Abstract
Critical limb ischemia (CLI) is a clinical syndrome of ischemic pain at rest or tissue loss, such as non-healing ulcers or gangrene, related to peripheral artery disease. CLI has a high short-term risk of limb loss and cardiovascular events. Non-invasive or invasive angiography help determine the feasibility and approach to arterial revascularization. An “endovascular-first” approach is often advocated based on a lower procedural risk, however, specific patterns of disease may be best treated by open surgical revascularization. Balloon angioplasty and stenting form the backbone of endovascular techniques, with drug-eluting stents and drug-coated balloons offering low rates of repeat revascularization. Combined antegrade and retrograde approaches can increase success in long total occlusions. Below the knee, angiosome-directed angioplasty may lead to greater wound healing, but failing this, any straight line flow into the foot is pursued. Hybrid surgical techniques such as iliac stenting and common femoral endarterectomy are commonly used to reduce operative risk. Lower extremity bypass grafting is most successful with a good quality, long, single-segment autogenous vein of at least 3.5mm diameter. Minor amputations are often required for tissue loss as part of the treatment strategy. Major amputations (at or above the ankle) limit functional independence and their prevention is a key goal of CLI therapy. Medical therapy after revascularization targets risk factors for atherosclerosis and assesses wound healing and new or recurrent flow limiting disease. The ongoing NIH sponsored BEST-CLI study is a randomized trial of the contemporary endovascular versus open surgical techniques in patients with CLI.
Keywords: peripheral artery disease, endovascular, vascular intervention, vascular disease extremities, vascular surgery, critical limb ischemia, drug coated balloons, drug-eluting stent
Critical limb ischemia is a clinical syndrome of ischemic pain at rest and/or ischemic tissue loss such as non-healing ulcers or gangrene, related to peripheral artery disease of the lower limbs. It differs from acute limb ischemia, which is a sudden loss of limb perfusion (defined as within 14 days) typically due to embolus or in-situ thrombus. In contrast, critical limb ischemia occurs over several weeks to months, but is at the extreme end of the spectrum of chronic limb ischemia (Rutherford classification 4–6, Fontaine III/IV – Table 1). Its importance is due to the much higher risks of limb loss and cardiovascular events than asymptomatic peripheral artery disease and intermittent claudication1, 2. The poor prognosis demands more rapid assessment, a greater role for wound care, and the earlier use of revascularization3. As a result, a multidiscipline approach involving specialists in endovascular revascularization, open surgical revascularization, podiatry, wound care, and other specialties is often required to maximize patient outcomes.
Table 1.
Rutherford and Fontaine Classifications of Chronic Peripheral Arterial Disease Severity2
| Symptom Complex | Rutherford Classification | Fontaine Classification | |
|---|---|---|---|
| Asymptomatic | Stage 0 | Stage I | |
| Intermittent Claudication | Mild Claudication | Stage 1 | Stage IIA (symptoms with >200 meters walking) |
| Moderate Claudication | Stage 2 | Stage IIB (symptoms with <200 meters walking) | |
| Severe Claudication | Stage 3 | ||
| Critical Limb Ischemia | Rest Pain | Stage 4 | Stage III |
| Ischemic Ulceration (limited to digits) | Stage 5 | Stage IV | |
| Severe Ischemic Ulceration or Frank Gangrene | Stage 6 |
Definitions
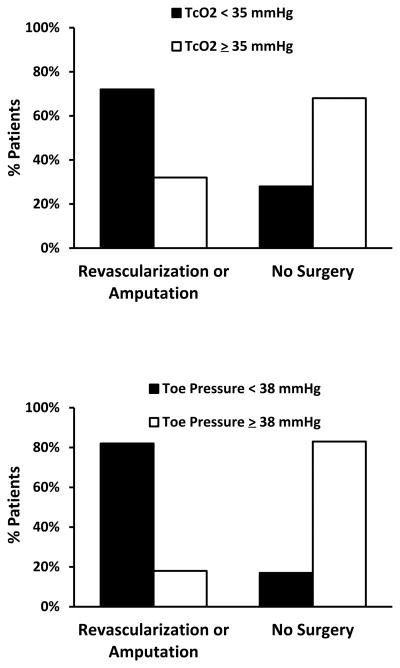
Definitions of CLI aim to identify patients who are risk of major limb amputation without specific treatment such as revascularization or wound care. Traditionally CLI is defined as rest pain or tissue loss (ulcers, or gangrene) supported by ischemia defined by the hemodynamic criteria of low ankle or toe pressures, or low transcutaneous oxygen (TcO2) values. Ankle pressure criteria range from less than 40–70mmHg, toe pressures less than 30–50mmHg, TcO2 less than 20–40mmHg. Higher cutpoints are often used for tissue loss on the assumption that greater perfusion is required for wound healing, but expert consensus on these hemodynamic criteria differs between guidelines2, 4–7. The original definitions were designed to standardize entry criteria for clinical trials of CLI in patients without diabetes to permit comparisons across studies4, 6, or to assess the likelihood of wound healing8. However, their value as diagnostic tests of CLI in clinical practice are more controversial2, 5, 9. Defining specific cut points of toe pressure or TcO2 for the clinical diagnosis of CLI is difficult because of the considerable overlap in values among CLI patients who do or do not progress to major amputation or cardiovascular events (Figure 1)10, 11. One trial suggests they don’t impact the decision for revascularization12. Other definitions of CLI incorporate wound infection and osteomyelitis in addition to ischemia13.
Figure 1.
Overlap in TcO2 and toe pressure results between patients requiring revascularization or amputation for CLI and patients managed medically. From data in Ubbink, et al11
For clinical purposes rest pain or non-healing wounds may suffice as a definition to justify the use of expensive technology (angiography and revascularization) which are fundamental to the clinical treatment of this condition.
Natural History of CLI
Patient outcomes in critical limb ischemia are largely determined by morbidity and mortality due to cardiovascular events and functional impairment due to limb loss. Although, over the whole spectrum of PAD, cardiovascular events such as myocardial infarction and stroke occur in 30–50% of subjects over a 5 year period, patients with CLI face this risk over a one year period1, 2, 14 – an outcome worse than many cancers or severe heart failure. Similarly, although the risk of major amputation (at or above the ankle) is less than 5% over 5–10 years in patients with claudication, it is at least 30–50% in the first year in patients with CLI who do not have revascularization2.
Assessment and Initial Treatment
The clinical presentation of CLI depends on the degree of ischemia, the presence of infection, and co-exisiting neuropathy1. Ischemic pain is usually worse when the patient is supine and often requires narcotics for analgesia. It may waken patients from sleep and prevent them from walking. Infection can increase pain even without severe ischemia. Neuropathy can contribute to tissue injury or mask pain from an ulcer.
Current guidelines recommend measuring the ankle pressure or ankle brachial index1, 2, 5, although medial calcinosis may yield artificially high values in which case toe pressures may indicate arterial obstruction. TcO2 or skin perfusion pressures may indicate the likelihood of wound healing.
The primary goal is to preserve limb function. Revascularization is a fundamental strategy to limb preservation, but in some patients, this does not improve limb function and mobility. For example, cognitive impairment, non-ambulatory status prior to CLI, and severe comorbidities portend a poor prognosis even with revascularization15. When revascularization is considered, arterial imaging identifies the targets and mode of revascularization.
Duplex ultrasound, and non-invasive angiography with computerized tomography (CTA) or magnetic resonance (MRA), can demonstrate arterial obstruction. Duplex ultrasound does not require contrast but requires specific training and may not image the tibial arteries very easily. In infra-inguinal disease, vein mapping is required to determine the feasibility of surgical bypass with autogenous vein.
