Abstract
Suicidality is exceedingly prevalent in pain patients. Although the pathophysiology of this link remains unclear, it may be potentially related to the partial congruence of physical and emotional pain systems. The latter system’s role in suicide is also conspicuous during setbacks and losses sustained in the context of social attachments. Here we propose a model based on the neural pathways mediating reward and anti-reward (i.e., allostatic adjustment to recurrent activation of the reward circuitry); both are relevant etiologic factors in pain, suicide and social attachments. A comprehensive literature search on neurobiology of pain and suicidality was performed. The collected articles were critically reviewed and relevant data were extracted and summarized within four key areas: (1) physical and emotional pain, (2) emotional pain and social attachments, (3) pain-and suicide-related alterations of the reward and anti-reward circuits as compared to addiction, which is the premier probe for dysfunction of these circuits and (4) mechanistically informed treatments of co-occurring pain and suicidality. Pain-, stress- and analgesic drugs-induced opponent and proponent states of the mesolimbic dopaminergic pathways may render reward and anti-reward systems vulnerable to sensitization, cross-sensitization and aberrant learning of contents and contexts associated with suicidal acts and behaviors. These findings suggest that pain patients exhibit alterations in the brain circuits mediating reward (depressed function) and anti-reward (sensitized function) that may affect their proclivity for suicide and support pain and suicidality classification among other “reward deficiency syndromes” and a new proposal for “enhanced anti-reward syndromes”. We suggest that interventions aimed at restoring the balance between the reward and anti-reward networks in patients with chronic pain may help decreasing their suicide risk.
Keywords: Anti-reward, Homeostasis, Allostasis, Habenula, Stress, Cross-sensitization, Aberrant learning
1. Background
1.1. Introduction
There is but one truly serious philosophical problem and that is suicide. Judging whether life is or is not worth living amounts to answering the fundamental question of philosophy.
Albert Camus. The Myth of Sisyphus (1942)
On October 21st 1805, the memorable day of the Battle of Trafalgar, Admiral Lord Horatio Nelson wrote his will and emerged on the flagship deck in full uniform decorated with military orders glittering in the warm sun that stood high in the clear skies of Spain. The Admiral emphatically rebuffed comrades’ pleas for a less flashy attire, so to avoid being a conspicuous target for the Napoleon’s fleet snipers. Unsurprisingly he was seriously wounded shortly before the conclusion of the Battle, but rejected receipt of the medical care immediately available on board of the flagship. Even though it may appear that Nelson was seeking to die, we will probably never find out what exactly was going through his mind. The hero’s glory was then at its zenith inevitably hinting to the only possible downward life trajectory that may be henceforth perceived as bland and unfulfilling in comparison to the fleeting triumph and exhilaration evoked by the grandiose victory. Nelson was also exasperated by the hypocritical moral values of the British monarchy insultingly denying societal recognition from his beloved lady and from their daughter. Furthermore, long-lasting and excruciating phantom-like pain caused by an amateurish amputation of the right arm performed a few years beforehand might have been yet another factor pushing Nelson to the precipice.
The link between pain and suicide has indeed been consistently documented in numerous epidemiological surveys (Fishbain, 1999; Ilgen et al., 2010; Ratcliffe et al., 2008; Tang and Crane, 2006). This link remains rather specific even after accounting for negative affective states (Blackburn-Munro and Blackburn-Munro, 2001; Edwards et al., 2006; Fishbain et al., 1997) and for the perceived severity of pain symptoms (Edwards et al., 2006; Fisher et al., 2001; Smith et al., 2004). For most people the act of suicide, defying instincts, fears and societal taboos is incomprehensible and even mysterious. Suicidality could be attributed to a constellation of developmental, environmental, social, psychological, psychiatric, medical, molecular, genetic, demographic and vocational factors (Durkheim, 1952; Ernst et al., 2009; Goldsmith, 2002; Van Orden et al., 2010). In the context of pain, low expectancy for subjective well-being (Meulders et al., 2012; Takahashi, 2011), conditioned fear (De Peuter et al., 2011) and avoidance behavior (Schrooten et al., 2012) are also considered among contributing causes. Nonetheless, the mere fact that the incidence of people’s willingness to part with the most precious possession they have, their life, remains by and large stable and unremitting over years despite at times extraordinary interventions and tight monitoring (Goldsmith, 2002) calls for further deepening of the insights into this enigmatic problem.
The current review will define interactions between pain and suicidality by examining the role of neural pathways mediating reward and anti-reward viz., allostatic adjustment to recurrent activation of the reward circuitry (Koob and Le Moal, 2005) that may have important implications for the two conditions (Apkarian, 2010; Blum et al., 2008; Niikura et al., 2010). Owing to well-defined disruptions of reward/anti-reward function in addiction, it serves as a comparison backdrop for understanding pain and suicidality. To that end, we attempt to bring together the prevailing and complementary theories on the nature of addiction. We will argue that addiction’s dimensional and interactive measures rather than a categorical adherence to a single theoretical framework are essential for a comprehensive clinical and scientific formulation of the complex biopsychosocial phenomena of pain and suicidality.
In addition to general introduction of the main papers’ themes, as noted above, Section 1 provides epidemiological and clinical context of the discussed entities. Section 2 discusses putative neurobiology of pain as it is involved in the sensory, emotional and reward/anti-reward function. Section 3, the main portion of the paper, pertains to the role played by reward and anti-reward systems in pain and in suicidality. Social attachment is used here to illustrate normative reward/anti-reward function and to compare it to that in pain, in suicidality and in addiction. In Section 4 specific evidence for testable hypotheses on therapeutic and preventive interventions based on mechanistically-informed psychopharmacological interventions is discussed. Section 5 presents final summary and conclusions.
Death and mortality are arguably the most integral problems of human condition, faced by any individual in the course of life and are inextricably linked to the questions dealing with the meaning of life and with how death may impact such a meaning. These questions are certainly far from ever been resolved. Also, because the awareness of life’s finality in conjunction with the freedom to choose whether “to be or not to be” are exclusive attributes of human nature, the present review does not draw heavily upon preclinical research. On the other hand, since literature and poetry is purported to help us surmising universal truths from individual examples (Aristotle, 1920), we selected several citations from countless volumes of literary and philosophical work that are intended to place the discussed themes into relevant scientific and humanistic contexts. This may inevitably generate an impression of reductionism and oversimplification. We are aware that inquiry into the mind-body conundrum, into philosophical meanings of life and death, into ethical aspects of voluntary life termination as well as into the origins of human behavior and its neural correlates has revealed substantial complexity (Elman, 2011), the discussion of which would go well beyond the scope of this paper.
1.2. Search terms and methodology
English language literature search of reward/motivation function in pain, mechanisms of normative hedonics and reward and their potential impairments in patients with suicidality was undertaken using PubMed (http://www.ncbi.nlm.nih.gov/pubmed) from inception until February 2013. Keywords used included pain plus reward, anti-reward, motivation, pleasure, suicide, self-injury, anhedonia, emotional numbing, stress, addiction, substance use disorders, dependence, striatum, nucleus accumbens (NAc), habenula, insula, prediction error, delay discounting, interoception, dopamine, opponent, proponent and aberrant learning. Each specific medication mentioned in the paper was combined with pain, suicide, reward and motivation terms. Data on reward, pain mechanisms and suicide neurobiology were also drawn from recent seminal reviews of these topics (Apkarian et al., 2011; Gardner, 2011; Koob and Volkow, 2010; Ribeiro and Joiner, 2009; Selby et al., 2010). Additional strategies included manual searches for relevant articles from the selected papers’ reference lists as well as utilization of PubMed’s related articles function.
1.3. Epidemiological and clinical evidence on the link between pain and suicide
No one ever lacks a good reason for suicide.
Cesare Pavese. Journal entry. The Burning Brand: Diaries 1935–1950 (1950)
Chronic pain is the most common problem encountered in primary care setting (Crook et al., 1984) affecting about 100 million Americans and its annual cost to the American society is estimated to be staggering $635 billion (Institute of Medicine, 2011) owing in part to the heightened occurrence of the most ominous outcome, namely death or injury due to suicidal acts. Indeed, about a third of pain patients contemplate or attempt suicide independent of psychiatric and medical comorbidities (Ilgen et al., 2008) and exceeding 2–3 times the prevalence in the general public (Edwards et al., 2006; Ilgen et al., 2008; Tang and Crane, 2006). The most comprehensive of the epidemiological studies, the University of Michigan National Comorbidity Survey – Replication (Edwards et al., 2006; Ilgen et al., 2008; Tang and Crane, 2006), accordingly reported an almost three-fold increased risk for suicidal attempts (for all types of pain) in a non-clinical sample after controlling for psychiatric/medical comorbidity and for demographic variables. The greatest increase (6.5 fold) was observed in the context of severe headaches followed by non-arthritic chronic pain (6.2 fold); in other studies back pain heightened the risk for completed suicide nine times (Penttinen, 1995) and generalized pain conditions as fibromyalgia and irritable bowel syndrome increased the risk up to 4 fold (Calandre et al., 2011; Spiegel et al., 2007). These figures likely underestimate the problem because about 20% of deaths (Carr et al., 2004; Bohnert et al., 2013) are misclassified as accidental overdoses by pain killers or by other unintentional causes (Cheatle, 2011; Rockett et al., 2010). Hence, in order to improve diagnosis and treatment of suicidality in pain patients it is important to identify its objective markers and their underlying neurobiology.
Presently, no clinical studies directly link pain neuropathology and suicidality. A cumulative body of evidence suggests, however, that there is a considerable overlap between brain circuitries that evolved in the service of physical and emotional pain and that predilection for suicidal behavior is ingrained in the pain neuropathology owing to emotional alterations that are similar to those driving suicidal behavior. The adaptation of these two ideas (discussed in the sections below) lends strength to the hypothesis that pain and suicidality may be potentially explained by recursive partly shared reward and anti-reward neural systems.
2. Pain
2.1. Neurobiology of pain
Illness is the doctor to whom we pay most heed; to kindness, to knowledge, we make promise only; pain we obey.
Marcel Proust. Remembrance of Things Past (1922)
The neurobiology of pain has been reviewed in detail elsewhere (Costigan et al., 2009; von Hehn et al., 2012) and some elements are briefly presented here. Acute painful sensation signals real or perceived tissue damage from environmental hazards. Chronic pain usually arises from neuropathic (damage to the peripheral or central nervous system e.g., diabetic neuropathy and post-herpetic neuralgia), chronic inflammatory (e.g., osteoarthritis) or idiosyncratic etiologies (e.g., fibromyalgia). Conceptually albeit not always clinically similar, chronic pain in cancer patients is also sometimes etiologically linked to direct tissue damage attributable to malignant neoplasms and/or to surgical, chemotherapy or radiotherapy treatments.
Etiology notwithstanding, chronic pain syndromes represent complex entities rooted in genetic, biological and psychosocial factors. Fig. 1 provides a schematic overview of the interface among sensory, reward, anti-reward, motivation, emotion, cognition and arousal neural systems that govern pain-related mood and behavior. Information about noxious stimuli from the skin, the internal organs and other tissues is detected by nociceptors and propagated along primary afferent neurons to converge at the level of the dorsal root ganglion, which is the origin of the spinothalamic tract. The lateral portions of the spinothalamic tract relay sensory-discriminatory dimensions of nociception (e.g., intensity, physical nature and topographic location) to the primary and secondary somatosensory cortices via the ventral postero-lateral and medial thalamic nuclei (Rome and Rome, 2000). The flow of painful stimuli is amplified or diminished at the level of the dorsal horn (Millan, 2002) by a system regulating pain signals through descending modulation from higher centers prior to their interpretation by the cortical and subcortical areas. Specifically, the periaqueductal gray matter (PAG) integrates nociceptive data arriving from the ascending pain pathways with an extensive contribution from the superior cortical and subcortical structures (e.g., cingulate gyrus, amygdala, NAc, habenula and hypothalamus) to regulate the descending pain modulation system by the inferior brainstem nuclei including nucleus tractus solitarius, parabrachial nucleus, locus coeruleus and raphe nuclei. Various neurotransmitters are involved in the descending pain control including opiates, dopamine, norepinephrine, cannabinoids and serotonin. Their action may be dependent on the type of receptors activated. For instance, opioid μ and δ receptors are antinociceptive while κ receptors actually promote pain. This is also respectively reported for the 5-HT1B/D and 5HT3 serotonin receptors (Stahl, 1998).
Fig. 1.

Schematic overview of the interface among sensory, reward, anti-reward, motivation, emotions, cognition and arousal neural systems that govern pain-related mood and behavior. Peripheral noxious stimuli are detected by nociceptors and propagated along primary afferent neurons to converge at the level of the dorsal root ganglion (DRG), which is the origin of the spinothalamic tract. The lateral portions of the spinothalamic tract relay sensory-discriminatory dimensions of nociception to the primary (S1) and secondary (S2) somatosensory cortices via the ventral postero-lateral and medial thalamic nuclei. The flow of painful stimuli is amplified or diminished at the DRG level by the Pain Modulatory System regulating pain signals flow prior to their interpretation by the cortical and subcortical areas. Specifically, the periaqueductal gray matter (PAG) integrates nociceptive data arriving from the ascending pain pathways with an extensive contribution from the superior cortical and subcortical structures including anterior cingulate gyrus (ACC), amygdala (AMY), nucleus accumbens (NAc), hypothalamus (HT) and Habenula (HB) to regulate the descending pain modulation system by the inferior brainstem nuclei including nucleus tractus solitarius, parabrachial nucleus, locus coeruleus (LC) and raphe nuclei (NR). Habenula modulates pain intensity, aversion and motor responses and is part of an anti-reward system that gets activated with exposure to a negative reinforcers (i.e., aversive stimuli) in contrast to the NAc which is part of the reward system that gets activated by positive reinforcers (i.e., reward). It receives projections from the spinothalamic tract, limbic system and the basal ganglia while its efferents are projected to the brainstem pain modulatory regions. Emotional–motivational aspects of pain are carried to the limbic structures (e.g., AMY, HT, striatum, INS and ACC) by the medial spinothalamic tract after synapsing at the medial thalamic and/or brainstem nuclei (e.g., PAG, LC and the NR). In addition to targeting sensory and limbic regions, ascending spinothalamic tract neurons also project to the reticular formation (RF) nuclei (arousal), superior colliculus (motoric orientation), parabrachial nucleus and HT (autonomic processes and neuroendocrine stress-like output). For the clarity of presentation, the scheme was rendered out-of-scale and simplified to reduce the numbers of the displayed links and structures to those of direct relevance to the main themes of this review.
Emotional–motivational aspects of pain (e.g., subjective unpleasantness prompting defensive responses, salience and fear) are carried to the limbic structures (e.g., amygdala, hypothalamus, striatum, insular cortex and anterior cingulate gyrus) by the medial spinothalamic tract after synapsing at the medial thalamic (Lumley et al., 2011; Rome and Rome, 2000) and/or brainstem nuclei (e.g., PAG, the locus coeruleus and the raphe nuclei). Importantly, emotions evoked by painful stimuli is only part of a broad biopsychosocial phenomenon (Lumley et al., 2011) that is comprised of complex interactions of interrelated attentional, contextual, cognitive, endocrine and environmental factors, each of which exhibits a unique role within the context of monitoring homeostasis as well as regulating consciousness and mood (Dunphy and Verne, 2001; Price et al., 2006; Rome and Rome, 2000). For that purpose, in addition to targeting sensory and limbic regions, ascending spinothalamic tract neurons also project (Rome and Rome, 2000) to the reticular formation nuclei (arousal), superior colliculus (motoric orientation), parabrachial nucleus and hypothalamus (autonomic processes and neuroendocrine stress-like output).
And so, chronic pain may become a stand-alone neuropsychopathologic entity occurring at the level of the spinal cord and involving supraspinal integrative circuits (Basbaum, 1999), respectively manifested as spontaneous pain or in exaggerated responses to painful (hyperalgesia) or normally nonpainful (allodynia) stimuli in combination with negative affective states and an overwhelming drive to eliminate pain via behavioral or pharmacologic means including compulsive seeking of opioid drugs driven by the desire to ameliorate inadequately treated pain, that is sometimes described as a pseudoaddiction state (Weissman and Haddox, 1989).
2.2. Emotional and physical pain: Are these two sides of the same coin?
Andrey Yefimitch lay and held his breath: he was expecting with horror to be struck again. He felt as though someone had taken a sickle, thrust it into him, and turned it round several times in his breast and bowels. He bit the pillow from pain and clenched his teeth, and all at once through the chaos in his brain there flashed the terrible unbearable thought that these people, who seemed now like black shadows in the moonlight, had to endure such pain day by day for years.
Anton Chekhov. Ward 6 (1892)
The primary affective unpleasantness of pain engendered simultaneously with the noxious sensory experience (Price, 1992) triggers secondary affective/cognitive processes in the cingulate and parahippocampal gyri attributing meaning and regulating the primary affect (Rome and Rome, 2000) as well as modifying it based on the environmental context and the general emotional state of an individual regardless of pain i.e., pain-unrelated affect (Gracely, 1992). Such a neuroanatomic representation may give an impression of independent pathways for neurobiological and psychological pain processing (Melzack and Wall, 1965, 1970), which may be a sweeping conclusion (Van Houdenhove and Luyten, 2005; Williamson et al., 2005) not entirely reflecting the intimate integration (Borsook et al., 2013) of the pain and emotion systems. To begin with, somatosensory cortex provides a massive input into the affective limbic pain areas (Friedman et al., 1986; Price et al., 2006) and is also involved in a host of both pain-related (Kross et al., 2011; Moulton et al., 2005) and unrelated (Bastiaansen et al., 2009; Rudrauf et al., 2009) emotions. Habenula, an epithalamic nucleus with projections from the spinothalamic tract (Cliffer et al., 1991; Craig, 2003), is yet another brain region with integrated sensory, emotional and motivational functions (Shelton et al., 2012a,b) that modulates pain intensity (Shelton et al., 2012a), aversion (Hikosaka, 2010) and motor responses (Hikosaka, 2010). The tight integration of sensory and emotional processing probably explains why stronger pain is associated with greater self-reported pain unpleasantness (Rainville et al., 2005; Wiech and Tracey, 2009) whereas elicitation of depressed vs. happy emotional states respectively enhances and diminishes the sensation of pain (Connelly et al., 2007; Pinerua-Shuhaibar et al., 2011; Tang et al., 2008).
Although the precise nature of the blurred functional distinctions, echoed by the interchanging use of the “pain” term in both physical and emotional contexts in at least 15 lay languages (Macdonald and Leary, 2005), is still a subject of an ongoing linguistic (Luyten and Van Houdenhove, 2005; Williamson et al., 2005) and scientific (Eisenberger, 2012b; Iannetti and Mouraux, 2011; Price et al., 2006) debate, it is a matter of consensus that “pain-related emotions” and “emotional pain” connote anatomically overlapping and potentially congruent entities (Eisenberger, 2012a,b; Kross et al., 2011; Meerwijk et al., 2012). In more simple terms, pain experience of either physical and/or psychological etiology is indeed hurtful on both levels. For instance, after receiving an announcement of her young brother’s death, the heroine of War and Peace felt “an electric shock” and that “some fearful pain seemed to stab her to the heart.” At the same time, pain is a very subjective experience that everybody understands its meaning but cries about it in the own voice.
In a like manner that grief-related emotional pain activates brain regions associated with physical pain including the PAG, insula and the anterior cingulate cortex (O’Connor et al., 2008), physical pain in humans activates reward/anti-reward circuits e.g., NAc, ventral tegmentum (VT), amygdala and habenula (Berridge, 2003; Borsook et al., 2007; Scott et al., 2006). Furthermore, acute emotional or physical pain in patients with complicated grief and chronic back pain robustly engages a central region of the reward circuit, namely the NAc, during the respective exposure to reminders of the deceased (O’Connor et al., 2008) or to thermal pain (Baliki et al., 2010). Another noteworthy analogy is that the same brain regions (NAc and medial prefrontal cortex; PFC) engaged by prediction of reward are also involved in a similar process with regard to prediction of pain (Atlas et al., 2010). From the evolutionary perspective, critical for the survival of the organisms, the pain system is embedded within extensive circuitry mediating emotions, reward/anti-reward and motivation, representing a neural network indispensable for preservation of individuals and species promoting behaviors necessary for survival (food, water and sex) and preventing those that jeopardize wellbeing (pain and fear) through learning and conditioning and their influence on decision making.
The interface between biopsychological factors governing pain-related affect is portrayed in Fig. 2. Primary pain affect, that is initial or ongoing unpleasantness associated with painful stimuli (Rome and Rome, 2000), is derived from interrelated factors involved in the homeostatic monitoring of physical integrity as part of the system determining emotions and conscious self (Price, 2000; Price et al., 2006). Thus, in addition to carrying the pain sensation in isolation to the somatosensory cortex or insula (Fig. 1), ascending spinal tracts also terminate in the amygdala (fear and emotion), cingulate (fear avoidance, unpleasantness, interoception and motor orientation), insula (subjective experience and interoception), reticular formation nuclei (arousal and vigilance), parabrachial nucleus and hypothalamus (autonomic and neuroendocrine stress responses), habenula (aversion and reduced motivation) to produce primary composite sensory/affective output (Isnard et al., 2011; Price et al., 2006; Rome and Rome, 2000; Vogt, 2005). This output incorporates contextual data in the form of environmental influences, memories, pain-unrelated emotions (e.g., anxiety, catastrophizing), cognitive constructs, personality characteristics and neuropsychopathology to generate the secondary pain affect that resets the primary affect via feedback mechanisms (Gracely, 1992; Price, 1992; Rome and Rome, 2000). The entire system is subject to modulation (facilitation or inhibition) by the descending pain control (Fig. 1) that affects the primary-, the secondary- and the pain-unrelated affects by screening pain information at the spinal cord level.
