Abstract
There are no previous studies of the association between prediagnostic serum vitamin D concentration and glioma. Vitamin D has immunosuppressive properties; as does glioma. It was, therefore, our hypothesis that elevated vitamin D concentration would increase glioma risk. We conducted a nested case–control study using specimens from the Janus Serum Bank cohort in Norway. Blood donors who were subsequently diagnosed with glioma (n = 592), between 1974 and 2007, were matched to donors without glioma (n = 1112) on date and age at blood collection and sex. We measured 25-hydroxyvitamin D (25(OH)D), an indicator of vitamin D availability, using liquid chromatography coupled with mass spectrometry. Seasonally adjusted odds ratios (ORs) and 95% confidence intervals (95%CIs) were estimated for each control quintile of 25(OH)D using conditional logistic regression. Among men diagnosed with high grade glioma >56, we found a negative trend (P=.04). Men diagnosed ≤ 56 showed a borderline positive trend (P=.08). High levels (>66 nmol/L) of 25(OH)D in men > 56 were inversely related to high grade glioma from ≥ 2 years before diagnosis (OR=0.59; 95%CI=0.38,0.91) to ≥ 15 years before diagnosis (OR=0.61; 95%CI=0.38,0.96). Our findings are consistent long before glioma diagnosis and are therefore unlikely to reflect preclinical disease.
Keywords: glioma, glioblastoma, 25(OH)D, vitamin D, estrogen, telomerase
Introduction
Vitamin D consists of a group of steroid prohormones that regulate approximately 900 genes (1). It is stored in the body in the form of 25-hydroxyvitamin D (25(OH)D) and transformed into its active form,1,25 hydroxyvitamin D (1,25(OH)D). Measurement of the serum concentration of 25(OH)D is widely accepted as the best indicator of an individual’s vitamin D status (2). Serum vitamin 25(OH)D has a long half-life (three weeks) that reflects vitamin D stores from both dietary intake and ultraviolet irradiation. Alternatively, the active form of vitamin D (1,25(OH)D) is only present in the blood for approximately 24 hours, representing the most recent exposure to solar radiation or vitamin D intake.
There is an extensive body of experimental literature suggesting that vitamin D inhibits cancer progression through many signaling pathways including those that result in apoptosis, cell re-differentiation and inhibition of cell proliferation or angiogenesis (3–8). In contrast to the experimental literature, results from the epidemiological are inconsistent with less variation among studies of certain cancer sites than of others. Colon cancer is most reliably inversely related to elevated 25(OH)D levels while studies of prostate, breast, and esophageal cancer show both positive and negative associations (9, 10). A recent meta-analysis (11) of 25(OH)D and total cancer incidence rates found reduced risk among people with elevated 25(OH)D levels (risk ratio (RR)=0.89, 95% CI=0.81, 0.97, RR of incidence per 50 nmol/L increase in circulating 25(OH)D concentration). Nonetheless authors of two recent papers (9, 12) conclude that the inverse association between 25(OH)D levels and ill health, found in observational studies but not in clinical trials, suggests that low levels of circulating vitamin D are indicators of preclinical disease rather than a reflection of the benefits of vitamin D. These two studies have been criticized for their disregard of problems of clinical trials including their low statistical power (13). In addition, in a cohort study of naturally randomly distributed genetic variants that affect plasma 25(OH)D levels, Afzal et al. (14) found an association between these variants and cancer mortality.
Glioma consists of a morphologically heterogeneous group of primary brain tumors possibly reflecting differences in germline genetic variation or etiology (15). Glioblastoma is the most common form of glioma among adults. There is presently no treatment that promotes long term survival among patients diagnosed with this tumor and, as a result, median survival time from glioblastoma diagnosis is only between 12 and 14 months (16).
Vitamin D metabolites cross the blood- brain barrier (17) and its receptors are found throughout the brain (18) thus it is not unreasonable to study the role of this vitamin in glioma etiology. It has recently been determined that vitamin D is of central importance in the immune system (19) and many of its functions are related to its anti-inflammatory and immunomodulatory roles. At the same time, glioma is an immune suppressive tumor. For example, it is inversely related to, IgE (20), a biomarker of immune hyperactivity (i.e., allergy), and glioblastoma -initiating cells inhibit T-cell growth and increase proliferation of immune suppressive regulatory T cells (21). It was therefore our hypothesis that people with elevated levels of circulating vitamin D would be at increased risk for glioma.
To investigate this hypothesis, we conducted a nested case-control study of prediagnostic 25(OH)D concentration using stored serum samples from the Janus Serum Bank (Norway). Serum samples for the present study were collected, on average, 15 years before glioma diagnosis and would therefore probably not be subject to effects of nascent tumors (9, 12). The relative absence of solar radiation during the Norwegian winter might lead one to predict that Norwegian vitamin D status would be atypically low. However, on average, Norwegians consume enough fatty fish, fish oil, vitamin D fortified food and dietary supplements (22, 23) to produce less seasonal fluctuation in their vitamin D status between summer and winter (24) than is observed among comparable populations (25). Furthermore, their average annual vitamin D levels are similar to those found in countries at lower latitudes including the United States (23, 26).
Methods
Study Population
The Janus Serum Bank was established in 1972 to conduct epidemiological studies of cancer (27–29). This biobank is now owned by the Cancer Registry of Norway and contains serum samples from approximately 167,000 men and 158,000 women. Approximately 90% of the serum donors were participants in routine cardiovascular health examinations in Norwegian counties, conducted by the National Health Screening Services. They used questionnaire and physical examination inclusive of a blood draw for cholesterol and lipids. The residual volumes were stored in the Janus Serum Bank. The majority of these donors were between ages 35 and 49 years old at the time of blood donation. Approximately 10% of the samples came from male and female Red Cross Blood Bank donors. Most of these donors were between ages 20 and 65 years old at the time of blood donation.
Serum samples were stored at −25°C. To prevent exposure to ultraviolet radiation the room in which they were stored had no windows. In addition, vials were kept in cardboard boxes with lids. When samples were initially collected they may have been exposed to lamp light for short periods before they were placed in the freezer. Workers used the room where samples were stored infrequently allowing the lights to be turned off most of the time. As an added precaution, ultraviolet light was not used in the lab. Samples underwent one thaw–freeze cycle in preparation for the present study.
