Abstract
Smokeless tobacco products, such as moist snuff or chewing tobacco, contain many of the same carcinogens as tobacco smoke; however the impact on children of indirect exposure to tobacco constituents via parental smokeless tobacco use is unknown. As part of the California Childhood Leukemia Study, dust samples were collected from 6 homes occupied by smokeless tobacco users, 6 homes occupied by active smokers, and 20 tobacco-free homes. To assess children’s potential for exposure to tobacco constituents, vacuum-dust concentrations of five tobacco-specific nitrosamines, including N′-nitrosonornicotine [NNN] and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone [NNK], as well as six tobacco alkaloids, including nicotine and myosmine were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS). We used generalized estimating equations derived from a multivariable marginal model to compare levels of tobacco constituents between groups, after adjusting for a history of parental smoking, income, home construction date, and mother’s age and race/ethnicity. The ratio of myosmine:nicotine was used as a novel indicator of the source of tobacco contamination, distinguishing between smokeless tobacco products and tobacco smoke. Median dust concentrations of NNN and NNK were significantly greater in homes with smokeless tobacco users compared to tobacco-free homes. In multivariable models, concentrations of NNN and NNK were 4.8-fold and 6.9-fold higher in homes with smokeless tobacco users compared to tobacco-free homes. Median myosmine:nicotine ratios were lower in homes with smokeless tobacco users (1.8%) compared to homes of active smokers (7.7%), confirming that cigarette smoke was not the predominant source of tobacco constituents in homes with smokeless tobacco users. Children living with smokeless tobacco users may be exposed to carcinogenic tobacco-specific nitrosamines via contact with contaminated dust and household surfaces.
Keywords: Alkaloids, Cigarette smoking, House dust, Nicotine, Nitrosamines, Smokeless tobacco
INTRODUCTION
Smokeless tobacco products contain tobacco as the primary constituent and are used either orally or nasally without combustion.1 The most commonly used smokeless tobacco product in the U.S. is moist snuff followed by loose-leaf chewing tobacco.2 In contrast to the declining prevalence of adult cigarette smoking in the U.S.3, the prevalence of smokeless tobacco use among Americans aged 12 and older has remained constant over the past decade at 3.0-3.5%.4 Smokeless tobacco products contain several suspected carcinogens, including tobacco-specific nitrosamines, N’-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), and smokeless tobacco is causally associated with cancers of the oral cavity and pancreas in adults.1 The impact on children of indirect exposure to tobacco constituents via parental smokeless tobacco use is unknown. Young children spend more time at home, especially near the floor, and are more likely to make hand-to-mouth contact than adults.5 Thus, young children potentially receive a relatively large portion of their total exposure to hazardous tobacco constituents via the ingestion of settled dust.
As part of the California Childhood Leukemia Study (CCLS), we previously collected vacuum-dust samples from homes occupied by smokeless tobacco users, homes occupied by active smokers, and tobacco-free homes and demonstrated that nicotine concentrations were higher in homes occupied by smokeless tobacco users than in tobacco-free homes.6 As a follow-up to our previous analysis, we assess the potential for a child sharing a home with a smokeless tobacco user to be exposed to several other tobacco constituents, including five tobacco-specific nitrosamines (NNN, NNK, 4-(methylnitrosamino)-4-(3-pyridyl)butanal [NNA], N′-nitrosoanabasine [NAB], and N′-nitrosoanatabine [NAT]) and five minor tobacco alkaloids (cotinine, myosmine, N-formylnornicotine, nicotelline, and 2,3’-bipyridine). Refer to Figure 1 for structural diagrams of the analytes and to Table 1 for properties of the analytes. We compare levels of these tobacco constituents in homes of smokeless tobacco users to levels in homes of active smokers and in tobacco-free homes. Additionally, we use an alkaloid ratio as a specific indicator of contamination from two distinct sources: smokeless tobacco products and tobacco smoke. To our knowledge, the current analysis is the first to characterize levels of NNA, myosmine, N-formylnornicotine, and 2,3’-bipyridine in settled dust
Figure 1.
Chemical structures of the tobacco-specific nitrosamines and tobacco alkaloids
Table 1.