Fig. 2.

Interface between biopsychological factors governing pain-related affect. Primary pain affect is derived from interrelated factors involved in the homeostatic monitoring of physical integrity as part of the system determining emotions and conscious self. Thus, in addition to carrying the pain sensation in isolation to the primary and secondary somatosensory cortex (S1 and S2), ascending spinal tracts also terminate in the habenula (HB), amygdala (AMY), anterior cingulate cortex (ACC), insula (INS), reticular formation nuclei (RF), parabrachial nucleus and hypothalamus (HT) to produce primary composite sensory/affective output. This output incorporates contextual data in the form of environmental influences, memories, pain-unrelated emotions, cognitive constructs, personality characteristics and neuropsychopathology to generate the secondary pain affect that resets the primary affect via feedback mechanisms.
The affects are further amplified through the long-term neuroplasticity process of sensitization at the cortical and subcortical limbic structures (Rome and Rome, 2000). This is an autonomous, self-sustaining feed-forward loop generated by a variety of intermittent sensory (e.g., pain), pharmacological (e.g., opioid and stimulant agents), emotional (e.g., depression and fear) and behavioral (e.g., addictive and suicidal acts) stimuli and characterized by conditionability, interchangeability as well as progressive intensification of the duration and magnitude of the responses (Pierce and Kalivas, 1997; Weiss and Post, 1994).
2.3. Pain and pleasure continuum
Her tender feet felt as if cut with sharp knives, but she cared not for it; a sharper pang had pierced through her heart. She knew this was the last evening she should ever see the prince, for whom she had forsaken her kindred and her home; she had given up her beautiful voice, and suffered unheard-of pain daily for him, while he knew nothing of it.
Hans Christian Andersen. The Little Mermaid (1836)
A key theory in outlining interfacing sensory and emotional pain components or more precisely the construct that integrates personally meaningful experiences with neuronal activity in the pain pathways is the pain–pleasure continuum (Borsook et al., 2007). This enthralling concept has permeated human cogitation for over 2000 years. Zeno of Citium included pain (as a form of grief) and pleasure among the four basic human passions (in addition to fear and desire) whereas pain and pleasure indifference was looked on as the crucial prerequisite for spiritual tranquility (Seddon and Yonge, 2008). Spinoza upheld the pain–pleasure continuum by designating them as opposite anchors of the perfection scale (de Spinoza, 2005), followed by Nietzsche’s perception of holistic and indivisible nature of the pain–pleasure amalgamation, that is to say, impossibility of gaining pleasure without sustaining pain (Nietzsche and Kaufmann, 1974) along with Bentham’s attribution to pain and pleasure the ultimate role in governing human cognitions and behaviors (Bentham, 1823). The latter idea, rooted in the ancient philosophical writings by Democritus, Aristippus and Aristotle (Aristotle and Roberts, 2004; Elliot, 2006), is supported by Freud’s structural model (Freud, 1917b, 2010) and by numerous other eminent scholars e.g., James (1890), Thorndike (1911), Pavlov (1927), Skinner (1938) and Lewin (1935) linking avoiding pain and seeking pleasure to opposing defense/avoidance vs. approach/motivational drives shaping the behavior of humans and other species.
The Motivation-Decision pain theory equates perceived pain to the actual pain less the simultaneous reward; the same logic defines perceived rewards by subtracting pain from the entire reward experience (Fields, 2007b; Leknes and Tracey, 2008; Sadowski et al., 1984). Such pain-reward tug of war is modulated by the contextual priority of the stimulus so subjective pain perception is diminished owing to the joint activity of the reward and of the descending pain control systems favoring rewards that are salient from the evolutionary or survival standpoints such as childbirth, for example (Fields, 2007b; Leknes and Tracey, 2008; Younger et al., 2010).
3. Reward and anti-reward system
3.1. The opponent process
There is no love which is not pain.
There is no love which does not bruise.
There is no love which does not wither.
Louis Aragon. There is no happy love (1946)
A great truth is a truth whose opposite is also a truth.
Thomas Mann. Essay on Freud (1929)
The valence-based approach/avoidance dichotomy does not seem to be universal, however, as some painful (Nixon et al., 2008) and/or aversive (Sapolsky, 1998; Selye, 1976) stimuli may be perceived as desirable (i.e., rewarding and reinforcing) across various species (Goeders, 2002) as corroborated by: (a) stress-induced place preference (Shen et al., 2011) and intracranial self-stimulation of the brain regions triggering aversive experiences (Cazala et al., 1985) in rodents; (b) self-administration of mild electric shocks in non-human primates (Barrett and Spealman, 1978; Malagodi et al., 1981); (c) a variety of stress-seeking behaviors in humans, e.g., roller coasters, automobile racing, skydiving and horror movies and (d) ‘traumatic bonding’ also known as Stockholm Syndrome or sympathy developed by victims toward the individuals causing their emotional and physical pain (Cantor and Price, 2007).
The Opponent-Process theory (Solomon and Corbit, 1973, 1974) addresses concomitant aversive and rewarding experiences by postulating an existence of powerful neural systems responsible for maintenance of hedonic equilibrium and for stabilization of emotional states and motivational drives. In view of that, the hedonic tone is derived from two valuationally opposite components arbitrarily termed “a” and “b” processes. The former refers to the stimulus’ primary emotional and motivational qualities, be they aversive, appetitive, pleasant or unpleasant; the latter is an antagonistic central nervous system’s response aimed at minimizing the intensity of the initially evoked affect, arousal and drives. Although low and sluggish at their onset, “b” processes dampen affective and motivational characteristics of the active initial input and may later grow to define the intensity and valence of the overall affective and motivational states (Solomon and Corbit, 1973, 1974). For example, initially aversive qualities of nicotine (Sellings et al., 2008) or opioids (Coluzzi et al., 2012) rapidly subside, giving raise to profoundly reinforcing experiences. Then again, cocaine-related dysphoria emerges shortly (minutes) after the drug’s reportedly pleasant high and rush effects (Yamamoto et al., 2007). Pain is likewise associated with the opponent loop evident in pain-induced analgesia (Gear et al., 1999; Tambeli et al., 2012). Such (and other) hedonically opposite of pain experiences, although overshadowed by the ongoing powerful pain stimuli, become conspicuous with pain termination in the form of pleasurable feeling, joy and reward (Becerra and Borsook, 2008; Leknes et al., 2011). In sum, short-term hedonic pain control apparatus is tightly regulated via antagonistic yin- and yang-type negative feedback processes that may be adaptive from a phylogenetic perspective as they improve coping mechanisms by reducing acute aversive effects of the primary challenge.
Neuroanatomy, neurophysiology, and neurochemistry of the opponent processes have been at the outset investigated using drug addiction paradigms. While pain is obviously a divergent neuropsychopathological entity, its comparison to addiction may be meaningful because of several reasons other than the high prevalence of various addictions (e.g., nicotine, alcohol and opioid analgesics) among patients with pain (Brown et al., 1996; Compton and Volkow, 2006; Dersh et al., 2002). Probably the most important is the neural pathways mediating reward and anti-reward since acute pain (Boutelle et al., 1990; Scott et al., 2006) and euphorogenic drugs (Hyman et al., 2006; Koob and Volkow, 2010) activate dopamine transmission in the brain reward circuitry, including the NAc, whereas prolonged periods of pain (Geha et al., 2008; Pais-Vieira et al., 2009; Wood et al., 2007) or of drug consumption (Koob and Le Moal, 2001; Rossetti et al., 1992) produce the opposite anti-reward effect (Kalivas and Volkow, 2005; Karoly and Lecci, 1997; Karoly and Ruehlman, 1996; Leite-Almeida et al., 2009). The dopaminergic system does not exist in isolation, though; it is integrated with a complex network of interrelated opioidergic (MacDonald et al., 2004), cannabinoid (El Khoury et al., 2012), cholinergic (Leslie et al., 2012) and GABAergic (Barrot et al., 2012) mechanisms that are not only involved in reward-related behavior but are also targeted by analgesic therapy e.g., morphine, tetrahydrocannabinol (Guindon and Hohmann, 2009), nicotine (Vincler, 2005) and benzodiazepines (Leslie et al., 2012), all of which have analgesic properties. The following sections discuss neurobiology of reward/anti-reward circuits and of addictive disorders along with their relevance for pain and analgesia.
3.2. Opponent process and “the anti-reward brain”
Dark, profound it was, and cloudy, so that though I fixed my sight on the bottom I did not discern anything there.
Dante Alighieri. The Divine Comedy – Inferno, Canto IV (1321)
As discussed above, the opponent theory of motivation postulates maintenance of hedonic homeostasis in the face of various neurochemical and emotional proponent perturbations (Koob and Le Moal, 2008). Dopamine neurons in the ventral tegmental area and their projections into the NAc are central to the brain’s reward system (Carlezon and Thomas, 2009) and constitute the putative ‘homeostat’ (c.f., Goldstein and McEwen, 2002) comparing hedonic information with a pre-set point. As any homeostatic system (Cannon, 1929), reward is regulated by negative feedback. The common element of the rewarding effects of natural rewards as well as drugs of abuse relates to increases in dopamine levels in the NAc (Carlezon and Thomas, 2009) including but not limited to pleasure or “high” (reward). The intervening variable of subjective pleasure is sensed in the medial PFC (Berridge and Kringelbach, 2013; Goldstein and Volkow, 2002), which exerts top down control of dopaminergic activity in the NAc via the corticostriatal segment of the cortico-striato-thalamocortical circuit (Bimpisidis et al., 2013; Volkow et al., 2011). Increase or decreases in NAc’s dopamine concentrations prompt respective habituation (Di Chiara et al., 2004) or sensitization (Tremblay et al., 2002) in the key effector systems (Goldstein, 1995) consisting of (to name a few) pre- and post-synaptic dopamine receptors, presynaptic dopamine transporters and enzymes involved in dopamine’s synthesis and metabolism; all oppose and thereby balance abrupt reward changes (Fig. 3).
Fig. 3.
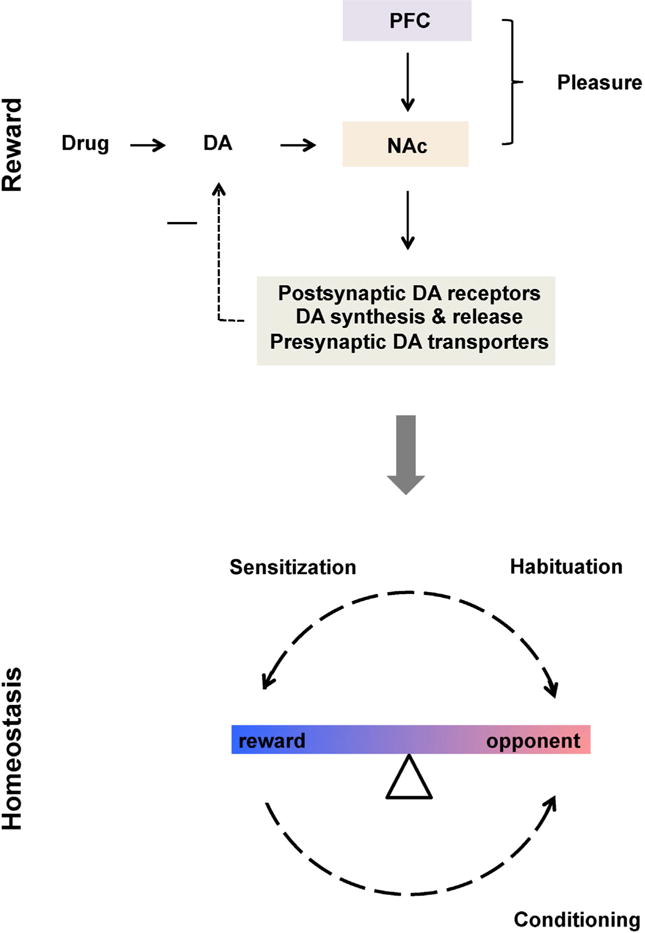
Homeostatic function of reward circuitry. Prefrontal cortex exerts top down control for bottom up dopamine (DA) signals coming from the nucleus accumbens (NAc) in response to natural and pharmacological rewards. The integrated information is compared to the regulated set point and in case of discrepancy is opposed via negative feedback loop by regulating postsynaptic DA receptors, DA synthesis and release as well as presynaptic DA transporters (among other mechanisms). Opponent processes clinically evident in depressive symptomatology may enhance drug use or natural reward via amplification of their rewarding and reinforcing properties i.e., sensitization. Backward conditioning further enhances opponent processes by evoking withdrawal symptomatology. “−” sign represent inhibition.
Recurrent dopaminergic trafficking consequent to eupohorogenic drugs consumption eventually results in hypodopminergic state in the NAc and the medial PFC (i.e., within system adaptation). The initially homeostatic adjustments to diminished resting state tonic dopaminergic neurotransmission in the NAc and PFC set in motion robust augmentations of phasic dopamine responses to drugs and via Pavlovian conditioning to drug-related cues (Kalivas and Volkow, 2011; Volkow et al., 2011) and/or to interoceptive bodily states (Verdejo-Garcia et al., 2012). Prefrontal activations caused by these cues profoundly increase PFC’ glutamatergic output (Kalivas and Volkow, 2005) to already hypofunctional NAc (Volkow et al., 2005), thus further decreasing its dopaminergic activity. This triggers the drive to seek and consume drugs (i.e., craving) with secondary drug-induced phasic dopaminergic bombardment (Grace, 2000) that eventually overpowers the homeostatic restraint (Koob and Le Moal, 2001) and gives rise to the between system adaptation recruiting central and basolateral amygdala nuclei, the bed nucleus of the stria terminalis, the lateral tegmental noradrenergic nuclei of the brain stem, the hippocampus and the habenula that in concert contribute to massive outpouring of stressogenic corticotropin-releasing factor (CRF), norepinephrine and dynorphin manifested in negative affective states, anhedonia and profound craving (Koob and Le Moal, 2001). The consequent adaptation in the form allostatic “anti-reward” loop (Fig. 4) is metaphorically termed the “dark side of addiction” (Koob and Le Moal, 2005) as it is unchecked by physiological negative feedback mechanisms with drugs (and/or natural rewards) providing only transient symptomatic relief while worsening neuroadaptations to the point of systematic collapse (Goldstein and Volkow, 2011) and subsequent end-stage outcomes of addictive disorders including suicide (Darke and Ross, 2002).
Fig. 4.

Allostatic dysregulation of anti-reward. Interference with homeostatic functioning triggers between system adaptation recruiting central and basolateral amygdala (BLA) nuclei, the bed nucleus of the stria terminalis (BNST), the lateral tegmental noradrenergic nuclei of the brain stem, the hippocampus and the habenula that in concert contribute to massive outpouring of stressogenic CRF, norepinephrine and dynorphin manifested in negative affective states, anhedonia and profound craving. This results in an unstable positive feedback loop wherein anti-reward systems activation drives further drug consumption that provides momentary relief but eventually increases aversiveness and craving and thus contributes to progressive worsening of the clinical condition. “+” sign represent stimulation and “−” inhibition.
Such positive feedback loops are relatively uncommon yet possible in clinical medicine (Goldstein, 1995). A similar example is weight gain and insulin resistance that render normal satiety signals ineffective leading to impairments in physiologic mechanisms regulating food intake and shifting the set point for energy homeostasis towards the development of overweight and obesity (Elman et al., 2006). This may be further worsened by concurrent drug use that elicits stress-like responses countering insulin action (Rott et al., 2008).
It is quite difficult to stop and reverse the developing vicious cycle (schematically illustrated in Fig. 4) as attempted abstinence by restriction of the drug intake results in the opponent hypodopaminergic condition triggering robust activation of drug relapse pathways (see below) in the face of suppressed cognitive control; the magnitude of these responses exceeds by far that during proponent stimulation by natural non-drug rewards.
In some instances it might be possible to postulate specificity of the opponent and anti-reward mechanisms. Their dissociability is supported by qualitatively different temporal and neuroadaptaional characteristics summarized in Table 1. An alternative continuum interpretation is that anti-reward is an exaggerated form of the opponent process. Compatible with the latter assumption, a key element of anti-reward namely, craving, has been shown to increase (incubate) over time even in the absence of recurrent drug exposure (Bedi et al., 2011). Also, termination of conditioned cue-induced effects (related to emotions, interoceptions and executive control) can evoke both opponent and anti-reward symptomatology caused by the backward conditioning (i.e., linked to the conclusion of the unconditioned stimulus) and evidenced in depression, anxiety, apathy and anhedonia symptoms (O’Brien et al., 1977; Solomon and Corbit, 1973, 1974). The existing preclinical and human data are far from conclusive on the dissociability score, and the theoretical considerations are not unambiguous. For these reasons, when appropriate, “opponent” and “anti-reward” are used as a combined term (i.e., “opponent/anti-reward”).
Table 1.
Characteristics of opponent and anti-reward processes.
| Characteristic | Opponent | Anti-reward |
|---|---|---|
| Conceptual frame | Mind | Brain |
| Compensatory mechanism | Homeostasis | Allostasis |
| Stimulus | Not well-defined: there are states “which have little or no opponent process” (Solomon and Corbit, 1974) | Recurrent activation of reward circuitry |
| Effector system | DA, opiates, cannabinoids and acetylcholine | DA (within system); Glutamate, NE, CRF and dynorphin (between system) |
| Adaptation | Habituation and sensitization | Stress sensitization and tolerance |
| Valence | Opposite to proponent; can be positive or negative | Negative |
| Time frame | Reversible and time limited; phasic changes | Chronic; tonic changes |
Proponent reward signals in the brain motivate actions that increase the probability that the behavior will be performed and repeated in the future whereas negative reward signals inhibit actions that are likely to result in fruitless pursuits or in aversive consequences (Wickens, 2008). It is now recognized that dopamine neurons in addition to encoding for reward per se also encode for motivational salience (McClure et al., 2003), for conditioned learning of stimulus–reward association (Berridge, 2004; Gardner, 2011; Koob and Volkow, 2010) and for discrepancy between actual and anticipated reward (Monterosso et al., 2012; Schultz, 2007; Schultz and Dickinson, 2000), that is to say, reward prediction error.
Prediction error is generated in response to a novel or unexpected reward whereas with repeated exposure when the reward becomes expected, DA cells stop firing and instead activate with increasingly distant and thus novel cues (Wise, 2002) predicting the reward. Attribution of incentive salience to rewarding stimuli (McClure et al., 2003) ‘gated’ by the NAc occurs in the ventromedial PFC (Montague et al., 2004). The same region integrates other peripheral and environmental stimuli to exercise cognitive control over drives and emotions (Goldstein and Volkow, 2002) and to perform (together with more lateral cortical areas) contextual framing of timing i.e., discounting of reward valuation in proportion to its delay (Ballard and Knutson, 2009). Additional regions also participate in the DA modulated circuitry including the olfactory tubercle (Ikemoto, 2010), which is the extension of the NAc’s shell and the hypothalamus interfacing between the motivational/emotional, motor and homeostatic reward components (Elman et al., 2006).
Cortical processing of homeostatic states (i.e., interoception) is carried out by the insula (Verdejo-Garcia et al., 2012) in concert with the anterior cingulate cortex (expectancy, cognitive appraisal and top down control) and the dorsolateral and ventromedial PFC (emotional attribution). Interoception is reflected in the resting state spontaneous brain activity that is helpfully assessed by the default-mode network (DMN) analytic technique. The DMN is reciprocally interrelated with the salience system (Bonnelle et al., 2012; Borsook et al., 2013). Of particular interest here is that the DMN insular connectivity aberrations observed in patients with pain (Xue et al., 2012), addiction (Verdejo-Garcia et al., 2012) and depression (Hamilton et al., 2011) may explain failure to restrain endogenous excitation as well as perceptions evoked by exogenous stimuli manifested in heightened prediction errors and salience attributed to bodily and environmental stimuli so giving rise to catastrophizing, craving and somatization.
Another key functional reciprocity is between dopamine neurons and neurons in the habenula. The latter increase their firing when an expected reward does not materialize or to predictors of no-reward (Ji and Shepard, 2007; Kimura et al., 2007) and decrease their firing when an expected reward occurs (Hong and Hikosaka, 2008; Matsumoto and Hikosaka, 2009). The habenula (with the input from the limbic system and basal ganglia) provides inhibitory tone to dopamine neurons through interpeduncular and the rostromedial tegmental (i.e., respective medial and lateral habenular) nuclei (Lee and Goto, 2011) projections resulting in decreased dopamine transmission in the PFC and in the NAc (Lecourtier et al., 2008; Lee and Goto, 2011) clinically visible as decreased motivation and anhedonia (Elman et al., 2009). Other major neurotransmitter systems contributing to aversive anti-reward states (Hikosaka, 2010) including serotonin (Kalen et al., 1989; Nishikawa and Scatton, 1985), norepinephrine (Lecourtier and Kelly, 2007) and acetylcholine (Lecourtier and Kelly, 2007) are also regulated by the habenula via respective raphe nuclei, locus coeruleus and nucleus of Meynert. And so, habenula is involved in the aversive anti-reward circuitry that when sensitized (Hikosaka, 2010) may contribute to the transition from the controlled to compulsive drug intake seen in addiction (Fig. 4). The involvement of the habenula as a central node in the anti-reward network is therefore in accord with the theoretical model of addiction postulating sensitized anti-reward responses mediated through enhanced sensitivity of the amygdala and increased CRF signaling (Koob and Le Moal, 2008).
