure implicit learning. With the exception of the cerebral peduncles, the tractography protocols have been published.23–25 The protocol for the cerebral peduncles is detailed below.
Cerebral peduncles
Two ROIs were placed on axial images of the FA map to isolate the right and left cerebral peduncle pathways using protocols illustrated below. The first ROI was selected at the upper brainstem level as the cerebral peduncle appeared in an oval shape. The second ROI was traced in the same area, but two or three slices inferior to the first one.
All DTI data were analyzed twice by a single rater to establish intrarater reliability using intraclass correlational coefficients (ICCs). A subset of the images was analyzed by two raters to establish inter-rater reliability. ICCs for all measurements were above 0.95.
Magnetic resonance imaging
A senior neuroradiologist (J.V.H.) reviewed and coded the structural MRI findings without knowledge of the participant's group. The ventricle-brain ratio was calculated by dividing the area of the lateral ventricles by the total intracranial area. We also measured the Evan's Index26 by dividing a linear measure of the maximum width of the anterior horns of the lateral ventricles by the maximal width of the internal diameter of the skull at the same level. A higher index value is interpreted as a rough biomarker of ventriculomegaly, possibly owing to normal pressure hydrocephalus or cerebral atrophy. Evans Index varies with age and sex, so interpretation depends on taking these characteristics into account.
Cognitive testing
Verbal Selective Reminding Test27
The Verbal Selective Reminding Test (VSRT) is a verbal learning and memory test that is sensitive to early dementia, TBI, and memory disorders of various etiologies.28 After the examiner serially presented each of the 12 words on trial 1, the participant was asked to recall the list.27 After each recall attempt, the examiner selectively presented on the next trial only those words that the participant failed to recall during the preceding trial. VSRT differs from other verbal learning tests in which the examiner presents the entire list of words on each trial. A delayed recall trial was administered without warning 30 min after the sixth and final learning trial.29 Dependent variables were: 1) total number of words consistently recalled after having been recalled on two consecutive trials, that is, consistent long-term retrieval, and 2) the total number of words recalled after a 30-min delay (delayed recall).
Serial Reaction Time Test30
This test evaluates implicit (procedural) memory, a form of memory that is often robust to mesial temporal pathology and dissociable from declarative memory.31 Subjects make a speeded response to a random number displayed on a screen by pressing the corresponding number on a keypad. There are 120 trials, but embedded within the random number trials is a repeating sequence of numbers that is not mentioned to the participant. RT for the random numbers remains stable across trials, but RT on the repeating sequence decreases.30 The difference in RT between the random and repeating sequences is interpreted as an index of implicit learning. Because participants are frequently unaware of the embedded nonrandom sequence, the decrease in RT has been attributed to implicit memory processes. Patients with mesial temporal lesions who have a declarative memory disorder as measured on the VSRT or similar tests often exhibit normal implicit learning,30 whereas motor skill learning may be impaired by lesions in cerebellum and basal ganglia.21,32 Implicit or procedural learning on the serial RT task is also thought to also depend on a network involving cortical motor regions, a cortico-striato-thalamo-cortical loop, and a cortico-cerebellar-thalamo-cortical loop.22 Variables analyzed include 1) mean RT for responding to the random sequences of numbers, 2) mean RT for responding to the repeating sequence, and 3) the difference in RT between random and repeating sequences (corresponding to implicit learning).
Statistical analysis
For this pilot study, we used nonparametric methods to compare the groups and assess association between variables. Wilcoxon's tests were used to examine group differences on continuous variables. Fisher's exact tests were used to test group differences on categorical variables, such as sex and ethnicity. Spearman's rank correlations were calculated within the boxer group to examine the relation between the DTI metrics and 1) cognitive scores as well as 2) variables related to boxing history. Effect sizes in terms of Cohen's d and f were used to assess group differences on DTI variables and cognitive measures. Statistical analyses were performed with the SAS software (version 9.4; SAS Institute Inc., Cary, NC), and the threshold for statistical significance was set at p < 0.05. Given the exploratory nature of the present study, corrections for multiple comparisons were not applied and exact p values along with appropriate effect-size measures are provided.
Results
Demographic characteristics and current status
Demographic information for both groups is summarized in Table 1 as are measures of boxing exposure for the boxers. All participants were living independently and none had been diagnosed with dementia. However, 1 boxer had post-traumatic parkinsonism with slurred speech and slowed movement, but no cognitive symptoms. In addition, 4 boxers reported chronic headaches.
Wilcoxon's tests revealed no significant group differences for age (rank sum = 84.5; p = 0.68) or education (rank sum = 104; p = 0.257). Fisher's exact tests indicated no significant between-group differences for ethnicity (p = 0.629) or race (p = 0.175).
