In light of the rapidly shifting landscape regarding the legalization of marijuana for medical and recreational purposes, patients may be more likely to ask physicians about its potential adverse and beneficial effects on health. The popular notion seems to be that marijuana is a harmless pleasure, access to which should not be regulated or considered illegal. Currently, marijuana is the most commonly used “illicit” drug in the United States, with about 12% of people 12 years of age or older reporting use in the past year and particularly high rates of use among young people.1 The most common route of administration is inhalation. The greenish-gray shredded leaves and flowers of the Cannabis sativa plant are smoked (along with stems and seeds) in cigarettes, cigars, pipes, water pipes, or “blunts” (marijuana rolled in the tobacco-leaf wrapper from a cigar). Hashish is a related product created from the resin of marijuana flowers and is usually smoked (by itself or in a mixture with tobacco) but can be ingested orally. Marijuana can also be used to brew tea, and its oil-based extract can be mixed into food products.
The regular use of marijuana during adolescence is of particular concern, since use by this age group is associated with an increased likelihood of deleterious consequences2 (Table 1). Although multiple studies have reported detrimental effects, others have not, and the question of whether marijuana is harmful remains the subject of heated debate. Here we review the current state of the science related to the adverse health effects of the recreational use of marijuana, focusing on those areas for which the evidence is strongest.
Table 1.
Adverse Effects of Short-Term Use and Long-Term or Heavy Use of Marijuana.
| Effects of short-term use |
| Impaired short-term memory, making it difficult to learn and to retain information |
| Impaired motor coordination, interfering with driving skills and increasing the risk of injuries |
| Altered judgment, increasing the risk of sexual behaviors that facilitate the transmission of sexually transmitted diseases |
| In high doses, paranoia and psychosis |
| Effects of long-term or heavy use |
| Addiction (in about 9% of users overall, 17% of those who begin use in adolescence, and 25 to 50% of those who are daily users)* |
| Altered brain development* |
| Poor educational outcome, with increased likelihood of dropping out of school* |
| Cognitive impairment, with lower IQ among those who were frequent users during adolescence* |
| Diminished life satisfaction and achievement (determined on the basis of subjective and objective measures as compared with such ratings in the general population)* |
| Symptoms of chronic bronchitis |
| Increased risk of chronic psychosis disorders (including schizophrenia) in persons with a predisposition to such disorders |
The effect is strongly associated with initial marijuana use early in adolescence.
ADVERSE EFFECTS
RISK OF ADDICTION
Despite some contentious discussions regarding the addictiveness of marijuana, the evidence clearly indicates that long-term marijuana use can lead to addiction. Indeed, approximately 9% of those who experiment with marijuana will become addicted3 (according to the criteria for dependence in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition [DSM-IV]). The number goes up to about 1 in 6 among those who start using marijuana as teenagers and to 25 to 50% among those who smoke marijuana daily.4 According to the 2012 National Survey on Drug Use and Health, an estimated 2.7 million people 12 years of age and older met the DSM-IV criteria for dependence on marijuana, and 5.1 million people met the criteria for dependence on any illicit drug1 (8.6 million met the criteria for dependence on alcohol1). There is also recognition of a bona fide cannabis withdrawal syndrome5 (with symptoms that include irritability, sleeping difficulties, dysphoria, craving, and anxiety), which makes cessation difficult and contributes to relapse. Marijuana use by adolescents is particularly troublesome. Adolescents’ increased vulnerability to adverse long-term outcomes from marijuana use is probably related to the fact that the brain, including the endocannabinoid system, undergoes active development during adolescence.6 Indeed, early and regular marijuana use predicts an increased risk of marijuana addiction, which in turn predicts an increased risk of the use of other illicit drugs.7 As compared with persons who begin to use marijuana in adulthood, those who begin in adolescence are approximately 2 to 4 times as likely to have symptoms of cannabis dependence within 2 years after first use.8
EFFECT ON BRAIN DEVELOPMENT
The brain remains in a state of active, experience-guided development from the prenatal period through childhood and adolescence until the age of approximately 21 years.9 During these developmental periods, it is intrinsically more vulnerable than a mature brain to the adverse long-term effects of environmental insults, such as exposure to tetrahydrocannabinol, or THC, the primary active ingredient in marijuana. This view has received considerable support from studies in animals, which have shown, for example, that prenatal or adolescent exposure to THC can recalibrate the sensitivity of the reward system to other drugs10 and that prenatal exposure interferes with cytoskeletal dynamics, which are critical for the establishment of axonal connections between neurons.11
As compared with unexposed controls, adults who smoked marijuana regularly during adolescence have impaired neural connectivity (fewer fibers) in specific brain regions. These include the precuneus, a key node that is involved in functions that require a high degree of integration (e.g., alertness and self-conscious awareness), and the fimbria, an area of the hippocampus that is important in learning and memory.12 Reduced functional connectivity has also been reported in the prefrontal networks responsible for executive function (including inhibitory control) and the subcortical networks, which process habits and routines.13 In addition, imaging studies in persons who use cannabis have revealed decreased activity in prefrontal regions and reduced volumes in the hippocampus.14 Thus, certain brain regions may be more vulnerable than others to the long-term effects of marijuana. One study showed that selective down-regulation of cannabinoid-1 (CB1) receptors in several cortical brain regions in long-term marijuana smokers was correlated with years of cannabis smoking and was reversible after 4 weeks of abstinence.15 Changes in CB1 receptors were not seen in subcortical regions.