Notably, similar anti-reward responses contribute to anhedonia in depression, as demonstrated in the preclinical model of learned helplessness, an animal model of depression (Amat et al., 2001; Shumake and Gonzalez-Lima, 2003). Clinical studies alike show evidence of structural changes in the habenula of depressed patients (Ranft et al., 2010; Savitz et al., 2011) and in a patient with refractory depression deep brain stimulation (DBS) of the habenula was associated with significant clinical improvement (Sartorius et al., 2010). The interactions between the reward and anti-reward networks (central nodes in the NAc and habenula respectively) is likely the reason to why DBS in the treatment of resistant depression appears to be effective both when electrodes are placed in the NAc as well as when placed in the habenula. Their interactions are also likely to be relevant to the neurobiology of the co-morbidity between major depression and substance use disorders and particularly nicotine dependence. This is because the habenula’s α5 nicotinic receptors modulate aversive responses to nicotine (Fowler et al., 2011) and the α5 and α2 nicotine receptors mediate nicotine withdrawal (Salas et al., 2009). Smoking may decrease the activity of the habenula by desensitizing these receptors and therefore depressed patients may use of nicotine as a mechanism of self-medication.
3.3. Reward and addiction are not homogenous entities
All kinds of beauty do not inspire love; there is a kind which only pleases the sight, but does not captivate the affections.
Miguel de Cervantes Saavedra. Don Quixote (1615)
The precise psychological nature of the mechanisms driving addictive disorders remains a subject of complementary theories. According to the Incentive Motivation theory brain reward function is not a homogenous entity but can be rather dissected, using neurochemical, neuroanatomical, and functional criteria, into core processes, namely ‘liking’ and ‘wanting’ (Berridge and Robinson, 2003). ‘Liking’ is conveyed to the frontotemporal cortical structures (Berridge, 2003; Kelley, 2004) through μ-opioid neurotransmission within the scattered network of subcortical and brainstem nuclei (Kelley, 2004; Pecina et al., 2003; Robinson and Berridge, 2003) including the NAc, VT, ventral pallidum, nucleus of the solitary tract, parabrachial nucleus and the amygdala.
Drug-induced changes in the mesolimbic dopaminergic circuitry including dopamine terminal fields (e.g., striatum, amygdala, and PFC) underlying the wanting but not liking purposes is purportedly responsible for transforming regular wanting responses into heightened incentive salience assigned to drugs or drug-related cues which is construed to be an animal homolog of human craving (Robinson and Berridge, 2003). Such hypersensitive state is further amplified through the Cross-Sensitization (Elman et al., 2012; George et al., 2012; Robinson and Berridge, 2003) when prior exposure to one stimulus (e.g., drug) increases subsequent response to itself (Basso et al., 1999; Self and Nestler, 1998) and to a different stimulus (e.g., stress) and in the reversed order, enhancement of drug motivational states e.g., craving following prior stress exposure (George et al., 2012). This anti-reward loop is termed “spiraling distress cycle” (Goldstein and McEwen, 2002; Koob and Le Moal, 2001) whereby a trivial event (e.g., psychosocial stress) can mount escalating dopamine releases in the striatum causing craving and further worsening symptoms of addiction.
Addictive drugs and behaviors introduce sustained and short-term up/down regulation in gene expression together with long-term potentiation and depression of synaptic strength with ensuing profound alterations in neuronal signaling evidenced in distorted learning and memory formation (Gurden et al., 2000; Hyman et al., 2006). Learned association is a basic tenant of the sensitization construct so that novel links are forged between emotions, motivations and behaviors in order to be reenacted as an ensemble by a seemingly desecrate stimulation (Rome and Rome, 2000; Weiss and Post, 1994). A closely related concept, derived from primate work, is the Aberrant Learning theory suggesting that learning of new rewards or expectancy of the old ones is encoded via interactions between tonic (baseline) and phasic spikes in dopaminegic neurons; phasic firing predicts new rewards (Kalivas and Volkow, 2005; Schultz, 2001). Hence, neural adaptations to excessive dopaminergic trafficking in response to drugs may lead to low signal-to-noise detection capability for natural rewards along with overlearning of the motivational significance of cues that predict delivery of drugs i.e., drugs are constantly perceived to be better than expected (Hyman, 2005; Hyman et al., 2006). Eventually addictive drugs establish habit-based valuation system mediated mostly by the dorsal rather than ventral striatum and driving compulsive behavioral patterns (Volkow et al., 2006) that are not goal-directed and therefore not amenable to modulation by adverse outcomes (Rangel et al., 2008).
Notwithstanding the theoretical school of thought on psychological mechanisms underlying addictions, the general consensus is that relapse to drug consumptions and/or to addictive behaviors after some period of abstinence constitutes the key factor responsible for the chronic course of the illness (Sinha, 2011). A widely used behavioral probe of relapse is an event when a laboratory animal reinitiates lever press responding after abstaining from drug self-administration (Epstein et al., 2006). Three types of stimuli are known to induce reinstatement i.e., low doses of drug itself, cues conditioned by drugs and stress (Gardner, 2011; Self and Nestler, 1998) and so relapse is usefully divided into subtypes based on the provocation technique (Table 2). Separate inquiry into each subtype provides evidence for the common obligatory circuitry, comprised of the glutamaterigic pathways extending from the PFC to the NAc core (Kalivas and Volkow, 2005), in conjunction with different neuroanatomic substrate engaged by cues vs. drugs or stress.
Table 2.
Characteristics of drug-, cue- and stress-induced relapse (prefrontal cortex, PFC; nucleus accumbens, NAC; ventral tegmentum, VT; basolateral complex of the amygdala, BLA; central nucleus of the amygdala, CEA; bed nucleus the stria terminalis, BNST).
| Brain region | Relapse
|
Role in pain | |||||
|---|---|---|---|---|---|---|---|
| Drug-induced (dopamine/glutamate) | Cue-induced (glutamate) | Stress-induced
|
|||||
| (NE) | (CRF) | ||||||
| PFC | x | x | x | x | Assessment of meaning and implications (Coghill et al., 1999) | ||
| NAC | x | x | x | x | Salience, analgesia, expectation and outcomes prediction | ||
| VT | x | ||||||
| Ventral subiculum | x | Conditioning (Maren, 1999) | |||||
| BLA | x | Emotional processing, memory (Minami, 2009) and conditioning (Scarlet et al., 2012) | |||||
| Hypothalamus | x | Descending modulation (Cortelli and Pierangeli, 2007) | |||||
| Lateral tegmentum | x | Stress, negative affective states, nociception and aversion | |||||
| CEA | x | (Kiser and German, 1978; Minami, 2009; Neugebauer et al., 2004) | |||||
| BNST | x | ||||||
| Habenula | x | x | Aversion, anhedonia and inhibition of motor activities (Creutzig et al., 1990; Hikosaka, 2010; Shelton et al., 2012a) | ||||
Since reward and craving produce similar drug seeking behaviors and consequent relapse, increased dopamine neurotransmission in the same medial forebrain bundle connecting the VT to the NAc has been assumed to subserve both phenomena (Gardner, 2011; Self and Nestler, 1998), though, some evidence suggests an important role for other monoaminergic systems besides dopamine (Rocha et al., 1998). Regions beyond the VT and Nac have been implicated in the stress-induced relapse (Table 2). One pathway starts out with in the lateral tegmentum noradrenergic cell bodies and terminates in the hypothalamus, NAc, amygdala and the bed nucleus the stria terminalis (BNST). Another stress relapse pathway connects the central nucleus of the amygdala with the BNST (Gardner, 2011; Moore and Bloom, 1979). Cue-induced relapse involves the ventral subiculum of the hippocampus, the basolateral complex of the amygdala and the NAc (Gardner, 2011; Koob and Le Moal, 2008; Vorel et al., 2001). Habenula’s role in addictive disorders (Lecca et al., 2011) is supported by its involvement in drug aversion (Fowler et al., 2011) along with drug- (Brown et al., 2010) and cue-induced (Madsen et al., 2012) relapse.
From the above evidence (Table 2) it is clear that addiction is not a unitary entity defined by a single type motivation, emotion or neural mechanism. On the contrary, it is both hierarchical and multidimensional, consisting of higher order reward and anti-reward factors and lower order sensitization, cross-sensitization and aberrant learning dimensions, each of which plays a specific role in relapse to drug use and seeking behavior. Modulation by the reward vs. anti-reward states (Table 3) as well as by such clinical variables as comorbid psychopathology, stress levels, expectancy states my produce a complex pattern of interactions. Such conceptualization offers structured algorithms for diagnostic, preventive and therapeutic pursuits in patients with addictive disorders. Clinical interviews and psychometric assessments may define opponent/anti-reward neuroadaptational states, while sensitization, cross-sensitization and aberrant learning could be demonstrated via neuroimaging in conjunction with cognitive and biochemical challenges procedures (Elman et al., 2005, 2009, 2012; Hopper et al., 2008).
Table 3.
Modulation by reward vs. anti-reward states of the effects of drug-, cues- and stress-related stimuli in patients with addiction.
| Stimulus | Reward
|
Opponent/anti-reward
|
||||
|---|---|---|---|---|---|---|
| Sensitization | Cross-sensitization | Learning | Sensitization | Cross-sensitization | Learning | |
| Drug | Drug-primed craving(Elman et al., 2002) | Cross-priming relapse by different classes of drugs e.g., cocaine and heroin (Bossert et al., 2005; Shalev et al., 2002) Drug primed exaggerated increase in sexual drive in addicts (Volkow et al., 2007) |
Exaggerated prediction errori.e., discrepancy between actual and anticipated drug effects (Schultz, 2011) | Exaggeration of rewarding and reinforcing drug effects during withdrawal i.e., “deprivation amplification” (Blum et al., 2012; Elman et al., 2002; Volkow et al., 2002) | Increased motivational appeal of food during drug withdrawal(Edge and Gold, 2011) | Backward conditioning of withdrawal symptomatology to termination of drug like effect (Solomon and Corbit, 1973, 1974) |
| Cue | Incentive sensitization of drug-related cues (Robinson and Berridge, 2003) | Spillover of incentive sensitization to non-drug rewards (Berridge, 2007) | Conditioning of drug-related cues e.g., modulation of euphoria and craving by perceived availability of the drug (McBride et al., 2006; Yamamoto et al., 2007) | Exaggerated drug cue brain reactivity during withdrawal (Langleben et al., 2008; Volkow and Fowler, 2000) | Food deprivation increases drug craving (Leeman et al., 2010) and drug cue reactivity (Jenks and Higgs, 2011) | Craving primed by conditioned withdrawal-related cues e.g., dungeon, running out of money, locked unit, etc. (Solomon and Corbit, 1973, 1974) |
| Stress | Heightened stress responsivity (Elman et al., 2001; Goeders, 2002; Karlsgodt et al., 2003) | Heightened drug-induced stress like effects (Elman et al., 1999; Koob and Kreek, 2007) | Stress hormone elevations repeatedly paired with drug use become a conditioned stimulus eliciting craving (Elman et al., 2003; Sinha et al., 2011) | Withdrawal-related activation of brain stress systems (NE, CRF) i.e., between system adaptation (George et al., 2012; Koob, 2009) Withdrawal-related negative affective states (Elman et al., 2010a,b) |
Withdrawal (Edge and Gold, 2011) stress-induced (Cottone et al., 2009) hyperphagia | Stress repeatedly paired with drug withdrawal become a conditioned stimulus eliciting craving (Sinha, 2007) |
Moreover, although it is hypothesized by some that addiction develops via negative reinforcement mechanisms viz., the drug is used to ameliorate the unpleasantness of the withdrawal state (Khantzian, 1997), the view presented here suggests an adaptation of this idea in the form of a positive reinforcement hypothesis, explicitly, that opponent and anti-reward processes clinically present as withdrawal and depressive symptomatologies enhance drug use through amplification of its rewarding and reinforcing properties (Elman et al., 2002). Drug use may even paradoxically produce a seemingly advantageous clinical action in individuals with reward deficits by sensitizing previously non- or under-responsive reward circuitry not only to drugs, but via cross-sensitization processes to natural rewards including social function (Berridge, 2007). The next section further emphasizes the heuristic value of addiction neuroscience for understanding neurobiology of social attachments and how they share the same brain structures and mechanisms with addictive drugs and pain.
3.4. Social attachment and addiction: Shared neurobiology and analogous presentation
Masturbation is the primal addiction.
Sigmund Freud. Sexuality in the etiology of neurosis (1898)
I joined the ring of players, while the rest of the crowd massed itself around me. At this distance of time I cannot remember whether I ever gave a thought to Polina; I seemed only to be conscious of a vague pleasure in seizing and raking in the banknotes which kept massing themselves in a pile before me.
Fyodor Dostoyevsky. The Gambler (1867)
One of the most consistent findings in basic and clinical neuroscience of addiction is the presence of neuroadaptations that are driving continuous drug intake and/or engagement in behavioral addictions e.g., gambling (Elman et al., 2012, 2013) and overeating (Elman et al., 2006). An important question that logically follows is whether addiction-like phenomena necessarily develop secondary to brain dysfunction elicited by a drug or a behavior or they are a reflection of the primary factors inherent in normal function. This question is supported by the general notion that chemical and behavioral addictions usurp the same neural systems involved in the fulfillment of the food, security and procreation needs that are vital for the continued existence of individual and species (Hyman and Malenka, 2001).
“Unsociable species … are doomed to decay” (Kropotkin, 1902) so historically human beings were destined for demise from starvation and/or from acts of violence in the absence of affiliation with one another. Accordingly group affiliation is an evolutionary need forming the basis for the establishment of (extended) families, tribes, states, nations and religions. This may be why we have evolved to be so compelled to seek social bonding and to experience respective pleasure and grief upon its attainment and painful loss so that “the simplest pattern, that in which a man was born, worked, married, had children, and died, was likewise the most perfect” (Maugham, 1915). At the fundamental level people may be really seeking human warmth, care and an ability to experience love for another soul, whereas addiction, like masturbation (Freud, 1898), presents a seemingly similar but an ill-fated pursuit of the longed emotions naturally evoked by genuine social connectedness (Zellner et al., 2011).
Social attachment is certainly purported to be the “primary form of addiction” (Burkett and Young, 2012; Insel, 2003; Zellner et al., 2011). Several lines of evidence support this sort of conceptualization, including heightened incentive salience assigned to heterosexual beauty, the term typically refers to a situation where the motivational targets that are ‘wanted’ more than could be explained by their hedonic properties, that is to say, ‘liking’, which may be akin to the urges to seek drugs despite their diminished (or absent) hedonic qualities (Levy, 2008). In addition, euphoria of early encounters with the love object is indistinguishable from drug-induced high. The same is true for tolerance, withdrawal, craving and giving up important activities with regard to respective estrangement, separation persistent desire to be with the loved one and loss of sense of time with realization of intimacy (Burkett and Young, 2012; Zellner et al., 2011).
Owing to understanding that addictive drugs tap the same neural mechanisms that are evolutionary designated for mediation of social attachments (Insel, 2003), reward and motivational systems, initially investigated in the context of drug addiction models, are now becoming a premier probe for addressing fundamental issues pertinent to social neuroscience. In fact, from a neuro-chemical/anatomical standpoints, motivation for pair bonding (Gingrich et al., 2000; Hansen et al., 1993) and for maternal care (Curtis et al., 2006), like euphorogenic drugs (Hernandez et al., 1987), induces dopamine release within mesolimbic dopaminergic reward and motivational pathways. At the behavioral level, such motivation can be demonstrated in preclinical models using the same procedures borrowed from the addiction field namely, conditioned place preference (Mattson et al., 2001), operant conditioning bar-pressing procedures (Wilsoncraft, 1968) and choice tasks (Mattson et al., 2001). The association between social attachment and addiction is further supported by extensive clinical literature on poor couple (Ghitza et al., 2007) and parental (Coyer, 2003; Eiden et al., 2006) functioning in individuals afflicted with substance use disorders. Hence, addiction entails taking over motivational and emotional systems by switching evolutionary programmed and normatively sensitized incentive targets (e.g., sexual attractions and maternal care) for the ones bearing absolutely no benefits for an individual. Emotional pain inevitably accompanying losses and setbacks of social attachments (Shneidman, 1998) may prompt suicide while an expression of a genuine compassionate concern even indirectly through letters have been shown to reduce suicidal deaths in high risk population more so than elaborate but less personable interventions (Motto, 1976; Motto and Bostrom, 2001).
3.5. Suicide can be an elusive for definition concept
I am reaching a conviction that the instinct of goal attainment is a part of human nature and the awareness of this instinct and its correct practice is one of the tasks of human life and conditions of human happiness…. So, I find, turning to the root of things, that the suicide phenomenon presents itself in the form of a fall in the goal attainment instinct.
Ivan Pavlov. About Suicides. Lecture at the Russian Academy of Military Medicine (1913; translation IE)
He had never looked forward to the wisdom and other vaunted benefits of old age. Would he be able to die young—and if possible free of all pain? A graceful death—as a richly patterned kimono, thrown carelessly across a polished table, slides unobtrusively down into the darkness of the floor beneath. A death marked by elegance.
Yukio Mishima. Spring Snow (1969)
As expressed so eloquently in the above quotations suicidality is comprised from motivational and hedonic and even esthetic pieces. It may vary in the intensity from vague wondering about the worthiness and meaning of the existence to self-inflicted damage of an escalating degree of lethality to a “successfully” completed act defined as “all cases of death resulting directly or indirectly from a positive or negative act of the victim himself, which he knows will produce this result” (Durkheim, 1952). Suicidality tops the public agenda not only as “physician-assisted suicide” but also in the form of ostensibly suicidal behaviors such as reckless driving (while texting or using cell phone), unprotected sex, dangerous sports and hobbies (e.g., car racing) that are becoming both integral and ubiquitous (though not readily acknowledged as such) elements of social life, entertainment, business and leisure activities. The answer to the question concerning the legitimacy of suicide and whether it violates the basic laws of existence cannot be simple as is it comprised from divergent emotional, rational and idiosyncratic considerations compounded by a multifaceted mixture of scientific, religious and philosophical principles.
Although suicide is commonly understood to be not only an integral part but the utmost menacing feature of mental illnesses with up to 90% of individuals who commit suicide suffer from psychiatric disorders (Nock et al., 2010, 2013), it is an intricate condition with symptoms that extend beyond psychopathology (Linehan, 2008). The mere valuation of the suicidal act for itself encompasses the entire range from abominable to neutral and admirable features (Table 4). An attempt for a rational examination of the suicide phenomenon therefore faces a first major challenge: how to define and approach the underlying mechanism. This elusive concept has been given nearly as many definitions as there are treatises that have dealt with it.
Table 4.
Examples of context-dependent perception of suicide.
| Theme | Negative | Neutral | Positive |
|---|---|---|---|
| Societal edict | Do you not know that your body is a temple of the Holy Spirit, who is in you, whom you have received from God? You are not your own. 1 Corinthians. Holy Bible, The New International Version | Self-punishment for gross violation of organizational (e.g., mafia, intelligence services) values, taboos and proscriptions e.g., Socrates’ choice to die rather than to violate the social contract with the Athens’ citizens. Plato. 360 BCE, Phaedo | Ridding of elderly and disabled people when community faces potential extinction due to paucity of food and other essential resources (e.g., Obasuteyama Mountain in Japan) |
| Individual choice | If a person knowingly destroys himself, we do not involve ourselves with him for anything. We do not mourn for him, we do not eulogize him, but we do form a condolence line and recite the blessing for mourners and perform all other practices which honor the living. Mosheh Ben Maimon (Rambam or Maimonides). circa 1170–1180, Mishneh Torah, Hilchot Avel | Above all, remember that the door stands open. Epictetus. circa 55-135, Golden Sayings | Abundant sources of ancient Greek mythology posit that it is preferable for a human being not to be born at all and if the birth nonetheless occurs it is preferable to return to the place where we came from as soon as possible. The happy person is the one that dies at the peak of life regardless of age (see, for example, conversation of Solon and Croesus as recorded in Herodotus. 440 BC, The Histories) |
| Let us here endeavor to restore men to their native liberty, by examining all the common arguments against suicide, and showing that that action may be free from every imputation of guilt or blame, according to the sentiments of all the ancient philosophers. David Hume. 1783, Essays on Suicide and the Immortality of the Soul | Freezing the moment by choosing to die at the peak of happiness is also exemplified in the Goethe’s Faust who loved what the devil bestowed upon him to the point of wishing to turn that moment to eternity (see also Nelson’s vignette above) | ||
| “Seven years and six months!” Humpty Dumpty repeated thoughtfully. “An uncomfortable sort of age. Now if you’d asked my advice, I’d have said, “Leave off at seven’ – but it’s too late now.” Lewis Carroll. 1872, Through the Looking-Glass and What Alice Found There | To kill myself, when I shall not want to live – constitutes my most sacred right. This right – the only genuine assurance of freedom, is the solid basis of my human dignity. Leonid Andreyev. 1912, Suicide (translation IE) | ||
| ‘Honorable escape’ | You are certainly wrong to compare suicide … with great accomplishments, since it cannot be considered as anything but a weakness. After all, it is easier to die than to endure a harrowing life with fortitude. Johann Wolfgang von Goethe. 1787, The Sorrows of Young Werther | Stoic and epicureans call for living life as long as it brings happiness and harmony and for parting from life when such arrangement becomes unattainable. | The most voluntary death is the finest. Life depends upon the pleasure of other; death upon our own. Michel De Montaigne. 1686, A Custom Of The Isle Of Cea |
| It is for the same reason that suicide is also immoral. Life in its entirety, and the possibility of living until natural death, have been given to man only on the condition that he serve the life of the Universe. But, having profited by life so long as it was pleasant, he refuses to serve the Universe as soon as life becomes unpleasant: whereas, in all probability, his service commenced precisely when life began to appear unpleasant. All work appears at first unpleasant. Lev Tolstoy. 1898, Letter on Suicide | If suicide is allowed then everything is allowed. If anything is not allowed then suicide is not allowed. This throws a light on the nature of ethics, for suicide is, so to speak, the elementary sin. <> It may also be that in and of itself suicide is not good or bad. Ludwig Wittgenstein. 1961, Notebooks 1914–1916 | If it were impossible to live, then one would kill oneself; and consequently one cannot speak of life as being unbearable. The possibility of killing himself has been given to man, and therefore he may (he has the right to) kill himself, and he continually uses this right – when he kills himself in duels, in war, by dissipation, wine, tobacco, opium, etc. Lev Tolstoy. 1898, Letter on Suicide | |
| Suicide is man’s greatest sin <> but God alone can judge it, for only God knows what and how much a man can bear. Fyodor Dostoyevsky. 1875, The Adolescent | To die:–to sleep: No more; and, by a sleep to say we end The heart-ache and the thousand natural shocks That flesh is heir to, ‘tis a consummation Devoutly to be wished. William Shakespeare. 1601, Hamlet | ||
| Relief of emotional and physical pain | Life is a joke because of all its pleasures and pains do not touch a person’s inner and better self. There are those, however, who fail to realize this, and they take life too seriously. So when misfortune occurs, some live a life full of vices and therefore seek to abuse their inner and better selves. Others show that by committing suicide, they cannot take a joke: Therefore as mauvais jouneur he does not take the loss with composure, but when bed cards are dealt to him he peevishly and impatiently refuses to go on playing, throws down the cards, and breaks up the game. Arthur Schopenhauer. 1819, Manuscript Remains |
There was a man that killed himself, whom I very much esteemed and loved and reckoned one of the finest of people. The reason for his suicide was hopeless sickness. It is not for me to judge him. When a man kills himself, because he is facing torture and fears being broken into betrayal, then this essentially is not even suicide.