Structural magnetic resonance imaging findings
The most frequent findings in both groups included T2-weighted hyperintensities in both the frontal and temporal regions (3 boxers, 1 control) and the parietal lobe (3 boxers, and no control). Mild volume loss in the frontal lobe (1 boxer) and a tear in the right anterior temporal region (1 boxer) were noted. Thinning of the corpus callosum (2 boxers, 1 control) was also present. Mild volume loss was noted in the cerebellum (4 boxers, 2 controls), brainstem (1 boxer, no control), anterior commissure (7 boxers, 4 controls), olfactory bulbs (4 boxers, 1 control), pituitary (1 boxer, 1 control), and the posterior pituitary (4 boxers, 1 control). A cavum septum pellucidum (5 boxers, 2 controls) was also a frequent finding. However, the hippocampus was small in only a single boxer and in no control. Increased perivascular spaces, especially in the basal ganglia (7 boxers, 7 controls) and large and/or asymmetric lateral ventricles (6 boxers, 3 controls), were present. Structural abnormalities were generally more frequent in boxers than in controls.
Cerebral atrophy and ventricular enlargement
Although the VBR tended to be larger in the boxers (mean = 1.70; standard deviation [SD] = 1.26) than the comparison group (mean = 1.25; SD = 0.55), the difference was not significant (Wilcoxon's rank sum = 56; p = 0.751). However, the Evan's Index significantly differed between the groups (F = 4.52; p = 0.050; Cohen's f = 0.531), with the boxers having a higher index score, even when age was controlled (Fig. 1A,B).
FIG. 1.
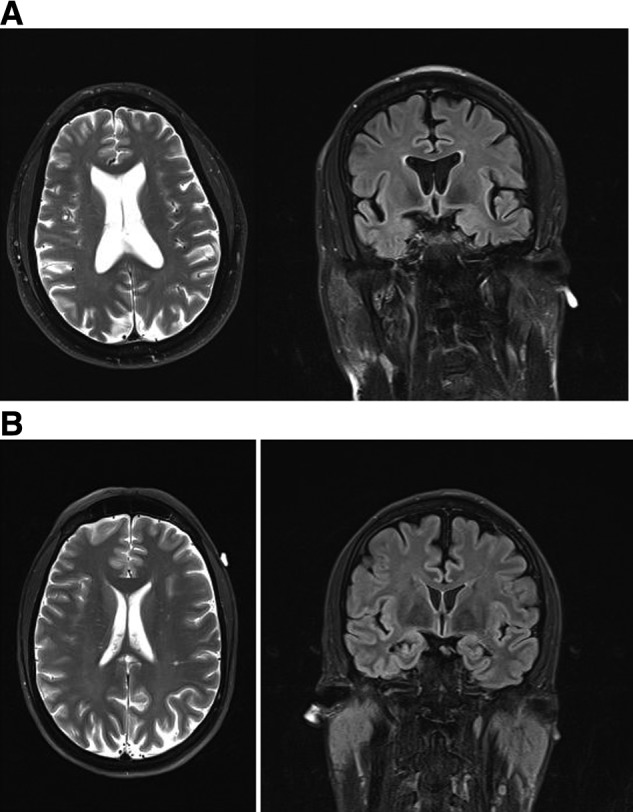
(A) Axial T2-weighted and coronal T2-weighted fluid-attenuated inversion recovery sequence magnetic resonance imaging of a 49-year-old ex-boxer who had 8 years of amateur experience (150 bouts) and 3 years as a professional boxer (21 bouts). He sustained 1 knockout as a professional. The Evans Index was 37.19%. (B). Axial T2-weighted and coronal T2-weighted fluid-attenuated inversion recovery sequence magnetic resonance imaging MRI of a 47-year-old comparison subject who had played soccer, football, and cycled frequently without sustaining a traumatic brain injury. His Evans Index was 26.05%.
Group differences on diffusion tensor imaging
Table 2A shows the mean FA by region for the boxer and comparison groups; results for ADC are summarized in Table 2B. Although there were no significant group differences in FA for any region, FA tended to be higher in the right uncinate fasciculus of the boxers (large effect size) and in the right cerebral peduncle of the comparison group (Table 2A). No between-group differences in ADC were significant, but boxers tended to have higher values in the right and left inferior longitudinal fasciculi and right ventral striatum (large effect sizes) and in the right cerebral peduncle (medium effect size; see Table 2B). In contrast, ADC was higher in the right and left uncinate fasciculi of the comparison group with large and medium effect sizes, respectively.
Table 2A.