The negative effect of marijuana use on the functional connectivity of the brain is particularly prominent if use starts in adolescence or young adulthood,12 which may help to explain the finding of an association between frequent use of marijuana from adolescence into adulthood and significant declines in IQ.16 The impairments in brain connectivity associated with exposure to marijuana in adolescence are consistent with preclinical findings indicating that the cannabinoid system plays a prominent role in synapse formation during brain development.17
POSSIBLE ROLE AS GATEWAY DRUG
Epidemiologic and preclinical data suggest that the use of marijuana in adolescence could influence multiple addictive behaviors in adulthood. In rodents exposed to cannabinoids during adolescence, there is decreased reactivity of the dopamine neurons that modulate the brain’s reward regions.18 The exposure of rodents to cannabis in utero alters the developmental regulation of the mesolimbic dopamine system of affected offspring.19 If reduced dopamine reactivity in the brain’s reward regions does follow early exposure to marijuana, this effect could help to explain the increased susceptibility to drug abuse and addiction to several drugs later in life, which has been reported in most epidemiologic studies.20 This theory is also consistent with animal models showing that THC can prime the brain for enhanced responses to other drugs.21 Although these findings support the idea that marijuana is a gateway drug, other drugs, such as alcohol and nicotine, can also be categorized as gateway drugs, since they also prime the brain for a heightened response to other drugs.22 However, an alternative explanation is that people who are more susceptible to drug-taking behavior are simply more likely to start with marijuana because of its accessibility and that their subsequent social interactions with other drug users would increase the probability that they would try other drugs.
RELATION TO MENTAL ILLNESS
Regular marijuana use is associated with an increased risk of anxiety and depression,23 but causality has not been established. Marijuana is also linked with psychoses (including those associated with schizophrenia), especially among people with a preexisting genetic vulnerability,24 and exacerbates the course of illness in patients with schizophrenia. Heavier marijuana use, greater drug potency, and exposure at a younger age can all negatively affect the disease trajectory (e.g., by advancing the time of a first psychotic episode by 2 to 6 years).25
However, it is inherently difficult to establish causality in these types of studies because factors other than marijuana use may be directly associated with the risk of mental illness. In addition, other factors could predispose a person to both marijuana use and mental illness. This makes it difficult to confidently attribute the increased risk of mental illness to marijuana use.
EFFECT ON SCHOOL PERFORMANCE AND LIFETIME ACHIEVEMENT
In the 2013 Monitoring the Future survey of high-school students,26 6.5% of students in grade 12 reported daily or near-daily marijuana use, and this figure probably represents an underestimate of use, since young people who have dropped out of school may have particularly high rates of frequent marijuana use.27 Since marijuana use impairs critical cognitive functions, both during acute intoxication and for days after use,28 many students could be functioning at a cognitive level that is below their natural capability for considerable periods of time. Although acute effects may subside after THC is cleared from the brain, it nonetheless poses serious risks to health that can be expected to accumulate with long-term or heavy use. The evidence suggests that such use results in measurable and long-lasting cognitive impairments,16 particularly among those who started to use marijuana in early adolescence. Moreover, failure to learn at school, even for short or sporadic periods (a secondary effect of acute intoxication), will interfere with the subsequent capacity to achieve increasingly challenging educational goals, a finding that may also explain the association between regular marijuana use and poor grades.29
The relationship between cannabis use by young people and psychosocial harm is likely to be multifaceted, which may explain the inconsistencies among studies. For example, some studies suggest that long-term deficits may be reversible and remain subtle rather than disabling once a person abstains from use.30 Other studies show that long-term, heavy use of marijuana results in impairments in memory and attention that persist and worsen with increasing years of regular use31 and with the initiation of use during adolescence.32 As noted above, early marijuana use is associated with impaired school performance and an increased risk of dropping out of school,27,29 although reports of shared environmental factors that influence the risks of using cannabis at a young age and dropping out of school33 suggest that the relationship may be more complex. Heavy marijuana use has been linked to lower income, greater need for socioeconomic assistance, unemployment, criminal behavior, and lower satisfaction with life.2,34