CTA requires iodinated contrast and may cause contrast nephropathy in patients with impaired renal function. Heavily arterial calcification can create artefacts that limit CTA particularly in distal disease. Non-contrast time-of-flight MRA is prone to artifact with non-laminar flow typical of atherosclerotic plaque, and concerns of nephrogenic systemic fibrosis from gadolinium contrast limit its use in advanced kidney disease16. CTA and MRA are sometimes inadequate to assess the smaller tibial arteries. Nevertheless, CTA and MRA can help localize disease targets and help plan the mode and approach to revascularization.
Due to limitations in imaging distal arteries non-invasively, invasive angiography is often used to clarify the potential for revascularization and should be considered prior to major amputation. Invasive angiography uses iodinated contrast and provides the highest spatial resolution. Diagnostic cases can use as little as 30mL of contrast for both legs with conventional and digital subtraction angiography.
Initial treatments include control of pain, which may require narcotics, pressure relief of ulcers, sheepskin boots to increase superficial collateral supply, and tilting the bed downward to increase limb dependency and perfusion2. Pain relief may reverse sympathetic-mediated vasoconstriction. Although some of these measures only marginally improve perfusion they may reduce the discomfort associated with CLI while planning definitive treatment.
Endovascular Revascularization
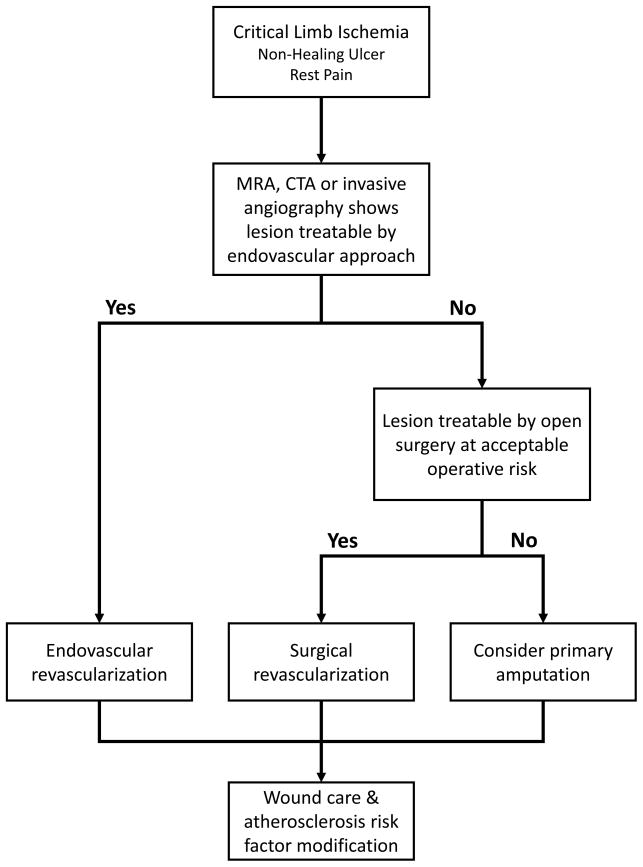
In many centers, endovascular revascularization is the favored approach to CLI, because of lower morbidity and mortality compared to open surgery (Figure 2). The optimal treatment strategy (endovascular versus open surgery) will depend on anatomical factors, comorbidities, patient preference and operator experience and skill. Although claudication can be relieved by inflow revascularization (aorto-iliac and femoral), CLI is often associated with multilevel disease and usually requires outflow (tibial) revascularization as well as treating inflow disease. Much of the evidence for endovascular treatment of inflow disease is based on studies of patients with claudication or a mix of claudication and CLI.
Figure 2.
Suggested algorithm for the approach to revascularization in patients with critical limb ischemia.
Inflow and Femoral-Popliteal Disease
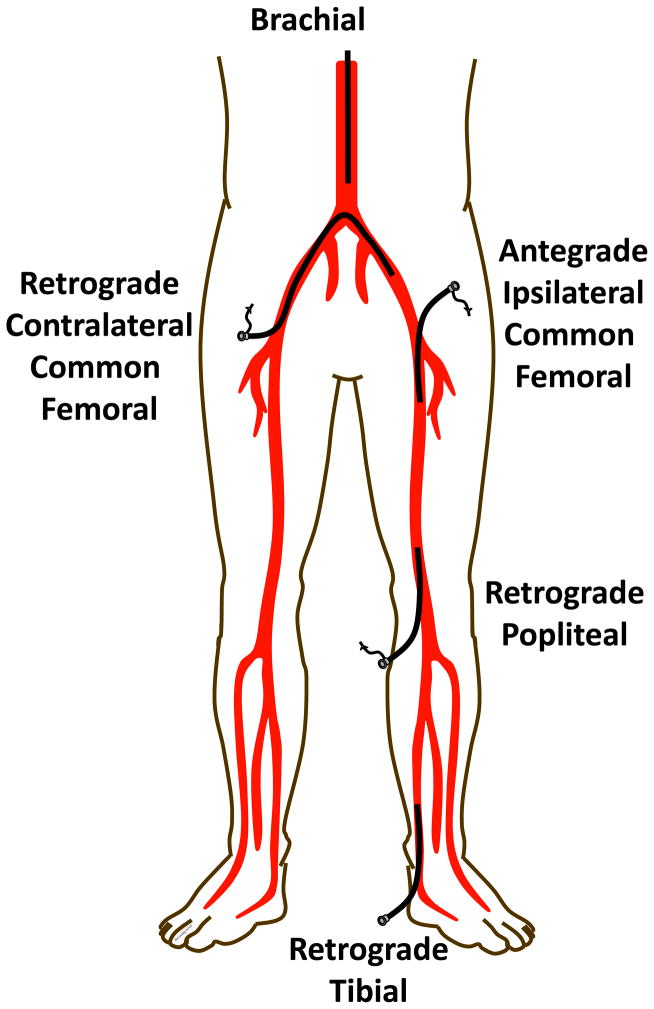
Aorto-iliac disease can be approached from the ipsilateral or contralateral common femoral arteries, or brachial and radial arteries. Rarely a retrograde approach from the popliteal artery can assist crossing superficial femoral artery occlusions which cannot be traversed antegrade3 (Figure 3). The retrograde popliteal approach requires access from above from the contralateral or antegrade common femoral artery, then turning the patient prone on the table and using ultrasound with a micropuncture needle to access the popliteal artery at or just above the knee joint. Small sheaths (4–5 French) provide access for a wire which can be snared from above once it traverses the occlusion. A wire that is exteriorized above and below and occlusion provides a rigid rail to assist pushing catheters and balloons through an occlusion (the “dental floss” technique).
Figure 3.
Approach for arterial access for endovascular revascularization of the lower limbs.
A variety of systems are used to cross lesions including 0.035″, 0.025″, 0.018″, and 0.014″ diameter wires and balloons. Concerns of recoil of ostial lesions and dissections associated with occluded or calcified disease has led to the almost universal practice of primary stenting in iliac disease17. Balloon expandable stents offer greater radial force and a more precise deployment (especially useful in ostial locations), whereas nitinol self-expanding stents may useful in long tapered lesions. Covered stents are useful for life-threatening perforations of the iliac artery during endovascular treatment. Their value in preventing restenosis is uncertain3, due to concerns of increased rates of stent thrombosis and the potential to jail and occlude branch vessels.
Common femoral disease often involves the profunda and SFA origins. Endovascular treatment alone can achieve durable results with acute dissection of the common femoral artery from arterial closure devices. Stents are avoided in this region due to the repeated flexion and extension of this artery and potential for stent fracture, as well as jailing the profunda artery – an important collateral in the event of SFA occlusion. Preservation of both branches with balloon angioplasty alone can be difficult with complex calcified plaques, and often surgical endarterectomy with patch angioplasty offers a more durable result. Hybrid endovascular-surgical approaches using endovascular approaches for iliac or superficial femoral disease and endarterectomy for common femoral disease are increasingly used18. New developments in atherectomy and drug coated balloons have renewed interest in endovascular approaches for common femoral disease, although this paradigm needs formal testing in clinical trials.