A recent Finnish study of the effects of long-term serum sample storage on the stability of 25(OH)D concludes that storage for as long as 24 years at −25 degrees C does not affect 25(OH)D levels (30). These results are consistent with those from a previous study showing 25(OH)D stability in serum stored for as long as 40 years (31).
Personal identification numbers were initially used to link Janus Serum Bank project blood donors to the Cancer Registry of Norway. However, data sets created by this link and used in the present study, contained no personal identifiers. We found 594 blood donors who were subsequently diagnosed with glioma (International Classification of Disease, Oncology, Third Edition [ICD-O-3] morphology codes 9380–9411, 9420–9480, and 9505) between January 1, 1974 and December 31, 2007. Two of the cases were unsuccessfully analyzed for vitamin D, leaving 592 cases with glioma, 403 of them with high grade glioma (ICD-O-3 morphology codes 9440 and 9401).
When available, two control participants for each glioma case were randomly selected according to an incidence density sampling scheme among blood donors. Controls were individually matched to cases on date of blood collection (±3 months), date of birth (±1 year), county of residence at blood collection, and sex. As were cases, matched control participants were required to be alive and free from any cancer except non-melanoma skin cancer on the date of glioma diagnosis of the case to which they were matched. In addition, to save valuable serum for use in subsequent biobank studies, potential controls diagnosed with rare tumors (i.e., all tumors other than breast, prostate, and colorectal) after the case’s date of glioma diagnosis were rejected from the study. Of the 1114 control subjects identified, two were not included in the study because their vitamin D values for the case to which they were matched were missing. The total number of control participants in matched sets with glioma cases was 1112 and 756 of them were to high grade glioma cases.
The Regional Ethics Committee of Southern Norway and the Data Inspectorate of Norway approved all consent produces and the present research plan. During the Janus Serum Bank’s first years (1972–1996), in line with current Norwegian legislation at the time, participants gave their broad verbal consent for samples to be used for cancer research (32). Verbal consent was considered sufficient under Norwegian law at the time and therefore before 1997 written consent was not obtained. Written and signed consent are archived by the Cancer Registry of Norway for participants from 1997 onwards, according to new regulations. As a general license for the biobank, the Norwegian Data Inspectorate has approved the use of all data and biological samples collected between 1972 and 2004, while requiring that participants may unconditionally withdraw their consent at any time. Should they wish to do so, their serum samples would be destroyed and their descriptive data deleted. All study data were de-identified.
Measurement of 25-Hydroxyvitamin D
We sent 100 μL of serum from each of 1,708 biobank samples to Vitas Laboratory in Oslo, Norway for analysis of 25(OH)D levels (www.vitas.no). Laboratory personnel, unaware of the case-control status of the samples, used stable isotope dilution liquid chromatography coupled with tandem mass spectrometry to measure serum 25-OH-vitamin D2 and 25-OH-vitamin D3 levels. 25-OH-vitamin D3 better represents vitamin D availability than 25-OH vitamin D2, and is therefore most often used as an indicator of vitamin D status in the cancer epidemiology literature (2). For this reason, we restricted the analysis to 25-OH-vitamin D3, and 25-OH-vitamin D2 was not available for analysis, as only 282 of the 1,708 samples yielded values.
Statistical Methods
We compared case and control median and interquartile ranges of the matching variables and age and sex stratified serum 25(OH)D levels serum by inspection. We then identified the subgroup of cases diagnosed with high grade glioma (anaplastic astrocytoma (grade 3), glioblastoma (grade 4) and total glioma) and their matched controls. Next, we stratified this subgroup and the total sample by sex and the control median age at diagnosis (56 years) based on the distribution of genetic and histologic factors suggested by previous literature (33). To further evaluate our data we stratified cases into seven histologic subtypes (e.g., anaplastic astrocytoma, oligodendroglioma) and compared their median ages at diagnosis and 25(OH)D levels.
Exposure to ultraviolet radiation induces seasonal patterns in serum 25(OH)D concentration (34). To account for these effects we estimated seasonally adjusted values by first calculating residuals from a linear regression model with serum 25(OH)D level as the dependent variable and month of blood collected as independent indicator variables. Next, we added the residuals to the overall population mean thus producing seasonally adjusted values.
To estimate odds ratios and 95% confidence intervals for control quintiles of seasonally adjusted serum 25(OH)D, we used conditional logistic regression, conditioned on sets matched on age within two years, date of serum collection and sex. To test for linear trend over levels of histologic type, sex and age at diagnosis stratified quintiles; we used the median value of seasonally adjusted 25(OH)D for each quintile as a continuous variable (34). We also used odds ratios based on the upper two and lower three quintiles to determine whether there were associations between 25(OH)D levels and glioma risk within the seven refined histologic categories.
To determine whether the association between seasonally adjusted 25(OH)D and high grade or total glioma risk changes with proximity of date of blood collection to date of diagnosis, we divided time between blood collection and diagnosis into overlapping intervals. The category closest to time of diagnosis was at least two years before diagnosis and the subsequent five year intervals extended from five to 25 years before diagnosis. Analyses were conducted using SAS statistical software, version 9.3 (SAS Institute Inc, Cary, NC).