RISK OF MOTOR-VEHICLE ACCIDENTS
Both immediate exposure and long-term exposure to marijuana impair driving ability; marijuana is the illicit drug most frequently reported in connection with impaired driving and accidents, including fatal accidents.35 There is a relationship between the blood THC concentration and performance in controlled driving-simulation studies,36 which are a good predictor of real-world driving ability. Recent marijuana smoking and blood THC levels of 2 to 5 ng per milliliter are associated with substantial driving impairment.37 According to a meta-analysis, the overall risk of involvement in an accident increases by a factor of about 2 when a person drives soon after using marijuana.37 In an accident culpability analysis, persons testing positive for THC (typical minimum level of detection, 1 ng per milliliter), and particularly those with higher blood levels, were 3 to 7 times as likely to be responsible for a motor-vehicle accident as persons who had not used drugs or alcohol before driving.38 In comparison, the overall risk of a vehicular accident increases by a factor of almost 5 for drivers with a blood alcohol level above 0.08%, the legal limit in most countries, and increases by a factor of 27 for persons younger than 21 years of age.39 Not surprisingly, the risk associated with the use of alcohol in combination with marijuana appears to be greater than that associated with the use of either drug alone.37
RISK OF CANCER AND OTHER EFFECTS ON HEALTH
The effects of long-term marijuana smoking on the risk of lung cancer are unclear. For example, the use of marijuana for the equivalent of 30 or more joint-years (with 1 joint-year of marijuana use equal to 1 cigarette [joint] of marijuana smoked per day for 1 year) was associated with an increased incidence of lung cancer and several cancers of the upper aerodigestive tract; however, the association disappeared after adjustment for potential confounders such as cigarette smoking.40 Although the possibility of a positive association between marijuana smoking and cancer cannot be ruled out,41 the evidence suggests that the risk is lower with marijuana than with tobacco.40 However, the smoking of cigarettes that contain both marijuana and tobacco products is a potential confounding factor with a prevalence that varies dramatically among countries.
Marijuana smoking is also associated with inflammation of the large airways, increased airway resistance, and lung hyperinflation, associations that are consistent with the fact that regular marijuana smokers are more likely to report symptoms of chronic bronchitis than are nonsmokers42; however, the long-term effect of low levels of marijuana exposure does not appear to be significant.43 The immunologic competence of the respiratory system in marijuana smokers may also be compromised, as indicated by increased rates of respiratory infections and pneumonia.44 Marijuana use has also been associated with vascular conditions that increase the risks of myocardial infarction, stroke, and transient ischemic attacks during marijuana intoxication.45 The actual mechanisms underlying the effects of marijuana on the cardiovascular and cerebrovascular systems are complex and not fully understood. However, the direct effects of cannabinoids on various target receptors (i.e., CB1 receptors in arterial blood vessels) and the indirect effects on vasoactive compounds46 may help explain the detrimental effects of marijuana on vascular resistance and coronary microcirculation.47
LIMITATIONS OF THE EVIDENCE AND GAPS IN KNOWLEDGE
Most of the long-term effects of marijuana use that are summarized here have been observed among heavy or long-term users, but multiple (often hidden) confounding factors detract from our ability to establish causality (including the frequent use of marijuana in combination with other drugs). These factors also complicate our ability to assess the true effect of intrauterine exposure to marijuana. Indeed, despite the use of marijuana by pregnant women,48 and animal models suggesting that cannabis exposure during pregnancy may alter the normal processes and trajectories of brain development,49 our understanding of the long-term effects of prenatal exposure to marijuana in humans is very poor.
The THC content, or potency, of marijuana, as detected in confiscated samples, has been steadily increasing from about 3% in the 1980s to 12% in 201250 (Fig. 1A). This increase in THC content raises concerns that the consequences of marijuana use may be worse now than in the past and may account for the significant increases in emergency department visits by persons reporting marijuana use51 (Fig. 1B) and the increases in fatal motor-vehicle accidents.35 This increase in THC potency over time also raises questions about the current relevance of the findings in older studies on the effects of marijuana use, especially studies that assessed long-term outcomes.
Figure 1. Increases over Time in the Potency of Tetrahydrocannabinol (THC) in Marijuana and the Number of Emergency Department Visits Involving Marijuana, Cocaine, or Heroin.