The SFA is the longest artery in the leg and subject to flexion, compression and torsion. These forces are particularly important close to the knee and the common femoral artery. Balloon angioplasty offers similar results to stenting in short lesions (<100mm) when there is good arterial expansion without flow limiting dissections19. Minor dissections often heal without long-term sequela. Nitinol self-expanding stents offer better long-term patency in longer lesions20 and re-expand after external radial compression. Stent fracture is thought to increase instent restenosis, but is much rarer with the newer self-expanding stent platforms20–23. Recent drug-eluting stents designs offer a lower rate of restenosis compared to bare-metal self-expanding stents24.
Drug-coated balloons offer lower rates of restenosis than balloon angioplasty alone in patients with SFA disease and claudication25–27. Drug-coated balloons also prevent restenosis when used prior to bare-metal stent deployment28, and offer more durable treatment of instent restenosis of the femoral artery29. The evidence supporting drug-eluting stents and drug-coated balloons is much stronger than for covered-self expanding stents, which have uncertain effects on restenosis and stent thrombosis30.
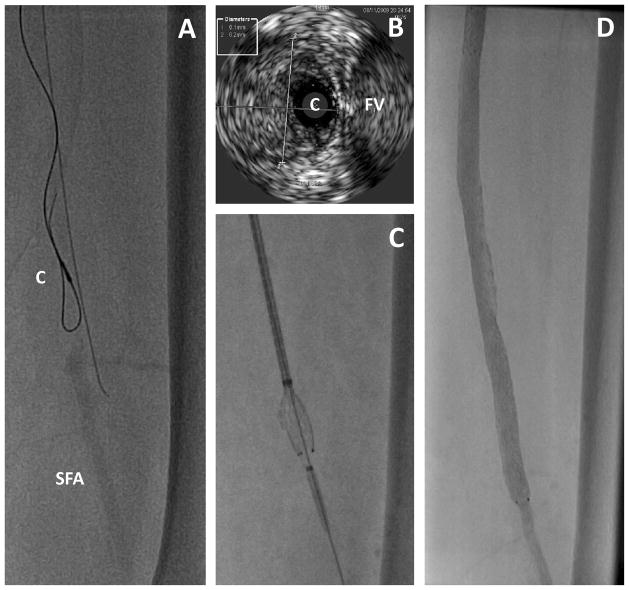
Chronic total occlusions of the SFA are common in symptomatic PAD. A variety of techniques and devices for crossing total occlusions and re-entering the true lumen in the distal artery are available, but few have been tested in randomized trials. These include hydrophilic wires to dissect through the intima or the medial (“subintimal”) layers of the artery. Specialty catheters include those with dissection devices, vibrational energy, drilling heads, and laser capabilities to penetrate the fibrous cap and length of occluded plaque. Intravascular ultrasound can confirm an intraluminal location of a wire in an occlusion (Figure 4), and other devices to redirect a 0.014″ wire from a dissection plane into the distal true lumen can facilitate crossing femoral artery occlusions. These devices are reviewed elsewhere in detail3. A number of atherectomy devices are also available to debulk lesions and may have utility in niche areas such as heavily calcified lesions resistant to balloon and stent dilation3. However a meta-analysis suggested no clear benefit from using atherectomy devices alone compared to balloon angioplasty31. Recent interest in the use of atherectomy combined with drug-coated balloons requires further testing, particularly in areas where stents are avoided (over the knee and hip joints). Given the high risks of major amputation, stenting over the knee joint is sometimes required to maintain patency. A number of specialty stents with greater durability to repeated flexion are designed for the popliteal artery in particular23.
Figure 4.
Intravascular ultrasound used to assess the intra-arterial location while traversing a long occlusion. A. The IVUS catheter (C) is seen over a looped wire in an occluded segment of the mid superficial femoral artery. An adjacent wire is extra-arterial. SFA indicates the distal superficial femoral artery beyond the occlusion. B. IVUS image showing the catheter (C) in the middle of the artery and adjacent to the femoral vein (FV). The diameter of the artery was 6.1 × 6.2 mm, which represents to media and intima and likely overestimates the reference lumen diameter. C. Stent deployment after successfully traversing the occluded artery. D. Final result on angiography.
Tibial Disease
There is rarely a justification for tibial interventions in claudication. However, wound healing and relief of CLI is more dependent on establishing straight-line flow into the foot. Therefore below-knee popliteal and tibial artery interventions are more commonly pursued in CLI.
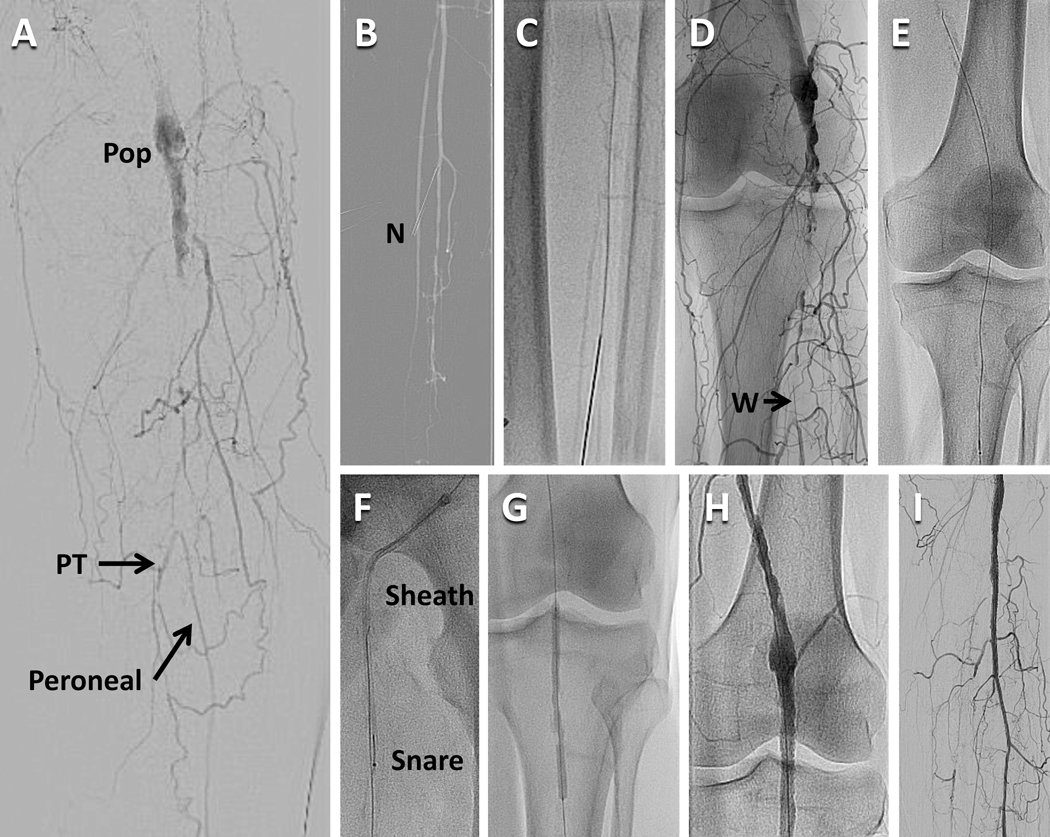
Access is more limited for distal tibial disease as a contralateral common femoral approach or brachial approach are often too distant for most equipment based on 130–150mm shaft lengths. An antegrade femoral approach also gives more “pushability” to drive through long occlusions. The retrograde tibial approach can be used for tibial and popliteal occlusions which cannot be crossed antegrade (Figure 3), but if unsuccessful may create a non-healing ulcer at the access site. The retrograde pedal or tibial artery approach uses ultrasound and a micropuncture needle for access and the dilator of the micropuncture kit or a small sheath for wire access. Access from above (e.g. antegrade femoral) allows a retrograde wire to be snared and exteriorized above and below the tibial or popliteal occlusion to provide a rigid rail to drive catheters and balloons through an occlusion (Figure 5).