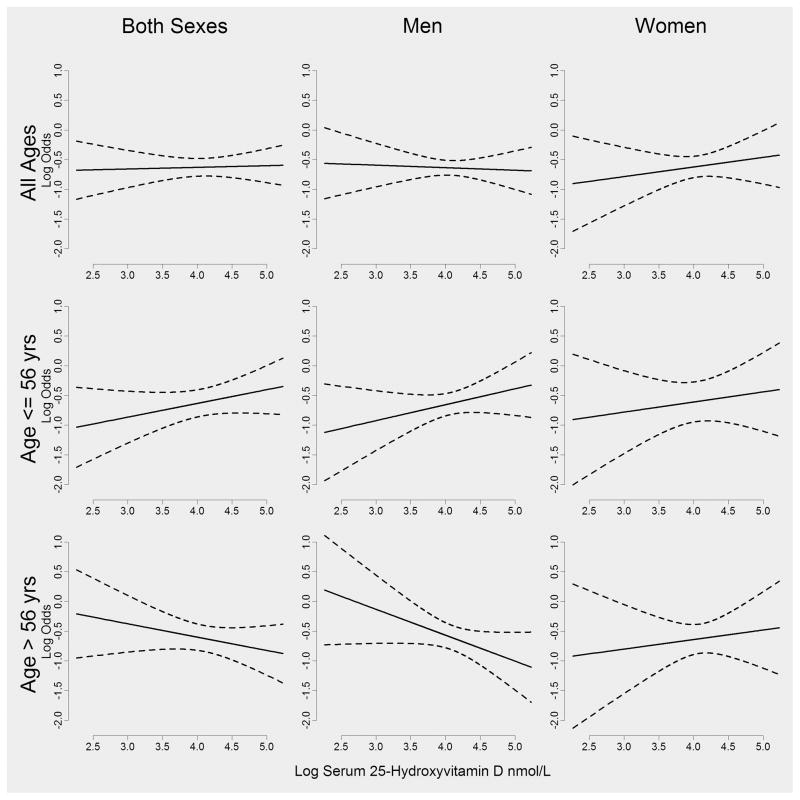
Figure 2 and Supplemental Figure 1 are based on graphs shown in Vinceti et al. (35) and were constructed to emphasize patterns in the data that are not readily apparent in the tables. For each of the subplots in these figures, a separate logistic regression model was fitted. For example, to create the plot in the first row and column of Figure 2 data were retained from both sexes and all ages, and a logistic regression model was fitted using high-grade glioma as the dependent variable and the natural log of 25(OH)D as the independent variable. From this model, we extracted the fitted value and 95% confidence intervals for the fitted value across the range of the observed natural log 25(OH)D. These fitted values are represented by solid lines and the 95% confidence intervals for the mean by dotted lines. All graphs were constructed using R (R Core Team (2013)). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.)
FIG. 2.
Log 25(OH)D against log odds of high grade glioma (astrocytoma and glioblastoma) by sex and median age at diagnosis.
Results
Characteristics of Study Population
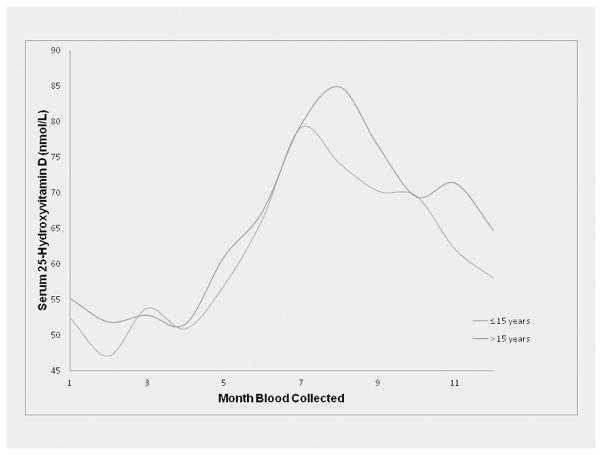
Table 1 indicates that matching was successful, resulting in almost identical values of the matching variables for cases and controls for high grade glioma (cases=403, controls=756) and total glioma (cases=592, controls=1112). Figure 1 shows two similar curves, representing the distribution of mean values of 25(OH)D over month of blood collection among study participants whose time from blood collection to diagnosis (or time of diagnosis of the case to which the controls were matched) was 15 years (the median) or less and greater than 15 years.
Table 1.
Demographic variables that characterize high grade1 and total glioma case and control study participants2
| High Grade Glioma (Anaplastic Astrocytoma (n=31) and Glioblastoma (n=372))
| ||
|---|---|---|
| Variable | Cases | Controls |
| Number | 403 | 756 |
| Percent men, IQR3 | 70 (65, 74) | 70 (67, 73) |
| Median age in years at blood collection, IQR | 42 (40, 43) | 42 (40, 43) |
| Median age in years at glioma diagnosis, IQR | 57 (50, 65) | ---4 |
| Median years from blood collection to diagnosis, IQR | 15 (9,21) | --- |
| Median year of blood collection, IQR | 1985 (1975, 1989) | 1986 (1975, 1989) |
| Median year of birth, IQR | 1944 (1932, 1948) | 1945 (1932, 1948) |
|
| ||
|
Total Glioma
| ||
| Number | 592 | 1112 |
| Percent men, IQR | 67 (63, 71) | 67 (64, 70) |
| Median age in years at blood collection, IQR | 41 (40, 43) | 41 (40, 43) |
| Median age in years at glioma diagnosis, IQR | 56 (49, 63) | --- |
| Median years from blood collection to diagnosis, IQR | 15 (9,21) | --- |
| Median year of blood collection, IQR | 1986 (1975, 1989) | 1986 (1975, 1989) |
| Median year of birth, IQR | 1945 (1934, 1948) | 1945 (1934, 1948) |
Anaplastic astrocytoma and glioblastoma
Case study participants were blood donors (1974–2007) to the Janus Serum Bank, Oslo, Norway who were subsequently diagnosed with high grade or other histologic subtypes of glioma. Control participants were individually matched to case participants on date of blood collection, 2-year age interval at blood collection, and sex.
IQR =Interquartile range
--- Not applicable
FIG. 1.
25(OH)D by month of blood collection for both glioma cases and controls combined separately by median time (15 years) between blood collection and tumor diagnosis.
Sex and Age-Specific Associations between High Grade Glioma, Total Glioma and Seasonally-Adjusted 25(OH)D
We found a statistically significant (P=.04) inverse association between quintiles of 25(OH)D concentration and high grade glioma risk (Table 2) in men older than age 56 years at diagnosis. In contrast, we observed a positive trend of borderline statistical significance among younger men with high grade glioma (P=.08). Therefore, due to opposing directions of dose-response trends, when men were evaluated independently of age at diagnosis there was no association between 25(OH)D levels and high grade glioma risk. When all histologic subtypes were analyzed together (Table 3), results for men were comparable but non-statistically significant. Supplemental Table 1 shows that median serum 25(OH)D levels for men with high grade glioma reflect a similar pattern. The odds ratio for men with high grade glioma diagnosed when they were older than age 56 is the only statistically significant odds ratio in this the table.