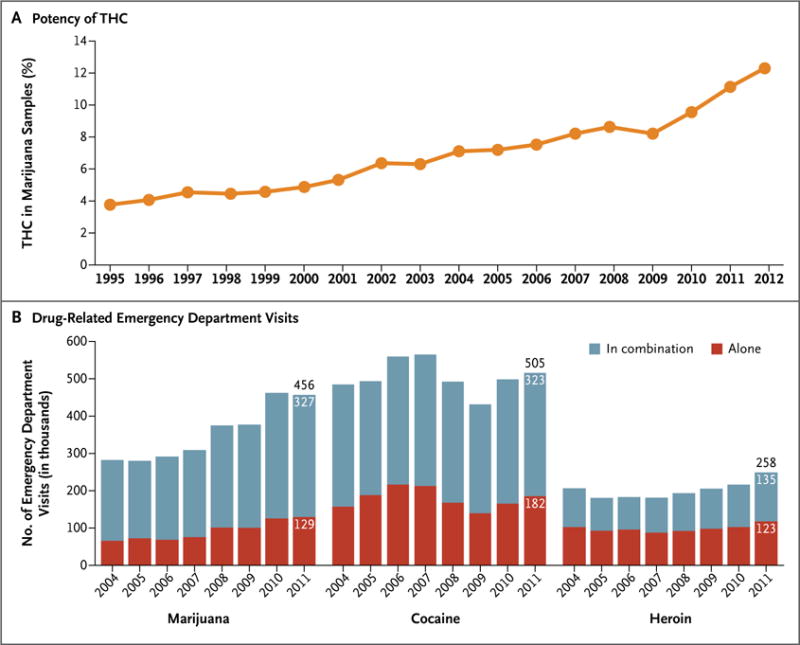
Panel A shows the increasing potency of marijuana (i.e., the percentage of THC) in samples seized by the Drug Enforcement Administration (DEA) between 1995 and 2012.50 Panel B provides estimates of the number of emergency department visits involving the use of selected illicit drugs (marijuana, cocaine, and heroin) either singly or in combination with other drugs between 2004 and 2011.51 Among these three drugs, only marijuana, used either in combination with other drugs or alone, was associated with significant increases in the number of visits during this period (a 62% increase when used in combination with other drugs and a 100% increase when used alone, P<0.05 for the two comparisons).
There is also a need to improve our understanding of how to harness the potential medical benefits of the marijuana plant without exposing people who are sick to its intrinsic risks. The authoritative report by the Institute of Medicine, Marijuana and Medicine,52 acknowledges the potential benefits of smoking marijuana in stimulating appetite, particularly in patients with the acquired immunodeficiency syndrome (AIDS) and the related wasting syndrome, and in combating chemotherapy-induced nausea and vomiting, severe pain, and some forms of spasticity. The report also indicates that there is some evidence for the benefit of using marijuana to decrease intraocular pressure in the treatment of glaucoma. Nonetheless, the report stresses the importance of focusing research efforts on the therapeutic potential of synthetic or pharmaceutically pure cannabinoids.52 Some physicians continue to prescribe marijuana for medicinal purposes despite limited evidence of a benefit (see box). This practice raises particular concerns with regard to long-term use by vulnerable populations. For example, there is some evidence to suggest that in patients with symptoms of human immunodeficiency virus (HIV) infection or AIDS, marijuana use may actually exacerbate HIV-associated cognitive deficits.75 Similarly, more research is needed to understand the potential effects of marijuana use on age-related cognitive decline in general and on memory impairment in particular.
Clinical Conditions with Symptoms That May Be Relieved by Treatment with Marijuana or Other Cannabinoids.*.
Glaucoma
Early evidence of the benefits of marijuana in patients with glaucoma (a disease associated with increased pressure in the eye) may be consistent with its ability to effect a transient decrease in intraocular pressure,53,54 but other, standard treatments are currently more effective. THC, cannabinol, and nabilone (a synthetic cannabinoid similar to THC), but not cannabidiol, were shown to lower intraocular pressure in rabbits.55,56 More research is needed to establish whether molecules that modulate the endocannabinoid system may not only reduce intraocular pressure but also provide a neuroprotective benefit in patients with glaucoma.57
Nausea
Treatment of the nausea and vomiting associated with chemotherapy was one of the first medical uses of THC and other cannabinoids.58 THC is an effective antiemetic agent in patients undergoing chemotherapy,59 but patients often state that marijuana is more effective in suppressing nausea. Other, unidentified compounds in marijuana may enhance the effect of THC (as appears to be the case with THC and cannabidiol, which operate through different antiemetic mechanisms).60 Paradoxically, increased vomiting (hyperemesis) has been reported with repeated marijuana use.