Properties of the tobacco-specific nitrosamines and minor tobacco alkaloids.
| Tobacco Constituents | MWa | log Pa | log KOAb | Persistence Timeb, Days |
|---|---|---|---|---|
| Nicotine | 162 | −1.52 | 8.08 | 41 |
| Tobacco-specific nitrosamines | ||||
| NNN | 177 | −4.60 | 8.48 | 50 |
| NNK | 207 | −6.67 | 10.59 | 49 |
| NNA | 207 | −6.72 | 10.27 | 49 |
| NAB | 191 | −4.92 | 8.85 | 52 |
| NAT | 189 | −4.55 | 8.69 | 50 |
| Minor tobacco alkaloids | ||||
| Cotinine | 176 | −3.38 | 9.94 | 41 |
| Myosmine | 146 | −1.33 | 8.04 | 52 |
| N-formylnornicotine | 176 | −4.69 | 9.99 | 41 |
| Nicotelline | 233 | −6.04 | 13.50 | 78 |
| 2,3'-Bipyridine | 156 | −2.57 | 9.00 | 49 |
MW=molecular weight; log p=vapor pressure; log KOA=octanol-air partition coefficient; NNN=N′-nitrosonornicotine; NNK=4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNA=4-(methylnitrosamino)-4-(3-pyridyl)butanal; NAB=N′-nitrosoanabasine; NAT=N′-nitrosoanatabine
From Chemical Abstracts, Log of Vapor Pressure at 25°C calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2015 ACD/Labs)
From US EPA. [2015]. Estimation Programs Interface Suite™ for Microsoft® Windows, v7 professional]. United States Environmental Protection Agency, Washington, DC, USA.
EXPERIMENTAL PROCEDURES
Hazardous materials
NNK and NNN are carcinogens, and appropriate safety precautions should be taken.
Study population
The CCLS is a case–control study of childhood leukemia conducted in the San Francisco Bay area and California Central Valley designed to identify genetic and environmental risk factors for childhood leukemia. Case and control participants enrolled in the study from December 1999 to November 2007 were eligible for initial dust collection if they were 0-7 years-old. Subsequently, in 2010, participants in the initial dust collection that still lived in the same home were eligible for a second dust collection. Among 629 participants in the initial dust collection, 225 were eligible for a second dust collection and 204 took part. Of the 204 participating homes, 6 were occupied by a smokeless tobacco user. We analyzed two dust samples for tobacco constituents from each of 5 of these homes occupied by a smokeless tobacco user, but we were only able to analyze tobacco constituents in one dust sample from the remaining home. For comparison, we analyzed two dust samples for tobacco constituents from each of 6 randomly selected homes occupied by an active smoker and 20 randomly selected tobacco-free homes. None of the participating households reported dual use of smokeless tobacco products and cigarettes. We obtained written informed consent from the participating families in accordance with the institutional review boards’ requirements at the University of California, Berkeley.
Collection of vacuum dust
In the first round of dust sampling (2002-2007), we collected vacuum cleaner dust and administered a questionnaire during an in-home visit. In the second round of dust sampling (2010), we interviewed subjects via telephone and instructed them to mail their vacuum cleaner bags (or the contents of their vacuum cleaner canisters) to the study center in prepaid packages. The median interval between rounds for paired samples was 4.7 years (range of 2.9 – 8.2 years). We stored dust samples in the dark at or below 4°C before chemical analysis. We previously analyzed these dust samples for nicotine6; however, dust samples were re-extracted and re-analyzed for nicotine along with other tobacco constituents using new laboratory methods described below. This replicate nicotine analysis provided an opportunity to confirm our previous findings and ensured internal consistency when comparing levels of nicotine to other tobacco constituents in the current analysis.