Mean FA by Tract for Boxers and Comparison Group
| Boxers | Comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tract | Mean | SD | N | Mean | SD | N | t | p value | d |
| Right UF | 0.340 | 0.035 | 10 | 0.318 | 0.018 | 7 | 1.543 | 0.144 | 0.760 |
| Left UF | 0.323 | 0.033 | 9 | 0.324 | 0.025 | 6 | 0.019 | 0.985 | 0.010 |
| Right ILF | 0.361 | 0.044 | 9 | 0.357 | 0.033 | 7 | 0.202 | 0.843 | 0.102 |
| Left ILF | 0.402 | 0.044 | 10 | 0.393 | 0.036 | 7 | 0.461 | 0.651 | 0.227 |
| Right VS | 0.393 | 0.029 | 10 | 0.394 | 0.028 | 7 | 0.054 | 0.957 | 0.027 |
| Left VS | 0.404 | 0.027 | 10 | 0.401 | 0.034 | 7 | 0.222 | 0.827 | 0.110 |
| Right CP | 0.457 | 0.031 | 10 | 0.478 | 0.022 | 7 | 1.472 | 0.162 | 0.725 |
| Left CP | 0.483 | 0.022 | 10 | 0.476 | 0.032 | 7 | 0.524 | 0.608 | 0.258 |
Large effect size (d ≥ 0.5) is bolded.
FA, fractional anisotropy; CP, cerebral peduncle; ILF, inferior longitudinal fasciculus; UF, uncinate fasciculus; VS, ventral striatum; SD, standard deviation.
Table 2B.
Mean ADC by Tract for Boxers and Comparison Group
| Boxers | Comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tract | Mean | SD | N | Mean | SD | N | t | p value | d |
| Right UF | 0.790 | 0.035 | 10 | 0.831 | 0.085 | 7 | 1.413 | 0.178 | 0.696 |
| Left UF | 0.794 | 0.031 | 9 | 0.814 | 0.061 | 6 | 0.845 | 0.413 | 0.445 |
| Right ILF | 0.803 | 0.078 | 9 | 0.769 | 0.040 | 7 | 1.029 | 0.321 | 0.518 |
| Left ILF | 0.798 | 0.090 | 10 | 0.758 | 0.037 | 7 | 1.106 | 0.286 | 0.545 |
| Right VS | 0.789 | 0.033 | 10 | 0.771 | 0.032 | 7 | 1.123 | 0.279 | 0.554 |
| Left VS | 0.785 | 0.066 | 10 | 0.771 | 0.054 | 7 | 0.473 | 0.643 | 0.233 |
| Right CP | 0.783 | 0.036 | 10 | 0.768 | 0.034 | 7 | 0.857 | 0.405 | 0.423 |
| Left CP | 0.763 | 0.034 | 10 | 0.770 | 0.042 | 7 | 0.404 | 0.692 | 0.199 |
Large effect size (d ≥ 0.5) is bolded.
ADC, apparent diffusion coefficient; CP, cerebral peduncle; ILF, inferior longitudinal fasciculus; UF, uncinate fasciculus; VS, ventral striatum; SD, standard deviation.
Group differences on cognitive testing
Declarative learning and memory
On the VSRT, the boxers recalled fewer words consistently across trials (mean CLTR = 20.3; SD = 15.17) than the comparison group (mean = 35.78; SD = 15.56), with a potential difference at the trend level, that had a moderate effect size (F(1,14) = 3.79; p = 0.072; Cohen's f = 0.52). The boxers recalled significantly fewer words following a 30-min delay (mean = 4.7; SD = 2.87) than the comparison group (mean = 8.89; SD = 2.32; F(1,14) = 10.70; p = 0.006; Cohen's f = 0.84). These between-group differences of approximately 1 SD in declarative memory are clinically significant.
Implicit learning on serial reaction time task
The boxers (mean = 971.97; SD = 161.3) performed similarly as the comparison group (mean = 892.31; SD = 196.35; F(1,12) = 0.21; p = 0.660; Cohen's f = 0.13) on RT (ms) to random sequences when age was controlled. Boxers' performance on RT to the repeating sequence (mean = 952.09; SD = 152.16) was also comparable to that of the comparison group (mean = 866.31; SD = 202.72; F(1,12) = 0.42; p = 0.530; Cohen's f = 0.19). The reduction in RT to the repeating sequence was significant in the boxers (mean = 19.88; SD = 23.70; t = 2.52; p = 0.036) and in the comparison group (mean = 25.90; SD = 24.26; t = 3.02; p = 0.019). The between-group difference in reduction of RT to the repeating sequence showed a promising effect size (F(1,12) = 2.55; p = 0.136; Cohen's f = 0.46),,suggesting that the comparison group had a potentially greater reduction in RT.