Table 2.
The association between serum 25-hydroxyvitamin D 1 and high grade glioma2 by sex and age of case at diagnosis
| Odds Ratios and 95 % Confidence Intervals
|
PTrend4 | |||||
|---|---|---|---|---|---|---|
| Quintiles of serum 25(OH)D3 | ||||||
| 1 | 2 | 3 | 4 | 5 | ||
|
| ||||||
| Quintile (nmol/L) | ≤43.5 | 43.5–55.3 | 55.3–67.1 | 67.1–81.7 | ≥81.8 | |
| High Grade Glioma5 (Men) | ||||||
| Cases/Controls | 23/65 | 28/51 | 25/44 | 32/56 | 28/45 | |
| ≤ 56 years old, OR5 (95% CI6) | 1.00 | 1.84 (0.91, 3.75) | 1.95 (0.94, 4.04) | 1.96 (0.96, 4.01) | 2.10 (0.99, 4.48) | 0.08 |
| Cases/Controls | 19/35 | 38/46 | 33/58 | 25/57 | 29/72 | |
| > 56 years old, OR (95% CI) | 1.00 | 1.42 (0.68, 3.01) | 0.99 (0.49, 2.01) | 0.76 (0.35, 1.63) | 0.65 (0.30, 1.41) | 0.04 |
| Cases/Controls | 42/100 | 66/97 | 58/102 | 57/113 | 57/117 | |
| Total | 1.00 | 1.67 (1.01, 2.77) | 1.39 (0.84, 2.29) | 1.25 (0.75, 2.08) | 1.18 (0.69, 2.00) | 0.81 |
| High Grade Glioma (Women) | ||||||
| Cases/Controls | 12/28 | 14/24 | 11/21 | 8/17 | 11/17 | |
| ≤ 56 years old, OR (95% CI) | 1.00 | 1.33 (0.50, 3.52) | 1.12 (0.41, 3.04) | 0.87 (0.30, 2.55) | 1.67 (0.54, 5.18) | 0.58 |
| Cases/Controls | 18/23 | 11/30 | 13/28 | 13/21 | 12/18 | |
| > 56 years old, OR (95% CI) | 1.00 | 0.49 (0.19, 1.22) | 0.65 (0.27, 1.59) | 0.89 (0.37, 2.17) | 0.86 (0.32, 2.32) | 0.82 |
| Cases/Controls | 30/51 | 25/54 | 24/49 | 21/38 | 23/35 | |
| Total | 1.00 | 0.76 (0.39, 1.46) | 0.81 (0.42, 1.56) | 0.90 (0.46, 1.77) | 1.17 (0.56, 2.41) | 0.55 |
| Total High Grade Glioma | ||||||
| Cases/Controls | 35/93 | 42/75 | 36/65 | 40/73 | 39/62 | |
| ≤ 56 years old, OR (95% CI) | 1.00 | 1.61 (0.91, 2.83) | 1.61 (0.90, 2.87) | 1.53 (0.86, 2.74) | 1.85 (1.00, 3.42) | 0.08 |
| Cases/Controls | 37/58 | 49/76 | 46/86 | 38/78 | 41/90 | |
| > 56 years old, OR (95% CI) | 1.00 | 0.99 (0.57, 1.73) | 0.84 (0.49, 1.45) | 0.77 (0.43, 1.36) | 0.66 (0.36, 1.20) | 0.11 |
| Cases/Controls | 72/151 | 91/151 | 82/151 | 78/151 | 80/152 | |
| Total | 1.00 | 1.26 (0.85, 1.87) | 1.14 (0.77, 1.69) | 1.08 (0.72, 1.62) | 1.11 (0.72, 1.69) | 0.89 |
Adjusted by month of blood draw (see Methods Section)
Anaplastic astrocytoma and glioblastoma
Quintiles based on distribution of age, sex, and date of blood draw-matched control subjects
Tests for linear trend treated median exposure in each quintile as continuous
Table 3.