Laboratory analysis of tobacco alkaloids and tobacco-specific nitrosamines
Concentrations of tobacco alkaloids and tobacco-specific nitrosamines were determined using previously described analytical protocols7, 8, with a modified extraction procedure, described below and in the Supporting Information, Figure S1. Each dust sample was homogenized and fractionated using a mechanical shaker equipped with a 100-mesh sieve to obtain dust particles smaller than 150 μm. Fine dust samples were accurately weighed (66 ± 11 mg) into 16x125 mm culture tubes and to each sample was added 150 µL of aqueous internal standard solution (containing d4-nicotine, d4-NNN, d4-NNK, d3-NNA, d4-NAB, d4-NAT, d9-cotinine, d4-myosmine, d4-N-formylnornicotine, d8-nicotelline, and d4-2,3’-bipyridine; for details see Hang et al.8), 1.5 mL distilled water, and 0.5 mL of 1M sulfuric acid. The mixture was vortexted and 10 mL of 70:30 toluene:butanol was added. The tubes were then sonicated at 55°C for 1 hour with intermittent vortexing. The tubes were centrifuged, frozen in a dry ice-acetone bath and the toluene:butanol phase discarded. After the remaining aqueous phase was made basic with 1 mL of 45% potassium carbonate 5% tetrasodium EDTA, 10 mL of 45:45:10 dichloromethane:pentane:ethyl acetate was added. The samples were extracted using vortex mixing, centrifuged, and frozen again in a dry ice-acetone bath and the organic layer divided into two sets of 13x100 mm culture tubes for analysis by GC-MS (Agilent 6890N, for nicotine) and liquid chromatography-tandem mass spectrometry (Thermo Fisher Vantage LC-MS/MS, for all other analytes), as previously described.7, 8 Representative LC-MS/MS chromatograms are provided in the Supporting Information, Figures S2 and S3. Concentrations were calculated using the instrument data system software, aqueous standards spanning the measured concentration range, and calibration curves prepared from analyte/internal standard peak area ratios and analyte concentrations using linear regression with 1/X weighting. The precision of the analytical method was demonstrated using National Institute of Standards and Technology Standard Reference Material 2585 (Organic Contaminants in House Dust). Table S1 (Supporting Information) shows coefficients of variation for each analyte in nine analytical replicates of three extracts of the standard reference material ranging in sample mass from 31 to 110 mg.
Questionnaires
Parents who participated in the dust collection responded firstly to an in-home interview designed to ascertain information relevant to childhood leukemia, such as parental race/ethnicity, parental age, and household annual income. The initial interviews were conducted from 2001-2007, on average 6 months prior to the first round of dust collection (2002-2007). Subsequently, at the time of the second dust collection in 2010, participating parents completed a second questionnaire by telephone designed to ascertain information about sources of residential chemical exposures and residential characteristics, such as the construction date and residence type.
During both interviews (2001-2007 and 2010), parents were asked to report current and past household smoking habits. Specifically, during the initial questionnaire (2001-2007), respondents were asked to report the history of active smoking for each parent at specific times (i.e., at the time of the interview; before, during, and after the index pregnancy; lifetime) Additionally, respondents were asked to report the history of passive smoking exposures in the home, at work/childcare, in the car, and in public/social settings for the mother, father, and child. During the second interview (2010), respondents were asked to characterize household smoking habits during the previous year and the history of household smoking since moving into their current home, by reporting whether anyone had regularly smoked cigarettes, pipes or cigars inside the home and whether any resident had regularly smoked outside the home (e.g., on the deck, in the yard, in the car, or at work). During the second questionnaire (2010) respondents were also asked whether anyone used smokeless tobacco products such as dipping or chewing tobacco in the home once a week or more during the previous 12 months.
Statistical analysis
Based on questionnaire responses from the interviews conducted in 2010, we grouped households into three tobacco-use categories: homes occupied by smokeless tobacco users (i.e., regular use at home during the previous year), homes occupied by active smokers [i.e., regular smoking by a resident inside (N=1) or outside (N=5) of the home during the previous year], and tobacco-free homes (i.e., no smokeless tobacco use at home during the previous year and no active smoking by a resident since the family moved into the home). The characteristics of these three groups have been previously described.6 Briefly, none of the households with a smokeless tobacco user reported any history of smoking (by the parents or others) in the index home. Some of the households with a smokeless tobacco user reported a history of parental smoking at a prior home; however, smoking ceased in these families at least 6 years prior to the first dust collection. Each family occupied the index home for at least one year prior to the initial dust collection and for at least four years prior to the second dust collection. Compared to tobacco-free households, a significantly larger proportion of households with a smokeless tobacco user had a history of parental smoking at prior homes. Otherwise, there were no statistically significant differences in the characteristics of the households with a smokeless tobacco user compared to tobacco-free homes (i.e., household annual income, home construction date and type, mother’s age and race/ethnicity were similar in both groups).