Figure 5.
Combined antegrade access from the left femoral artery and retrograde access from the left peroneal artery in a patient with an occluded left popliteal artery and peroneal tibial trunk artery. A. Digital subtraction angiogram showing the occluded popliteal artery (Pop) with reconstitution of the peroneal artery. The posterior tibial artery (PT) is also reconstituted by is occluded shortly after its origin. The anterior tibial artery is completely occluded proximally. B. A road map image overlaying the distal peroneal artery to enable access of the peroneal artery in the mid calf with a needle (N). C. The needle and 0.014″ wire are advanced up the peroneal artery. D. The distal end of the wire (W) is penetrating the distal occlusion of the peroneal tibial trunk. E. A 0.025″ wire crosses the popliteal occlusion in a retrograde direction. F. The wire is snared from above through the femoral sheath. G. Both the proximal and distal ends of the wire were held taught to advance a balloon into the occlusion prior to dilation. I The popliteal artery after deployment of a self-expanding stent. J. Antegrade flow is restored in the peroneal artery after deployment of a short drug-eluting coronary stent at the distal margin of the popliteal stent.
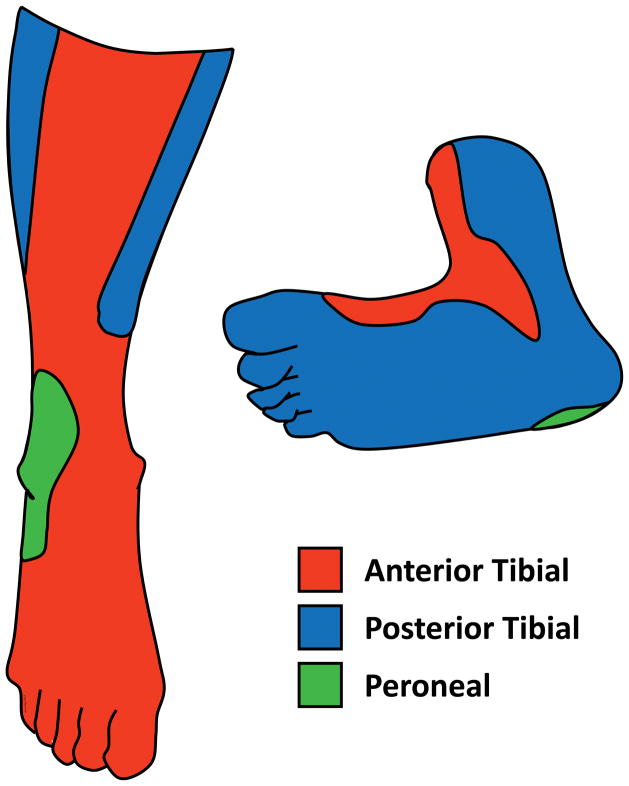
The value of angiosome directed revascularization versus restoring any straight-line flow into the foot is debated. The former assumes that revascularization of a tibial artery supplying the angiosome of the ulcer or gangrenous region (Figure 6) is more likely to promote healing than non-angiosome revascularization which relies on increased collateral flow to an ischemic region. In observational studies, wound healing was greater and amputation lower with angiosome-directed compared to indirect (non-angiosome) tibial revascularization32. However, these observations may be confounded. Indirect revascularization, may be a marker for more complex tibial disease, which may be associated both with no option for angiosome directed revascularization and poorer limb salvage. In one study, changes in foot microcirculation assessed by skin perfusion pressure improved regardless of whether the angiosome related tibial artery or the non-angiosome related artery was revascularized33. Although it makes intuitive sense to use an angiosome directed treatment wherever possible, if this is not successful, any straight-line flow should be better than none.
Figure 6.
Angiosome distributions showing regions supplied by each of the three below knee arteries.
Primary balloon angioplasty of tibial disease provides a good response in most situations. Long balloons are specifically designed to treat the often diffuse tibial disease with prolonged inflations. Stents are reserved for poor balloon results (re-occlusion, recoil to more than 50% stenosis, flow-limiting dissection). Tibial arteries are about 2.5–3.5mm in diameter, and are usually treated with balloon expandable coronary stents with a spot-stenting philosophy. Proximal lesions are somewhat protected by the bulk of the calf muscle, but can theoretically be crushed by external compression. Stent crush is more likely with extensive stenting and stents in the distal calf. Poor outflow theoretically increases the risk of stent thrombosis and may reduce the enthusiasm for stenting. Randomized studies in tibial arteries show better patency and less need for reintervention with drug-eluting compared to bare-metal coronary stents34–36, with one trial showing lower rates of amputation35.
Compared to conventional balloon angioplasty, drug-coated balloons for tibial interventions provided promising results in early series and single center trials37. However, restenosis rates were higher than drug-eluting stents in one small trial38, and the multicenter randomized IN.PACT DEEP study raised concerns because of a trend to more amputations in the drug-coated versus standard balloon angioplasty arms (8.8% versus 3.6%, p=0.08)39. Reasons for the lackluster results compared to femoral-popliteal disease include reduced drug delivery due to drug coating after balloon wrapping and poor drug-release characteristics. Further randomized trials will explore their value in tibial arteries.
Atherectomy in tibial arteries is of uncertain value beyond balloon angioplasty and stenting31. Long segment tibial atherectomy could cause embolization which decreases outflow and distal perfusion. One recent report showed greater acute success, but no difference in amputation, repeat revascularization or mortality with laser assisted versus conventional angioplasty40.
Wire perforation of the tibial arteries is usually easily treatable by low-pressure balloon angioplasty, but larger perforations may require longer balloon inflations or covered stents to avoid a compartment syndrome which can cause ischemic muscle and nerve injury and threaten the viability of the lower limb.
Open Surgical Revascularization
The goals of surgical revascularization are to provide straight-line flow into the foot, promote wound healing, and to limit the level of amputation. Open surgery has higher risks of peri-operative myocardial infarction, death and stroke than endovascular revascularization. However, in critical limb ischemia, the potential loss of limb and function may favor surgery when endovascular therapy is not possible or not successful and patients otherwise have a reasonable 2 year survival18. Risk scores can help risk-stratify CLI patients having infrainguinal bypass surgery. For example, the PREVENT III risk score includes dialysis, tissue loss, age ≥ 75 years, and coronary artery disease41. A higher score associates with a lower risk of survival free from amputation. In addition to patient risk, assessment includes vein mapping of saphenous vein to determine available autogenous conduit. This is particularly important in patients who may have had vein harvested for coronary artery bypass in the past.
Multilevel disease is often treated with hybrid revascularization using endovascular techniques to treat inflow disease (e.g. iliac stenting), and surgical revascularization for femoral or infrainguinal disease (e.g. common femoral endarterectomy, and femoral popliteal bypass)18. Rarely, occlusion of the distal aorta and iliac disease may require aorto-bifemoral bypass, contralateral femoral to femoral bypass, or axillary-femoral bypass18.
Common femoral endarterectomy may extend into the proximal SFA or profunda artery. Closure is usually achieved with a bovine or synthetic patch to reduce restenosis, or sometimes with primary closure without a patch18. Complications include wound infection (particularly in obese patients), hematoma and lymph leak. This procedure offers a high long-term patency rate (>90%) and considered superior to endovascular treatement particularly for heavily calcified disease involving the SFA and profunda orgins.
As with endovascular treatment, infrainguinal bypass relies on good inflow and outflow. The three types of saphenous vein bypass are reversed (translocated) vein, nonreversed vein, and in situ bypass where vein branches are ligated and the distal ends mobilized and anastomosed to the artery. The latter two configurations require excision of the valves with a valvulotome, which can sometimes injure the vein conduit. Observational studies suggest similar outcomes with all three configurations18, 42. Limb salvage and graft patency are best with good quality, long, single-segment, autogenous vein with a diameter of at least 3.5mm43–45. In the PREVENT III trial, bypass grafts with these characteristics had a low 30 day failure rate (less than 2%), and high secondary patency and limb salvage at one year (approximately 90%)45.