The association between serum 25-hydroxyvitamin D 1 and total glioma by sex and age of case at diagnosis
| Odds Ratios and 95 % Confidence Intervals
|
PTrend3 | |||||
|---|---|---|---|---|---|---|
| Quintiles of serum 25(OH) D2 | ||||||
| 1 | 2 | 3 | 4 | 5 | ||
|
| ||||||
| Quintile (nmol/L) | ≤42.2 | 42.2–54.1 | 54.1–65.5 | 65.5–79.7 | ≥79.7 | |
| Glioma (Men) | ||||||
| Cases/Controls | 41/93 | 40/85 | 42/72 | 44/84 | 43/68 | |
| ≤ 56 years old, OR4 (95% CI)5 | 1.00 | 1.20 (0.68, 2.10) | 1.45 (0.83, 2.52) | 1.31 (0.74, 2.32) | 1.55 (0.86, 2.82) | 0.15 |
| Cases/Controls | 27/45 | 41/61 | 39/75 | 40/76 | 38/86 | |
| > 56 years old, OR (95% CI) | 1.00 | 1.09 (0.57, 2.07) | 0.80 (0.42, 1.50) | 0.81 (0.43, 1.53) | 0.66 (0.33, 1.31) | 0.09 |
| Cases/Controls | 68/138 | 81/146 | 81/148 | 84/160 | 81/154 | |
| Total | 1.00 | 1.15 (0.76, 1.75) | 1.12 (0.74, 1.70) | 1.08 (0.71, 1.64) | 1.07 (0.68, 1.66) | 0.99 |
| Glioma (Women) | ||||||
| Cases/Controls | 21/50 | 25/38 | 15/34 | 20/33 | 18/35 | |
| ≤ 56 years old, OR (95% CI) | 1.00 | 1.57 (0.77, 3.19) | 1.06 (0.48, 2.34) | 1.29 (0.60, 2.78) | 1.26 (0.57, 2.83) | 0.71 |
| Cases/Controls | 22/34 | 13/37 | 24/42 | 22/29 | 17/34 | |
| > 56 years old, OR (95% CI) | 1.00 | 0.57 (0.25, 1.28) | 0.95 (0.47, 1.93) | 1.27 (0.59, 2.74) | 0.76 (0.33, 1.77) | 0.88 |
| Cases/Controls | 43/84 | 38/75 | 39/76 | 42/62 | 35/69 | |
| Total | 1.00 | 0.97 (0.58, 1.64) | 1.00 (0.59, 1.68) | 1.28 (0.75, 2.20) | 0.98 (0.56, 1.74) | 0.72 |
| Total Glioma | ||||||
| Cases/Controls | 62/143 | 65/123 | 57/107 | 64/117 | 61/103 | |
| ≤ 56 years old, OR (95% CI) | 1.00 | 1.32 (0.85, 2.05) | 1.32 (0.84, 2.07) | 1.31 (0.83, 2.06) | 1.46 (0.91, 2.34) | 0.17 |
| Cases/Controls | 49/79 | 54/98 | 63/117 | 62/105 | 55/120 | |
| > 56 years old, OR (95% CI) | 1.00 | 0.89 (0.55, 1.46) | 0.86 (0.54, 1.37) | 0.94 (0.58, 1.52) | 0.69 (0.41, 1.17) | 0.21 |
| Cases/Controls | 111/222 | 119/221 | 120/224 | 126/222 | 116/223 | |
| Total | 1.00 | 1.08 (0.78, 1.50) | 1.07 (0.78, 1.48) | 1.13 (0.82, 1.57) | 1.04 (0.73, 1.47) | 0.84 |
Adjusted by month of blood draw (see Methods Section)
Quintiles based on distribution of ages, sex and date of blood draw- matched controls
Tests for linear trend treated median exposure in each quintile as continuous
When men and women over age 56 years at diagnosis (the median age at diagnosis of the cases) were considered together, the inverse trend was of borderline significance (high grade glioma P=.08, total glioma P=.11). Figure 2 shows age and sex-specific relationships between the log odds of high grade glioma and the log of seasonally adjusted 25(OH)D. As in Table 2, among men age 56 years and younger at diagnosis, the trend with increasing levels of 25(OH)D is positive. Patterns in Table 3 and Supplemental Figure 1 (based on Table 3) for total glioma are consistent with those observed for high grade glioma which is to be expected because high grade glioma constitutes 68 percent of the total glioma cases in our sample.
Results for Analyses by Refined Histologic Subgroups
Supplemental Table 2 shows median ages and 25(OH)D levels and odds ratios for seven histologic types and controls for men old than 56 years when diagnosed. Only the odds ratio for glioblastoma (OR=0.60 (95%CI=0.39, 0.93)) is statistically significant while that for anaplastic astrocytoma, combined with glioblastoma in our analyses, is in the same direction relative to the null. For this sex and age-specific subgroup only glioblastoma has a sufficient number of cases to be analyzed separately. Supplemental Table 3 contains median values and odds ratios for both sexes and all ages combined by histologic subtype. None of the odds ratios are statistically significant.
Time between Blood Collection and High Grade Glioma Diagnosis
To determine whether, among older men, proximity of the date of diagnosis to the date of blood collection alters the association between 25(OH)D and high grade glioma risk, we further stratified the analyses by overlapping intervals between times of blood collection and tumor diagnosis (Table 4). Each time stratum included all subsequent but not previous periods. We found that odds ratios were similar and statistically significant until at least 15 years before diagnosis but continued to be consistent until at least 25 years before diagnosis.
Table 4.
Associations between 25-hydroxyvitamin D1 and risk of high-grade glioma2 and total glioma among men diagnosed after age 56 years3 and stratified by time between blood collection and tumor diagnosis
| Time from blood collection to tumor diagnosis and adjusted 25(OH)D level | High Grade Glioma | Total Glioma | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cases (n=147) | Controls (n=277) | OR4 (95% CI5) | Cases (n=190) | Controls (n=355) | OR (95% CI) | |
| At least 25 years | ||||||
| 25(OH)D ≤666 nmol/L | 25 | 38 | 1.00 (referent) | 28 | 49 | 1.00 (referent) |
| 25(OH)D >667 nmol/L | 21 | 47 | 0.71 (0.34, 1.47) | 28 | 55 | 0.93 (0.49, 1.77) |
| At least 20 years | ||||||
| 25(OH)D ≤66 nmol/L | 43 | 64 | 1.00 (referent) | 49 | 83 | 1.00 (referent) |
| 25(OH)D >66 nmol/L | 33 | 74 | 0.63 (0.36, 1.12) | 43 | 84 | 0.86 (0.51, 1.44) |
| At least 15 years | ||||||
| 25(OH)D ≤66 nmol/L | 78 | 120 | 1.00 (referent) | 90 | 154 | 1.00 (referent) |
| 25(OH)D >66 nmol/L | 49 | 122 | 0.61 (0.38, 0.96) | 68 | 144 | 0.81 (0.54, 1.21) |
| At least 10 years | ||||||
| 25(OH)D ≤66 nmol/L | 84 | 131 | 1.00 (referent) | 104 | 181 | 1.00 (referent) |
| 25(OH)D >66 nmol/L | 56 | 132 | 0.62 (0.40, 0.97) | 77 | 156 | 0.84 (0.57, 1.23) |
| At least 5 years | ||||||
| 25(OH)D ≤66 nmol/L | 88 | 137 | 1.00 (referent) | 108 | 188 | 1.00 (referent) |
| 25(OH)D >66 nmol/L | 57 | 136 | 0.62 (0.40, 0.95) | 79 | 161 | 0.83 (0.57, 1.21) |
| At least 2 years | ||||||
| 25(OH)D ≤66 nmol/L | 90 | 138 | 1.00 (referent) | 111 | 191 | 1.00 (referent) |
| 25(OH)D >66 nmol/L | 57 | 139 | 0.59 (0.38, 0.91) | 99 | 164 | 0.80 (0.55, 1.17) |
Adjusted by month of blood draw (see Methods Section)
Anaplastic astrocytoma and glioblastoma
Median age at diagnosis
Odds ratio
Confidence interval
Third quintile and below of control distribution
Upper two quintiles of control distribution
Discussion
Ours is the first study to evaluate a potential association between prediagnostic serum vitamin D and glioma risk. Although because of opposing age-specific trends, we found no overall effect of serum 25(OH)D concentration on glioma risk, we observed a reduced risk of both high grade and total glioma among men over age 56 years at diagnosis. This relationship is consistent at least 15 years before high grade glioma diagnosis. In contrast, among men and women diagnosed at age 56 years or younger, we observed a weak positive association of borderline statistical significance. Although these positive trends were similar for younger men and women, in neither case were they strong enough to confirm our hypothesis that the immunosuppressive effects of vitamin D increase the risk of glioma.