We compared concentrations of tobacco constituents between tobacco-use categories using the Wilcoxon two-sample Z-test (SAS v.9.3, Proc Npar1way). We also tested whether observed differences in logged concentrations of tobacco constituents by tobacco-use category remained significant after adjustment for a history of parental smoking at prior homes, household annual income, home construction date, and mother’s age and race/ethnicity using generalized estimating equations derived from a multivariable marginal model (SAS v.9.3, Proc Genmod). These contextual variables were previously related to nicotine concentrations in dust from CCLS homes.9 Demographic descriptors of mothers and fathers were mostly concordant within a household, so we used the more complete data describing mothers in regression models. For summary statistics, Z-tests, and regression models, observations below the lower limit of quantitation (LLOQ) were assigned a value of LLOQ/√2.
RESULTS
Table 2 shows detection frequencies and median concentrations of tobacco constituents in vacuum dust by tobacco-use category (see Supporting Information, Table S2 for additional summary statistics). Nicotine and the five minor tobacco alkaloids were detected in nearly every dust sample from homes of smokeless tobacco users and active smokers during both sampling rounds, with the exception of one 2,3’-bipyridine measurement that was below LLOQ in one home of a smokeless tobacco user in Sampling Round 1. Each of the minor tobacco alkaloids was also detected in at least 60% of the tobacco-free homes during both sampling rounds, with nicotine detected in over 90% of these homes. NNK was the most frequently detected of the tobacco-specific nitrosamines, with measurable quantities in both dust samples from each home of a smokeless tobacco user or active smoker. NNK was also detected in tobacco-free homes, with detection frequencies of 40% and 65% during Sampling Rounds 1 and 2, respectively. NNN was detected in at least two-thirds of the dust samples from homes of smokeless tobacco users and active smokers, but in no more than 10% of the dust samples from tobacco-free homes. Similarly, NNA and NAB were detected in some dust samples from homes of smokeless tobacco users and active smokers (33-67%), but in very few of the dust samples from tobacco-free homes (0-20%). NAT was detected in some dust samples from homes of smokeless tobacco users (50% and 40% for Sampling Rounds 1 and 2, respectively), but in very few of the dust samples from homes of active smokers (0% in both rounds) or tobacco free homes (0% and 5% for Sampling Rounds 1 and 2, respectively).
Table 2.
Detection frequencies and median concentrations (ng/g) of tobacco constituents in vacuum dust collected from homes participating in the California Childhood Leukemia Study during the first and second sampling rounds, by tobacco use at the index home.
| Tobacco constituent | 1st sampling round (2002-2007) |
2nd sampling round (2010) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smokeless tobacco users, N=6 |
Active Smokers, N=6 |
Tobacco-free homes, N=20 |
Smokeless tobacco users, N=5 |
Active Smokers, N=6 |
Tobacco-free homes, N=20 |
|||||||
|
|
|
|||||||||||
| % Det | Median | % Det | Median | % Det | Median | % Det | Median | % Det | Median | % Det | Median | |
| Nicotine | 100 | 14,000a | 100 | 7,000a | 90 | 520 | 100 | 15,000a | 100 | 7,800a | 95 | 510 |
| Tobacco-specific nitrosamines | ||||||||||||
| NNN | 67 | 5.7a | 67 | 1.6a | 5 | <1.4b | 80 | 4.3a | 83 | 2.9a | 10 | <1.4b |
| NNK | 100 | 6.3a | 100 | 3.7a | 40 | <0.45b | 100 | 3.4a | 100 | 5.8a | 65 | 0.51 |
| NNA | 50 | 1.2a | 67 | 0.46a | 0 | <0.45b | 40 | <0.45a, b | 50 | 0.60a | 5 | <0.45b |
| NAB | 50 | 0.28 | 33 | <0.15b | 20 | <0.15b | 60 | 0.29a | 50 | 0.18a | 10 | <0. 15b |
| NAT | 50 | 3.6a | 0 | <4.2b | 0 | <4.2b | 40 | <4.2b | 0 | <4.2b | 5 | <4.2b |