Relations between diffusion tensor imaging, age, and cognitive performance
We first calculated the correlations of the DTI metrics with age and found significant or nonsignificant, negative correlations with FA for all brain regions in the boxers (r = −0.616 to −0.757). For the comparison group, the only correlation of FA with age that approached significance was the right cerebral peduncle (r = −0.739; p = 0.058). The correlations of age with ADC in the boxers were significant only for the left ventral striatum (r = 0.671; p = 0.034). For the comparison group, age was significantly correlated with ADC in the left uncinate fasciculus, (r = 0.812; p = 0.049) and there was a promising trend for the left inferior longitudinal fasciculus (r = 0.739; p = 0.068).
Diffusion tensor imaging in relation to declarative learning and memory
These correlations were generally stronger in the boxers than in the comparison group. Several significant Spearman's correlations between FA and declarative memory on the VSRT were observed in the boxers. Poorer memory was related to low FA, suggestive of reduced microstructural integrity of the white matter tracts. As seen in Table 3A, the number of words consistently recalled across trials was significantly related to FA in the right cerebral peduncle. Delayed recall of the word list was also correlated with FA in the right cerebral peduncle, the right uncinate fasciculus, the left inferior longitudinal fasciculus, and the left ventral striatum. In contrast, the only significant correlation of FA with declarative learning and memory in the comparison group was the number of words consistently recalled over trials with FA in the left inferior longitudinal fasciculus (r = 0.764; p = 0.046). For ADC, Figure 2 shows that the number of words recalled by the boxers after a delay was negatively correlated with ADC in left ventral striatum (r = −0.740; p = 0.015). In the comparison group, potential negative trends were present in the correlation of right cerebral peduncle ADC with the number of words consistently recalled (r = −0.709; p = 0.074) and with delayed recall (r = −0.727; p = 0.064).
Table 3A.
Correlations in the Boxer Group of Declarative Learning and Memory on the VSRT with FA Values
| Right UF | Left UF | Right ILF | Left ILF | |||||
|---|---|---|---|---|---|---|---|---|
| CLTR | Delay | CLTR | Delay | CLTR | Delay | CLTR | Delay | |
| r | 0.616 | 0.629 | 0.563 | 0.193 | −0.092 | −0.018 | 0.573 | 0.649 |
| p | 0.058 | 0.051 | 0.115 | 0.620 | 0.813 | 0.964 | 0.083 | 0.042 |
| Right CP | Left CP | Right VS | Left VS | |||||
|---|---|---|---|---|---|---|---|---|
| CLTR | Delay | CLTR | Delay | CLTR | Delay | CLTR | Delay | |
| r | 0.628 | 0.662 | 0.348 | 0.428 | 0.201 | 0.370 | 0.573 | 0.616 |
| p | 0.052 | 0.037 | 0.325 | 0.217 | 0.577 | 0.293 | 0.083 | 0.056 |
VSRT, Verbal Selective Reminding Test; CLTR, consistent long-term retrieval; CP, cerebral peduncles; FA, fractional anisotropy; ILF, inferior longitudinal fasciculus; UF, uncinate fasciculus; VS, ventral striatum.
FIG. 2.
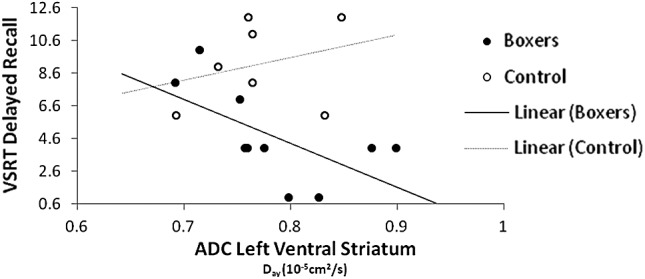
Relation of ADC left ventral striatum to VSRT delayed recall. VSRT, Verbal Selective Reminding Test; ADC, apparent diffusion coefficient.
Table 3B.
Correlations in the Boxer Group between Declarative Learning and Memory on the VSRT and ADC
| Right UF | Left UF | Right ILF | Left ILF | |||||
|---|---|---|---|---|---|---|---|---|
| CLTR | Delay | CLTR | Delay | CLTR | Delay | CLTR | Delay | |
| r | −0.390 | −0.311 | −0.529 | −0.227 | −0.286 | 0.043 | −0.573 | −0.363 |
| p | 0.265 | 0.381 | 0.143 | 0.556 | 0.456 | 0.911 | 0.083 | 0.302 |
| Right CP | Left CP | Right VS | Left VS | |||||
|---|---|---|---|---|---|---|---|---|
| CLTR | Delay | CLTR | Delay | CLTR | Delay | CLTR | Delay | |
| r | −0.366 | −0.175 | −0.311 | −0.208 | −0.494 | −0.539 | −0.604 | −0.740 |
| p | 0.299 | 0.628 | 0.382 | 0.565 | 0.147 | 0.1081 | 0.065 | 0.015 |
VSRT, Verbal Selective Reminding Test; ADC, apparent diffusion coefficient; UF, uncinate fasciculus; ILF, inferior longitudinal fasciculus; CP, cerebral peduncles; VS, ventral striatum; CLTR, continuous long-term retrieval; LTS, long-term storage.