In pooled analyses, autogenous saphenous vein provided better long-term patency than prosthetic grafts for above- and below-knee grafts44, 46, 47. Prosthetic grafts of heparin-bonded polytetrafluoroethylene may provide better outcomes than older prosthetic grafts48 with comparable results to autologous vein in a one retrospective study49. Cryopreserved cadaveric vein has poorer long-term patency results50
Comparisons of Open Surgical versus Endovascular Revascularization
The only randomized study comparing endovascular versus open surgical treatment of CLI patients is the BASIL study14. This trial published a decade ago, demonstrated no difference in major amputation or death over 5 years. Rates of myocardial infarction, wound infection, pulmonary complications were higher in the surgical group, and repeat revascularization was higher in the endovascular arm. However, over the last 10–15 years, perioperative mortality from open surgery has improved and there are more options for graft salvage with endovascular techniques. Similarly, endovascular options developed after the BASIL trial include bare-metal stents, drug-coated balloons and stents, and a variety of wires and devices to assist crossing long occlusions.
More recently, several proposals for endpoints important in CLI have converged on limb salvage (avoiding a major amputation), and the need for major re-intervention (a new bypass graft, or thrombolysis of graft or treated segment)4, 7, 51. As a result of these developments, the NIH sponsored the BEST-CLI study - a new randomized trial of open surgery versus endovascular revascularization in CLI52.
BEST-CLI will assess open surgery or endovascular revascularization in patients who are acceptable candidates for both techniques. The primary endpoint of BEST-CLI includes major limb amputation, repeat major intervention (thrombolysis or new bypass graft), and mortality. These reflect life and limb threatening events that have major effects on quality and quantity of life, as opposed to minor procedures to treat restenosis which are not included in the primary endpoint. BEST-CLI will also include assessments of quality-of-life and cost-effectiveness.
Amputation
Minor amputations such toe, ray (toe and metatarsal), or transmetatarsal amputations require an adequate blood supply into the foot to maximize healing and are usually part of the treatment plan for gangrene or tissue loss after successful revascularization. Generally, minor amputation does not limit functional independence or require a prosthesis.
Major amputations (at or above the knee) limit functional independence and require a prosthesis to walk. Although preventing major amputation is a key goal, amputation may be indicated for failed revascularization, patients with extensive tissue loss or infection, patients unfit for surgical revascularization with no endovascular options, and potentially non-ambulating patients. Up to one third of below-knee amputations may require further surgery or an above-knee amputation due to poor healing53. A patent popliteal pulse reduces the failure rate of healing to less than 10%. More than 90% of above-knee amputations heal, but only about 20% of amputees regain full mobility with a prosthesis compared to 60% with a below-knee prosthesis53, 54. Factors related to poor prosthesis use and function after major amputation include increasing age, bilateral or above-knee amputations, dementia, and poor function prior to amputation54.
Wound Care
Wound care principles include improving perfusion into the limb, treating infection, avoiding pressure on a wound, debridement, and adequate nutrition. Debridement of devitalized or infected tissue by scalpel, collagenases, or even maggots55, promotes wound healing. Antibiotics may be required to treat infection to prevent osteomyelitis. Avoiding pressure on the wound (e.g. off-loading the foot) also assists wound healing56. The local temperature of the limb can be increased using sheepskin (Rooke) boots and may improve superficial collateral flow to help perfuse a limb57. Negative pressure dressings (e.g. vacuum-assisted) increase capillary flow and help drain wounds58. Hyperbaric oxygen therapy offers no advantages for amputation prevention, but may improve the more subjective endpoint of wound healing in diabetes59.
In patients where there are no revascularization options, intermittent pneumatic compression may assist wound healing and prevent major amputation54. To date, cell based therapies such as infusion of bone marrow derived mononuclear cells have not prevented major amputation in patients with no revascularization options60.
Medical Therapy and Surveillance After Revascularization
Failure of endovascular and surgical treatment of CLI due to thrombosis, neointimal proliferation, or progression in atherosclerosis demands close surveillance of patients by providers with vascular expertise. Surveillance also includes intensively treating risk factors for atherosclerosis to reduce the high risk of cardiovascular events.
Recurrent ischemic pain in the leg, lack of progression in wound healing, or a decline in ABIs are indicators of restenosis or occlusion. Duplex ultrasound of bypass grafts is commonly practiced to identify graft stenoses for revision and preserve long-term patency. However, this practice was not associated with lower amputation or better patency in one randomized trial61, and there are no randomized trials of its value after endovascular therapy. Our practice after endovascular therapy includes a history and exam, and to use duplex ultrasound in the femoral artery particularly after treating long segment disease or when symptoms or poor wound healing raise concerns of patency62, 63.
Evidence for therapies to prevent thrombosis or restenosis after endovascular interventions is sparse and often extrapolated from studies of coronary artery interventions. Low-dose aspirin is usually given for life to prevent thrombosis of a treated segment, but also other cardiovascular events. The duration of clopidogrel to prevent occlusion of segments treated by endovascular techniques is uncertain. Most clinical trials of bare-metal stenting use dual-antiplatelet therapy for 1–3 months19–21, 24, but data extrapolated from medical studies of patients with peripheral artery disease, could justify longer treatment64. Clinical studies from Japan suggest that cilostazol may reduce in-stent restenosis65–67 but this is not yet incorporated in recent guidelines of revascularization for CLI.
The value of anticoagulation for lower extremity bypass is conflicting with some trials showing benefit over aspirin for autogenous vein versus prosthetic conduit and vice-versa18. Given the increased risk of bleeding, most surgeons reserve anticoagulation for graft thrombosis, or hypercoagulable disorders. There is no benefit of adding clopidogrel to aspirin for graft patency68.
Evidence for the value of intensive atherosclerosis risk factor reduction is derived largely from observational studies and subgroups of patients with PAD in clinical trials. For example, PAD patients who stop smoking have fewer cardiovascular events than those who continue smoking69, 70. Antiplatelet therapy with aspirin71, 72 or clopidogrel73, and angiotensin converting enzyme inhibitors74 decrease cardiovascular events in patients with PAD. Intensive statin therapy consistently lowers cardiovascular events in PAD patients compared to no statin or low intensity statins75–77. In observational studies of patients receiving revascularization for PAD, statin therapy is associated with lower risks of cardiovascular events63, 78–80, and limb loss63, 81, 82. In population studies, intensive risk factor modification in patients with peripheral artery disease is improving, but still lags behind its use in patients with symptomatic coronary disease83.
Conclusions
Patients with CLI have a high risk of limb loss without revascularization and a high short term risk of cardiovascular events compared to less severe forms of chronic peripheral artery disease. Revascularization is indicated if it will prevent limb loss and preserve ambulation and function, while intensive medical therapy targets the risk factors for atherosclerosis progression and cardiovascular events. Endovascular revascularization offers a lower initial risk than open surgery, but recurrent disease from restenosis or new de-novo disease is common in patients with CLI. New drug-eluting balloons and stents offer better longer term outcomes after some endovascular revascularizations, but further long-term data on durability is required in order to assess their overall benefit given the increased costs of initial treatment. Close follow-up focusing on wound care and prevention, risk factor management, and surveillance for new and recurrent disease is required.
Acknowledgments
Sources of Funding
VA Clinical Science Research and Development Awards 1/01CX000440 and 1/21CX000793, and by the Assistant Secretary of Defense for Health Affairs, through the Gulf War Illness Research Program under Award No. W81XWH-15-1-0216. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Footnotes
Disclosures
Dr. Kinlay has received research support for trials at VA Boston from Medtronic (SYMPLICITY III study) and The Medicines Company (ENDOMAX study).