| Minor tobacco alkaloids | ||||||||||||
| Cotinine | 100 | 680a | 100 | 430a | 80 | 54 | 100 | 450a | 100 | 460a | 70 | 26 |
| Myosmine | 100 | 310a | 100 | 440a | 85 | 54 | 100 | 140a | 100 | 700a | 80 | 45 |
| N-formylnornicotine | 100 | 370a | 100 | 480a | 90 | 43 | 100 | 200a | 100 | 660a | 75 | 30 |
| Nicotelline | 100 | 5.5 | 100 | 8.0a | 95 | 1.0 | 100 | 2.9a | 100 | 7.1a | 100 | 0.62 |
| 2,3'-Bipyridine | 83 | 76a | 100 | 74a | 60 | 6.2 | 100 | 52a | 100 | 72a | 75 | 5.9 |
%Det=Detection frequency; NNN=N′-nitrosonornicotine; NNK=4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNA=4-(methylnitrosamino)-4-(3-pyridyl)butanal; NAB=N′-nitrosoanabasine; NAT=N′-nitrosoanatabine
Significantly greater than concentrations of the tobacco constituent in dust samples collected during the same sampling round from tobacco-free homes; using the Wilcoxon two-sample Z-test, two-sided p<0.05
Average lower limit of quantitation, determined as the lowest calibration standard for which back-calculated values were within ±20% of the expected concentration, with units converted from ng/sample to ng/g using average sample mass of 0.066 g.
Table 2 shows that, in general, median concentrations of tobacco constituents in dust samples from homes with a smokeless tobacco user were greater than median concentrations of tobacco constituents in dust samples from tobacco-free homes, with two exceptions being NNA and NAT concentrations in Sampling Round 2 (infrequently detected in both groups). Differences in concentrations of tobacco constituents between dust samples from homes with a smokeless tobacco user and dust samples from tobacco-free homes were generally statistically significant (Wilcoxon two-sample Z-test, two-sided p<0.05), with three exceptions being NAB and nicotelline in Sampling Round 1 and NAT in Sampling Round 2. Similarly, median concentrations of tobacco constituents in dust samples from homes with an active smoker were generally greater than median concentrations of tobacco constituents in dust samples from tobacco-free homes, and differences in concentrations of tobacco constituents between these two groups were generally statistically significant, with three exceptions being NAB in Sampling Round 1 and NAT in both sampling rounds (infrequently detected in both groups). Concentrations did not differ significantly between homes with a smokeless tobacco user and homes with an active smoker for any tobacco constituent, during either sampling round. Small differences in concentrations of tobacco constituents within-households between the first and second sampling round were not statistically significant and these differences were not explained by reported smoking habits, which did not change between rounds.
Table 3 shows results from the multivariable regression models of 63 dust samples from 32 participating homes. In crude models, levels of tobacco constituents were estimated to be 1.6- to 31-fold higher in homes with a smokeless tobacco user compared to tobacco-free homes (P<0.05 for all models except NAT). After adjustment for a history of parental smoking at prior homes, household annual income, home construction date, and mother’s age and race/ethnicity, levels of tobacco constituents from homes with smokeless tobacco users remained elevated in comparison to levels of tobacco constituents found in tobacco-free homes (range of 1.6- to 41-fold higher, P<0.05 for all models). In both crude and adjusted models, the largest difference between the two groups was observed for nicotine and the smallest for NAT. Most tobacco constituents were also significantly higher in homes of active smokers compared to tobacco-free homes, although this was not the case for NAB (crude model) or NAT (crude and adjusted models), which were infrequently detected in both groups.
Table 3.