There are no previous studies of circulating vitamin D and glioma risk; however, we can compare our findings to those of a related tumor, malignant melanoma, which shares common features with glioma. Both melanocytes and glial cells originate in the neural tube (36) and Scarbrough et al. (37) recently found that the risk of glioma is greater among people diagnosed with melanoma than among those not so diagnosed. Scheurer et al. (38) report that, among first degree relatives of people diagnosed with glioma, there is an elevated risk of malignant melanoma again suggesting common mechanisms. Risk for both tumors is elevated by specific SNP TERT variants (39, 40). Furthermore, mutations in the TERT promoter are also common in both glioma and melanoma somatic tissue, suggesting that telomerase activity plays a role in melanogenesis as it apparently does in gliomagenesis.
In a study of dietary vitamin D consumption (adjusted for energy consumption, calcium intake, skin phototype, history of sunburns, skin sun reaction and education) and risk of malignant melanoma, Vinceti et al. (35) found age specific patterns for men similar to those in our study. That is, they report that an inverse association between vitamin D consumption and melanoma risk among men age 60 years and older. Furthermore, this association was weaker among older women than among older men. Whether the similarity of these age-specific patterns in the two studies is meaningful will have to be determined by subsequent research.
The previous glioma literature is also consistent with our age and sex-specific findings. For example, Walsh et al (41) observed that glioma characterized by risk alleles in telomerase-related genes, TERT (rs2736100) and RTEL1 (rs6010620) occurs most frequently among people diagnosed at older ages. Furthermore, TERT promoter mutations, present in 83% of primary glioblastoma somatic tissue (42) share a similar age distribution (43). These somatic promoter mutations are associated with increased telomerase activity (44, 45). Vitamin D down-regulates telomerase (6, 46–50) and may therefore inhibit growth of tumors associated with telomerase risk alleles. Thus subsequent studies should evaluate the effects of vitamin D on glioma risk by genetic subtype.
Suggesting a rationale for the differential distribution of the effects of vitamin D among men and women in our data set there are fewer women (n=74) than men (n=147) with high grade glioma over age 56 in our sample indicating that we had a higher probability of identifying associations between prediagnostic vitamin D and glioma among older men than older women. A more elaborate rationale for age and sex specific differences in our study is based on work by Correale et al. (51) who found that 17-β estradiol, in premenopausal women, enhances the immunomodulatory effects of activated vitamin D. Supporting these findings, in a cohort study of plasma 25(OH)D and risk of female breast cancer (52), the authors report an inverse association between 25(OH)D concentration and breast cancer risk only among hormone replacement therapy (HRT) users (OR=0.62, 95% CI= 0.42–0.90) and no association among HRT non-users (OR=1.14, 95% CI=0.80–1.62) or all women combined. Menopause results in a drastic lowering of plasma estradiol among women (53) but estradiol continues to be produced from testosterone in men (54). Thus older men have higher plasma estradiol levels than do menopausal women of the same age (e.g., (55)) and plasma estradiol crosses the blood-brain barrier (56). Therefore, in theory, older men have relatively greater access to the immunomodulatory effects of vitamin D than do menopausal women of the same age. These data and literature-derived hypothesis based on complex interactions between vitamin D and estrogen should be empirically examined in future studies.
The major limitation of the present study is that our statistically significant results are restricted to older men, while those in younger men are of borderline significance in the opposite direction. Although a positive association between vitamin D and glioma was hypothesized in advance, an age and sex specific inverse association between vitamin D and glioma risk among older men was not. In addition, our findings are based on a single value of vitamin D using serum collected from participants who were on average 41 years old at the time of blood donation. Unless we posit that vitamin D levels at age 41 years affect subsequent glioma risk, we implicitly assume that our single vitamin D value accurately represents that available during a critical period when it might inhibit or enhance gliomagenesis. Unfortunately, this critical period has not yet been identified. Supporting the assumption that our samples represent averages that extend beyond age 41 years is the similarity of our averages to those reported in large surveys of vitamin D status of the Norwegian population (23). Although Norwegian vitamin D status decreases with age and body mass index (57) if it does so equally among age-matched cases and controls this decrease this may pull the odds ratios toward their null values although this depends on additional conditions (58). Furthermore in the present study the similarity of the odds ratios from two to 25 years before high grade glioma diagnosis for men over age 56 years suggests that a single vitamin D value provides information about subsequent values. Nonetheless, our findings require replication to determine their validity.
In the first large study of the association between serum 25(OH)D and glioma risk, we found that older men with higher levels of serum 25(OH)D had a reduced risk of glioma. Vitamin D regulates approximately 900 genes (1) whose functions could reasonably be expected to either increase or decrease glioma risk. We therefore suggest that the complex relation between vitamin D and glioma development accounts for the restriction of the observed effects, in the present study, to one age and sex-specific subgroup. Given the functional complexity of vitamin D, we propose there may not be an average effect of vitamin D on cancer risk but rather a variety of effects depending on age at diagnosis, tumor heterogeneity and other factors resulting from vitamin D’s multiple biological functions. To test our hypotheses concerning interactions of vitamin D with telomerase and estrogen, future studies of the relation between vitamin D and glioma risk should, at a minimum, be evaluated by genetic subtype and include information about hormone replacement therapy use (59).