AIDS-associated anorexia and wasting syndrome
Reports have indicated that smoked or ingested cannabis improves appetite and leads to weight gain and improved mood and quality of life among patients with AIDS.61 However, there is no long-term or rigorous evidence of a sustained effect of cannabis on AIDS-related morbidity and mortality, with an acceptable safety profile, that would justify its incorporation into current clinical practice for patients who are receiving effective antiretroviral therapy.62 Data from the few studies that have explored the potential therapeutic value of cannabinoids for this patient population are inconclusive.62
Chronic pain
Marijuana has been used to relieve pain for centuries. Studies have shown that cannabinoids acting through central CB1 receptors, and possibly peripheral CB1 and CB2 receptors,63 play important roles in modeling nociceptive responses in various models of pain. These findings are consistent with reports that marijuana may be effective in ameliorating neuropathic pain,64,65 even at very low levels of THC (1.29%).66 Both marijuana and dronabinol, a pharmaceutical formulation of THC, decrease pain, but dronabinol may lead to longer-lasting reductions in pain sensitivity and lower ratings of rewarding effects.67
Inflammation
Cannabinoids (e.g., THC and cannabidiol) have substantial antiinflammatory effects because of their ability to induce apoptosis, inhibit cell proliferation, and suppress cytokine production.68 Cannabidiol has attracted particular interest as an antiinflammatory agent because of its lack of psychoactive effects.58 Animal models have shown that cannabidiol is a promising candidate for the treatment of rheumatoid arthritis58 and for inflammatory diseases of the gastrointestinal tract (e.g., ulcerative colitis and Crohn’s disease).69
Multiple sclerosis
Nabiximols (Sativex, GW Pharmaceuticals), an oromucosal spray that delivers a mix of THC and cannabidiol, appears to be an effective treatment for neuropathic pain, disturbed sleep, and spasticity in patients with multiple sclerosis. Sativex is available in the United Kingdom, Canada, and several other countries70,71 and is currently being reviewed in phase 3 trials in the United States in order to gain approval from the Food and Drug Administration.
Epilepsy
In a recent small survey of parents who use marijuana with a high cannabidiol content to treat epileptic seizures in their children,72 11% (2 families out of the 19 that met the inclusion criteria) reported complete freedom from seizures, 42% (8 families) reported a reduction of more than 80% in seizure frequency, and 32% (6 families) reported a reduction of 25 to 60% in seizure frequency. Although such reports are promising, insufficient safety and efficacy data are available on the use of cannabis botanicals for the treatment of epilepsy.73 However, there is increasing evidence of the role of cannabidiol as an antiepileptic agent in animal models.74
*AIDS denotes acquired immunodeficiency syndrome, CB1 cannabinoid-1 receptor, and CB2 cannabinoid-2 receptor, HIV human immunodeficiency virus, and THC tetrahydrocannabinol.
Research is needed on the ways in which government policies on marijuana affect public health outcomes. Our understanding of the effects of policy on market forces is quite limited (e.g., the allure of new tax-revenue streams from the legal sale of marijuana, pricing wars, youth-targeted advertising, and the emergence of cannabis-based medicines approved by the Food and Drug Administration), as is our understanding of the interrelated variables of perceptions about use, types of use, and outcomes. Historically, there has been an inverse correlation between marijuana use and the perception of its risks among adolescents (Fig. 2A). Assuming that this inverse relationship is causal, would greater permissiveness in culture and social policy lead to an increase in the number of young people who are exposed to cannabis on a regular basis? Among students in grade 12, the reported prevalence of regular marijuana smoking has been steadily increasing in recent years and may soon intersect the trend line for regular tobacco smoking (Fig. 2B). We also need information about the effects of second-hand exposure to cannabis smoke and cannabinoids. Second-hand exposure is an important public health issue in the context of tobacco smoking, but we do not have a clear understanding of the effects of second-hand exposure to marijuana smoking.76 Studies in states (e.g., Colorado, California, and Washington) and countries (e.g., Uruguay, Portugal, and the Netherlands) where social and legal policies are shifting may provide important data for shaping future policies.
Figure 2. Use of Marijuana in Relation to Perceived Risk and Daily Use of Tobacco Cigarettes or Marijuana among U.S. Students in Grade 12, 1975–2013.
