Abstract
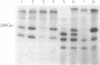
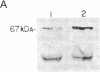
The eukaryotic initiation factor 2 (eIF-2)-associated 67-kDa polypeptide (p67) isolated from reticulocyte lysate protects the eIF-2 alpha subunit from eIF-2 kinase-catalyzed phosphorylation and promotes protein synthesis in the presence of active eIF-2 kinases. We have now studied the roles of p67 and eIF-2 kinases in regulation of protein synthesis using several animal cell lysates and an animal cell line (KRC-7) in culture under various growth conditions. The results are as follows. (i) Both p67 and eIF-2 kinase(s) are present in active forms in all animal cells under normal growth conditions and p67 protects the eIF-2 alpha subunit from eIF-2 kinase-catalyzed phosphorylation, thus promoting protein synthesis in the presence of active eIF-2 kinases. (ii) In heme-deficient reticulocyte lysates and in serum-starved KRC-7 cells in culture, p67 is deglycosylated and subsequently degraded. This leads to eIF-2 kinase-catalyzed eIF-2 alpha-subunit phosphorylation and thus to protein synthesis inhibition. (iii) Addition of a mitogen (namely, phorbol 12-myristate 13-acetate) to serum-starved KRC-7 cells in culture induces an increase of p67 and thus increases protein synthesis. These results suggest the following conclusions. (i) Protein synthesis inhibition in a heme-deficient reticulocyte lysate is not due to the activation of an eIF-2 kinase (heme-regulated inhibitor), as is generally believed, but is due to degradation of p67. The heme-regulated inhibitor is present in an active form and possibly in equal amounts in both heme-deficient and heme-supplemented reticulocyte lysates but cannot phosphorylate eIF-2 alpha subunit because of the presence of p67. (ii) p67 is essential for protein synthesis as it protects the eIF-2 alpha subunit from eIF-2 kinase-catalyzed phosphorylation and promotes protein synthesis in the presence of one or more active eIF-2 kinases present in all animal cells. (iii) p67 is both degradable and inducible. Only the p67 level correlates directly with the protein synthesis activity of the cell, indicating that p67 is a critical factor in protein synthesis regulation in animal cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akkaraju G. R., Whitaker-Dowling P., Youngner J. S., Jagus R. Vaccinia specific kinase inhibitory factor prevents translational inhibition by double-stranded RNA in rabbit reticulocyte lysate. J Biol Chem. 1989 Jun 15;264(17):10321–10325. [PubMed] [Google Scholar]
- Datta B., Chakrabarti D., Roy A. L., Gupta N. K. Roles of a 67-kDa polypeptide in reversal of protein synthesis inhibition in heme-deficient reticulocyte lysate. Proc Natl Acad Sci U S A. 1988 May;85(10):3324–3328. doi: 10.1073/pnas.85.10.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta B., Ray M. K., Chakrabarti D., Gupta N. K. Roles of eIF-2 and eIF-2-associated proteins in regulation of protein synthesis during growth of animal cells in culture. Indian J Biochem Biophys. 1988 Dec;25(6):478–482. [PubMed] [Google Scholar]
- Datta B., Ray M. K., Chakrabarti D., Wylie D. E., Gupta N. K. Glycosylation of eukaryotic peptide chain initiation factor 2 (eIF-2)-associated 67-kDa polypeptide (p67) and its possible role in the inhibition of eIF-2 kinase-catalyzed phosphorylation of the eIF-2 alpha-subunit. J Biol Chem. 1989 Dec 5;264(34):20620–20624. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Heat shock-induced translational alterations in HeLa cells. Initiation factor modifications and the inhibition of translation. J Biol Chem. 1984 Oct 10;259(19):11882–11889. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Covalent modification. J Biol Chem. 1985 May 10;260(9):5493–5497. [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Kudlicki W., Fullilove S., Read R., Kramer G., Hardesty B. Identification of spectrin-related peptides associated with the reticulocyte heme-controlled alpha subunit of eukaryotic translational initiation factor 2 kinase and of Mr 95,000 peptide that appears to be the catalytic subunit. J Biol Chem. 1987 Jul 15;262(20):9695–9701. [PubMed] [Google Scholar]
- Pain V. M., Lewis J. A., Huvos P., Henshaw E. C., Clemens M. J. The effects of amino acid starvation on regulation of polypeptide chain initiation in Ehrlich ascites tumor cells. J Biol Chem. 1980 Feb 25;255(4):1486–1491. [PubMed] [Google Scholar]
- Petryshyn R., Chen J. J., London I. M. Detection of activated double-stranded RNA-dependent protein kinase in 3T3-F442A cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1427–1431. doi: 10.1073/pnas.85.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransone L. J., Dasgupta A. A heat-sensitive inhibitor in poliovirus-infected cells which selectively blocks phosphorylation of the alpha subunit of eucaryotic initiation factor 2 by the double-stranded RNA-activated protein kinase. J Virol. 1988 Oct;62(10):3551–3557. doi: 10.1128/jvi.62.10.3551-3557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransone L. J., Dasgupta A. Activation of double-stranded RNA-activated protein kinase in HeLa cells after poliovirus infection does not result in increased phosphorylation of eucaryotic initiation factor-2. J Virol. 1987 Jun;61(6):1781–1787. doi: 10.1128/jvi.61.6.1781-1787.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R., Griffith J., Maniatis T. Purification and visualization of native spliceosomes. Cell. 1988 Jun 17;53(6):949–961. doi: 10.1016/s0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Reichel P. A., Merrick W. C., Siekierka J., Mathews M. B. Regulation of a protein synthesis initiation factor by adenovirus virus-associated RNA. Nature. 1985 Jan 17;313(5999):196–200. doi: 10.1038/313196a0. [DOI] [PubMed] [Google Scholar]
- Rice A. P., Kerr I. M. Interferon-mediated, double-stranded RNA-dependent protein kinase is inhibited in extracts from vaccinia virus-infected cells. J Virol. 1984 Apr;50(1):229–236. doi: 10.1128/jvi.50.1.229-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorsone K. A., Panniers R., Rowlands A. G., Henshaw E. C. Phosphorylation of eukaryotic initiation factor 2 during physiological stresses which affect protein synthesis. J Biol Chem. 1987 Oct 25;262(30):14538–14543. [PubMed] [Google Scholar]
- Trevillyan J. M., Kulkarni R. K., Byus C. V. Tumor-promoting phorbol esters stimulate the phosphorylation of ribosomal protein S6 in quiescent Reuber H35 hepatoma cells. J Biol Chem. 1984 Jan 25;259(2):897–902. [PubMed] [Google Scholar]
- Whitaker-Dowling P., Youngner J. S. Characterization of a specific kinase inhibitory factor produced by vaccinia virus which inhibits the interferon-induced protein kinase. Virology. 1984 Aug;137(1):171–181. doi: 10.1016/0042-6822(84)90020-5. [DOI] [PubMed] [Google Scholar]