Results from generalized estimating equations derived from marginal models; relative fold-differencea between tobacco-use groups in concentrations of tobacco constituents in vacuum dust collected from homes in the California Childhood Leukemia Study (2002-2010).
| Tobacco constituent | Crude models |
Adjusted modelsb |
||
|---|---|---|---|---|
| Smokeless tobacco vs. tobacco-free |
Active smokers vs. tobacco-free |
Smokeless tobacco vs. tobacco-free |
Active smokers vs. tobacco-free |
|
| Nicotine | 31* | 27* | 41* | 33* |
| Tobacco-specific nitrosamines | ||||
| NNN | 4.3* | 1.9* | 4.8* | 2.4* |
| NNK | 5.0* | 7.5* | 6.9* | 7.7* |
| NNA | 2.2* | 2.1* | 2.3* | 2.6* |
| NAB | 2.6* | 1.6 | 3.1* | 2.2* |
| NAT | 1.6 | 0.98 | 1.6* | 1.1 |
| Minor tobacco alkaloids | ||||
| Cotinine | 11* | 13* | 15* | 13* |
| Myosmine | 5.4* | 16* | 7.2* | 16* |
| N-formylnornicotine | 6.6* | 14* | 9.3* | 14* |
| Nicotelline | 3.6* | 9.8* | 5.7* | 9.4* |
| 2,3'-Bipyridine | 7.7* | 8.9* | 11* | 9.1* |
NNN=N′-nitrosonornicotine; NNK=4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNA=4-(methylnitrosamino)-4-(3-pyridyl)butanal; NAB=N′-nitrosoanabasine; NAT=N′-nitrosoanatabine
P< 0.05, based on 95% confidence intervals calculated using robust standard errors
Relative fold-difference = exp(β1); where β1 is the coefficient from the multivariable model, with tobacco-free homes as the referent group.
Adjusted for history of parental smoking in prior homes, household annual income, home construction date, mother’s age, and mother’s race/ethnicity.
Table S3 (Supporting Information) shows estimated effect sizes for the other explanatory variables that were included in the multivariable regression models described above (N=63 dust samples from 32 homes). After adjusting for reported tobacco use, households with non-Hispanic, White mothers (compared to Hispanic or Asian mothers) and households with annual income below $75,000 (compared to income ≥$75,000) had significantly higher concentrations of nicotine, NNK, cotinine, myosmine, N-formylnornicotine, nicotelline, and 2,3’-bipyridine. Households with younger mothers tended to have higher concentrations of nicotine, NNN, NNK, NAB, cotinine, myosmine, N-formylnornicotine, and 2,3’-bipyridine. Older homes tended to have higher concentrations of NNN, cotinine, nicotelline, and 2,3’-bipyridine. Tobacco-free homes and homes with a smokeless tobacco user that reported a history of parental smoking at previous homes did not have significantly higher concentrations of tobacco constituents in vacuum dust than lifetime non-smokers.
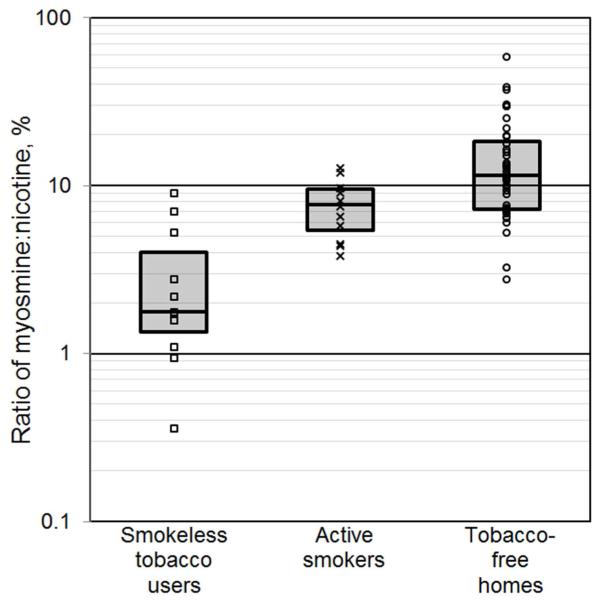
Figure 2 shows myosmine-to-nicotine ratios for the three tobacco-use categories. Ratios were smaller in dust samples from homes of smokeless tobacco users (median: 1.8%; interquartile range, IQR: 1.3-4.0%) than in homes of active smokers (median: 7.7%; IQR: 5.4-9.6%). Ratios were highest and most variable in dust samples from tobacco-free homes (median: 11%; IQR: 7.2-18%). Table S4 (Supporting Information) shows summary statistics of nicotelline-to-nicotine ratios for the three tobacco-use categories. The same conclusions can be drawn from the observed nicotelline-to-nicotine ratios as the analogous myosmine-to-nicotine ratios and, therefore, nicotelline-to-nicotine ratios are not discussed further.