Serial reaction time test and diffusion tensor imaging
In the following correlations, longer RT was associated with decreased white matter integrity (lower FA and higher ADC) in the boxers. RT to the random sequence of numbers was negatively related to FA in the right cerebral peduncle (r = −0.683; p = 0.042) and the left cerebral peduncle (r = −0.817; p = 0.007). RT to the repeating sequence was also negatively correlated with FA in the right (r = −0.667; p = 0.049) and left (r = −0.850; p = 0.004) cerebral peduncles in the boxers. In contrast to the findings in the boxers, the correlations with FA were less consistently negative in the comparison group, ranging from −0.60 (p = 0.210) for the right cerebral peduncle to 0.60 (p = 0.210) for the right uncinate fasciculus.
Convergent with the correlations for FA, ADC was significantly and positively correlated with RT for both the random and repeated sequences in the boxers, but not in the comparison group. In the boxers, left ventral striatum ADC was positively correlated with RT to the random sequence of numbers (r = 0.750; p = 0.019) and to the repeating sequence (r = 0.700; p = 0.036). In contrast, the direction of correlation of ADC with RT was inconsistent in the comparison group, ranging from correlational coefficients of −0.49 (p = 0.330) in the left ventral striatum to 0.771 (p = 0.072) in the right cerebral peduncle.
Relation of boxing exposure to diffusion tensor imaging metrics
In the following results, boxing exposure was negatively correlated with FA and positively correlated with ADC, after controlling for age. Of the measures of exposure, years of boxing had the most significant, negative correlations with FA, including for the right uncinate fasciculus (r = −0.807; p = 0.026) and the right cerebral peduncle (r = −0.827; p = 0.022). Figure 3 shows that right uncinate fasciculus FA was inversely related to the years of boxing. Convergent with the correlations for FA, years of boxing was positively related to ADC, reaching significance for the left inferior longitudinal fasciculus (r = 0.798; p = 0.032) and the left uncinate fasciculus (r = −0.763; p = 0.046). Number of bouts was positively related to ADC in the left cerebral peduncle in a promising trend (r = 0.736; p = 0.059; Fig. 4). However, we did not find any correlation between number of KOs and the DTI metrics.
FIG. 3.
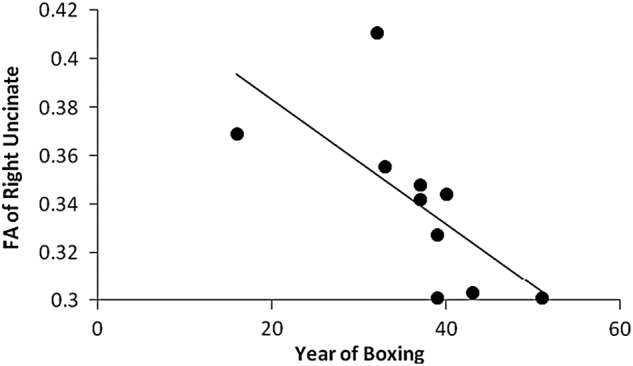
Relation of years of boxing to FA of right uncinate. FA, fractional anisotropy.
FIG. 4.
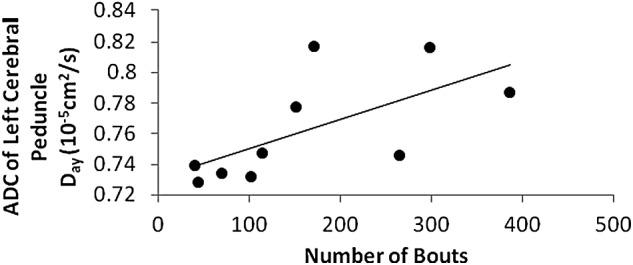
Relation of number of bouts to ADC of left cerebral peduncle. ADC, apparent diffusion coefficient.
Relation of boxing exposure to cognitive performance
For declarative memory, years of boxing was negatively related to the number of words consistently recalled over trials (r = −0.736; p = 0.015) and to delayed recall (r = −0.832; p = 0.003). For the serial RT task, years of boxing tended to be positively related to RT under the random condition (r = 0.664; p = 0.051) and for the repeating sequences (r = 0.622; p = 0.074), indicating slower response with longer exposure.