Nikolai Berdyaev.1931, On Suicide … the evil days come and the years draw near of which you will say, “I have no pleasure in them.” Ecclesiastes. The English Standard Version Bible |
The thought of suicide is a powerful solace: by means of it one gets through many a bad night. (Friedrich Nietzsche. 1886, Beyond Good and Evil) I think that I did not shoot myself this night only thanks to my firm decision to do it in any case if not today then tomorrow. Ivan Bunin. 1952. The Life of Arseniev (translation IE) Every minute of [GULAG] camp life is a poisoned minute. There is much there that a person should not know, should not see, and if he has seen it – it would be better for him to die. Varlam Shalamov. 1966, Kolyma Tales |
A sociologist views suicide as a consequence of disintegration of either society in its entirety or of an individual’s ties with the society (i.e., anomie). Altruistic suicide may conversely be a reflection of overly cohesive society exerting an undue pressure on an individual (Durkheim, 1952). A psychoanalyst formulates suicidal tendencies as a death instinct (Freud, 1917b, 2010) that may be conceptualized as the opponent process to the libidinal drive aimed to achieve pleasure. A physicist, on the other hand, may understand suicide as a marginal confirmation (of course, not the ultimate proof) of the second law of thermodynamics postulating that all closed (without supply of external energy) systems are striving to increase their entropy, that is to say, the measure of chaos and disorganization (Asimov, 1988) while living organisms are in stark contrast characterized by high level of order and organization and only start obeying the fundamental law of physics with their demise. Finally, a neurochemist’s view of suicide may entail low serotonin and norepinephrine concentrations throughout the brain (Linehan, 2008).
The present review responds to this question by considering suicide from the neuroanatomical standpoint since we adopted the neuroanatomical entity known as reward and anti-reward brain circuitry. Although only one of many legitimate ways that suicide might be to operationalized, this approach has several advantages. First, reward/anti-reward circuitry is clearly defined by the extensive investigations of rodent and primate models. Second, it rests on a firm clinical research foundation. Third, the relationship between reward/anti-reward and suicide may be intuitively apparent. So, in humans healthy capacities for experiencing pleasure (reward circuitry) drive motivational behaviors that are crucial for successful coping with life challenges whereas an enhanced sensitivity to negative stimuli (anti-reward circuitry) promotes isolation and withdrawal that jeopardize adequate coping strategies (Charney, 2004). In the form of reward, pleasure has evolved well beyond gratification of immediate needs to support a variety of social behaviors, including emotional attachments, community affiliation and patriotism while anti-reward is critical for avoidance potentially harmful situations, including fear, pain and losses. Pleasure touches almost every facet of life through enthusiasm, determination, affection, esthetic awareness, altruism and self-fulfillment buffering anti-reward systems. Consequently, lives of people with impaired hedonic capacity may be difficult particularly if they must cope with severe pain, but without many of the rewards and supportive social network, to the point that suicide may result (Blum et al., 2008). Hence, understanding the mechanisms underlying the balance of reward/anti-reward functions along with their impact on motivation may lead to therapeutic breakthroughs in the domain of suicide and pain. Note, that in this respect social attachments strongly stimulate reward circuitry helping buffer anti-reward processes associated with pain and depression.
3.6. Hierarchical and multidimensional addiction model’s utility for suicidality
Suicide cannot happen by chance. Here is a giant mistake when we say that a strong willpower has allowed ending a person’s life. This is undoubtedly not so. By and large, a suicide victim does not display any willpower. Simply put, there is a creation of such a physical state when self-destruction becomes a normal rather than a random act. The nature in this case is working toward the same end. The weakened and semi-paralyzed brain practically has no reaction to pain and therefore fear of death and the instinct of life preservation are substantially numbed.
Mikhail Zoshehenko. Youth Returned (1933; translation IE)
What is hell? I maintain that it is the suffering of being unable to love.
Fyodor Dostoyevsky. The Brothers Karamazov (1880)
Untreated severe pain can naturally cause suicide because unbearable suffering in combination with hopelessness obliterates the lust for living (Dees et al., 2011). As already noted, in addition to considerable overlap with the physical pain (Eisenberger, 2012a; Mee et al., 2006), its emotional counterpart or psychache (Schneidman, 1996), not only accompanying the former but also arising consequentially to troubled social attachments (Freud, 1917a; Insel, 2003), is another key component of suicidality (Reisch et al., 2010; Shneidman, 1993; Troister and Holden, 2012). The socially-mediated psychache is captured by the Interpersonal Psychological Suicide Theory of suicidality as “thwarted belongingness and perceived burdensomeness” (Van Orden et al., 2010) and may be the main reason to why people kill themselves when combined with the actual capability (not a mere desire) to execute the suicidal act as such while overcoming the pain and fear inescapably innate to such process (Selby et al., 2010). Alternatively or additionally, recent work also suggests that suicidality, pain and addiction share the “reward deficiency syndrome,” which is comprised of a broad range of personality traits and mental disorders characterized by hypofunctionality of the brain circuits mediating reward and motivation, clinically noticeable as diminution of drive/motivation and of capacity to enjoy or to experience pleasure (Blum et al., 2008; Comings and Blum, 2000). In parallel there is enhanced sensitivity of anti-reward circuits that lead to dysphoria/depression prompting a novel conceptualization of the “enhanced anti-reward syndrome”. Both a “reward deficit syndrome” along with an “enhanced anti-reward syndrome” may be driving forces behind suicidal acts.
Among psychiatric patients suicidality is most commonly diagnosed (Goldsmith, 2002; Nock et al., 2010) when reward/anti-reward abnormalities are also implicated, including major depression (Panksepp and Watt, 2011), bipolar disorder (Hasler et al., 2006), impulse control disorders (Karim and Chaudhri, 2012), posttraumatic stress disorder (Elman et al., 2009) and schizophrenia (Elman et al., 2006). Also, besides addiction being the most common diagnosis in completed suicides (Yoshimasu et al., 2008), there are clinical features of suicidality that are analogous to addiction and may thus point to reward/anti-reward system dysfunction. The most notable of these are addiction-like phenomena, which are encoded in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV TR) diagnostic criteria, viz., tolerance, loss of control and social impairments (Table 5). Without the presence of at least some of these addiction-like characteristics, it may be nearly impossible to diagnose and prevent suicidality in psychiatric patients and in mentally healthy people alike. Even though it may appear somewhat naive to reduce the entire suicidal puzzle to a single brain abnormality, modern clinical neuroscience research have documented a series of neuroadaptations in reward and anti-reward circuits impinging upon the motivational circuitry that may underlie social impairments and capability to engage in suicidal acts (Fig. 5).
Table 5.
Key clinical characteristics of addiction and suicidality.
| Substance dependence (DSM-IV TR criterion) | Suicidality (clinical characteristic) | Putative mechanism |
|---|---|---|
| Tolerance (increase in amount and decrease in effect) | While some die on the first attempt (Rudd et al., 1996), others show gradual progression from non-suicidal self-injury to more painful, damaging and lethal attempts (Franklin et al., 2011; Suominen et al., 2004; Van Orden et al., 2008) Physical pain tolerance is a consistent and specific (Orbach et al., 1996, 1997) clinical finding in suicidal patients (Bryan and Cukrowicz, 2011; Franklin et al., 2011; Smith et al., 2010). The degree of pain insensitivity tends to parallel the severity of suicidality (Berman, 2003) The number of past suicidal attempts correlates with diminished fear of death and with self-harm averseness with repeated attempts (Van Orden et al., 2008) |
Opponent/anti-reward process |
| Important social, occupational, or recreational activities given up or reduced | Social isolation reflected in thwarted belongingness and perceived burdensomeness are essential features of suicidal behavior (Kjolseth et al., 2010; Van Orden et al., 2010) | |
| Withdrawal symptoms; substance taken to relieve withdrawal | Improvement in the affective state following deliberate self-injury (Gordon et al., 2010; Van Orden et al., 2010) Suicide may be perceived as rewarding and reinforcing by people who do not realize the finality of death due to cognitive distortions (Morse, 1973) Failed lethal suicidal attempt may be an exhilarating experience (c.f., active avoidance of punishment in negative reinforcement). Such behavior may be perpetuated via backward conditioning |
Proponent process Aberrant learning |
| Use continues despite knowledge of adverse consequences (e.g., failure to fulfill role obligation, use when physically hazardous) | Lack of deterrence by clearly aversive and detrimental outcomes (Smith et al., 2010; Van Orden et al., 2010) i.e., habit-based actions | |
| Substance taken in larger amount and for longer period than intended | Loss of control (Schnyder et al., 1999) and impulsivity (Joiner et al., 2005) are important determinants of suicidal behavior | Drug or death become a sensitized motivational target |
| Much time spent to obtain, use and recover | Substantial amount of time spent is in suicide-related activities e.g., browsing know how websites, trying a noose, choosing a gun, imagining the aftermath of death, etc. (Van Orden et al., 2008, 2010) More time spent in planning an attempt leads to greater loss of fear and subsequently increased chances for a terminal outcome (Van Orden et al., 2010) |
|
| Persistent desire or repeated unsuccessful attempt to quit | Suicidal ideations persist for at least a year from the initial assessment (Zhang et al., 2011), which also appears to be the most vulnerable time for the second attempt suicide (Kessler et al., 1999). Nonetheless, suicides continue to accumulate up to 37 years after the index attempt (Suominen et al., 2004) |
Fig. 5.

Schematic overview of the neurobiological processes underlying manifestation of suicidal behavior and of the potential sites where pain and treatment with opioid analgesics predispose for suicidality. Hypodopaminergic state in the striatum and prefrontal cortex (PFC) is an illustration of the opponent/anti-reward process consequential to excessive stress and drug exposure and it is linked to suicidality. Clinical correlates of the dopaminergic striatal deficits in suicidal patients could be evident in diminished hedonic capacity, low motivation for social interactions and decrease in self-harm averseness, while the hypofunctional PFC involved in the inhibitory control over suicidal behavior corresponds to impulsive and reckless behavior combined with faulty decision making. At the same time, between systems opponent/anti-reward sensitization e.g., enhanced CRF, norepinephrine, dynorphin and glutamate activities further worsens anhedonia and other negative affective states. Another manifestation of sensitization is related to death as a motivational target and so the capability for suicide is acquired via the irresistible drive to die combined with the tolerance for the emotional and physical pain. The increase in the frequency and lethality of suicidal attempts as the condition advances is yet another expression of sensitization. Suicide-stress cross-sensitization is evident in the strong link between life adversities, physical abuse and a variety of stressful experiences with the acquired suicidal capability. Aberrant salience in suicidality limits the behavioral repertoire and creates habit-based rigid motivational states fixated on suicide-related content, in contravention to its devastating outcome and with diminution of mesolimbic neurons’ ability to detect signals for normal salience (social rewards) and loss of normal modulation of reinforcers’ values by non-suicidal contexts. Suicidal urges may be further enhanced owing to a hypofunctional PFC involved in the inhibitory control. Propensity for suicide could be worsened by pain and by excessive opioid use as both are associated with reward/motivation deficits. Pain- or opioid drug-induced effects in the mesolimbic dopaminergic circuitry are salient and motivate behavior, so persistent exposure to this type of stimuli dysregulates the system, increasing the incentive salience assigned to pain- or to drug-related cues along with habit-based learning and cross-sensitization.
Hypodopaminergic state in the striatum and PFC is an illustration of the opponent/anti-reward process consequential to excessive stress (Elman et al., 2009) and drug (Kalivas and Volkow, 2005) exposure and it is likewise linked (Jollant et al., 2010) to suicidality as demonstrated by convergent findings from postmortem neuropathology (Noga et al., 1997; Sequeira et al., 2012), in-vivo biochemical assays (Pitchot et al., 2001; Sher et al., 2006; Traskman et al., 1981) as well as structural (Dombrovski et al., 2012; Vang et al., 2010; Wagner et al., 2011) and functional (Amen et al., 2009; Desmyter et al., 2011; Reisch et al., 2010; Willeumier et al., 2011) neuroimaging. Notably, self-injurious behaviors (not necessarily bona fide suicidality) typically occur in illnesses characterized by low brain dopamine content such as Lesch-Nyhan, Prader-Willi and Cornelia de Lange Syndromes (reviewed in Devine, 2012). Clinical correlates of the dopaminergic striatal deficits in suicidal patients could be evident in diminished hedonic capacity and low motivation for social interactions (Fawcett et al., 1990; Guerra and Calhoun, 2011; Nordstrom et al., 1995). What is more, the hypofunctional PFC (Jollant et al., 2008) involved in the inhibitory control over suicidal acts corresponds to cognitive impairments (Keilp et al., 2012; Miranda et al., 2012), reckless behavior (Gao et al., 2011; Nordstrom et al., 1996) combined with faulty decision making (Dombrovski et al., 2011; Jollant et al., 2007; Takahashi, 2011), impulsivity (Goldstein and Volkow, 2002) and defects in delayed discounting (Dombrovski et al., 2012), aggravated by co-occurring pain (Bender et al., 2011; Brañas-Garza et al., 2010) and/or addiction (MacKillop et al., 2011).
At the same time, there are several instances that suicidal patients are actually sensitized rather than hyposensitive to various stimuli evidenced in the between systems anti-reward markers of enhanced CRF (Arato et al., 1989; Hiroi et al., 2001), norepinephrine (Chandley and Ordway, 2012; Pandey and Dwivedi, 2007), dynorphin (Hurd et al., 1997) and glutamate (Ernst et al., 2011; Nevin, 2012) activities further worsening anhedonia and other negative affective states (c.f., Table 3). Another manifestation of sensitization may be related to death as a motivational target. The capability for suicide is acquired via the irresistible drive to die combined with the tolerance for the emotional and physical pain associated with such an endeavor (Anestis et al., 2011a,b; Anestis and Joiner, 2011; Smith et al., 2010; Van Orden et al., 2010). Incentive sensitization, reminiscent of the irresistible urge to consume drugs by addicts in blatant disregard for the resultant pain and suffering, can be deduced from a heightened ratio of the motivational value assigned to the target and the absolute value of its hedonic qualities (Yamamoto et al., 2009). The increase in the frequency and lethality of suicidal attempts as the condition advances is yet another expression of sensitization, noted in a number of reports (Franklin et al., 2011; Joiner, 2002; Suominen et al., 2004; Van Orden et al., 2008). Although a possibility of suicide-stress cross-sensitization (Pettit et al., 2004) have received relatively less attention, it may be relevant that not only such life adversities as childhood maltreatment and sexual and physical abuse (Perez-Fuentes et al., 2012; Rhodes et al., 2012) but also a variety of stressful experiences e.g., combat exposure (Selby et al., 2010), engagement in physical violence, sexual promiscuity and shoplifting (Van Orden et al., 2008) impart a greater acquired suicidal capability.
Severe stress causes excessive release of dopamine in the mesolimbic pathways (Elman et al., 2010a,b) leading to consequential salience attribution, that is, aberrant conditioned learning (Mahan and Ressler, 2012). Similarly to pain and addiction that are two other conditions associated with erratic supraphysiological dopamine surges in the limbic system (Elman et al., 2011), aberrant salience in suicidality limits the behavioral repertoire and creates habit- rather than value-based rigid motivational states (Miranda et al., 2012) fixated on suicide-related content, in contravention to its devastating outcome and with diminution of mesolimbic neurons’ ability to detect signals for normal salience (social rewards) and loss of normal modulation of reinforcers’ values by non-suicidal contexts (Rangel et al., 2008). Suicidal urges may be further enhanced owing to the hypofunctional PFC (including the anterior cingulate) (Matthews et al., 2012) involved in the inhibitory control over obviously inappropriate acts (including processing their inappropriateness) involved in taking one’s own life (Dombrovski et al., 2012).
Given the reward/anti-reward and motivational abnormalities arising in suicidal people without identifiable co-occurring brain disorders, a compelling a fortiori argument could be that propensity for suicide is worsened by neuropathology afflicting the same factors involved in reward/anti-reward and motivation regulation. Pain could be considered such a condition and, as addiction, its association with reward/anti-reward deficits is supported by the typical symptomatology of anhedonia (Marbach et al., 1983), reduced motivation (Karoly and Lecci, 1997; Karoly and Ruehlman, 1996), social isolation (Nguyen et al., 2012) and apathy (Watson, 1982).
3.7. Suicidality and pain may be explained by shared neural systems
There are people who have an appetite for grief; pleasure is not strong enough and they crave pain.
Ralph Waldo Emerson. The Tragic (1844)
Algophilia: a morbid pleasure in the pain either of oneself or of others.
Algolagnia: a perversion in which pleasure and especially sexual gratification is obtained by inflicting or suffering pain.
Dictionary and Thesaurus. Merriam-Webster Online (www.merriam-webster.com)
Presently, no clinical studies directly link suicidality and pain neuropathology. A cumulative body of evidence suggests, however, that: a) pain neuropathology is characterized by reward/anti-reward and motivational changes that are similar to long-term dependence on addictive substances (see Tables 3 and 6 for comparison of these changes) but without the necessity of prior drug exposure (Apkarian, 2010; Egli et al., 2012; Elman et al., 2011) and b) there is a considerable overlap between brain circuitry that evolved for the maintenance of emotional attachments and is impaired by addictive drugs and brain circuitry that is affected by chronic pain conditions. The adaptation of these two ideas lends strength to the hypothesis that pain and suicidal tendencies may be potentially explained by recursive partly shared neural systems.
Table 6.
Modulation by opponent vs. proponent states of the effects of pain-, cues- and stress-related stimuli in patients with pain.
| Stimulus | Proponent
|
Opponent/anti-reward
|
||||
|---|---|---|---|---|---|---|
| Sensitization | Cross-sensitization | Learning | Sensitization | Cross-sensitization | Learning | |
| Pain | Acute pain primes opioid craving (Ren et al., 2009) | Pain-induced overeating (Foo and Mason, 2009) | Catastrophizing or exaggerated prediction error (Sullivan et al., 2001) i.e., discrepancy between actual and anticipated pain (Elsenbruch et al., 2012) | Increased pain or opioid-induced hyperalgesia (Tompkins and Campbell, 2011) | Opioids’ incentive sensitization in chronic pain patients (Wasan et al., 2012) | “Learned pain” behavior (Stein et al., 2000; Tyrer, 1986; Van Damme et al., 2006) |
| Increase in pain causes increase in smoking and vice versa (Ditre et al., 2010, 2011) | Nocebo effect (Mitsikostas, 2012) | Pseudo-addiction: compulsive seeking of opioids driven by the desire to ameliorate inadequately treated pain (Weissman and Haddox, 1989) | Flashbacks of pain episodes experienced during surgery (Salomons et al., 2004) | |||
| Cue | Enhanced brain and behavioral responses to pain-related cues (Eck et al., 2011; Schoth et al., 2012) | Pain response predicts cue-induced opioid craving (Ren et al., 2009) | Conditioning of nocebo effect by cues (Lorenz et al., 2005) | Incentive sensitization of pain-related cues (Eck et al., 2011) | Incentive sensitization of opioid-related cues (Garland et al., 2012; Wilsey et al., 2009) | Therapeutic dependence: seeking of opioid drugs to avoid a conditioned opioid withdrawal (Portenoy and Foley, 1986) |
| Stress | Stress and/or emotional trauma predispose for pain conditions (Davis et al., 2005; Kivimaki et al., 2004; Lumley et al., 2011) potentially mediated via glutamtergic neurotransmission (Coderre et al., 1993; Mao et al., 1995, 2002) | Acute pain, paired with emotional a conditioned stimulus evoking augment subjective pain perception et al., 2011) leading to the “mutual Harvey, 2001) reflected in avoidance pain- and trauma-related situations of both conditions (Liedl and | trauma and its recollections, becomes fear and anxiety responses that in turn and its neural correlates (Elman maintenance” cycle (Sharp and of both and consequent worsening Knaevelsrud, 2008) | Sustained pain leads to increased stress and negative affective states (Elman et al., 2011) | High pain prevalence in PTSD (Ciccone and Kline, 2012) and high PTSD prevalence in chronic pain patients (Gerber et al., 2012; Otis et al., 2003; Sherman et al., 2000) | Pseudo-opioid resistance: self-reported pain with adequate analgesia owing to stress resulting from a feared opioid dose reduction (Evers, 1997) |
The first of the above-mentioned ideas is supported by neuroimaging research showing that akin to euphorogenic drugs, acute pain (Karoly and Ruehlman, 1996; Leite-Almeida et al., 2009; Scott et al., 2006) activate dopamine transmission in the brain reward circuitry including the NAc, whereas prolonged periods of pain (Rodriguez-Raecke et al., 2009; Schmidt-Wilcke et al., 2007; Wartolowska et al., 2012), like drug consumption (Fields, 2007a; Portenoy and Foley, 1986; Weissman and Haddox, 1989), change the dopaminergic reward structures (Borsook et al., 2007) and create a dysfunctional neuroadaptational state characterized by reward and motivational disturbances as well as by heightened incentive salience attribution to pain and to pain-related stimuli in the face of impaired responsivity to natural reinforcers and enhanced sensitivity to stress and negative emotional states (Ballard, 1974; Narita et al., 2004; Wang et al., 2011). In addition, extensive preclinical work (Descalzi et al., 2009; Reissner and Kalivas, 2010) provides supportive evidence for distinct roles for tonic vs. phasic dopaminergic firing (Bilder et al., 2004) and glutamatergic signaling in clinical symptomatology, motivational states, and in the outcomes of pain and addictive disorders (Jarcho et al., 2012; Reissner and Kalivas, 2010).