Figure 2.
Myosmine-to-nicotine ratios (% nicotine) in vacuum dust samples collected from homes participating in the California Childhood Leukemia Study during the first (2002-2007) and second (2010) sampling rounds, by tobacco-use category. Vertical axis is shown on a logarithmic scale. Box plots represent the 25th, 50th, and 75th percentiles.
DISCUSSION
To our knowledge, the current analysis is the first to characterize levels of NNA, myosmine, N-formylnornicotine, and 2,3’-bipyridine in settled dust. The current analysis is also the first to characterize levels of carcinogenic tobacco-specific nitrosamines, NNN and NNK, in dust from the homes of smokeless tobacco users. Our findings demonstrate that levels of NNN and NNK are significantly higher in homes of smokeless tobacco users compared to tobacco-free homes. Fundamentally, our findings raise concerns about the safety of smokeless tobacco use in homes with young children.
In the absence of coincident exposure to cigarette smoke, the health risks posed by exposure to settled dust contaminated with tobacco constituents are not well known. Using in vitro assays, we previously reported the genotoxic potential of residual tobacco contamination on settled dust and household surfaces.8 Human cells were exposed to a mixture of tobacco constituents (i.e., NNN, NNK, NNA, cotinine, N-formylnornicotine, nicotelline, and 2,3’-bipyridine) that were sorbed on to model surfaces using a laboratory system and the resultant genotoxic effect was characterized by increased DNA strand breaks in the alkaline Comet assay as well as increased oxidative DNA damage in the long amplicon-quantitative PCR assay.8 One estimate suggests that the excess lifetime cancer risk attributable to non-dietary ingestion of NNK in settled dust at a concentration of 20 ng/g (compared to a maximum of 15 ng/g found in homes of smokeless tobacco users in this analysis) would exceed one case per one million exposed.10 Elevated concentrations of tobacco constituents in settled dust would be of greatest concern in homes with young children, because children spend more time at home, spend more time near the floor, and are more likely to make hand-to-mouth contact than adults.5 Matt et al.11 reported that, in non-smoking households, children with elevated dust-nicotine concentrations (10,900 and 11,000 ng/g for living rooms and bedrooms, respectively) had significantly higher urinary cotinine levels than children with background dust-nicotine concentrations, suggesting that, even without coincident exposure to cigarette smoke, exposure to settled dust contaminated with tobacco constituents could have a measurable biological effect. Likewise, da Silva et al.12 reported that non-smoking farmers exposed to high levels of tobacco constituents via dermal contact with tobacco leaves had elevated cotinine levels as well as increased markers of DNA damage. Moreover, because tobacco constituents in settled dust persist in the indoor environment11, are readily transported throughout the indoor environment13, and partition between settled dust and other surfaces14, children living with tobacco users are likely continuously exposed to tobacco constituents in every room of their home. The potential health impacts of exposure to tobacco constituents via dust and household surfaces for young children living with smokeless tobacco users warrant further investigation.
We have previously suggested two mechanisms by which constituents of smokeless tobacco are released into the residential environment (e.g., the carpet) – (1) a spill of smokeless tobacco by the user directly onto the floor and (2) volatilization of tobacco constituents to the indoor air from the mouth of a smokeless tobacco user, and subsequent contamination of settled dust on the floor. We observed that non-volatile constituents of smokeless tobacco, including NNK (estimated log KOA = 10.59, see Table 1), were present at much higher levels in dust from homes of smokeless tobacco users compared to tobacco-free homes. This observation supports our hypothesis, based on mass balance equations6, that tobacco spillage was probably the predominant mechanism for the transfer of smokeless tobacco constituents to settled dust, with volatilization likely playing a minor role.