Supplementary Material
Acknowledgments
Funding
This work was funded by the National Cancer Institute, National Institutes of Health (grant number R01CA122163 to JS) and a Research Enhancement and Assistance Program grant from the Ohio State University Comprehensive Cancer Center (to JS).
References
- 1.Kongsbak M, Levring TB, Geisler C, von Essen MR. The vitamin d receptor and T cell function. Front Immunol. 2013;4:148. doi: 10.3389/fimmu.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087S–91S. doi: 10.1093/ajcn/87.4.1087S. [DOI] [PubMed] [Google Scholar]
- 3.Nemazannikova N, Antonas K, Dass CR. Vitamin D: Metabolism, molecular mechanisms, and mutations to malignancies. Mol Carcinog. 2013 doi: 10.1002/mc.21999. [DOI] [PubMed] [Google Scholar]
- 4.Diesel B, Radermacher J, Bureik M, Bernhardt R, Seifert M, et al. Vitamin D(3) metabolism in human glioblastoma multiforme: functionality of CYP27B1 splice variants, metabolism of calcidiol, and effect of calcitriol. Clin Cancer Res. 2005;11:5370–80. doi: 10.1158/1078-0432.CCR-04-1968. [DOI] [PubMed] [Google Scholar]
- 5.Elias J, Marian B, Edling C, Lachmann B, Noe CR, et al. Induction of apoptosis by vitamin D metabolites and analogs in a glioma cell line. Recent Results Cancer Res. 2003;164:319–32. doi: 10.1007/978-3-642-55580-0_22. [DOI] [PubMed] [Google Scholar]
- 6.Hansen CM, Binderup L, Hamberg KJ, Carlberg C. Vitamin D and cancer: effects of 1,25(OH)2D3 and its analogs on growth control and tumorigenesis. Front Biosci. 2001;6:D820–48. doi: 10.2741/hansen. [DOI] [PubMed] [Google Scholar]
- 7.Kyritsis AP, Bondy ML, Levin VA. Modulation of glioma risk and progression by dietary nutrients and antiinflammatory agents. Nutr Cancer. 2011;63:174–84. doi: 10.1080/01635581.2011.523807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–5. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 9.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 10.Toner CD, Davis CD, Milner JA. The vitamin D and cancer conundrum: aiming at a moving target. J Am Diet Assoc. 2010;110:1492–500. doi: 10.1016/j.jada.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Yin L, Ordonez-Mena JM, Chen T, Schottker B, Arndt V, et al. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: A systematic review and meta-analysis. Prev Med. 2013;57:753–64. doi: 10.1016/j.ypmed.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:307–20. doi: 10.1016/S2213-8587(13)70212-2. [DOI] [PubMed] [Google Scholar]
- 13.Gille O. Controlled trials of vitamin D, causality and type 2 statistical error. Public Health Nutrition. 2014 doi: 10.1017/S1368980014002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: mendelian randomisation analysis in three large cohorts. Bmj. 2014;349:g6330. doi: 10.1136/bmj.g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins RB, Wrensch MR, Johnson D, Fridley BL, Decker PA, et al. Distinct germ line polymorphisms underlie glioma morphologic heterogeneity. Cancer Genet. 2011;204:13–8. doi: 10.1016/j.cancergencyto.2010.10.002. S0165-4608(10)00557-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koshy M, Villano JL, Dolecek TA, Villano JL, Howard A, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107:207–212. doi: 10.1007/s11060-011-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stumpf WE. Drugs in the brain--cellular imaging with receptor microscopic autoradiography. Prog Histochem Cytochem. 2012;47:1–26. doi: 10.1016/j.proghi.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Eyles DW, Liu PY, Josh P, Cui X. Intracellular distribution of the vitamin D receptor in the brain: Comparison with classic target tissues and redistribution with development. Neuroscience. 2014;268:1–9. doi: 10.1016/j.neuroscience.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 19.Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014;5:151. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartzbaum J, Ding B, Johannesen TB, Osnes LT, Karavodin L, et al. Association between prediagnostic IgE levels and risk of glioma. J Natl Cancer Inst. 2012;104:1251–9. doi: 10.1093/jnci/djs315. djs315 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J, Barr J, Kong LY, Wang Y, Wu A, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16:461–73. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Brustad M, Sandanger T, Aksnes L, Lund E. Vitamin D status in a rural population of northern Norway with high fish liver consumption. Public Health Nutr. 2004;7:783–9. doi: 10.1079/phn2004605. [DOI] [PubMed] [Google Scholar]
- 23.Holvik K, Brunvand L, Brustad M, Meyer HE. Solar Radiation and Human Health. The Norwegian Academy of Science and Letters; Oslo: 2008. Vitamin D status in the Norwegian population; pp. 216–228. [Google Scholar]
- 24.Christensen MH, Lien EA, Hustad S, Almas B. Seasonal and age-related differences in serum 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone in patients from Western Norway. Scand J Clin Lab Invest. 2010;70:281–6. doi: 10.3109/00365511003797172. [DOI] [PubMed] [Google Scholar]
- 25.Vieth R, Cole DE, Hawker GA, Trang HM, Rubin LA. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr. 2001;55:1091–7. doi: 10.1038/sj.ejcn.1601275. [DOI] [PubMed] [Google Scholar]
- 26.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, et al. Vitamin D status: United States, 2001–2006. NCHS Data Brief. 2011:1–8. [PubMed] [Google Scholar]
- 27.Jellum E, Andersen A, Lund-Larsen P, Theodorsen L, Orjasaeter H. The JANUS serum bank. Sci Total Environ. 1993;139–140:527–35. doi: 10.1016/0048-9697(93)90049-c. [DOI] [PubMed] [Google Scholar]
- 28.Jellum E, Andersen A, Lund-Larsen P, Theodorsen L, Orjasaeter H. Experiences of the Janus Serum Bank in Norway. Environ Health Perspect. 1995;103(Suppl 3):85–8. doi: 10.1289/ehp.95103s385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langseth H, Gislefoss R, Martinsen JI, Stornes A, Lauritzen M, et al. The Janus Serum Bank-From sample collection to cancer research. Oslo: Cancer Registry of Norway; 2009. [Google Scholar]