References
- 1.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Rutherford RB. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26:81–157. [PubMed] [Google Scholar]
- 3.Thukkani AK, Kinlay S. Endovascular intervention for peripheral artery disease. Circulation research. 2015;116:1599–613. doi: 10.1161/CIRCRESAHA.116.303503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, Nehler MR, Powell RJ, Sidawy AN. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. Journal of vascular surgery. 2009;50:1462–73. e1–3. doi: 10.1016/j.jvs.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 5.European Stroke O; Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Rother J, Sievert H, van Sambeek M, Zeller T Guidelines ESCCfP. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC) European heart journal. 2011;32:2851–906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 6.Labs KH, Dormandy JA, Jaeger KA, Stuerzebecher CS, Hiatt WR. Transatlantic Conference on Clinical Trial Guidelines in Peripheral Arterial Disease: clinical trial methodology. Basel PAD Clinical Trial Methodology Group. Circulation. 1999;100:e75–81. doi: 10.1161/01.cir.100.17.e75. [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Conte MS, Cutlip DE, Dib N, Geraghty P, Gray W, Hiatt WR, Ho M, Ikeda K, Ikeno F, Jaff MR, Jones WS, Kawahara M, Lookstein RA, Mehran R, Misra S, Norgren L, Olin JW, Povsic TJ, Rosenfield K, Rundback J, Shamoun F, Tcheng J, Tsai TT, Suzuki Y, Vranckx P, Wiechmann BN, White CJ, Yokoi H, Krucoff MW. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC) J Am Coll Cardiol. 2015;65:931–41. doi: 10.1016/j.jacc.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ubbink DT, Spincemaille GH, Reneman RS, Jacobs MJ. Prediction of imminent amputation in patients with non-reconstructible leg ischemia by means of microcirculatory investigations. Journal of vascular surgery. 1999;30:114–21. doi: 10.1016/s0741-5214(99)70183-7. [DOI] [PubMed] [Google Scholar]
- 9.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. Journal of vascular surgery. 1997;26:517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 10.Apelqvist J, Castenfors J, Larsson J, Stenstrom A, Agardh CD. Prognostic value of systolic ankle and toe blood pressure levels in outcome of diabetic foot ulcer. Diabetes Care. 1989;12:373–8. doi: 10.2337/diacare.12.6.373. [DOI] [PubMed] [Google Scholar]
- 11.Ubbink DT, Tulevski II, de Graaff JC, Legemate DA, Jacobs MJ. Optimisation of the non-invasive assessment of critical limb ischaemia requiring invasive treatment. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2000;19:131–7. doi: 10.1053/ejvs.1999.0953. [DOI] [PubMed] [Google Scholar]
- 12.de Graaff JC, Ubbink DT, Legemate DA, Tijssen JG, Jacobs MJ. Evaluation of toe pressure and transcutaneous oxygen measurements in management of chronic critical leg ischemia: a diagnostic randomized clinical trial. Journal of vascular surgery. 2003;38:528–34. doi: 10.1016/s0741-5214(03)00414-2. [DOI] [PubMed] [Google Scholar]
- 13.Mills JL, Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G Society for Vascular Surgery Lower Extremity Guidelines C. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) Journal of vascular surgery. 2014;59:220–34. e1–2. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 15.Oresanya L, Zhao S, Gan S, Fries BE, Goodney PP, Covinsky KE, Conte MS, Finlayson E. Functional outcomes after lower extremity revascularization in nursing home residents: a national cohort study. JAMA Intern Med. 2015;175:951–7. doi: 10.1001/jamainternmed.2015.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis: predictor of early mortality and association with gadolinium exposure. Arthritis and rheumatism. 2007;56:3433–41. doi: 10.1002/art.22925. [DOI] [PubMed] [Google Scholar]
- 17.AbuRahma AF, Hayes JD, Flaherty SK, Peery W. Primary iliac stenting versus transluminal angioplasty with selective stenting. Journal of vascular surgery. 2007;46:965–970. doi: 10.1016/j.jvs.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 18.Slovut DP, Lipsitz EC. Surgical technique and peripheral artery disease. Circulation. 2012;126:1127–38. doi: 10.1161/CIRCULATIONAHA.111.059048. [DOI] [PubMed] [Google Scholar]
- 19.Krankenberg H, Schluter M, Steinkamp HJ, Burgelin K, Scheinert D, Schulte KL, Minar E, Peeters P, Bosiers M, Tepe G, Reimers B, Mahler F, Tubler T, Zeller T. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST) Circulation. 2007;116:285–92. doi: 10.1161/CIRCULATIONAHA.107.689141. [DOI] [PubMed] [Google Scholar]
- 20.Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, Schlager O, Cejna M, Lammer J, Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–88. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 21.Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ, Goldstein J, Jaff MR. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circulation Cardiovascular interventions. 2010;3:267–76. doi: 10.1161/CIRCINTERVENTIONS.109.903468. [DOI] [PubMed] [Google Scholar]
- 22.Scheinert D, Scheinert S, Sax J, Piorkowski C, Braunlich S, Ulrich M, Biamino G, Schmidt A. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol. 2005;45:312–5. doi: 10.1016/j.jacc.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 23.George JC, Rosen ES, Nachtigall J, VanHise A, Kovach R. SUPERA interwoven nitinol Stent Outcomes in Above-Knee IntErventions (SAKE) study. J Vasc Interv Radiol. 2014;25:954–61. doi: 10.1016/j.jvir.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, Snyder SA, Ragheb AO, White JK, Machan LS. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circulation Cardiovascular interventions. 2011;4:495–504. doi: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- 25.Cassese S, Byrne RA, Ott I, Ndrepepa G, Nerad M, Kastrati A, Fusaro M. Paclitaxel-coated versus uncoated balloon angioplasty reduces target lesion revascularization in patients with femoropopliteal arterial disease: a meta-analysis of randomized trials. Circulation Cardiovascular interventions. 2012;5:582–9. doi: 10.1161/CIRCINTERVENTIONS.112.969972. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R, Muller-Hulsbeck S, Nehler MR, Benenati JF, Scheinert D, Investigators L. Trial of a Paclitaxel-Coated Balloon for Femoropopliteal Artery Disease. N Engl J Med. 2015;373:145–53. doi: 10.1056/NEJMoa1406235. [DOI] [PubMed] [Google Scholar]
- 27.Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR, Investigators IPST. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liistro F, Grotti S, Porto I, Angioli P, Ricci L, Ducci K, Falsini G, Ventoruzzo G, Turini F, Bellandi G, Bolognese L. Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery) JACC Cardiovascular interventions. 2013;6:1295–302. doi: 10.1016/j.jcin.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Krankenberg H, Tubler T, Ingwersen M, Schluter M, Scheinert D, Blessing E, Sixt S, Kieback A, Beschorner U, Zeller T. Drug-Coated Balloon Versus Standard Balloon for Superficial Femoral Artery In-Stent Restenosis: The Randomized Femoral Artery In-Stent Restenosis (FAIR) Trial. Circulation. 2015;132:2230–6. doi: 10.1161/CIRCULATIONAHA.115.017364. [DOI] [PubMed] [Google Scholar]
- 30.Lammer J, Zeller T, Hausegger KA, Schaefer PJ, Gschwendtner M, Mueller-Huelsbeck S, Rand T, Funovics M, Wolf F, Rastan A, Gschwandtner M, Puchner S, Ristl R, Schoder M. Heparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: the randomized VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease) J Am Coll Cardiol. 2013;62:1320–7. doi: 10.1016/j.jacc.2013.05.079. [DOI] [PubMed] [Google Scholar]