Decrease in cerebral metabolism and blood flow have been observed in both chronic pain (Becker et al., 2012; Glass et al., 2011; Tagliazucchi et al., 2010; Tracey, 2007) and in addictive disorders (Goldstein and Volkow, 2002; Volkow and Fowler, 2000) at baseline, when challenged by cognitive tasks and when exposed to natural reinforcers. This hypofrontality phenomenon has been related to pain- (Jarcho et al., 2012) or drug-related (Kalivas and Volkow, 2005) diminished dopaminergic tone with corresponding decrease in the tonic glutamatergic activity (West et al., 2003). Against the background of this diminished activity, acute pain (Kupers et al., 2011; Mathew, 2011; Ringel et al., 2008) and/or stress (Elsenbruch et al., 2010; Stoeter et al., 2007) in chronic pain patients or exposure to drugs (Volkow and Li, 2005) or drug-related cues (Childress et al., 1999; Garavan et al., 2000) in drug addicts produce strong activations in the PFC. Involvement of stress in this phenomenon is clinically manifested by a contribution to the “spiraling distress cycle” (Koob and Le Moal, 1997) whereby stress and negative affective states producing via cross-sensitization (Rome and Rome, 2000; Woolf and Thompson, 1991) additional deterioration of pain problems (Alba-Delgado et al., 2012).
In consequence, pain disrupts the normal function of the structures involved in stress and reward processing thereby reducing the ability to restrain suicidal urges and the impact of painful stimuli. More studies are needed to uncover potential competitive, independent (i.e., additive) or synergistic nature of the pain and suicidality interactions. Synergism may be the most notable type because sensitized stress responses in pain may confer greater motivational salience to suicide-related cues, whereas suicidal attempts cause more stress and additional worsening of pain symptoms. On the other hand, competition may also be possible as at times intentional self-inflicted harm may provide a sense of relief and reduction in tension (Gordon et al., 2010). Such likely interactions may be tested in neuroimaging research by administration of corresponding stimuli to the same four groups of subjects, viz., healthy, pain, suicide, and comorbid pain with suicide, in a 2×2 factorial design, with the presence or absence of pain and suicidality as two independent factors. It is a suggestion of this article that a better understanding of the brain correlates of pain-related reward deficits, in addition to providing insights into the pathophysiology of both pain and suicidality, will offer new leads for the development of heretofore purely empirical therapeutic interventions aimed at enhancing reward and decreasing anti-reward reactivity and at increasing activation of motivational system involved with healthy coping styles as strategies for the prevention of suicidality in chronic pain patients.
4. Therapeutic considerations
But he who is joined with all the living has hope, for a living dog is better than a dead lion. For the living know that they will die, but the dead know nothing, and they have no more reward, for the memory of them is forgotten. Their love and their hate and their envy have already perished, and forever they have no more share in all that is done under the sun.
Ecclesiastes. The English Standard Version Bible (1971)
Suicide- and pain neuropsychopathologies are indisputably enormous entities with biological, psychological and societal aspects modulating physical and emotional pain and suffering. Still, some key specific symptoms are presumably derived from alterations in the brain circuits that may not only be characterized by the hierarchical multidimensional model outlined here but also treated in accordance with individualized formulations, based on thorough clinical assessments combined with the application of methods from functional neuroimaging, psychopharmacology and cognitive psychology. This is because opponent, anti-reward, proponent, sensitization, cross-sensitization and aberrant learning mechanisms correspond to neuroanatomically-defined regions that may be targeted by therapeutic agents remedying the relevant malfunction and both alleviating symptoms and improving general processing of information by the brain. An expected variability in the magnitude of each dimension impairment (or lack of thereof) could guide the dosing and type of appropriate therapeutic agent(s).
Relatively little data are available regarding the effects of pharmacotherapeutic interventions on suicidal behavior (Linehan, 2008; Perlis, 2011). Yet, practicing psychiatry without adequate comprehension of suicide neurobiology and treatment may be paraphrased to John Searle’s metaphor of “studying the stomach without studying digestion, or studying genetics without studying the inheritance of traits” (Searle, 2000). Suicidality research in humans is limited in part by a lack of animal model and laboratory-based formulation introducing testable hypotheses and informing rigorously designed trials that can be controlled with respect to the type and “amount” of the baseline neurobiological impairment underlying the targeted problem. Be that as it may, suicidality remains the most critical problem within the psychiatric discipline as well as a grave concern for the entire field of medicine and for society at large.
The hallmark of the within system opponent/anti-reward neuroadaptations are anhedonia, numbing, apathy and decreased motivation for normally pleasurable objects and goals, linked to diminished mesolimbic dopaminergic neurotransmission (Gardner, 2011; Solomon and Corbit, 1974), which may be targeted by psychopharmacological intervention (Gorelick et al., 2004; Schroeder et al., 2013). Such approach may also secondarily address additional symptoms caused by sensitization and learning mechanisms (Tables 3 and 6).
Inasmuch the within system opponent neuroadaptation mainly diminishes positive moods and motivations, the between systems anti-reward process adds to that type of effects via dynorphin on top of increasing negative affective states through the CRF and noradrenergic action (Koob and Le Moal, 1997, 2001, 2008). These could be addressed by the respective (but not yet approved) k and CRF antagonists (Bruijnzeel et al., 2010; Bruijnzeel et al., 2012; Chartoff et al., 2012), which may also stabilize aberrant learning mechanisms since elevations in stress hormones and neurotransmitters (e.g., cortisol) repeatedly paired with stressful situations and consequent suicidality may become a conditioned stimulus and independently elicit suicidal psychological state of mind c.f., cortisol-primed drug craving (Elman et al., 2003).
Proponent mechanisms remain the most extensively investigated entity in both pain and addiction. However, seemingly obvious analgesic therapy of the proponent pain process could further deteriorate opponent/anti-reward processes and worsen pre-existing suicidality. As a result, changes in the mesolimbic dopaminergic circuitry induced by opioids, administered at the doses exceeding the homeostatic need for pain alleviation, may be responsible for the amplification of “hyperkatifeia” or negative affective states and of physical pain itself (Shurman et al., 2010). Hence is the significance of cognitive and behavioral strategies (e.g., cognitive restructuring, stress management and systemic desensitization) alongside non-addictive alternatives to opioids with substantial analgesic properties such as antidepressants, neuroleptics and anticonvulsant agents, which have been related to clinical improvement in suicidal patient (Ahearn et al., 2012; Kirino and Gitoh, 2011; Yerevanian et al., 2007).
Since anti-reward type sensitization across numerous conditions is determined by similar neurobiological adaptation notwithstanding the primary source, as it was demonstrated for pain (Rome and Rome, 2000), addiction (Robinson and Berridge, 2003) and mood disorders (Post, 1990), suicidality-related sensitization may also map onto the same circuits identified for those syndromes (Pettit et al., 2006; Wingate et al., 2004). A way to test this idea is to treat suicidal patients with pharmacological agents that mitigate sensitization. For example, mood stabilizing anticonvulsants (carbamazepine and divalproex sodium) exhibit superior efficacy for suicidality in bipolar disorder, which is the prototype psychiatric illness where sensitization is implicated (Yerevanian et al., 2007) with about a quarter of patients prone to commit suicide (Merikangas et al., 2011). Moreover, the gold standard non-anticonvulsant mood stabilizer, lithium, also mitigates sensitization (Ago et al., 2012) and possesses unique anti-suicidal properties not only in bipolar patients (Ahearn et al., 2012; Yerevanian et al., 2007; but see Oquendo et al., 2011) but also in mood disorders overall (Cipriani et al., 2005) and even in the general public (Ohgami et al., 2009). Remarkably, lithium has beneficial features as an analgesic drug (Banafshe et al., 2012; Tfelt-Hansen and Jensen, 2012). This is also the case for another exceptional anti-suicidal drug, clozapine, that likewise reduces pain (Schreiber et al., 1999) and sensitization (Park et al., 2010) phenomena. Unfortunately such advantageous therapeutic properties of clozapine are tarnished by unusually severe side effects profile. Because chronic pain-induced activation of mesolimbic dopaminergic pathways leads to sensitization of the excitatory (e.g., glutamatergic) neurotransmission (Coderre et al., 1993; Mao et al., 2002) involved in self-injurious behavior (Muehlmann and Devine, 2008) glutamate inhibition is an additional therapeutic strategy. Antiglutametergic agents reduce self-injurious behavior in laboratory animals (Devine, 2012). Less direct reduction of glutamatergic activity in humans by lamotrigine (Davanzo and King, 1996), riluzole (Sasso et al., 2006) or topiramate (Smathers et al., 2003) also improves self-injurious behavior (but see (Pugh et al., 2012) and provides effective pain relief (Hama and Sagen, 2011; Johannessen Landmark, 2008; Webb and Kamali, 1998).
With regard to cross-sensitization, stress is a strong candidate for cross-reactivity with pain (Gibson, 2012) and suicidality (Currier and Mann, 2008; Turecki et al., 2012; van Heeringen, 2012). As discussed above, the neuroanatomic substrate for such an effect may involve two closely linked and interacting networks, namely dopaminergic reward pathways and extrahypothalamic norepinephrine/CRF system. Change in the mesolimbic dopaminergic circuitry subsequently to suicidal behaviors may be responsible for transforming regular motivational processes into heightened incentive salience assigned to death and to ecologically valid cues resulting in irresistible urges, analogous to drug craving. Involvement of pain and stress is clinically noticeable by their role in suicidality that is in all likelihood mediated by: (a) stimulation of dopaminergic neurotransmission within the reward and motivational circuitry and (b) by sensitized norepinephrine and consequent extrahypothalamic CRF hypersecretion within the extended amygdala structures underlying anxiety (Koob, 2009) and through the habenula that inhibits DA cell activity (Chandley and Ordway, 2012). Thus, negative affective states secondary to suicidality and/or environmental stress produce additional deterioration of emotional and behavioral problems, leading via negative reinforcement processes to suicidal attempts that may further impair neurobiological systems and emotional states. Because mesolimbic dopaminergic and extrahypothalamic norepinephrine/CRF systems are intimately linked, dopaminergic antagonists block norepinephrine/CRF anxiogenic responses and vice versa (Saigusa et al., 2012; Sommermeyer et al., 1995). Serious adverse effects of antidopaminergic agents may render them less than an optimal choice for the maintenance of chronically suicidal patients. On the other hand, given the role of noradrenergic mechanisms and decrease of the α2 gene expression in suicide victims (Fukutake et al., 2008; Sequeira et al., 2004), blockade of adrenergic neurotransmission via α2 adrenoceptors’ agonists or α1 adrenoceptors’ antagonists (e.g., clonidine, guanfacine or prazosin) could evolve as more tolerable therapeutic alternative for these patients (Fig. 6). In this respect it is interesting that such agents were tried with some success in addiction (Kleber, 2003), pain (Blaudszun et al., 2012) and impaired impulse control conditions (Arnsten and Pliszka, 2011) that are quite ubiquitous in suicide attempters (Keilp et al., 2012).
Fig. 6.

Blockade of adrenergic neurotransmission as a potential treatment option for patients with pain and suicidality. Blockade of adrenergic neurotransmission via α2 adrenoceptors’ agonists or α1 adrenoceptors’ antagonists (e.g., clonidine, guanfacine or prazosin) could be a well-tolerated therapeutic option for patients with pain and suicidality as they improve negative affective states and stabilize the sensitized motivational system.
It is a matter of general agreement that associative learning mechanisms are inherent in incentive sensitization (Hyman, 2005), but the aberrant learning theory emphasizes the specific nature of the stimulus–cues interaction, which is not conceptualized as such by the proponents of the former theory (Berridge, 2007; Robinson and Berridge, 2003). Apart from such theoretical differences adrenergic agents may be helpful as well as they block reconsolidation processes instrumental for the stimulus–cues memories formations (Gamache et al., 2012) and have been tried with some degree of success in PTSD patients (Jeffreys et al., 2012; Poundja et al., 2012). Due to their essential features e.g., persistent relieving of stress (Blackburn-Munro and Blackburn-Munro, 2001), responding with hopelessness (Beck et al., 1975, 1985; Joiner et al., 2001), catastrophizing (Quartana et al., 2009) or horror (Haugh, 2005) and avoidance of pain-related situations (Asmundson and Katz, 2009; Liedl and Knaevelsrud, 2008), chronic pain conditions may be viewed as a form of PTSD (Gibson, 2012; Liedl et al., 2010) suggesting additional rationale for the use of adrenergic antagonists. Evidently, various forms of psychotherapy is another critical modality for reversing aberrant learning and for emotional reconditioning (Harned et al., 2012; Jeffries and Davis, 2013).
5. Summary and conclusions
Any knowledge that doesn’t lead to new questions quickly dies out: it fails to maintain the temperature required for sustaining life.
Wislawa Szymborska. Nobel Lecture (1996)
Suicide is a question occupying human mind since the dawn of history and its ethical, philosophical, logical or even clinical legitimacy is certainly far from been addressed by the present paper. A compilation of balanced views on this conundrum is presented in the middle column of Table 4.
Pain patients are vulnerable for the development of suicidality due to neuropsychopathological alterations inherent in their illness. These alterations may be worsened by excessive or inadequate opioid analgesics as demonstrated in previous clinical studies and larger epidemiologic surveys (Elman, 2011; National Center for Health Statistics, 2007). Currently there is no available consensus in the field on optimal therapeutic strategies for the associated suicidality perhaps because little is known about the specific mechanisms that lead to suicidality after initiation of a pain condition, or whether a target pharmacologic approach might be able to specifically identify the underlying cause. In this paper we have pointed to a potential system involved in pain, which may be targeted in the clinical and in research on pain patients with co-occurring suicidality.
The coalescence of preclinical and clinical research suggests marked alterations of reward/anti-reward and motivational functions. To further advance our understanding of this effect, we have synthesized seemingly disparate themes of emotional pain, social attachments and addiction with sensory and pain neuroscience into a clinically relevant theory and suggested a series of testable hypotheses. In view of the high prevalence of suicidality among pain patients, we anticipate that identification of effective treatments that specifically target the underlying pathophysiologic defects will provide substantial clinical benefit to this at risk population.
If confirmed in clinical studies, our insights could have important implications for the primary and secondary prevention of suicidality in pain patients. If neurobiologic vulnerability factors for suicidality could be identified, they might be used to screen patients at risk for the development of such condition. Patients found to possess high vulnerability for the development of suicidality owing to pain-related alterations in sensory or reward function might be counseled to avoid excessive opioid consumption (primary prevention), or targeted for early intervention even in the presence of mild suicidal ideation (secondary prevention).
The proposed model of suicidality in pain conditions could also have treatment implications, as it implies use of several classes of pharmacological agents including anticonvulsants, lithium and adrenergic blockers. Clinical experience suggests that due to the rareness of psychiatrists engaged in the care of patients with chronic pain (Elman et al., 2011) psychological interventions and non-opioid analgesics utilization in pain patients is lower than what could be expected from positive outcomes of clinical trials. We therefore believe that this review will equip practitioners with the essential knowledge base for understanding the rationale for non-opioid therapy and will sharpen their sensitivity to the unmet mental health needs of the pain patients. Lastly, this model may also provide important leads for recognition and treatment of suicidality in general public as well as in patients with other disorders where reward/anti-reward and motivational functions are strongly implicated such as depression, schizophrenia and PTSD.
Acknowledgments
The authors gratefully acknowledge Ms. Adriana Johnson for her help with the preparation of the manuscript and support from the Mayday Fund/Herlands Fund for Pain Systems Neuroscience Research (DB).