Interval since most recent bout
Correlations of years since the most recent bout with FA were generally negative and reached significance only for the left cerebral peduncle (r = −0.756; p = 0.011). Although the correlations were less consistently related to ADC, there was a promising trend for the right ventral striatum (r = 0.604; p = 0.065). Number of years since the most recent bout was significantly correlated with RT for random (r = 0.787; p = 0.012) and repeating (r = 0.745; p = 0.021) sequences; responses were slower as a function of the time since the most recent bout (which was the final bout for 8 of the 10 boxers). In no case was the post-exposure interval positively related to microstructural integrity of the white matter tracts or to cognitive performance.
Discussion
DTI metrics were related to cognitive performance in boxers, whereas these relations were weaker in the comparison group. Consistent with the findings reported by Chappell and colleagues,12 the correlation of DTI metrics with age tended to be stronger in the boxers than in the comparison group, suggesting the possibility of accelerated degeneration of the white matter in the former group. Moreover, boxing exposure was negatively related to the microstructural integrity of white matter tracts and to cognitive performance. The direction of correlations was as predicted; low FA and high ADC indicative of reduced microstructural integrity of the white matter tracts were correlated with poorer cognitive performance and more extensive exposure to boxing. For example, the significant correlations of response latencies on the serial RT test with diffusivity in the cerebral peduncles of the boxers are consistent with the relevance of corticospinal and -bulbar fibers and other motor pathways to implicit learning of motor skills. Similarly, declarative learning was related to DTI findings, including diffusivity in the left ventral striatum of the boxers. These relations were relatively inconsistent and weaker in the comparison group.
We found that declarative learning and memory on a word list recall test were impaired in the boxers relative to a comparison group who had similar demographic features and had engaged in predominantly noncontact sports. The between-group difference in declarative memory of 1 SD is also clinically significant. In contrast, performance on the serial RT task did not differ between the groups under the random and repeating sequence conditions; the boxers exhibited preserved implicit learning indexed by faster RT to repeating than random sequences. This dissociation between impaired declarative memory and preserved implicit memory in the boxers is consistent with their frequent frontotemporal hyperintensities on MRI, which may have contributed to poor strategic memory on the VSRT.
Using the Evans Index, we confirmed that cerebral atrophy and expansion of the frontal horns of the lateral ventricles in the boxers were greater than in the comparison group. However, our hypothesis concerning between-group differences in DTI metrics was not supported. The direction of the differences in diffusivity between the boxers and comparison group was inconsistent, and we did not detect significant differences in FA. These findings are not in accord with previous studies that reported significantly reduced FA and increased diffusivity in boxers as compared to nonboxers.12,14 However, three of the four between-group differences in diffusivity that had a large effect size in the present study were in the direction of higher values in the boxers. The wide range of age and boxing exposure in the small sample we recruited in this pilot study may have contributed to the lack of significant between-group differences in the DTI metrics. Recruiting comparison subjects who had participated in noncontact sports and had demographic features similar to the boxers might have also been a factor.
Our hypothesis concerning the relation of DTI metrics to boxing exposure found strong support; duration of boxing career was negatively related to FA in the right uncinate fasciculus and right cerebral peduncle, whereas ADC was positively correlated with years of boxing (left inferior longitudinal fasciculus and left ventral striatum). The number of bouts was positively related to ADC in the right ventral striatum, and a trend was found for ADC in the left cerebral peduncle. Although duration of boxing career was confounded with age in this small sample, we confirmed the correlation of years of boxing with DTI metrics while controlling for age. Our DTI findings support the cumulative, adverse effects of boxing exposure on the microstructure of the cerebral white matter. These relations are also consistent with Chappell and colleagues'12 study, which identified brain regions wherein reduced FA and increased ADC were more strongly related to age than in a comparison group. Although Chappell and colleagues did not report exposure variables in their professional boxers, these were presumably age related.
Although preliminary, we also found that the interval since the last boxing bout was negatively related to FA (right and left cerebral peduncles) and positively related to ADC (right ventral striatum), suggesting a self-perpetuating neurodegenerative process. This interpretation is supported by McKee and colleagues'1 neuropathology report showing that proliferation of phosphorylated tau (p-tau) pathology was a function of the interval after retirement from professional football. Moreover, we found that time since the last bout was directly related to RT for random and repeating sequences (i.e., longer post-exposure interval related to slower RT).
In the present study, declarative learning and memory indexed by the number of words consistently recalled across trials was impaired in the boxers relative to the comparison group. Delayed recall of the word list was also reduced in the boxers, lending further support for compromise of declarative memory after extensive exposure to boxing. Moreover, declarative memory was negatively related to boxing exposure, including years of boxing.