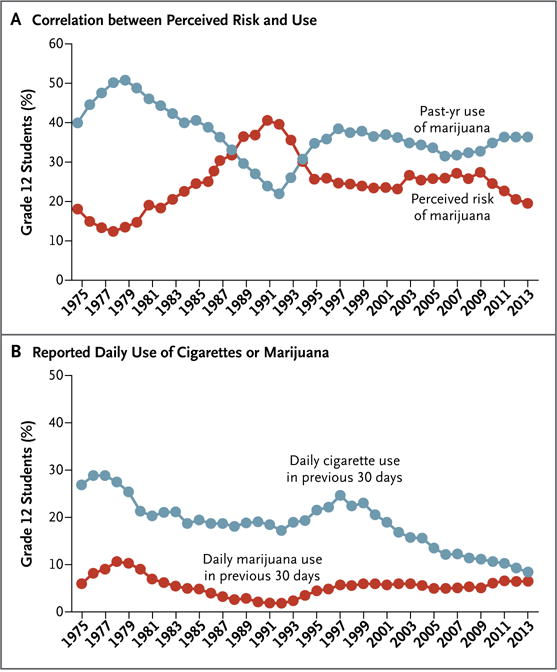
Panel A shows the inverse correlation between the perception of the risk associated with marijuana use and actual use. Perceived risk corresponds to the percentage of teenagers who reported that the use of marijuana is dangerous. Panel B shows the percentage of students who reported daily use of tobacco cigarettes or marijuana in the previous 30 days. Data for both graphs are from Johnston et al.26
CONCLUSIONS
Marijuana use has been associated with substantial adverse effects, some of which have been determined with a high level of confidence (Table 2). Marijuana, like other drugs of abuse, can result in addiction. During intoxication, marijuana can interfere with cognitive function (e.g., memory and perception of time) and motor function (e.g., coordination), and these effects can have detrimental consequences (e.g., motor-vehicle accidents). Repeated marijuana use during adolescence may result in long-lasting changes in brain function that can jeopardize educational, professional, and social achievements. However, the effects of a drug (legal or illegal) on individual health are determined not only by its pharmacologic properties but also by its availability and social acceptability. In this respect, legal drugs (alcohol and tobacco) offer a sobering perspective, accounting for the greatest burden of disease associated with drugs77 not because they are more dangerous than illegal drugs but because their legal status allows for more widespread exposure. As policy shifts toward legalization of marijuana, it is reasonable and probably prudent to hypothesize that its use will increase and that, by extension, so will the number of persons for whom there will be negative health consequences.
Table 2.
Level of Confidence in the Evidence for Adverse Effects of Marijuana on Health and Well-Being.
| Effect | Overall Level of Confidence* |
|---|---|
| Addiction to marijuana and other substances | High |
| Abnormal brain development | Medium |
| Progression to use of other drugs | Medium |
| Schizophrenia | Medium |
| Depression or anxiety | Medium |
| Diminished lifetime achievement | High |
| Motor vehicle accidents | High |
| Symptoms of chronic bronchitis | High |
| Lung cancer | Low |
The indicated overall level of confidence in the association between marijuana use and the listed effects represents an attempt to rank the strength of the current evidence, especially with regard to heavy or long-term use and use that starts in adolescence.
Footnotes
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Center for Behavioral Health Statistics and Quality. National survey on drug use and health. Rockville, MD: Substance Abuse & Mental Health Services Administration; 2013. [Google Scholar]
- 2.Fergusson DM, Boden JM. Cannabis use and later life outcomes. Addiction. 2008;103:969–76. doi: 10.1111/j.1360-0443.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115:120–30. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–91. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick DA, Levin KH, Copersino ML, et al. Diagnostic criteria for cannabis withdrawal syndrome. Drug Alcohol Depend. 2012;123:141–7. doi: 10.1016/j.drugalcdep.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 7.Hall W, Degenhardt L. Prevalence and correlates of cannabis use in developed and developing countries. Curr Opin Psychiatry. 2007;20:393–7. doi: 10.1097/YCO.0b013e32812144cc. [DOI] [PubMed] [Google Scholar]
- 8.Chen CY, Storr CL, Anthony JC. Early-onset drug use and risk for drug dependence problems. Addict Behav. 2009;34:319–22. doi: 10.1016/j.addbeh.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinieri JA, Hurd YL. Rat models of prenatal and adolescent cannabis exposure. Methods Mol Biol. 2012;829:231–42. doi: 10.1007/978-1-61779-458-2_14. [DOI] [PubMed] [Google Scholar]
- 11.Tortoriello G, Morris CV, Alpar A, et al. Miswiring the brain: Δ9-tetrahydro-cannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 2014;33:668–85. doi: 10.1002/embj.201386035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalesky A, Solowij N, Yücel M, et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135:2245–55. doi: 10.1093/brain/aws136. [DOI] [PubMed] [Google Scholar]
- 13.Filbey F, Yezhuvath U. Functional connectivity in inhibitory control networks and severity of cannabis use disorder. Am J Drug Alcohol Abuse. 2013;39:382–91. doi: 10.3109/00952990.2013.841710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirvonen J, Goodwin RS, Li C-T, et al. Reversible and regionally selective down-regulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–9. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657–E2564. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaffuri AL, Ladarre D, Lenkei Z. Type-1 cannabinoid receptor signaling in neuronal development. Pharmacology. 2012;90:19–39. doi: 10.1159/000339075. [DOI] [PubMed] [Google Scholar]