This study was supported with resources and the use of facilities at the Providence VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Abbreviations
- ACC
anterior cingulate gyrus
- AMY
amygdala
- BLA
basolateral complex of the amygdala
- BNST
bed nucleus the striaterminalis
- CEA
central nucleus of the amygdala
- CRF
corticotropin-releasing factor
- DA
dopamine
- DBS
deep brain stimulation
- DMN
default-mode network
- DSM-IV TR
Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision
- DRG
dorsal root ganglion
- GABA
gamma-aminobutyricacid
- HB
habenula
- HT
hypothalamus
- INS
insula
- LC
locus coeruleus
- NAc
nucleus accumbens
- NE
norepinephrine
- NR
raphe nuclei
- PAG
periaqueductal gray matter
- PFC
prefrontal cortex
- PTSD
posttraumatic stress disorder
- RF
reticular formation nuclei
- S1
primary somatosensory cortex
- S2
secondary somatosensory cortex
- VT
ventral tegmentum
References
- Ago Y, Tanaka T, Kita Y, Tokumoto H, Takuma K, Matsuda T. Lithium attenuates methamphetamine-induced hyperlocomotion and behavioral sensitization via modulation of prefrontal monoamine release. Neuropharmacology. 2012;62:1634–1639. doi: 10.1016/j.neuropharm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Ahearn EP, Chen P, Hertzberg M, Cornette M, Suvalsky L, Cooley-Olson D, Swanlund J, Eickhoff J, Becker T, Krahn D. Suicide attempts in veterans with bipolar disorder during treatment with lithium, divalproex, and atypical antipsychotics. J Affect Disord. 2012;145:77–82. doi: 10.1016/j.jad.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba-Delgado C, Llorca-Torralba M, Horrillo I, Ortega JE, Mico JA, Sanchez-Blazquez P, Meana JJ, Berrocoso E. Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biol Psychiatry. 2012;73:54–62. doi: 10.1016/j.biopsych.2012.06.033. [DOI] [PubMed] [Google Scholar]
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- Amen DG, Prunella JR, Fallon JH, Amen B, Hanks C. A comparative analysis of completed suicide using high resolution brain SPECT imaging. J Neuropsychiatry Clin Neurosci. 2009;21:430–439. doi: 10.1176/jnp.2009.21.4.430. [DOI] [PubMed] [Google Scholar]
- Anestis MD, Bagge CL, Tull MT, Joiner TE. Clarifying the role of emotion dysregulation in the interpersonal-psychological theory of suicidal behavior in an undergraduate sample. J Psychiatr Res. 2011a;45:603–611. doi: 10.1016/j.jpsychires.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Anestis MD, Bender TW, Selby EA, Ribeiro JD, Joiner TE. Sex and emotion in the acquired capability for suicide. Arch Suicide Res. 2011b;15:172–182. doi: 10.1080/13811118.2011.566058. [DOI] [PubMed] [Google Scholar]
- Anestis MD, Joiner TE. Examining the role of emotion in suicidality: negative urgency as an amplifier of the relationship between components of the interpersonal-psychological theory of suicidal behavior and lifetime number of suicide attempts. J Affect Disord. 2011;129:261–269. doi: 10.1016/j.jad.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Apkarian AV. Human brain imaging studies of chronic pain: translational opportunities. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. CRC Press; Boca Raton, FL: 2010. [PubMed] [Google Scholar]
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–S64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry. 1989;25:355–359. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- Aristotle . On the Art of Poetry. Oxford University Press; Oxford: 1920. [Google Scholar]
- Aristotle, Roberts WR. Rhetoric. Dover Publications; Mineola, NY: 2004. [Google Scholar]
- Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99:211–216. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimov I. Understanding Physics. Dorset Press; New York: 1988. [Google Scholar]
- Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26:888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard BE. Pharmacokinetics and temperature. J Pharm Sci. 1974;63:1345–1358. doi: 10.1002/jps.2600630903. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banafshe HR, Mesdaghinia A, Arani MN, Ramezani MH, Heydari A, Hamidi GA. Lithium attenuates pain-related behavior in a rat model of neuropathic pain: possible involvement of opioid system. Pharmacol Biochem Behav. 2012;100:425–430. doi: 10.1016/j.pbb.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Barrett JE, Spealman RD. Behavior simultaneously maintained by both presentation and termination of noxious stimuli. J Exp Anal Behav. 1978;29:375–383. doi: 10.1901/jeab.1978.29-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci. 2012;32:14094–14101. doi: 10.1523/JNEUROSCI.3370-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI. Distinct neurochemical features of acute and persistent pain. Proc Natl Acad Sci U S A. 1999;96:7739–7743. doi: 10.1073/pnas.96.14.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Keysers C. Evidence for mirror systems in emotions. Philos Trans R Soc Lond B Biol Sci. 2009;364:2391–2404. doi: 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain. 2008;12:866–869. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A. Hopelessness and suicidal behaviour. An overview JAMA. 1975;234:1146–1149. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Kovacs M, Garrison B. Hopelessness and eventual suicide: a 10-year prospective study of patients hospitalized with suicidal ideation. Am J Psychiatry. 1985;142:559–563. doi: 10.1176/ajp.142.5.559. [DOI] [PubMed] [Google Scholar]
- Becker S, Gandhi W, Schweinhardt P. Cerebral interactions of pain and reward and their relevance for chronic pain. Neurosci Lett. 2012;520:182–187. doi: 10.1016/j.neulet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender TW, Gordon KH, Bresin K, Joiner TE., Jr Impulsivity and suicidality: the mediating role of painful and provocative experiences. J Affect Disord. 2011;129:301–307. doi: 10.1016/j.jad.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Bentham J. Principles of Morals and Legislation: Jeremy Bentham Oxford at the. Clarendon Press; London: 1823. [Google Scholar]
- Berman MEWJC. Imitation of self-aggressive behavior: an experimental test of the contagion hypothesis. J Appl Social Psychol. 2003;33:1036–1057. [Google Scholar]
- Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol. 2013;23:294–303. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bimpisidis Z, De Luca MA, Pisanu A, Di Chiara G. Lesion of medial prefrontal dopamine terminals abolishes habituation of accumbens shell dopamine responsiveness to taste stimuli. Eur J Neurosci. 2013;37:613–622. doi: 10.1111/ejn.12068. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence? J Neuroendocrinol. 2001;13:1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, Blum SH, Cassel K, Downs BW, Waite RL, Williams L, Prihoda TJ, Kerner MM, Palomo T, Comings DE, Tung H, Rhoades P, Oscar-Berman M. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model. 2008;5:24. doi: 10.1186/1742-4682-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Gardner E, Oscar-Berman M, Gold M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Curr Pharm Des. 2012;18:113–118. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert AS, McCarthy JF, Ignacio RV, Ilgen MA, Eisenberg A, Blow FC. Misclassification of suicide deaths: examining the psychiatric history of overdose decedents. Inj Prev (Epub ahead of print) 2013 doi: 10.1136/injuryprev-2012-040631. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA, Jr, Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain. 2007;11:7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: the value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Boutelle MG, Zetterstrom T, Pei Q, Svensson L, Fillenz M. In vivo neurochemical effects of tail pinch. J Neurosci Methods. 1990;34:151–157. doi: 10.1016/0165-0270(90)90053-i. [DOI] [PubMed] [Google Scholar]
- Brañas-Garza P, Espinosa MP, Pro MR. Discounting Future Pain: Effects on Self-reported Pain. University of the Basque Country; Bilbao: 2010. [Google Scholar]
- Brown RL, Patterson JJ, Rounds LA, Papasouliotis O. Substance abuse among patients with chronic back pain. J Fam Pract. 1996;43:152–160. [PubMed] [Google Scholar]
- Brown RM, Short JL, Lawrence AJ. Identification of brain nuclei implicated in cocaine-primed reinstatement of conditioned place preference: a behaviour dissociable from sensitization. PLoS One. 2010;5:e15889. doi: 10.1371/journal.pone.0015889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Bishnoi M, van Tuijl IA, Keijzers KF, Yavarovich KR, Pasek TM, Ford J, Alexander JC, Yamada H. Effects of prazosin, clonidine, and propranolol on the elevations in brain reward thresholds and somatic signs associated with nicotine withdrawal in rats. Psychopharmacology (Berl) 2010;212:485–499. doi: 10.1007/s00213-010-1970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Ford J, Rogers JA, Scheick S, Ji Y, Bishnoi M, Alexander JC. Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. Pharmacol Biochem Behav. 2012;101:62–68. doi: 10.1016/j.pbb.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan CJ, Cukrowicz KC. Associations between types of combat violence and the acquired capability for suicide. Suicide Life Threat Behav. 2011;41:126–136. doi: 10.1111/j.1943-278X.2011.00023.x. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224:1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandre EP, Vilchez JS, Molina-Barea R, Tovar MI, Garcia-Leiva JM, Hidalgo J, Rodriguez-Lopez CM, Rico-Villademoros F. Suicide attempts and risk of suicide in patients with fibromyalgia: a survey in Spanish patients. Rheumatology (Oxford) 2011;50:1889–1893. doi: 10.1093/rheumatology/ker203. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]
- Cantor C, Price J. Traumatic entrapment, appeasement and complex post-traumatic stress disorder: evolutionary perspectives of hostage reactions, domestic abuse and the Stockholm syndrome. Aust N Z J Psychiatry. 2007;41:377–384. doi: 10.1080/00048670701261178. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JR, Hoge CW, Gardner J, Potter R. Suicide surveillance in the U.S. Military – reporting and classification biases in rate calculations. Suicide Life Threat Behav. 2004;34:233–241. doi: 10.1521/suli.34.3.233.42785. [DOI] [PubMed] [Google Scholar]
- Cazala P, Bendani T, Zielinski A. Self-stimulation of an ‘aversive’ brain structure: the mesencephalic central gray area. Brain Res. 1985;327:53–60. doi: 10.1016/0006-8993(85)91498-2. [DOI] [PubMed] [Google Scholar]
- Chandley MJ, Ordway GA. Noradrenergic dysfunction in depression and suicide. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. CRC Press; Boca Raton, FL: 2012. [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(Suppl 2):S43–S48. doi: 10.1111/j.1526-4637.2011.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone DS, Kline A. A longitudinal study of pain and pain catastrophizing in a cohort of National Guard troops at risk for PTSD. Pain. 2012;153:2055–2060. doi: 10.1016/j.pain.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162:1805–1819. doi: 10.1176/appi.ajp.162.10.1805. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Burstein R, Giesler GJ., Jr Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci. 1991;11:852–868. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Coluzzi F, Rocco A, Mandatori I, Mattia C. Non-analgesic effects of opioids: opioid-induced nausea and vomiting: mechanisms and strategies for their limitation. Curr Pharm Des. 2012;18:6043–6052. doi: 10.2174/138161212803582540. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Connelly M, Keefe FJ, Affleck G, Lumley MA, Anderson T, Waters S. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain. 2007;131:162–170. doi: 10.1016/j.pain.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortelli P, Pierangeli G. Hypothalamus and headaches. Neurol Sci. 2007;28(Suppl 2):S198–S202. doi: 10.1007/s10072-007-0776-2. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyer SM. Women in recovery discuss parenting while addicted to cocaine. MCN Am J Matern Child Nurs. 2003;28:45–49. doi: 10.1097/00005721-200301000-00013. [DOI] [PubMed] [Google Scholar]
- Craig AD. Distribution of trigeminothalamic and spinothalamic lamina I terminations in the cat. Somatosens Mot Res. 2003;20:209–222. doi: 10.1080/08990220310001623013. [DOI] [PubMed] [Google Scholar]
- Creutzig U, Niederbiermann G, Ritter J, Harbott J, Loffler H, Schellong G. Prognostic significance of eosinophilia in acute myelomonocytic leukemia in relation to induction treatment. Haematol Blood Transfus. 1990;33:226–232. doi: 10.1007/978-3-642-74643-7_41. [DOI] [PubMed] [Google Scholar]
- Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18:299–314. doi: 10.1016/0304-3959(84)90824-8. [DOI] [PubMed] [Google Scholar]
- Currier D, Mann JJ. Stress, genes and the biology of suicidal behavior. Psychiatr Clin N Am. 2008;31:247–269. doi: 10.1016/j.psc.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and monogamy. Brain Res. 2006;1126:76–90. doi: 10.1016/j.brainres.2006.07.126. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J. Suicide among heroin users: rates, risk factors and methods. Addiction. 2002;97:1383–1394. doi: 10.1046/j.1360-0443.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- Davanzo PA, King BH. Open trial lamotrigine in the treatment of self-injurious behavior in an adolescent with profound mental retardation. J Child Adolesc Psychopharmacol. 1996;6:273–279. doi: 10.1089/cap.1996.6.273. [DOI] [PubMed] [Google Scholar]
- Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature Clin J Pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- De Peuter S, Van Diest I, Vansteenwegen D, Van den Bergh O, Vlaeyen JW. Understanding fear of pain in chronic pain: interoceptive fear conditioning as a novel approach. Eur J Pain. 2011;15:889–894. doi: 10.1016/j.ejpain.2011.03.002. [DOI] [PubMed] [Google Scholar]
- de Spinoza B. Ethics Penguin Classics. New York: 2005. [Google Scholar]
- Dees MK, Vernooij-Dassen MJ, Dekkers WJ, Vissers KC, van Weel C. ’Unbearable suffering’: a qualitative study on the perspectives of patients who request assistance in dying. J Med Ethics. 2011;37:727–734. doi: 10.1136/jme.2011.045492. [DOI] [PubMed] [Google Scholar]
- Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: research findings and theoretical considerations. Psychosom Med. 2002;64:773–786. doi: 10.1097/01.psy.0000024232.11538.54. [DOI] [PubMed] [Google Scholar]
- Descalzi G, Kim S, Zhuo M. Presynaptic and postsynaptic cortical mechanisms of chronic pain. Mol Neurobiol. 2009;40:253–259. doi: 10.1007/s12035-009-8085-9. [DOI] [PubMed] [Google Scholar]
- Desmyter S, van Heeringen C, Audenaert K. Structural and functional neuroimaging studies of the suicidal brain. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:796–808. doi: 10.1016/j.pnpbp.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Devine DP. Animal models of self-injurious behaviour: an overview. Methods Mol Biol. 2012;829:65–84. doi: 10.1007/978-1-61779-458-2_4. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull. 2011;137:1065–1093. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Butts EA, Brandon TH. Effects of expectancies and coping on pain-induced motivation to smoke. J Abnorm Psychol. 2010;119:524–533. doi: 10.1037/a0019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychol Med. 2012;42:1203–1215. doi: 10.1017/S0033291711002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Szanto K, Siegle GJ, Wallace ML, Forman SD, Sahakian B, Reynolds CF, 3rd, Clark L. Lethal forethought: delayed reward discounting differentiates high- and low-lethality suicide attempts in old age. Biol Psychiatry. 2011;70:138–144. doi: 10.1016/j.biopsych.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy RC, Verne GN. Drug treatment options for irritable bowel syndrome: managing for success. Drugs Aging. 2001;18:201–211. doi: 10.2165/00002512-200118030-00005. [DOI] [PubMed] [Google Scholar]
- Durkheim E. Suicide: A Study in Sociology. Routledge; London: 1952. [Google Scholar]
- Eck J, Richter M, Straube T, Miltner WH, Weiss T. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain. 2011;152:1104–1113. doi: 10.1016/j.pain.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Edge PJ, Gold MS. Drug withdrawal and hyperphagia: lessons from tobacco and other drugs. Curr Pharm Des. 2011;17:1173–1179. doi: 10.2174/138161211795656738. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Smith MT, Kudel I, Haythornthwaite J. Pain-related catastrophizing as a risk factor for suicidal ideation in chronic pain. Pain. 2006;126:272–279. doi: 10.1016/j.pain.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Stevens A, Schuetze P, Dombkowski LE. Conceptual model for maternal behavior among polydrug cocaine-using mothers: the role of postnatal cocaine use and maternal depression. Psychol Addict Behav. 2006;20:1–10. doi: 10.1037/0893-164X.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The neural bases of social pain: evidence for shared representations with physical pain. Psychosom Med. 2012a;74:126–135. doi: 10.1097/PSY.0b013e3182464dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012b;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- El Khoury MA, Gorgievski V, Moutsimilli L, Giros B, Tzavara ET. Interactions between the cannabinoid and dopaminergic systems: evidence from animal studies. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:36–50. doi: 10.1016/j.pnpbp.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Elliot AJ. The hierarchical model of approach-avoidance motivation. Motiv Emot. 2006;30:111–116. [Google Scholar]
- Elman I. Integrating psychology research and behavioral management. Psychol Res Behav Manag. 2011;4:11–12. doi: 10.2147/PRBM.S16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Breiter HC, Gollub RL, Krause S, Kantor HL, Baumgartner WA, Gastfriend DR, Rosen BR. Depressive symptomatology and cocaine-induced pituitary–adrenal axis activation in individuals with cocaine dependence. Drug Alcohol Depend. 1999;56:39–45. doi: 10.1016/s0376-8716(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Elman I, D’Ambra MN, Krause S, Breiter H, Kane M, Morris R, Tuffy L, Gastfriend DR. Ultrarapid opioid detoxification: effects on cardiopulmonary physiology, stress hormones and clinical outcomes. Drug Alcohol Depend. 2001;61:163–172. doi: 10.1016/s0376-8716(00)00139-3. [DOI] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR, Chabris CF, Breiter HC. Cocaine-primed craving and its relationship to depressive symptomatology in individuals with cocaine dependence. J Psychopharmacol. 2002;16:163–167. doi: 10.1177/026988110201600207. [DOI] [PubMed] [Google Scholar]
- Elman I, Lukas SE, Karlsgodt KH, Gasic GP, Breiter HC. Acute cortisol administration triggers craving in individuals with cocaine dependence. Psychopharmacol Bull. 2003;37:84–89. [PubMed] [Google Scholar]
- Elman I, Ariely D, Mazar N, Aharon I, Lasko NB, Macklin ML, Orr SP, Lukas SE, Pitman RK. Probing reward function in post-traumatic stress disorder with beautiful facial images. Psychiatry Res. 2005;135:179–183. doi: 10.1016/j.psychres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31:2091–2120. doi: 10.1038/sj.npp.1301051. [DOI] [PubMed] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Tschibelu E, Lowen S, Borsook D. Neurobiology of Post-Traumatic Stress Disorder. Nova Science Publishers; Happauge: 2010a. Reward and motivational systems in post-traumatic stress disorder. [Google Scholar]
- Elman I, Tschibelu E, Borsook D. Psychosocial stress and its relationship to gambling urges in individuals with pathological gambling. Am J Addict. 2010b;19:332–339. doi: 10.1111/j.1521-0391.2010.00055.x. [DOI] [PubMed] [Google Scholar]
- Elman I, Zubieta JK, Borsook D. The missing p in psychiatric training: why it is important to teach pain to psychiatrists. Arch Gen Psychiatry. 2011;68:12–20. doi: 10.1001/archgenpsychiatry.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Becerra L, Tschibelu E, Yamamoto R, George E, Borsook D. Yohimbine-induced amygdala activation in pathological gamblers: a pilot study. PLoS One. 2012;7:e31118. doi: 10.1371/journal.pone.0031118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Gurvits TV, Tschibelu E, Spring JD, Lasko NB, Pitman RK. Neurological soft signs in individuals with pathological gambling. PLoS One. 2013;8:e60885. doi: 10.1371/journal.pone.0060885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Schmid J, Basler M, Cesko E, Schedlowski M, Benson S. How positive and negative expectations shape the experience of visceral pain: an experimental pilot study in healthy women. Neurogastroenterol Motil. 2012;24:914–e460. doi: 10.1111/j.1365-2982.2012.01950.x. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst C, Mechawar N, Turecki G. Suicide neurobiology. Prog Neurobiol. 2009;89:315–333. doi: 10.1016/j.pneurobio.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Ernst C, Nagy C, Kim S, Yang JP, Deng X, Hellstrom IC, Choi KH, Gershenfeld H, Meaney MJ, Turecki G. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol Psychiatry. 2011;70:312–319. doi: 10.1016/j.biopsych.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Evers GC. Pseudo-opioid-resistant pain. Support Care Cancer. 1997;5:457–460. doi: 10.1007/s005200050114. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Scheftner WA, Fogg L, Clark DC, Young MA, Hedeker D, Gibbons R. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147:1189–1194. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- Fields HL. Should we be reluctant to prescribe opioids for chronic nonmalignant pain? Pain. 2007a;129:233–234. doi: 10.1016/j.pain.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Fields HL. Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med. 2007b;32:242–246. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Fishbain DA. The association of chronic pain and suicide. Semin Clin Neuropsychiatry. 1999;4:221–227. doi: 10.153/SCNP00400221. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review Clin J Pain. 1997;13:116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Fisher BJ, Haythornthwaite JA, Heinberg LJ, Clark M, Reed J. Suicidal intent in patients with chronic pain. Pain. 2001;89:199–206. doi: 10.1016/s0304-3959(00)00363-8. [DOI] [PubMed] [Google Scholar]
- Foo H, Mason P. Analgesia accompanying food consumption requires ingestion of hedonic foods. J Neurosci. 2009;29:13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JC, Hessel ET, Prinstein MJ. Clarifying the role of pain tolerance in suicidal capability. Psychiatry Res. 2011;189:362–367. doi: 10.1016/j.psychres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Freud S. Collected Papers. Hogarth; London: 1898. Sexuality in the etiology of neurosis. [Google Scholar]
- Freud S. Mourning and melancholia. In: Strachey J, editor. The Standard Edition of the Complete Psychological Works of Sigmund Freud, Volume XIV (1914–1916): On the History of the Psycho-Analytic Movement, Papers on Metapsychology and Other Works. Hogarth Press; London: 1917a. pp. 237–258. [Google Scholar]
- Freud S. Repression. In: Strachey J, editor. The Standard Edition of the Complete Psychological Works of Sigmund Freud, Volume XIV (1914–1916): On the History of the Psycho-Analytic Movement, Papers on Metapsychology and Other Works. Hogarth Press; London: 1917b. pp. 143–158. [Google Scholar]
- Freud S. Beyond the Pleasure Principle. Independent Publishing Platform, Seattle 2010 [Google Scholar]
- Friedman DP, Murray EA, O’Neill JB, Mishkin M. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J Comp Neurol. 1986;252:323–347. doi: 10.1002/cne.902520304. [DOI] [PubMed] [Google Scholar]
- Fukutake M, Hishimoto A, Nishiguchi N, Nushida H, Ueno Y, Shirakawa O, Maeda K. Association of alpha2A-adrenergic receptor gene polymorphism with susceptibility to suicide in Japanese females. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1428–1433. doi: 10.1016/j.pnpbp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Gamache K, Pitman RK, Nader K. Preclinical evaluation of reconsolidation blockade by clonidine as a potential novel treatment for posttraumatic stress disorder. Neuropsychopharmacology. 2012;37:2789–2796. doi: 10.1038/npp.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Zhang J, Jia C. Psychometric properties of the Dickman Impulsivity Instrument in suicide victims and living controls of rural China. J Affect Disord. 2011;132:368–374. doi: 10.1016/j.jad.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger BE, Passik SD, Howard MO. Attentional bias for prescription opioid cues among opioid dependent chronic pain patients. J Behav Med. 2012 doi: 10.1007/s10865-012-9455-8. http://dx.doi.org/10.1007/s10865-012-9455-8. [DOI] [PMC free article] [PubMed]