- 30.Agborsangaya C, Toriola AT, Grankvist K, Surcel HM, Holl K, et al. The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr Cancer. 2010;62:51–7. doi: 10.1080/01635580903191460. [DOI] [PubMed] [Google Scholar]
- 31.Bodnar LM, Catov JM, Wisner KL, Klebanoff MA. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr. 2009;101:278–84. doi: 10.1017/S0007114508981460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helgesson G, Dillner J, Carlson J, Bartram CR, Hansson MG. Ethical framework for previously collected biobank samples. Nat Biotechnol. 2007;25:973–6. doi: 10.1038/nbt0907-973b. nbt0907-973b [pii] [DOI] [PubMed] [Google Scholar]
- 33.Bozdag S, Li A, Riddick G, Kotliarov Y, Baysan M, et al. Age-specific signatures of glioblastoma at the genomic, genetic, and epigenetic levels. PLoS ONE. 2013;8:e62982. doi: 10.1371/journal.pone.0062982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, et al. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100:796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinceti M, Malagoli C, Fiorentini C, Longo C, Crespi CM, et al. Inverse association between dietary vitamin D and risk of cutaneous melanoma in a northern Italy population. Nutr Cancer. 2011;63:506–13. doi: 10.1080/01635581.2011.539314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crane JF, Trainor PA. Neural Crest Stem and Progenitor Cells. Annual Review of Cell and Developmental Biology. 2006;22:267–286. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- 37.Scarbrough PM, Akushevich I, Wrensch M, Il’yasova D. Exploring the association between melanoma and glioma risks. Ann Epidemiol. 2014;24:469–74. doi: 10.1016/j.annepidem.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheurer ME, Etzel CJ, Liu M, Barnholtz-Sloan J, Wiklund F, et al. Familial aggregation of glioma: a pooled analysis. Am J Epidemiol. 2010;172:1099–107. doi: 10.1093/aje/kwq261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–61. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 40.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–9. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh KM, Rice T, Decker PA, Kosel ML, Kollmeyer T, et al. Genetic variants in telomerase-related genes are associated with an older age at diagnosis in glioma patients: evidence for distinct pathways of gliomagenesis. Neuro Oncol. 2013;15:1041–7. doi: 10.1093/neuonc/not051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koelsche C, Sahm F, Capper D, Reuss D, Sturm D, et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013;126:907–15. doi: 10.1007/s00401-013-1195-5. [DOI] [PubMed] [Google Scholar]
- 44.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arita H, Narita Y, Takami H, Fukushima S, Matsushita Y, et al. TERT promoter mutations rather than methylation are the main mechanism for TERT upregulation in adult gliomas. Acta Neuropathol. 2013 doi: 10.1007/s00401-013-1203-9. [DOI] [PubMed] [Google Scholar]
- 46.Chiang KC, Yeh CN, Hsu JT, Chen LW, Kuo SF, et al. MART-10, a novel vitamin D analog, inhibits head and neck squamous carcinoma cells growth through cell cycle arrest at G0/G1 with upregulation of p21 and p27 and downregulation of telomerase. J Steroid Biochem Mol Biol. 2013;138:427–34. doi: 10.1016/j.jsbmb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Jiang F, Bao J, Li P, Nicosia SV, Bai W. Induction of ovarian cancer cell apoptosis by 1,25-dihydroxyvitamin D3 through the down-regulation of telomerase. J Biol Chem. 2004;279:53213–21. doi: 10.1074/jbc.M410395200. [DOI] [PubMed] [Google Scholar]
- 48.Kasiappan R, Shen Z, Tse AK, Jinwal U, Tang J, et al. 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J Biol Chem. 2012;287:41297–309. doi: 10.1074/jbc.M112.407189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Semin Cancer Biol. 2011;21:349–53. doi: 10.1016/j.semcancer.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubis B, Holysz H, Gladych M, Toton E, Paszel A, et al. Telomerase downregulation induces proapoptotic genes expression and initializes breast cancer cells apoptosis followed by DNA fragmentation in a cell type dependent manner. Mol Biol Rep. 2013;40:4995–5004. doi: 10.1007/s11033-013-2600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Correale J, Ysrraelit MC, Gaitan MI. Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol. 2010;185:4948–58. doi: 10.4049/jimmunol.1000588. [DOI] [PubMed] [Google Scholar]
- 52.Kuhn T, Kaaks R, Becker S, Eomois PP, Clavel-Chapelon F, et al. Plasma 25-hydroxyvitamin D and the risk of breast cancer in the European prospective investigation into cancer and nutrition: a nested case-control study. Int J Cancer. 2013;133:1689–700. doi: 10.1002/ijc.28172. [DOI] [PubMed] [Google Scholar]
- 53.Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2011;32:604–13. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. Aging Male. 2002;5:98–102. [PubMed] [Google Scholar]
- 55.Lin JH, Zhang SM, Rexrode KM, Manson JE, Chan AT, et al. Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol. 2013;11:419–424 e1. doi: 10.1016/j.cgh.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johann S, Beyer C. Neuroprotection by gonadal steroid hormones in acute brain damage requires cooperation with astroglia and microglia. J Steroid Biochem Mol Biol. 2013;137:71–81. doi: 10.1016/j.jsbmb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–20. [PubMed] [Google Scholar]
- 58.Jurek AM, Greenland S, Maldonado G. How far from non-differential does exposure or disease misclassification have to be to bias measures of association away from the null? Int J Epidemiol. 2008;37:382–5. doi: 10.1093/ije/dym291. [DOI] [PubMed] [Google Scholar]
- 59.Qi ZY, Shao C, Zhang X, Hui GZ, Wang Z. Exogenous and endogenous hormones in relation to glioma in women: a meta-analysis of 11 case-control studies. PLoS ONE. 2013;8:e68695. doi: 10.1371/journal.pone.0068695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.