- 18.Pistis M, Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 19.DiNieri JA, Wang X, Szutorisz H, et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry. 2011;70:763–9. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agrawal A, Neale MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med. 2004;34:1227–37. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- 21.Panlilio LV, Zanettini C, Barnes C, Solinas M, Goldberg SR. Prior exposure to THC increases the addictive effects of nicotine in rats. Neuropsychopharmacology. 2013;38:1198–208. doi: 10.1038/npp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine A, Huang Y, Drisaldi B, et al. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3:107ra109. doi: 10.1126/scitranslmed.3003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325:1195–8. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–27. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Di Forti M, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014 Mar 19; doi: 10.1093/schbul/sbt181. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future: national survey results on drug use, 1975–2013 — overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan; 2014. http://monitoringthefuture.org/pubs/monographs/mtf-overview2013.pdf. [Google Scholar]
- 27.Bray JW, Zarkin GA, Ringwalt C, Qi J. The relationship between marijuana initiation and dropping out of high school. Health Econ. 2000;9:9–18. doi: 10.1002/(sici)1099-1050(200001)9:1<9::aid-hec471>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 28.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95:1621–30. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- 30.Macleod J, Oakes R, Copello A, et al. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet. 2004;363:1579–88. doi: 10.1016/S0140-6736(04)16200-4. [DOI] [PubMed] [Google Scholar]
- 31.Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–31. doi: 10.1001/jama.287.9.1123. [Erratum, JAMA 2002;287:1651.] [DOI] [PubMed] [Google Scholar]
- 32.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verweij KJ, Huizink AC, Agrawal A, Martin NG, Lynskey MT. Is the relationship between early-onset cannabis use and educational attainment causal or due to common liability? Drug Alcohol Depend. 2013;133:580–6. doi: 10.1016/j.drugalcdep.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brook JS, Lee JY, Finch SJ, Seltzer N, Brook DW. Adult work commitment, financial stability, and social environment as related to trajectories of marijuana use beginning in adolescence. Subst Abus. 2013;34:298–305. doi: 10.1080/08897077.2013.775092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brady JE, Li G. Trends in alcohol and other drugs detected in fatally injured drivers in the United States, 1999–2010. Am J Epidemiol. 2014;179:692–9. doi: 10.1093/aje/kwt327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenné MG, Dietze PM, Triggs TJ, Walmsley S, Murphy B, Redman JR. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859–66. doi: 10.1016/j.aap.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 37.Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013;59:478–92. doi: 10.1373/clinchem.2012.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramaekers JG, Berghaus G, van Laar M, Drummer OH. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73:109–19. doi: 10.1016/j.drugalcdep.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Peck RC, Gebers MA, Voas RB, Romano E. The relationship between blood alcohol concentration (BAC), age, and crash risk. J Safety Res. 2008;39:311–9. doi: 10.1016/j.jsr.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 40.Hashibe M, Morgenstern H, Cui Y, et al. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2006;15:1829–34. doi: 10.1158/1055-9965.EPI-06-0330. [DOI] [PubMed] [Google Scholar]
- 41.Callaghan RC, Allebeck P, Sidorchuk A. Marijuana use and risk of lung cancer: a 40-year cohort study. Cancer Causes Control. 2013;24:1811–20. doi: 10.1007/s10552-013-0259-0. [DOI] [PubMed] [Google Scholar]
- 42.Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239–47. doi: 10.1513/AnnalsATS.201212-127FR. [DOI] [PubMed] [Google Scholar]
- 43.Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012;307:173–81. doi: 10.1001/jama.2011.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol. 2014;46:65–81. doi: 10.1007/s12016-013-8374-y. [DOI] [PubMed] [Google Scholar]
- 45.Thomas G, Kloner RA, Rezkalla S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: what cardiologists need to know. Am J Cardiol. 2014;113:187–90. doi: 10.1016/j.amjcard.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 46.Stanley C, O’Sullivan SE. Vascular targets for cannabinoids: animal and human studies. Br J Pharmacol. 2014;171:1361–78. doi: 10.1111/bph.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rezkalla SH, Sharma P, Kloner RA. Coronary no-flow and ventricular tachycardia associated with habitual marijuana use. Ann Emerg Med. 2003;42:365–9. doi: 10.1016/s0196-0644(03)00426-8. [DOI] [PubMed] [Google Scholar]
- 48.Brown HL, Graves CR. Smoking and marijuana use in pregnancy. Clin Obstet Gynecol. 2013;56:107–13. doi: 10.1097/GRF.0b013e318282377d. [DOI] [PubMed] [Google Scholar]