- Gear RW, Aley KO, Levine JD. Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci. 1999;19:7175–7181. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiol Behav. 2012;106:58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber MR, Fried LE, Pineles SL, Shipherd JC, Bernstein CA. Posttraumatic stress disorder and intimate partner violence in a women’s headache center. Women Health. 2012;52:454–471. doi: 10.1080/03630242.2012.684088. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Epstein DH, Preston KL. Psychosocial functioning and cocaine use during treatment: strength of relationship depends on type of urine-testing method. Drug Alcohol Depend. 2007;91:169–177. doi: 10.1016/j.drugalcdep.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CA. Review of posttraumatic stress disorder and chronic pain: the path to integrated care. J Rehabil Res Dev. 2012;49:753–776. doi: 10.1682/jrrd.2011.09.0158. [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behav Neurosci. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- Glass JM, Williams DA, Fernandez-Sanchez ML, Kairys A, Barjola P, Heitzeg MM, Clauw DJ, Schmidt-Wilcke T. Executive function in chronic pain patients and healthy controls: different cortical activation during response inhibition in fibromyalgia. J Pain. 2011;12:1219–1229. doi: 10.1016/j.jpain.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goldsmith SK. Reducing Suicide: A National Imperative. National Academies Press; Washington, D.C: 2002. [PubMed] [Google Scholar]
- Goldstein DS. Stress, Catecholamines, and Cardiovascular Disease. Oxford University Press; New York: 1995. [Google Scholar]
- Goldstein DS, McEwen B. Allostasis, homeostats, and the nature of stress. Stress. 2002;5:55–58. doi: 10.1080/102538902900012345. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KH, Selby EA, Anestis MD, Bender TW, Witte TK, Braithwaite S, Van Orden KA, Bresin K, Joiner TE., Jr The reinforcing properties of repeated deliberate self-harm. Arch Suicide Res. 2010;14:329–341. doi: 10.1080/13811118.2010.524059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Gracely RH. Affective dimensions of pain How many and how measured? APS J. 1992;1:243–247. [Google Scholar]
- Guerra VS, Calhoun PS. Examining the relation between posttraumatic stress disorder and suicidal ideation in an OEF/OIF veteran sample. J Anxiety Disord. 2011;25:12–18. doi: 10.1016/j.janxdis.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Sagen J. Antinociceptive effect of riluzole in rats with neuropathic spinal cord injury pain. J Neurotrauma. 2011;28:127–134. doi: 10.1089/neu.2010.1539. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Harned MS, Korslund KE, Foa EB, Linehan MM. Treating PTSD in suicidal and self-injuring women with borderline personality disorder: development and preliminary evaluation of a Dialectical Behavior Therapy Prolonged Exposure Protocol. Behav Res Ther. 2012;50:381–386. doi: 10.1016/j.brat.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Haugh R. Hospitals and clinicians confront a new imperative: pain management. Hosp Health Netw. 2005;79:51–52. 54–56, 52. [PubMed] [Google Scholar]
- Hernandez L, Lee F, Hoebel BG. Simultaneous microdialysis and amphetamine infusion in the nucleus accumbens and striatum of freely moving rats: increase in extracellular dopamine and serotonin. Brain Res Bull. 1987;19:623–628. doi: 10.1016/0361-9230(87)90047-5. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Wong ML, Licinio J, Park C, Young M, Gold PW, Chrousos GP, Bornstein SR. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001;6:540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper JW, Pitman RK, Su Z, Heyman GM, Lasko NB, Macklin ML, Orr SP, Lukas SE, Elman I. Probing reward function in posttraumatic stress disorder: expectancy and satisfaction with monetary gains and losses. J Psychiatr Res. 2008;42:802–807. doi: 10.1016/j.jpsychires.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Herman MM, Hyde TM, Bigelow LB, Weinberger DR, Kleinman JE. Prodynorphin mRNA expression is increased in the patch vs matrix compartment of the caudate nucleus in suicide subjects. Mol Psychiatry. 1997;2:495–500. doi: 10.1038/sj.mp.4000319. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A. Can the functional MRI responses to physical pain really tell us why social rejection “hurts”? Proc Natl Acad Sci U S A. 2011;108:E343. doi: 10.1073/pnas.1105451108. (author reply E344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgen MA, Zivin K, Austin KL, Bohnert AS, Czyz EK, Valenstein M, Kilbourne AM. Severe pain predicts greater likelihood of subsequent suicide. Suicide Life Threat Behav. 2010;40:597–608. doi: 10.1521/suli.2010.40.6.597. [DOI] [PubMed] [Google Scholar]
- Ilgen MA, Zivin K, McCammon RJ, Valenstein M. Pain and suicidal thoughts, plans and attempts in the United States. Gen Hosp Psychiatry. 2008;30:521–527. doi: 10.1016/j.genhosppsych.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Isnard J, Magnin M, Jung J, Mauguiere F, Garcia-Larrea L. Does the insula tell our brain that we are in pain? Pain. 2011;152:946–951. doi: 10.1016/j.pain.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Relieving Pain in America A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; Washington, D.C: 2011. [PubMed] [Google Scholar]
- James W. The Principles of Psychology. Henry Holt & Co; New York: 1890. [Google Scholar]
- Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain. 2012;153:744–754. doi: 10.1016/j.pain.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys M, Capehart B, Friedman MJ. Pharmacotherapy for posttraumatic stress disorder: Review with clinical applications. J Rehabil Res Dev. 2012;49:703–716. doi: 10.1682/jrrd.2011.09.0183. [DOI] [PubMed] [Google Scholar]
- Jeffries FW, Davis P. What is the role of eye movements in eye movement desensitization and reprocessing (EMDR) for post-traumatic stress disorder (PTSD)? A review Behav Cogn Psychother. 2013;41(3):290–300. doi: 10.1017/S1352465812000793. [DOI] [PubMed] [Google Scholar]
- Jenks RA, Higgs S. Reactivity to smoking- and food-related cues in currently dieting and non-dieting young women smokers. J Psychopharmacol. 2011;25:520–529. doi: 10.1177/0269881109359093. [DOI] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen Landmark C. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22:27–47. doi: 10.2165/00023210-200822010-00003. [DOI] [PubMed] [Google Scholar]
- Joiner TE., Jr The trajectory of suicidal behavior over time. Suicide Life Threat Behav. 2002;32:33–41. doi: 10.1521/suli.32.1.33.22187. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Jr, Brown JS, Wingate LR. The psychology and neurobiology of suicidal behavior. Annu Rev Psychol. 2005;56:287–314. doi: 10.1146/annurev.psych.56.091103.070320. [DOI] [PubMed] [Google Scholar]
- Joiner TE, Jr, Steer RA, Abramson LY, Alloy LB, Metalsky GI, Schmidt NB. Hopelessness depression as a distinct dimension of depressive symptoms among clinical and non-clinical samples. Behav Res Ther. 2001;39:523–536. doi: 10.1016/s0005-7967(00)00024-3. [DOI] [PubMed] [Google Scholar]
- Jollant F, Guillaume S, Jaussent I, Castelnau D, Malafosse A, Courtet P. Impaired decision-making in suicide attempters may increase the risk of problems in affective relationships. J Affect Disord. 2007;99:59–62. doi: 10.1016/j.jad.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Jollant F, Lawrence NS, Giampietro V, Brammer MJ, Fullana MA, Drapier D, Courtet P, Phillips ML. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am J Psychiatry. 2008;165:740–748. doi: 10.1176/appi.ajp.2008.07081239. [DOI] [PubMed] [Google Scholar]
- Jollant F, Lawrence NS, Olie E, O’Daly O, Malafosse A, Courtet P, Phillips ML. Decreased activation of lateral orbitofrontal cortex during risky choices under uncertainty is associated with disadvantageous decision-making and suicidal behavior. Neuroimage. 2010;51:1275–1281. doi: 10.1016/j.neuroimage.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Kalen P, Strecker RE, Rosengren E, Bjorklund A. Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 1989;492:187–202. doi: 10.1016/0006-8993(89)90901-3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim R, Chaudhri P. Behavioral addictions: an overview. J Psychoactive Drugs. 2012;44:5–17. doi: 10.1080/02791072.2012.662859. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Lukas SE, Elman I. Psychosocial stress and the duration of cocaine use in non-treatment seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2003;29:539–551. doi: 10.1081/ada-120023457. [DOI] [PubMed] [Google Scholar]
- Karoly P, Lecci L. Motivational correlates of self-reported persistent pain in young adults. Clin J Pain. 1997;13:104–109. doi: 10.1097/00002508-199706000-00004. [DOI] [PubMed] [Google Scholar]
- Karoly P, Ruehlman LS. Motivational implications of pain: chronicity, psychological distress, and work goal construal in a national sample of adults. Health Psychol. 1996;15:383–390. doi: 10.1037//0278-6133.15.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Russell M, Oquendo MA, Burke AK, Harkavy-Friedman J, Mann JJ. Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med. 2012;43:1–13. doi: 10.1017/S0033291712001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56:617–626. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kimura M, Satoh T, Matsumoto N. What does the habenula tell dopamine neurons? Nat Neurosci. 2007;10:677–678. doi: 10.1038/nn0607-677. [DOI] [PubMed] [Google Scholar]
- Kirino E, Gitoh M. Rapid improvement of depressive symptoms in suicide attempters following treatment with milnacipran and tricyclic antidepressants – a case series. Neuropsychiatr Dis Treat. 2011;7:723–728. doi: 10.2147/NDT.S27718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser RS, German DC. Opiate effects on aversive midbrain stimulation in rats. Neurosci Lett. 1978;10:197–202. doi: 10.1016/0304-3940(78)90035-6. [DOI] [PubMed] [Google Scholar]
- Kivimaki M, Leino-Arjas P, Virtanen M, Elovainio M, Keltikangas-Jarvinen L, Puttonen S, Vartia M, Brunner E, Vahtera J. Work stress and incidence of newly diagnosed fibromyalgia: prospective cohort study. J Psychosom Res. 2004;57:417–422. doi: 10.1016/j.jpsychores.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Kjolseth I, Ekeberg O, Steihaug S. Why suicide? Elderly people who committed suicide and their experience of life in the period before their death. Int Psychogeriatr. 2010;22:209–218. doi: 10.1017/S1041610209990949. [DOI] [PubMed] [Google Scholar]
- Kleber HD. Pharmacologic treatments for heroin and cocaine dependence. Am J Addict. 2003;12(Suppl 2):S5–S18. [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropotkin P. Mutual Aid: A Factor of Evolution. Forgotten Books; Charleston: 1902. [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci U S A. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Lonsdale MN, Aasvang E, Kehlet H. A positron emission tomography study of wind-up pain in chronic postherniotomy pain. Eur J Pain. 2011;15 (698):e691–616. doi: 10.1016/j.ejpain.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–394. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL, Pistis M. Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology. 2011;36:589–602. doi: 10.1038/npp.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Defrancesco A, Moghaddam B. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur J Neurosci. 2008;27:1755–1762. doi: 10.1111/j.1460-9568.2008.06130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lee YA, Goto Y. Neurodevelopmental disruption of cortico-striatal function caused by degeneration of habenula neurons. PLoS One. 2011;6:e19450. doi: 10.1371/journal.pone.0019450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, O’Malley SS, White MA, McKee SA. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology (Berl) 2010;212:25–32. doi: 10.1007/s00213-010-1902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite-Almeida H, Almeida-Torres L, Mesquita AR, Pertovaara A, Sousa N, Cerqueira JJ, Almeida A. The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats. Pain. 2009;144:57–65. doi: 10.1016/j.pain.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: hedonic and neural responses to safety from pain. PLoS One. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Mojica CY, Reynaga DD. Nicotinic receptors in addiction pathways. Mol Pharmacol. 2012;83:753–758. doi: 10.1124/mol.112.083659. [DOI] [PubMed] [Google Scholar]
- Levy B. Gender differences in the motivational processing of facial beauty. Learn Motiv. 2008;39:136–145. doi: 10.1016/j.lmot.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin K. A Dynamic Theory of Personality. McGraw-Hill; New York: 1935. [Google Scholar]
- Liedl A, Knaevelsrud C. Chronic pain and PTSD: the Perpetual Avoidance Model and its treatment implications. Torture. 2008;18:69–76. [PubMed] [Google Scholar]
- Liedl A, O’Donnell M, Creamer M, Silove D, McFarlane A, Knaevelsrud C, Bryant RA. Support for the mutual maintenance of pain and post-traumatic stress disorder symptoms. Psychol Med. 2010;40:1215–1223. doi: 10.1017/S0033291709991310. [DOI] [PubMed] [Google Scholar]
- Linehan MM. Suicide intervention research: a field in desperate need of development. Suicide Life Threat Behav. 2008;38:483–485. doi: 10.1521/suli.2008.38.5.483. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Hauck M, Paur RC, Nakamura Y, Zimmermann R, Bromm B, Engel AK. Cortical correlates of false expectations during pain intensity judgments – a possible manifestation of placebo/nocebo cognitions. Brain Behav Immun. 2005;19:283–295. doi: 10.1016/j.bbi.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol. 2011;67:942–968. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten P, Van Houdenhove B. Pain: in search of a common language. Pain. 2005;116:170–171. doi: 10.1016/j.pain.2005.04.009. [DOI] [PubMed] [Google Scholar]
- MacDonald AF, Billington CJ, Levine AS. Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Res. 2004;1018:78–85. doi: 10.1016/j.brainres.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull. 2005;131:202–223. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, Brown RM, Short JL, Lawrence AJ. Investigation of the neuroanatomical substrates of reward seeking following protracted abstinence in mice. J Physiol. 2012;590:2427–2442. doi: 10.1113/jphysiol.2011.225219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagodi EF, Gardner ML, Ward SE, Magyar RL. Responding maintained under intermittent schedules of electric-shock presentation: “Safety” or schedule effects? J Exp Anal Behav. 1981;36:171–190. doi: 10.1901/jeab.1981.36-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbach JJ, Richlin DM, Lipton JA. Illness behavior, depression and anhedonia in myofascial face and back pain patients. Psychother Psychosom. 1983;39:47–54. doi: 10.1159/000287720. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113:283–290. doi: 10.1037//0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- Mathew NT. Pathophysiology of chronic migraine and mode of action of preventive medications. Headache. 2011;51(Suppl 2):84–92. doi: 10.1111/j.1526-4610.2011.01955.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews S, Spadoni A, Knox K, Strigo I, Simmons A. Combat-exposed war veterans at risk for suicide show hyperactivation of prefrontal cortex and anterior cingulate during error processing. Psychosom Med. 2012;74:471–475. doi: 10.1097/PSY.0b013e31824f888f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Maugham WS. Of Human Bondage. George H. Doran Company; New York: 1915. [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClure SM, Daw ND, Montague PR. A computational substrate for incentive salience. Trends Neurosci. 2003;26(8):423–428. doi: 10.1016/s0166-2236(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Mee S, Bunney BG, Reist C, Potkin SG, Bunney WE. Psychological pain: a review of evidence. J Psychiatr Res. 2006;40:680–690. doi: 10.1016/j.jpsychires.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Meerwijk EL, Ford JM, Weiss SJ. Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging Behav. 2012;7:1–14. doi: 10.1007/s11682-012-9179-y. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Evolution of pain theories. Int Anesthesiol Clin. 1970;8:3–34. doi: 10.1097/00004311-197000810-00003. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulders A, Vansteenwegen D, Vlaeyen JW. Women, but not men, report increasingly more pain during repeated (un)predictable painful electrocutaneous stimulation: evidence for mediation by fear of pain. Pain. 2012;153:1030–1041. doi: 10.1016/j.pain.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Minami M. Neuronal mechanisms for pain-induced aversion behavioral studies using a conditioned place aversion test. Int Rev Neurobiol. 2009;85:135–144. doi: 10.1016/S0074-7742(09)85010-1. [DOI] [PubMed] [Google Scholar]
- Miranda R, Gallagher M, Bauchner B, Vaysman R, Marroquin B. Cognitive inflexibility as a prospective predictor of suicidal ideation among young adults with a suicide attempt history. Depress Anxiety. 2012;29:180–186. doi: 10.1002/da.20915. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD. Nocebo in headaches: implications for clinical practice and trial design. Curr Neurol Neurosci Rep. 2012;12:132–137. doi: 10.1007/s11910-011-0245-4. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431(7010):760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Piray P, Luo S. Neuroeconomics and the study of addiction. Biol Psychiatry. 2012;72:107–112. doi: 10.1016/j.biopsych.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Morse SJ. The after-pleasure of suicide. Br J Med Psychol. 1973;46:227–238. doi: 10.1111/j.2044-8341.1973.tb02247.x. [DOI] [PubMed] [Google Scholar]
- Motto JA. Suicide prevention for high-risk persons who refuse treatment. Suicide Life Threat Behav. 1976;6:223–230. [PubMed] [Google Scholar]
- Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv. 2001;52:828–833. doi: 10.1176/appi.ps.52.6.828. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD. Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. J Neurophysiol. 2005;93:2183–2193. doi: 10.1152/jn.01025.2004. [DOI] [PubMed] [Google Scholar]
- Muehlmann AM, Devine DP. Glutamate-mediated neuroplasticity in an animal model of self-injurious behaviour. Behav Brain Res. 2008;189:32–40. doi: 10.1016/j.bbr.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Imai S, Ozaki S, Kishimoto Y, Oe K, Yajima Y, Yamazaki M, Suzuki T. Molecular mechanism of changes in the morphine-induced pharmacological actions under chronic pain-like state: suppression of dopaminergic transmission in the brain. Life Sci. 2004;74:2655–2673. doi: 10.1016/j.lfs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics (U.S.) With Chartbook on Trends in the Health of Americans. Ed. CDC; Hyattsville, MD: 2007. [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Nevin RL. Mefloquine blockade of connexin 36 and connexin 43 gap junctions and risk of suicide. Biol Psychiatry. 2012;71:e1–e2. doi: 10.1016/j.biopsych.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Nguyen RH, Ecklund AM, Maclehose RF, Veasley C, Harlow BL. Co-morbid pain conditions and feelings of invalidation and isolation among women with vulvodynia. Psychol Health Med. 2012;17:589–598. doi: 10.1080/13548506.2011.647703. [DOI] [PubMed] [Google Scholar]
- Nietzsche FW, Kaufmann WA. The Gay Science; with a Prelude in Rhymes and an Appendix of Songs. Vintage Books; New York: 1974. [Google Scholar]
- Niikura K, Narita M, Butelman ER, Kreek MJ, Suzuki T. Neuropathic and chronic pain stimuli downregulate central mu-opioid and dopaminergic transmission. Trends Pharmacol Sci. 2010;31:299–305. doi: 10.1016/j.tips.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Scatton B. Inhibitory influence of GABA on central serotonergic transmission. Involvement of the habenulo-raphe pathways in the GABAergic inhibition of ascending cerebral serotonergic neurons. Brain Res. 1985;331:81–90. doi: 10.1016/0006-8993(85)90717-6. [DOI] [PubMed] [Google Scholar]
- Nixon MK, Cloutier P, Jansson SM. Nonsuicidal self-harm in youth: a population-based survey. CMAJ. 2008;178:306–312. doi: 10.1503/cmaj.061693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, Kessler RC. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. 2013;70:300–310. doi: 10.1001/2013.jamapsychiatry.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Hwang I, Sampson NA, Kessler RC. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:868–876. doi: 10.1038/mp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga JT, Hyde TM, Herman MM, Spurney CF, Bigelow LB, Weinberger DR, Kleinman JE. Glutamate receptors in the postmortem striatum of schizophrenic, suicide, and control brains. Synapse. 1997;27:168–176. doi: 10.1002/(SICI)1098-2396(199711)27:3<168::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Nordstrom P, Gustavsson P, Edman G, Asberg M. Temperamental vulnerability and suicide risk after attempted suicide. Suicide Life Threat Behav. 1996;26:380–394. [PubMed] [Google Scholar]
- Nordstrom P, Schalling D, Asberg M. Temperamental vulnerability in attempted suicide. Acta Psychiatr Scand. 1995;92:155–160. doi: 10.1111/j.1600-0447.1995.tb09560.x. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Testa T, O’Brien TJ, Brady JP, Wells B. Conditioned narcotic withdrawal in humans. Science. 1977;195:1000–1002. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD. Craving love? Enduring grief activates brain’s reward center. Neuroimage. 2008;42:969–972. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami H, Terao T, Shiotsuki I, Ishii N, Iwata N. Lithium levels in drinking water and risk of suicide. Br J Psychiatry. 2009;194:464–465. doi: 10.1192/bjp.bp.108.055798. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy HC, Currier D, Grunebaum MF, Sher L, Sullivan GM, Burke AK, Harkavy-Friedman J, Sublette ME, Parsey RV, Mann JJ. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168:1050–1056. doi: 10.1176/appi.ajp.2011.11010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach I, Mikulincer M, King R, Cohen D, Stein D. Thresholds and tolerance of physical pain in suicidal and nonsuicidal adolescents. J Consult Clin Psychol. 1997;65:646–652. doi: 10.1037//0022-006x.65.4.646. [DOI] [PubMed] [Google Scholar]
- Orbach I, Stein D, Palgi Y, Asherov J, Har-Even D, Elizur A. Perception of physical pain in accident and suicide attempt patients: self-preservation vs self-destruction. J Psychiatr Res. 1996;30:307–320. doi: 10.1016/0022-3956(96)00008-8. [DOI] [PubMed] [Google Scholar]
- Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and post-traumatic stress disorder. J Rehabil Res Dev. 2003;40:397–405. doi: 10.1682/jrrd.2003.09.0397. [DOI] [PubMed] [Google Scholar]
- Pais-Vieira M, Mendes-Pinto MM, Lima D, Galhardo V. Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience. 2009;161:671–679. doi: 10.1016/j.neuroscience.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y. Noradrenergic function in suicide. Arch Suicide Res. 2007;11:235–246. doi: 10.1080/13811110701402587. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Watt D. Why does depression hurt? Ancestral primary-process separation-distress (PANIC/GRIEF) and diminished brain reward (SEEKING) processes in the genesis of depressive affect. Psychiatry. 2011;74:5–13. doi: 10.1521/psyc.2011.74.1.5. [DOI] [PubMed] [Google Scholar]
- Park HJ, Cui FJ, Hwang JY, Kang UG. Effects of clozapine on behavioral sensitization induced by cocaine. Psychiatry Res. 2010;175:165–170. doi: 10.1016/j.psychres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned Reflexes: An Investigation into the Physiological Activity of the Cortex. Dover, New York: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “’wanting” but not “liking” for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen J. Back pain and risk of suicide among Finnish farmers. Am J Public Health. 1995;85:1452–1453. doi: 10.2105/ajph.85.10.1452-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Fuentes G, Olfson M, Villegas L, Morcillo C, Wang S, Blanco C. Prevalence and correlates of child sexual abuse: a national study. Compr Psychiatry. 2012;54:16–27. doi: 10.1016/j.comppsych.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH. Hard outcomes: clinical trials to reduce suicide. Am J Psychiatry. 2011;168:1009–1011. doi: 10.1176/appi.ajp.2011.11081250. [DOI] [PubMed] [Google Scholar]