- 31.Ambler GK, Radwan R, Hayes PD, Twine CP. Atherectomy for peripheral arterial disease. The Cochrane database of systematic reviews. 2014;3:CD006680. doi: 10.1002/14651858.CD006680.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Biancari F, Juvonen T. Angiosome-targeted lower limb revascularization for ischemic foot wounds: systematic review and meta-analysis. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2014;47:517–22. doi: 10.1016/j.ejvs.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Kawarada O, Yasuda S, Nishimura K, Sakamoto S, Noguchi M, Takahi Y, Harada K, Ishihara M, Ogawa H. Effect of single tibial artery revascularization on microcirculation in the setting of critical limb ischemia. Circulation Cardiovascular interventions. 2014;7:684–91. doi: 10.1161/CIRCINTERVENTIONS.113.001311. [DOI] [PubMed] [Google Scholar]
- 34.Bosiers M, Scheinert D, Peeters P, Torsello G, Zeller T, Deloose K, Schmidt A, Tessarek J, Vinck E, Schwartz LB. Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. Journal of vascular surgery. 2012;55:390–8. doi: 10.1016/j.jvs.2011.07.099. [DOI] [PubMed] [Google Scholar]
- 35.Rastan A, Brechtel K, Krankenberg H, Zahorsky R, Tepe G, Noory E, Schwarzwalder U, Macharzina R, Schwarz T, Burgelin K, Sixt S, Tubler T, Neumann FJ, Zeller T. Sirolimus-eluting stents for treatment of infrapopliteal arteries reduce clinical event rate compared to bare-metal stents: long-term results from a randomized trial. J Am Coll Cardiol. 2012;60:587–91. doi: 10.1016/j.jacc.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 36.Scheinert D, Katsanos K, Zeller T, Koppensteiner R, Commeau P, Bosiers M, Krankenberg H, Baumgartner I, Siablis D, Lammer J, Van Ransbeeck M, Qureshi AC, Stoll HP, Investigators A. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol. 2012;60:2290–5. doi: 10.1016/j.jacc.2012.08.989. [DOI] [PubMed] [Google Scholar]
- 37.Liistro F, Porto I, Angioli P, Grotti S, Ricci L, Ducci K, Falsini G, Ventoruzzo G, Turini F, Bellandi G, Bolognese L. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation. 2013;128:615–21. doi: 10.1161/CIRCULATIONAHA.113.001811. [DOI] [PubMed] [Google Scholar]
- 38.Siablis D, Kitrou PM, Spiliopoulos S, Katsanos K, Karnabatidis D. Paclitaxel-coated balloon angioplasty versus drug-eluting stenting for the treatment of infrapopliteal long-segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC Cardiovascular interventions. 2014;7:1048–56. doi: 10.1016/j.jcin.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Zeller T, Baumgartner I, Scheinert D, Brodmann M, Bosiers M, Micari A, Peeters P, Vermassen F, Landini M, Snead DB, Kent KC, Rocha-Singh KJ. Drug-Eluting Balloon Versus Standard Balloon Angioplasty for Infrapopliteal Arterial Revascularization in Critical Limb Ischemia: 12-Month Results From the IN.PACT DEEP Randomized Trial. J Am Coll Cardiol. 2014;64:1568–76. doi: 10.1016/j.jacc.2014.06.1198. [DOI] [PubMed] [Google Scholar]
- 40.Piyaskulkaew C, Parvataneni K, Ballout H, Szpunar S, Sharma T, Almahmoud M, LaLonde T, Davis T, Mehta RH, Yamasaki H. Laser in infrapopliteal and popliteal stenosis 2 study (LIPS2): Long-term outcomes of laser-assisted balloon angioplasty versus balloon angioplasty for below knee peripheral arterial disease. Catheter Cardiovasc Interv. 2015;86:1211–8. doi: 10.1002/ccd.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schanzer A, Mega J, Meadows J, Samson RH, Bandyk DF, Conte MS. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. Journal of vascular surgery. 2008;48:1464–71. doi: 10.1016/j.jvs.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh N, Sidawy AN, DeZee KJ, Neville RF, Akbari C, Henderson W. Factors associated with early failure of infrainguinal lower extremity arterial bypass. Journal of vascular surgery. 2008;47:556–61. doi: 10.1016/j.jvs.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 43.Conte MS. Critical appraisal of surgical revascularization for critical limb ischemia. Journal of vascular surgery. 2013;57:8S–13S. doi: 10.1016/j.jvs.2012.05.114. [DOI] [PubMed] [Google Scholar]
- 44.Pereira CE, Albers M, Romiti M, Brochado-Neto FC, Pereira CA. Meta-analysis of femoropopliteal bypass grafts for lower extremity arterial insufficiency. Journal of vascular surgery. 2006;44:510–517. doi: 10.1016/j.jvs.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 45.Schanzer A, Hevelone N, Owens CD, Belkin M, Bandyk DF, Clowes AW, Moneta GL, Conte MS. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. Journal of vascular surgery. 2007;46:1180–90. doi: 10.1016/j.jvs.2007.08.033. discussion 1190. [DOI] [PubMed] [Google Scholar]
- 46.Twine CP, McLain AD. Graft type for femoro-popliteal bypass surgery. The Cochrane database of systematic reviews. 2010:CD001487. doi: 10.1002/14651858.CD001487.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Pomposelli FB, Kansal N, Hamdan AD, Belfield A, Sheahan M, Campbell DR, Skillman JJ, Logerfo FW. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. Journal of vascular surgery. 2003;37:307–15. doi: 10.1067/mva.2003.125. [DOI] [PubMed] [Google Scholar]
- 48.Lumsden AB, Morrissey NJ Comparison of S, Primary Patency Between the FBH-CVG and Co-investigators ESeT. Randomized controlled trial comparing the safety and efficacy between the FUSION BIOLINE heparin-coated vascular graft and the standard expanded polytetrafluoroethylene graft for femoropopliteal bypass. Journal of vascular surgery. 2015;61:703–12. e1. doi: 10.1016/j.jvs.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 49.Daenens K, Schepers S, Fourneau I, Houthoofd S, Nevelsteen A. Heparin-bonded ePTFE grafts compared with vein grafts in femoropopliteal and femorocrural bypasses: 1- and 2-year results. Journal of vascular surgery. 2009;49:1210–6. doi: 10.1016/j.jvs.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Chang CK, Scali ST, Feezor RJ, Beck AW, Waterman AL, Huber TS, Berceli SA. Defining utility and predicting outcome of cadaveric lower extremity bypass grafts in patients with critical limb ischemia. Journal of vascular surgery. 2014;60:1554–64. doi: 10.1016/j.jvs.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinlay S. Outcomes for clinical studies assessing drug and revascularization therapies for claudication and critical limb ischemia in peripheral artery disease. Circulation. 2013;127:1241–50. doi: 10.1161/CIRCULATIONAHA.112.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menard MT, Farber A. The BEST-CLI trial: a multidisciplinary effort to assess whether surgical or endovascular therapy is better for patients with critical limb ischemia. Seminars in vascular surgery. 2014;27:82–4. doi: 10.1053/j.semvascsurg.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Dormandy J, Heeck L, Vig S. Major amputations: clinical patterns and predictors. Seminars in vascular surgery. 1999;12:154–61. [PubMed] [Google Scholar]
- 54.Taylor SM, Kalbaugh CA, Blackhurst DW, Hamontree SE, Cull DL, Messich HS, Robertson RT, Langan EM, 3rd, York JW, Carsten CG, 3rd, Snyder BA, Jackson MR, Youkey JR. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. Journal of vascular surgery. 2005;42:227–35. doi: 10.1016/j.jvs.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Enoch S, Grey JE, Harding KG. ABC of wound healing. Non-surgical and drug treatments. BMJ. 2006;332:900–3. doi: 10.1136/bmj.332.7546.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Healy A, Naemi R, Chockalingam N. The effectiveness of footwear and other removable off-loading devices in the treatment of diabetic foot ulcers: a systematic review. Current diabetes reviews. 2014;10:215–30. doi: 10.2174/1573399810666140918121438. [DOI] [PubMed] [Google Scholar]