In contrast to declarative memory, the nonsignificant between-group differences in serial RT for random and repeated sequences of numbers indicates that implicit learning was relatively spared in the boxers. Similar to the comparison group, the boxers' RTs were significantly decreased for a repeating sequence embedded in the random sequence of numbers, an index of implicit learning. The dissociation between declarative and implicit learning in the boxers is consistent with literature on memory disorder in patients with mesial temporal pathology.32
From the perspective of CTE, it is plausible that the sample of boxers included individuals who were in a latent stage or stage 1 of this neurodegenerative condition.1 This view is supported by their impaired declarative memory and the strong relations between duration of exposure to boxing and both DTI metrics and cognitive performance. We also found that the interval since the last bout was related to reduced white matter integrity on DTI and to worse cognitive performance, a finding consistent with neuropathological evidence for proliferation of p-tau pathology as a function of the interval after retirement from football.1 Finally, 4 of the boxers reported chronic headaches despite having long retired. Headaches are one of the phenotypic features associated with CTE.1 Although a single boxer had symptoms of post-traumatic parkinsonism, no boxer had been diagnosed with dementia and they were all living independently.
Limitations of this pilot study include the small sample size, as well as heterogeneity in boxing exposure and age. In our sample, age and years of boxing represent a confound because older boxers also had longer exposure to boxing. However, a subset of the correlations between exposure and DTI metrics were still significant after controlling for age. The convergent findings for FA and ADC in relation to boxing exposure and cognitive performance underscore the implications for a neurodegenerative process. Moreover, our investigation of brain regions using DTI was not exhaustive. We did not apply strict correction for multiple comparisons, and this represents another limitation. On the positive side, we utilized a comparison group of predominantly noncontact athletes with a range and distribution of demographic features similar to the boxers. Clinical coding of the MRIs and analysis of the image data were performed independently of the group identification. Additionally, we demonstrated impaired declarative memory and a dissociation of intact implicit memory in boxers who were living independently and had not received a diagnosis of dementia despite their extensive exposure to repetitive head trauma. This dissociation in the boxers is characteristic of memory disorder associated with frontal temporal pathology.
Acknowledgments
This work was supported by the National Institutes of Health (grants 5R21-NS086714 and 5P01-NS056202). The funding sources had no role in the interpretation of data, writing of this article, or in the decision to submit for publication. The contents of this article are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health. The authors thank James Montier, Baylor College of Medicine (BCM), for his assistance in the preparation of this manuscript, Dr. Paolo Moretti (BCM), Gary Simmons, and Ron Collins for their help with recruitment, and Krista Runge (BCM) for her assistance in MRI acquisition.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H., Lee H.S., Wojtowicz S.M., Hall G., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., and Cantu R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan B.D. (2013). The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 9, 222–230 [DOI] [PubMed] [Google Scholar]
- 3.Unterharnscheidt F., and Unterharnscheidt J.T. (2003). Boxing: Medical Aspects. Academic: London, San Diego [Google Scholar]
- 4.Martland H.S. (1928). Punch drunk. JAMA 91, 1103–1107 [Google Scholar]
- 5.Corsellis J.A., Bruton C.J., and Freeman-Browne D. (1973). The aftermath of boxing. Psychol. Med. 3, 270–303 [DOI] [PubMed] [Google Scholar]
- 6.Roberts A.H. (1969). Brain Damage in Boxers: A Study of the Prevalence of Traumatic Encephalopathy Among Ex-Professional Boxers. Pitman Medical & Scientific: London [Google Scholar]
- 7.Orrison W.W., Hanson E.H., Alamo T., Watson D., Sharma M., Perkins T.G., and Tandy R.D. (2009). Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J. Neurotrauma 26, 689–701 [DOI] [PubMed] [Google Scholar]
- 8.Huisman T.A., Schwamm L.H., Schaefer P.W., Koroshetz W.J., Shetty-Alva N., Ozsunar Y., Wu O., and Sorensen A.G. (2004). Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am. J. Neuroradiol. 25, 370–376 [PMC free article] [PubMed] [Google Scholar]
- 9.Benson R.R., Meda S.A., Vasudevan S., Kou Z., Govindarajan K.A., Hanks R.A., Millis S.R., Makki M., Latif Z., Coplin W., Meythaler J., and Haacke E.M. (2007). Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J. Neurotrauma 24, 446–459 [DOI] [PubMed] [Google Scholar]