- 49.Jutras-Aswad D, DiNieri JA, Harkany T, Hurd YL. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur Arch Psychiatry Clin Neurosci. 2009;259:395–412. doi: 10.1007/s00406-009-0027-z. [DOI] [PubMed] [Google Scholar]
- 50.ElSohly MA. Potency Monitoring Program quarterly report no 123 — reporting period: 09/16/2013-12/15/2013. Oxford: University of Mississippi, National Center for Natural Products Research; 2014. [Google Scholar]
- 51.Drug Abuse Warning Network, 2011: national estimates of drug-related emergency department visits. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. http://www.samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. [PubMed] [Google Scholar]
- 52.Joy JE, Watson SJ Jr, Benson JA Jr, editors. Marijuana and medicine: assessing the science base. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 53.Merritt JC, Crawford WJ, Alexander PC, Anduze AL, Gelbart SS. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology. 1980;87:222–8. doi: 10.1016/s0161-6420(80)35258-5. [DOI] [PubMed] [Google Scholar]
- 54.Hepler RS, Frank IR. Marihuana smoking and intraocular pressure. JAMA. 1971;217:1392. [PubMed] [Google Scholar]
- 55.Chen J, Matias I, Dinh T, et al. Finding of endocannabinoids in human eye tissues: implications for glaucoma. Biochem Biophys Res Commun. 2005;330:1062–7. doi: 10.1016/j.bbrc.2005.03.095. [DOI] [PubMed] [Google Scholar]
- 56.Song ZH, Slowey CA. Involvement of cannabinoid receptors in the intraocular pressure-lowering effects of WIN55212-2. J Pharmacol Exp Ther. 2000;292:136–9. [PubMed] [Google Scholar]
- 57.Nucci C, Bari M, Spanò A, et al. Potential roles of (endo) cannabinoids in the treatment of glaucoma: from intraocular pressure control to neuroprotection. Prog Brain Res. 2008;173:451–64. doi: 10.1016/S0079-6123(08)01131-X. [DOI] [PubMed] [Google Scholar]
- 58.Zuardi AW. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev Bras Psiquiatr. 2008;30:271–80. doi: 10.1590/s1516-44462008000300015. [DOI] [PubMed] [Google Scholar]
- 59.Sallan SE, Zinberg NE, Frei E., III Antiemetic effect of delta-9-tetrahydrocanna-binol in patients receiving cancer chemotherapy. N Engl J Med. 1975;293:795–7. doi: 10.1056/NEJM197510162931603. [DOI] [PubMed] [Google Scholar]
- 60.Parker LA, Kwiatkowska M, Burton P, Mechoulam R. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew) Psychopharmacology (Berl) 2004;171:156–61. doi: 10.1007/s00213-003-1571-2. [DOI] [PubMed] [Google Scholar]
- 61.D’Souza G, Matson PA, Grady CD, et al. Medicinal and recreational marijuana use among HIV-infected women in the Women’s Interagency HIV Study (WIHS) cohort, 1994–2010. J Acquir Immune Defic Syndr. 2012;61:618–26. doi: 10.1097/QAI.0b013e318273ab3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;4:CD005175. doi: 10.1002/14651858.CD005175.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiou LC, Hu SS, Ho YC. Targeting the cannabinoid system for pain relief? Acta Anaesthesiol Taiwan. 2013;51:161–70. doi: 10.1016/j.aat.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506–21. doi: 10.1016/j.jpain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallace M, Schulteis G, Atkinson JH, et al. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology. 2007;107:785–96. doi: 10.1097/01.anes.0000286986.92475.b7. [DOI] [PubMed] [Google Scholar]
- 66.Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136–48. doi: 10.1016/j.jpain.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooper ZD, Comer SD, Haney M. Comparison of the analgesic effects of dronabinol and smoked marijuana in daily marijuana smokers. Neuropsychopharmacology. 2013;38:1984–92. doi: 10.1038/npp.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1:1333–49. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esposito G, Filippis DD, Cirillo C, et al. Cannabidiol in inflammatory bowel diseases: a brief overview. Phytother Res. 2013;5:633–6. doi: 10.1002/ptr.4781. [DOI] [PubMed] [Google Scholar]
- 70.Collin C, Davies P, Mutiboko IK, Ratcliffe S. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J. 2007;14:290–6. doi: 10.1111/j.1468-1331.2006.01639.x. [DOI] [PubMed] [Google Scholar]
- 71.Centonze D, Mori F, Koch G, et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurol Sci. 2009;30:531–4. doi: 10.1007/s10072-009-0136-5. [DOI] [PubMed] [Google Scholar]
- 72.Porter BE, Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013;29:574–7. doi: 10.1016/j.yebeh.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kogan NM, Mechoulam R. Cannabinoids in health and disease. Dialogues Clin Neurosci. 2007;9:413–30. doi: 10.31887/DCNS.2007.9.4/nkogan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill TD, Cascio MG, Romano B, et al. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol. 2013;170:679–92. doi: 10.1111/bph.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci. 2004;16:330–5. doi: 10.1176/jnp.16.3.330. [DOI] [PubMed] [Google Scholar]
- 76.Niedbala S, Kardos K, Salamone S, Fritch D, Bronsgeest M, Cone EJ. Passive cannabis smoke exposure and oral fluid testing. J Anal Toxicol. 2004;28:546–52. doi: 10.1093/jat/28.7.546. [DOI] [PubMed] [Google Scholar]
- 77.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]