- 57.Rooke TW, Hollier LH, Osmundson PJ. The influence of sympathetic nerves on transcutaneous oxygen tension in normal and ischemic lower extremities. Angiology. 1987;38:400–10. doi: 10.1177/000331978703800508. [DOI] [PubMed] [Google Scholar]
- 58.Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative-pressure wound dressings for diabetic foot wounds. Annals of vascular surgery. 2003;17:645–9. doi: 10.1007/s10016-003-0065-3. [DOI] [PubMed] [Google Scholar]
- 59.Kranke P, Bennett M, Roeckl-Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. The Cochrane database of systematic reviews. 2004:CD004123. doi: 10.1002/14651858.CD004123.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Teraa M, Sprengers RW, Schutgens RE, Slaper-Cortenbach IC, van der Graaf Y, Algra A, van der Tweel I, Doevendans PA, Mali WP, Moll FL, Verhaar MC. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) trial. Circulation. 2015;131:851–60. doi: 10.1161/CIRCULATIONAHA.114.012913. [DOI] [PubMed] [Google Scholar]
- 61.Davies AH, Hawdon AJ, Sydes MR, Thompson SG, Participants V. Is duplex surveillance of value after leg vein bypass grafting? Principal results of the Vein Graft Surveillance Randomised Trial (VGST) Circulation. 2005;112:1985–91. doi: 10.1161/CIRCULATIONAHA.104.518738. [DOI] [PubMed] [Google Scholar]
- 62.Sobieszczyk P, Eisenhauer A. Management of patients after endovascular interventions for peripheral artery disease. Circulation. 2013;128:749–57. doi: 10.1161/CIRCULATIONAHA.113.001560. [DOI] [PubMed] [Google Scholar]
- 63.Todoran TM, Connors G, Engelson BA, Sobieszczyk PS, Eisenhauer AC, Kinlay S. Femoral artery percutaneous revascularization for patients with critical limb ischemia: outcomes compared to patients with claudication over 2.5 years. Vasc Med. 2012;17:138–44. doi: 10.1177/1358863X12440141. [DOI] [PubMed] [Google Scholar]
- 64.Wong PF, Chong LY, Mikhailidis DP, Robless P, Stansby G. Antiplatelet agents for intermittent claudication. The Cochrane database of systematic reviews. 2011:CD001272. doi: 10.1002/14651858.CD001272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iida O, Nanto S, Uematsu M, Morozumi T, Kitakaze M, Nagata S. Cilostazol reduces restenosis after endovascular therapy in patients with femoropopliteal lesions. Journal of vascular surgery. 2008;48:144–9. doi: 10.1016/j.jvs.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 66.Soga Y, Iida O, Hirano K, Suzuki K, Yokoi H, Nobuyoshi M. Restenosis after stent implantation for superficial femoral artery disease in patients treated with cilostazol. Catheter Cardiovasc Interv. 2012;79:541–8. doi: 10.1002/ccd.23304. [DOI] [PubMed] [Google Scholar]
- 67.Soga Y, Iida O, Kawasaki D, Hirano K, Yamaoka T, Suzuki K. Impact of cilostazol on angiographic restenosis after balloon angioplasty for infrapopliteal artery disease in patients with critical limb ischemia. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2012;44:577–81. doi: 10.1016/j.ejvs.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 68.Belch JJ, Dormandy J, Committee CW, Biasi GM, Cairols M, Diehm C, Eikelboom B, Golledge J, Jawien A, Lepantalo M, Norgren L, Hiatt WR, Becquemin JP, Bergqvist D, Clement D, Baumgartner I, Minar E, Stonebridge P, Vermassen F, Matyas L, Leizorovicz A. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. Journal of vascular surgery. 2010;52:825–33. 833 e1–2. doi: 10.1016/j.jvs.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 69.Faulkner KW, House AK, Castleden WM. The effect of cessation of smoking on the accumulative survival rates of patients with symptomatic peripheral vascular disease. The Medical journal of Australia. 1983;1:217–9. doi: 10.5694/j.1326-5377.1983.tb99395.x. [DOI] [PubMed] [Google Scholar]
- 70.Jonason T, Bergstrom R. Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221:253–60. [PubMed] [Google Scholar]
- 71.2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;124:2020–45. doi: 10.1161/CIR.0b013e31822e80c3. [DOI] [PubMed] [Google Scholar]
- 72.Berger JS, Hiatt WR. Medical therapy in peripheral artery disease. Circulation. 2012;126:491–500. doi: 10.1161/CIRCULATIONAHA.111.033886. [DOI] [PubMed] [Google Scholar]
- 73.A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) CAPRIE Steering Committee. Lancet. 1996;348:1329–39. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 74.Klein AJ, Pinto DS, Gray BH, Jaff MR, White CJ, Drachman DE. SCAI expert consensus statement for femoral-popliteal arterial intervention appropriate use. Catheter Cardiovasc Interv. 2014;84:529–38. doi: 10.1002/ccd.25504. [DOI] [PubMed] [Google Scholar]
- 75.Klein AJ, Feldman DN, Aronow HD, Gray BH, Gupta K, Gigliotti OS, Jaff MR, Bersin RM, White CJ. SCAI expert consensus statement for aorto-iliac arterial intervention appropriate use. Catheter Cardiovasc Interv. 2014;84:520–8. doi: 10.1002/ccd.25505. [DOI] [PubMed] [Google Scholar]
- 76.Bonvini RF, Rastan A, Sixt S, Noory E, Schwarz T, Frank U, Roffi M, Dorsaz PA, Schwarzwalder U, Burgelin K, Macharzina R, Zeller T. Endovascular treatment of common femoral artery disease: medium-term outcomes of 360 consecutive procedures. J Am Coll Cardiol. 2011;58:792–8. doi: 10.1016/j.jacc.2011.01.070. [DOI] [PubMed] [Google Scholar]
- 77.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 78.Armstrong EJ, Chen DC, Westin GG, Singh S, McCoach CE, Bang H, Yeo KK, Anderson D, Amsterdam EA, Laird JR. Adherence to guideline-recommended therapy is associated with decreased major adverse cardiovascular events and major adverse limb events among patients with peripheral arterial disease. Journal of the American Heart Association. 2014;3:e000697. doi: 10.1161/JAHA.113.000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Connors G, Todoran TM, Engelson BA, Sobieszczyk PS, Eisenhauer AC, Kinlay S. Percutaneous revascularization of long femoral artery lesions for claudication: patency over 2.5 years and impact of systematic surveillance. Catheter Cardiovasc Interv. 2011;77:1055–62. doi: 10.1002/ccd.22802. [DOI] [PubMed] [Google Scholar]
- 80.O’Neil-Callahan K, Katsimaglis G, Tepper MR, Ryan J, Mosby C, Ioannidis JP, Danias PG. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol. 2005;45:336–42. doi: 10.1016/j.jacc.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 81.Henke PK, Blackburn S, Proctor MC, Stevens J, Mukherjee D, Rajagopalin S, Upchurch GR, Jr, Stanley JC, Eagle KA. Patients undergoing infrainguinal bypass to treat atherosclerotic vascular disease are underprescribed cardioprotective medications: effect on graft patency, limb salvage, and mortality. Journal of vascular surgery. 2004;39:357–65. doi: 10.1016/j.jvs.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 82.Vogel TR, Dombrovskiy VY, Galinanes EL, Kruse RL. Preoperative statins and limb salvage after lower extremity revascularization in the Medicare population. Circulation Cardiovascular interventions. 2013;6:694–700. doi: 10.1161/CIRCINTERVENTIONS.113.000274. [DOI] [PubMed] [Google Scholar]
- 83.Subherwal S, Patel MR, Kober L, Peterson ED, Jones WS, Gislason GH, Berger J, Torp-Pedersen C, Fosbol EL. Missed opportunities: despite improvement in use of cardioprotective medications among patients with lower-extremity peripheral artery disease, underuse remains. Circulation. 2012;126:1345–54. doi: 10.1161/CIRCULATIONAHA.112.108787. [DOI] [PubMed] [Google Scholar]