- 10.Hulkower M.B., Poliak D.B., Rosenbaum S.B., Zimmerman M.E., and Lipton M.L. (2013). A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am. J. Neuroradiol. 34, 2064–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., and Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6, 137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chappell M.H., Ulug A.M., Zhang L., Heitger M.H., Jordan B.D., Zimmerman R.D., and Watts R. (2006). Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study. J. Magn. Reson. Imaging 24, 537–542 [DOI] [PubMed] [Google Scholar]
- 13.Zhang L., Ravdin L.D., Relkin N., Zimmerman R.D., Jordan B., Lathan W.E., and Ulug A.M. (2003). Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? AJNR Am. J. Neuroradiol. 24, 52–57 [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L., Heier L.A., Zimmerman R.D., Jordan B., and Ulug A.M. (2006). Diffusion anisotropy changes in the brains of professional boxers. AJNR Am. J. Neuroradiol. 27, 2000–2004 [PMC free article] [PubMed] [Google Scholar]
- 15.Shin W., Mahmoud S.Y., Sakaie K., Banks S.J., Lowe M.J., Phillips M., Modic M.T., and Bernick C. (2014). Diffusion measures indicate fight exposure-related damage to cerebral white matter in boxers and mixed martial arts fighters. AJNR Am. J. Neuroradiol. 35, 285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zazryn T., Cameron P., and McCrory P. (2006). A prospective cohort study of injury in amateur and professional boxing. Br. J. Sports Med. 40, 670–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahnel S., Stippich C., Weber I., Darm H., Schill T., Jost J., Friedmann B., Heiland S., Blatow M., and Meyding-Lamade U. (2008). Prevalence of cerebral microhemorrhages in amateur boxers as detected by 3T MR imaging. AJNR Am. J. Neuroradiol. 29, 388–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin H.S., Lippold S.C., Goldman A., Handel S., High W.M., Jr., Eisenberg H.M., and Zelitt D. (1987). Neurobehavioral functioning and magnetic resonance imaging findings in young boxers. J. Neurosurg. 67, 657–667 [DOI] [PubMed] [Google Scholar]
- 19.Mori S., Crain B.J., Chacko V.P., and van Zijl P.C. (1999). Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 45, 265–269 [DOI] [PubMed] [Google Scholar]
- 20.Catani M., and Thiebaut de Schotten M. (2008). A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44, 1105–1132 [DOI] [PubMed] [Google Scholar]
- 21.Mattfeld A.T., and Stark C.E. (2015). Functional contributions and interactions between the human hippocampus and subregions of the striatum during arbitrary associative learning and memory. Hippocampus 25, 900–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dayan E., and Cohen L.G. (2011). Neuroplasticity subserving motor skill learning. Neuron 72, 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilde E.A., McCauley S.R., Chu Z., Hunter J.V., Bigler E.D., Yallampalli R., Wang Z.J., Hanten G., Li X., Ramos M.A., Sabir S.H., Vasquez A.C., Menefee D., and Levin H.S. (2009). Diffusion tensor imaging of hemispheric asymmetries in the developing brain. J. Clin. Exp. Neuropsychol. 31, 205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin H.S., Wilde E.A., Chu Z., Yallampalli R., Hanten G.R., Li X., Chia J., Vasquez A.C., and Hunter J.V. (2008). Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J. Head Trauma Rehabil. 23, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah S., Yallampalli R., Merkley T.L., McCauley S.R., Bigler E.D., Macleod M., Chu Z., Li X., Troyanskaya M., Hunter J.V., Levin H.S., and Wilde E.A. (2012). Diffusion tensor imaging and volumetric analysis of the ventral striatum in adults with traumatic brain injury. Brain Inj. 26, 201–210 [DOI] [PubMed] [Google Scholar]
- 26.Evans W. (1942). An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch. Neurol. Psychiatry 47, 931–937 [Google Scholar]
- 27.Buschke H., and Fuld P.A. (1974). Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 24, 1019–1025 [DOI] [PubMed] [Google Scholar]
- 28.Levin H.S., Mattis S., Ruff R.M., Eisenberg H.M., Marshall L.F., Tabaddor K., High W.M., Jr., and Frankowski R.F. (1987). Neurobehavioral outcome following minor head injury: a three-center study. J. Neurosurg. 66, 234–243 [DOI] [PubMed] [Google Scholar]
- 29.Larrabee G.J., Trahan D.E., and Levin H.S. (2000). Normative data for a six-trial administration of the Verbal Selective Reminding Test. Clin. Neuropsychol. 14, 110–118 [DOI] [PubMed] [Google Scholar]
- 30.Nissen M.J., and Bullemer P. (1987). Attentional requirements of learning: evidence from performance measures. Cogn. Psychol. 19, 1–32 [Google Scholar]
- 31.Gobel E.W., Blomeke K., Zadikoff C., Simuni T., Weintraub S., and Reber P.J. (2013). Implicit perceptual-motor skill learning in mild cognitive impairment and Parkinson's disease. Neuropsychology 27, 314–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reber P.J. (2013). The neural basis of implicit learning and memory: a review of neuropsychological and neuroimaging research. Neuropsychologia 51, 2026–2042 [DOI] [PubMed] [Google Scholar]