- Pettit JW, Joiner TE, Jr, Rudd MD. Kindling and behavioral sensitization: are they relevant to recurrent suicide attempts? J Affect Disord. 2004;83:249–252. doi: 10.1016/j.jad.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Pettit JW, Paukert AL, Joiner TE, Jr, Rudd MD. Pilot sample of very early onset bipolar disorder in a military population moderates the association of negative life events and non-fatal suicide attempt. Bipolar Disord. 2006;8:475–484. doi: 10.1111/j.1399-5618.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pinerua-Shuhaibar L, Villalobos N, Delgado N, Rubio MA, Suarez-Roca H. Enhanced central thermal nociception in mildly depressed nonpatients and transiently sad healthy subjects. J Pain. 2011;12:360–369. doi: 10.1016/j.jpain.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Pitchot W, Hansenne M, Ansseau M. Role of dopamine in non-depressed patients with a history of suicide attempts. Eur Psychiatry. 2001;16:424–427. doi: 10.1016/s0924-9338(01)00601-0. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171–186. doi: 10.1016/0304-3959(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Post RM. Sensitization and kindling perspectives for the course of affective illness: toward a new treatment with the anticonvulsant carbamazepine. Pharmacopsychiatry. 1990;23:3–17. doi: 10.1055/s-2007-1014476. [DOI] [PubMed] [Google Scholar]
- Poundja J, Sanche S, Tremblay J, Brunet A. Trauma reactivation under the influence of propranolol: an examination of clinical predictors. Eur J Psychotraumatol. 2012;3 doi: 10.3402/ejpt.v3i0.15470. http://dx.doi.org/10.3402/ejpt.v3i0.15470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. The affective-motivational dimension of pain A two-stage model. APS J. 1992;1:229–239. [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Price DD, Verne GN, Schwartz JM. Plasticity in brain processing and modulation of pain. Prog Brain Res. 2006;157:333–352. doi: 10.1016/s0079-6123(06)57020-7. [DOI] [PubMed] [Google Scholar]
- Pugh MJ, Copeland LA, Zeber JE, Wang CP, Amuan ME, Mortensen EM, Tabares JV, Van Cott AC, Cooper TL, Cramer JA. Antiepileptic drug monotherapy exposure and suicide-related behavior in older veterans. J Am Geriatr Soc. 2012;60:2042–2047. doi: 10.1111/j.1532-5415.2012.04207.x. [DOI] [PubMed] [Google Scholar]
- Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9:745–758. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Bao QV, Chretien P. Pain-related emotions modulate experimental pain perception and autonomic responses. Pain. 2005;118:306–318. doi: 10.1016/j.pain.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B, Bernstein HG. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med. 2010;40:557–567. doi: 10.1017/S0033291709990821. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe GE, Enns MW, Belik SL, Sareen J. Chronic pain conditions and suicidal ideation and suicide attempts: an epidemiologic perspective. Clin J Pain. 2008;24:204–210. doi: 10.1097/AJP.0b013e31815ca2a3. [DOI] [PubMed] [Google Scholar]
- Reisch T, Seifritz E, Esposito F, Wiest R, Valach L, Michel K. An fMRI study on mental pain and suicidal behavior. J Affect Disord. 2010;126:321–325. doi: 10.1016/j.jad.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol. 2010;21:514–522. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZY, Shi J, Epstein DH, Wang J, Lu L. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology (Berl) 2009;204:423–429. doi: 10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes AE, Boyle MH, Bethell J, Wekerle C, Goodman D, Tonmyr L, Leslie B, Lam K, Manion I. Child maltreatment and onset of emergency department presentations for suicide-related behaviors. Child Abuse Negl. 2012;36:542–551. doi: 10.1016/j.chiabu.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Ribeiro JD, Joiner TE. The interpersonal-psychological theory of suicidal behavior: current status and future directions. J Clin Psychol. 2009;65:1291–1299. doi: 10.1002/jclp.20621. [DOI] [PubMed] [Google Scholar]
- Ringel Y, Drossman DA, Leserman JL, Suyenobu BY, Wilber K, Lin W, Whitehead WE, Naliboff BD, Berman S, Mayer EA. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134:396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Rockett IR, Hobbs G, De Leo D, Stack S, Frost JL, Ducatman AM, Kapusta ND, Walker RL. Suicide and unintentional poisoning mortality trends in the United States, 1987–2006: two unrelated phenomena? BMC Public Health. 2010;10:705. doi: 10.1186/1471-2458-10-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome HP, Jr, Rome JD. Limbically augmented pain syndrome (LAPS): kindling, corticolimbic sensitization, and the convergence of affective and sensory symptoms in chronic pain disorders. Pain Med. 2000;1:7–23. doi: 10.1046/j.1526-4637.2000.99105.x. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Rott D, Langleben DD, Elman I. Cocaine decreases plasma insulin concentrations in non-diabetic subjects: a randomized double-blind study. Diabet Med. 2008;25:510–511. doi: 10.1111/j.1464-5491.2008.02412.x. [DOI] [PubMed] [Google Scholar]
- Rudd MD, Joiner T, Rajab MH. Relationships among suicide ideators, attempters, and multiple attempters in a young-adult sample. J Abnorm Psychol. 1996;105:541–550. doi: 10.1037//0021-843x.105.4.541. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, Lachaux JP, Damasio A, Baillet S, Hugueville L, Martinerie J, Damasio H, Renault B. Enter feelings: somatosensory responses following early stages of visual induction of emotion. Int J Psychophysiol. 2009;72:13–23. doi: 10.1016/j.ijpsycho.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Sadowski B, Marek P, Panocka I. Enhancement of performance for brain stimulation reward after footshock in rats. Acta Neurobiol Exp (Wars) 1984;44:51–59. [PubMed] [Google Scholar]
- Saigusa T, Aono Y, Uchida T, Takada K, Verheij MM, Koshikawa N, Cools AR. The alpha(1)-, but not alpha(2)-, adrenoceptor in the nucleus accumbens plays an inhibitory role upon the accumbal noradrenaline and dopamine efflux of freely moving rats. Eur J Pharmacol. 2012;688:35–41. doi: 10.1016/j.ejphar.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Osterman JE, Gagliese L, Katz J. Pain flashbacks in post-traumatic stress disorder. Clin J Pain. 2004;20:83–87. doi: 10.1097/00002508-200403000-00004. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why zebras don’t get ulcers: an updated guide to stress, stress-related diseases, and coping. W.H. Freeman and Co; New York: 1998. [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Sasso DA, Kalanithi PS, Trueblood KV, Pittenger C, Kelmendi B, Wayslink S, Malison RT, Krystal JH, Coric V. Beneficial effects of the glutamate-modulating agent riluzole on disordered eating and pathological skin-picking behaviors. J Clin Psychopharmacol. 2006;26:685–687. doi: 10.1097/01.jcp.0000245567.29531.d6. [DOI] [PubMed] [Google Scholar]
- Savitz JB, Nugent AC, Bogers W, Roiser JP, Bain EE, Neumeister A, Zarate CA, Jr, Manji HK, Cannon DM, Marrett S, Henn F, Charney DS, Drevets WC. Habenula volume in bipolar disorder and major depressive disorder: a high-resolution magnetic resonance imaging study. Biol Psychiatry. 2011;69:336–343. doi: 10.1016/j.biopsych.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlet J, Delamater AR, Campese V, Fein M, Wheeler DS. Differential involvement of the basolateral amygdala and orbitofrontal cortex in the formation of sensory-specific associations in conditioned flavor preference and magazine approach paradigms. Eur J Neurosci. 2012;35:1799–1809. doi: 10.1111/j.1460-9568.2012.08113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia – a voxel-based morphometry study. Pain. 2007;132(Suppl 1):S109–S116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Schneidman ES. The Suicidal Mind. Oxford UNiversity Press; Oxford: 1996. [Google Scholar]
- Schnyder U, Valach L, Bichsel K, Michel K. Attempted suicide. Do we understand the patients’ reasons? Gen Hosp Psychiatry. 1999;21:62–69. doi: 10.1016/s0163-8343(98)00064-4. [DOI] [PubMed] [Google Scholar]
- Schoth DE, Nunes VD, Liossi C. Attentional bias towards pain-related information in chronic pain; a meta-analysis of visual-probe investigations. Clin Psychol Rev. 2012;32:13–25. doi: 10.1016/j.cpr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Getslev V, Backer MM, Weizman R, Pick CG. The atypical neuroleptics clozapine and olanzapine differ regarding their antinociceptive mechanisms and potency. Pharmacol Biochem Behav. 1999;64:75–80. doi: 10.1016/s0091-3057(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Epps SA, Grice TW, Weinshenker D. The selective dopamine beta-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrooten MG, Van Damme S, Crombez G, Peters ML, Vogt J, Vlaeyen JW. Nonpain goal pursuit inhibits attentional bias to pain. Pain. 2012;153:1180–1186. doi: 10.1016/j.pain.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W. Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron. 2011;69:603–617. doi: 10.1016/j.neuron.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle JR. Consciousness. Annu Rev Neurosci. 2000;23:557–578. doi: 10.1146/annurev.neuro.23.1.557. [DOI] [PubMed] [Google Scholar]
- Seddon K, Yonge CD. A Summary of Stoic Philosophy: Seno of Citium in Diogenes Laertius Book Seven. Keith Seddon 2008 [Google Scholar]
- Selby EA, Anestis MD, Bender TW, Ribeiro JD, Nock MK, Rudd MD, Bryan CJ, Lim IC, Baker MT, Gutierrez PM, Joiner TE., Jr Overcoming the fear of lethal injury: evaluating suicidal behavior in the military through the lens of the Interpersonal-Psychological Theory of Suicide. Clin Psychol Rev. 2010;30:298–307. doi: 10.1016/j.cpr.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Sellings LH, Baharnouri G, McQuade LE, Clarke PB. Rewarding and aversive effects of nicotine are segregated within the nucleus accumbens. Eur J Neurosci. 2008;28:342–352. doi: 10.1111/j.1460-9568.2008.06341.x. [DOI] [PubMed] [Google Scholar]
- Selye H. The Stress of Life. McGraw-Hill; New York: 1976. [Google Scholar]
- Sequeira A, Mamdani F, Lalovic A, Anguelova M, Lesage A, Seguin M, Chawky N, Desautels A, Turecki G. Alpha 2A adrenergic receptor gene and suicide. Psychiatry Res. 2004;125:87–93. doi: 10.1016/j.psychres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Morgan L, Walsh DM, Cartagena PM, Choudary P, Li J, Schatzberg AF, Watson SJ, Akil H, Myers RM, Jones EG, Bunney WE, Vawter MP. Gene expression changes in the prefrontal cortex, anterior cingulate cortex and nucleus accumbens of mood disorders subjects that committed suicide. PLoS One. 2012;7:e35367. doi: 10.1371/journal.pone.0035367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev. 2001;21:857–877. doi: 10.1016/s0272-7358(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Shelton L, Becerra L, Borsook D. Unmasking the mysteries of the habenula in pain and analgesia. Prog Neurobiol. 2012a;96:208–219. doi: 10.1016/j.pneurobio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton L, Pendse G, Maleki N, Moulton EA, Lebel A, Becerra L, Borsook D. Mapping pain activation and connectivity of the human habenula. J Neurophysiol. 2012b;107:2633–2648. doi: 10.1152/jn.00012.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YL, Chen JC, Liao RM. Place conditioning and neurochemical responses elicited by the aftereffect of acute stressor exposure involving an elevated stand. Neurosci Lett. 2011;504:156–159. doi: 10.1016/j.neulet.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Sher L, Mann JJ, Traskman-Bendz L, Winchel R, Huang YY, Fertuck E, Stanley BH. Lower cerebrospinal fluid homovanillic acid levels in depressed suicide attempters. J Affect Disord. 2006;90:83–89. doi: 10.1016/j.jad.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sherman JJ, Turk DC, Okifuji A. Prevalence and impact of posttraumatic stress disorder-like symptoms on patients with fibromyalgia syndrome. Clin J Pain. 2000;16:127–134. doi: 10.1097/00002508-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Shneidman ES. Suicide as psychache. J Nerv Ment Dis. 1993;181:145–147. doi: 10.1097/00005053-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Shneidman ES. Perspectives on suicidology. Further reflections on suicide and psychache. Suicide Life Threat Behav. 1998;28:245–250. [PubMed] [Google Scholar]
- Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav Cogn Neurosci Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyper-katifeia: a framework for the rational use of opioids for pain. Pain Med. 2010;11:1092–1098. doi: 10.1111/j.1526-4637.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF. The Behavior of Organisms: An Experimental Analysis. Prentice-Hall; Englewood Cliffs: 1938. [Google Scholar]
- Smathers SA, Wilson JG, Nigro MA. Topiramate effectiveness in Prader-Willi syndrome. Pediatr Neurol. 2003;28:130–133. doi: 10.1016/s0887-8994(02)00490-3. [DOI] [PubMed] [Google Scholar]
- Smith MT, Edwards RR, Robinson RC, Dworkin RH. Suicidal ideation, plans, and attempts in chronic pain patients: factors associated with increased risk. Pain. 2004;111:201–208. doi: 10.1016/j.pain.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Smith PN, Cukrowicz KC, Poindexter EK, Hobson V, Cohen LM. The acquired capability for suicide: a comparison of suicide attempters, suicide ideators, and non-suicidal controls. Depress Anxiety. 2010;27:871–877. doi: 10.1002/da.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81:158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Sommermeyer H, Frielingsdorf J, Knorr A. Effects of prazosin on the dopaminergic neurotransmission in rat brain. Eur J Pharmacol. 1995;276:267–270. doi: 10.1016/0014-2999(95)00062-p. [DOI] [PubMed] [Google Scholar]
- Spiegel B, Schoenfeld P, Naliboff B. Systematic review: the prevalence of suicidal behaviour in patients with chronic abdominal pain and irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:183–193. doi: 10.1111/j.1365-2036.2007.03357.x. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51:215–235. doi: 10.1016/s0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- Stein C, Schafer M, Machelska H. Why is morphine not the ultimate analgesic and what can be done to improve it? J Pain. 2000;1:51–56. doi: 10.1054/jpai.2000.9820. [DOI] [PubMed] [Google Scholar]
- Stoeter P, Bauermann T, Nickel R, Corluka L, Gawehn J, Vucurevic G, Vossel G, Egle UT. Cerebral activation in patients with somatoform pain disorder exposed to pain and stress: an fMRI study. Neuroimage. 2007;36:418–430. doi: 10.1016/j.neuroimage.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Rodgers WM, Kirsch I. Catastrophizing, depression and expectancies for pain and emotional distress. Pain. 2001;91:147–154. doi: 10.1016/s0304-3959(00)00430-9. [DOI] [PubMed] [Google Scholar]
- Suominen K, Isometsa E, Suokas J, Haukka J, Achte K, Lonnqvist J. Completed suicide after a suicide attempt: a 37-year follow-up study. Am J Psychiatry. 2004;161:562–563. doi: 10.1176/appi.ajp.161.3.562. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. Neuroeconomics of suicide. Neuro Endocrinol Lett. 2011;32:400–404. [PubMed] [Google Scholar]
- Tambeli CH, Fischer L, Monaliza SL, Menescal-de-Oliveira L, Parada CA. The functional role of ascending nociceptive control in defensive behavior. Brain Res. 2012;1464:24–29. doi: 10.1016/j.brainres.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Tang NK, Crane C. Suicidality in chronic pain: a review of the prevalence, risk factors and psychological links. Psychol Med. 2006;36:575–586. doi: 10.1017/S0033291705006859. [DOI] [PubMed] [Google Scholar]
- Tang NK, Salkovskis PM, Hodges A, Wright KJ, Hanna M, Hester J. Effects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patients. Pain. 2008;138:392–401. doi: 10.1016/j.pain.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen PC, Jensen RH. Management of cluster headache. CNS Drugs. 2012;26:571–580. doi: 10.2165/11632850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Thorndike EL. Animal Intelligence. The Macmillan Company; New York: 1911. [Google Scholar]
- Tompkins DA, Campbell CM. Opioid-induced hyperalgesia: clinically relevant or extraneous research phenomenon? Curr Pain Headache Rep. 2011;15:129–136. doi: 10.1007/s11916-010-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I. Neuroimaging of pain mechanisms. Curr Opin Support Palliat Care. 2007;1:109–116. doi: 10.1097/SPC.0b013e3282efc58b. [DOI] [PubMed] [Google Scholar]
- Traskman L, Asberg M, Bertilsson L, Sjostrand L. Monoamine metabolites in CSF and suicidal behavior. Arch Gen Psychiatry. 1981;38:631–636. doi: 10.1001/archpsyc.1981.01780310031002. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: altered response to dextroamphetamine. Arch Gen Psychiatry. 2002;59:409–416. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- Troister T, Holden RR. A two-year prospective study of psychache and its relationship to suicidality among high-risk undergraduates. J Clin Psychol. 2012;68:1019–1027. doi: 10.1002/jclp.21869. [DOI] [PubMed] [Google Scholar]
- Turecki G, Ernst C, Jollant F, Labonte B, Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends Neurosci. 2012;35:14–23. doi: 10.1016/j.tins.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Tyrer SP. Learned pain behaviour. Br Med J. 1986;292:1–2. doi: 10.1136/bmj.292.6512.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Eccleston C, Koster EH. Hypervigilance to learned pain signals: a componential analysis. J Pain. 2006;7:346–357. doi: 10.1016/j.jpain.2005.12.006. [DOI] [PubMed] [Google Scholar]
- van Heeringen K. Stress-diathesis model of suicidal behavior. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. CRC Press; Boca Raton, FL: 2012. [Google Scholar]
- Van Houdenhove B, Luyten P. Beyond dualism: the role of life stress in chronic pain. Pain. 2005;113:238–239. doi: 10.1016/j.pain.2004.10.010. (discussion 232–240) [DOI] [PubMed] [Google Scholar]
- Van Orden KA, Witte TK, Cukrowicz KC, Braithwaite SR, Selby EA, Joiner TE., Jr The interpersonal theory of suicide. Psychol Rev. 2010;117:575–600. doi: 10.1037/a0018697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Orden KA, Witte TK, Gordon KH, Bender TW, Joiner TE., Jr Suicidal desire and the capability for suicide: tests of the interpersonal-psychological theory of suicidal behavior among adults. J Consult Clin Psychol. 2008;76:72–83. doi: 10.1037/0022-006X.76.1.72. [DOI] [PubMed] [Google Scholar]
- Vang FJ, Ryding E, Traskman-Bendz L, van Westen D, Lindstrom MB. Size of basal ganglia in suicide attempters, and its association with temperament and serotonin transporter density. Psychiatry Res. 2010;183:177–179. doi: 10.1016/j.pscychresns.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Vincler M. Neuronal nicotinic receptors as targets for novel analgesics. Expert Opin Investig Drugs. 2005;14:1191–1198. doi: 10.1517/13543784.14.10.1191. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F, Jayne M, Wong C. Stimulant-induced enhanced sexual desire as a potential contributing factor in HIV transmission. Am J Psychiatry. 2007;164:157–160. doi: 10.1176/ajp.2007.164.1.157. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlosser RG. Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? Neuroimage. 2011;54:1607–1614. doi: 10.1016/j.neuroimage.2010.08.082. [DOI] [PubMed] [Google Scholar]
- Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, Zou AH, Blanck TJ, Ziff EB. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115:812–821. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartolowska K, Hough MG, Jenkinson M, Andersson J, Wordsworth BP, Tracey I. Structural changes of the brain in rheumatoid arthritis. Arthritis Rheum. 2012;64:371–379. doi: 10.1002/art.33326. [DOI] [PubMed] [Google Scholar]
- Wasan AD, Ross EL, Michna E, Chibnik L, Greenfield SF, Weiss RD, Jamison RN. Craving of prescription opioids in patients with chronic pain: a longitudinal outcomes trial. J Pain. 2012;13:146–154. doi: 10.1016/j.jpain.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. Neurotic tendencies among chronic pain patients: an MMPI item analysis. Pain. 1982;14:365–385. doi: 10.1016/0304-3959(82)90145-2. [DOI] [PubMed] [Google Scholar]
- Webb J, Kamali F. Analgesic effects of lamotrigine and phenytoin on cold-induced pain: a crossover placebo-controlled study in healthy volunteers. Pain. 1998;76:357–363. doi: 10.1016/S0304-3959(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Weiss SR, Post RM. Caveats in the use of the kindling model of affective disorders. Toxicol Ind Health. 1994;10:421–447. [PubMed] [Google Scholar]
- Weissman DE, Haddox JD. Opioid pseudoaddiction – an iatrogenic syndrome. Pain. 1989;36:363–366. doi: 10.1016/0304-3959(89)90097-3. [DOI] [PubMed] [Google Scholar]
- West AR, Floresco SB, Charara A, Rosenkranz JA, Grace AA. Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann N Y Acad Sci. 2003;1003:53–74. doi: 10.1196/annals.1300.004. [DOI] [PubMed] [Google Scholar]
- Wickens J. Toward an anatomy of disappointment: reward-related signals from the globus pallidus. Neuron. 2008;60:530–531. doi: 10.1016/j.neuron.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009;47:987–994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
- Willeumier K, Taylor DV, Amen DG. Decreased cerebral blood flow in the limbic and prefrontal cortex using SPECT imaging in a cohort of completed suicides. Transl Psychiatry. 2011;1:e28. doi: 10.1038/tp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson OD, Buchanan DA, Quintner JL, Cohen ML. Pain beyond monism and dualism. Pain. 2005;116:169–170. doi: 10.1016/j.pain.2005.04.011. (author reply 171) [DOI] [PubMed] [Google Scholar]
- Wilsey BL, Fishman S, Li CS, Storment J, Albanese A. Markers of abuse liability of short- vs long-acting opioids in chronic pain patients: a randomized cross-over trial. Pharmacol Biochem Behav. 2009;94:98–107. doi: 10.1016/j.pbb.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsoncraft WE. Babies by bar-press: maternal behavior in the rat. Behavior Res Methods. 1968;1:229–230. [Google Scholar]
- Wingate LR, Joiner TE, Jr, Walker RL, Rudd MD, Jobes DA. Empirically informed approaches to topics in suicide risk assessment. Behav Sci Law. 2004;22:651–665. doi: 10.1002/bsl.612. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Xue T, Yuan K, Zhao L, Yu D, Zhao L, Dong T, Cheng P, von Deneen KM, Qin W, Tian J. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One. 2012;7:e52927. doi: 10.1371/journal.pone.0052927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Ariely D, Chi W, Langleben DD, Elman I. Gender differences in the motivational processing of babies are determined by their facial attractiveness. PLoS One. 2009;4:e6042. doi: 10.1371/journal.pone.0006042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto RT, Karlsgodt KH, Rott D, Lukas SE, Elman I. Effects of perceived cocaine availability on subjective and objective responses to the drug. Subst Abuse Treat Prev Policy. 2007;2:30. doi: 10.1186/1747-597X-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerevanian BI, Koek RJ, Mintz J. Bipolar pharmacotherapy and suicidal behavior. Part I: Lithium, divalproex and carbamazepine. J Affect Disord. 2007;103:5–11. doi: 10.1016/j.jad.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Yoshimasu K, Kiyohara C, Miyashita K. Suicidal risk factors and completed suicide: meta-analyses based on psychological autopsy studies. Environ Health Prev Med. 2008;13:243–256. doi: 10.1007/s12199-008-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: involvement of neural reward systems. PLoS One. 2010;5:e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner MR, Watt DF, Solms M, Panksepp J. Affective neuroscientific and neuropsychoanalytic approaches to two intractable psychiatric problems: why depression feels so bad and what addicts really want. Neurosci Biobehav Rev. 2011;35:2000–2008. doi: 10.1016/j.neubiorev.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Law CK, Yip PS. Psychological factors associated with the incidence and persistence of suicidal ideation. J Affect Disord. 2011;133:584–590. doi: 10.1016/j.jad.2011.05.003. [DOI] [PubMed] [Google Scholar]