While none of the households with a smokeless tobacco user reported any history of smoking in the index home and the history of parental smoking at prior homes was accounted for in regression models; in our previous analysis6, we could not eliminate cigarette smoke (e.g., smoke infiltration or unreported smoking by guests in the home) as a potential source of elevated nicotine concentrations in homes of smokeless tobacco users. To address this limitation, in the current analysis, we used the ratio of myosmine:nicotine in dust samples as a novel indicator of the source of tobacco constituents in each home. Myosmine is a minor tobacco alkaloid that is found in cigarette tobacco and smokeless tobacco at a level of less than 0.5% of the nicotine content.15, 16 However, during cigarette combustion, additional mysomine is generated as a result of pyrolysis, and mysomine has been measured in cigarette smoke in levels up to 7% of the nicotine content.17, 18 Thus, we propose that the ratio of myosmine:nicotine in dust samples can be used as an indicator of the source of the tobacco contamination, with settled dust contaminated by tobacco smoke expected to have a higher ratio than settled dust contaminated by smokeless tobacco products. Accordingly, we generally observed smaller myosmine:nicotine ratios in dust samples from homes of smokeless tobacco users compared to dust samples from homes of active smokers. However, in three dust samples from two homes of smokeless tobacco users, myosmine concentrations were at least 5% as high as nicotine concentrations, suggesting that the contamination derived from tobacco smoke rather than smokeless tobacco products. Correspondingly, these three dust samples had low nicotine concentrations (1,500-3,200 ng/g), in the range of background contamination found in tobacco-free homes. The observed myosmine:nicotine ratios minimize the likelihood that the elevated levels of tobacco constituents found in dust samples from homes of smokeless tobacco users were the result of contamination from cigarette smoke.
Tobacco constituents can be tracked inside a home on clothes, shoes, or skin contaminated by cigarette smoke or they can enter a home when cigarette smoke infiltrates doors or windows.19 This type of indirect contamination from cigarette smoke is likely the major source of myosmine and nicotine in most tobacco-free homes. Accordingly, the observed median myosmine:nicotine ratio for tobacco-free homes was similar to the ratio for homes of active smokers. However, the variability in myosmine:nicotine ratios differed between tobacco-free homes and homes of active smokers. In homes of active smokers, it is likely that the short distance (in space and time) between cigarette smoking and adsorption of the tobacco constituents onto settled dust resulted in a limited range of observed myosmine:nicotine ratios that was reflective of nearby sources.
In contrast, in tobacco-free homes, the longer distances (in space and time) between cigarette smoking and adsorption of the tobacco constituents onto settled dust allowed for changes in the relative abundance of tobacco constituents (e.g., transformation of nicotine into myosmine via ultraviolet light20, 21 or ozone22), and differences in these chemical aging processes between homes resulted in a wider range of observed myosmine:nicotine ratios.
In a previous analysis of the CCLS population, we reported that contextual factors, such as household annual income, home construction date, parental age, and (to a lesser extent) mother’s race/ethnicity were determinants of background nicotine contamination in settled dust.9 In the present analysis, we confirmed that some of these factors were related to concentrations of tobacco constituents using a multivariable model, but adjusting for these covariates did not explain the observed differences in levels of tobacco constituents between homes of smokeless tobacco users and tobacco-free homes.
As previously noted6, the observed range of nicotine concentrations in tobacco-free homes (maximum of 3,400 ng/g) was consistent with previous investigations of nicotine in floor dust from other non-smoking California homes (maximums of 4,700 and 4,400 ng/g, respectively).11, 23 Likewise, the observed range of NNN (maximum of 10 ng/g), NNK (maximum of 8.7 ng/g), and nicotelline (maximum of 13 ng/g) in tobacco-free homes, was consistent with a previous investigation of vacuum cleaner dust from five other non-smoking California homes (maximums of 1.83, 6.14, and 8.13 ng/g for NNN, NNK, and nicotelline, respectively)23; whereas the central tendencies for concentrations of these tobacco constituents were lower in our study. When comparing concentrations of tobacco constituents found in dust from active smokers in our study to those reported in previous studies, it is important to note that 5 of 6 households in the active smoking group of our study reported exclusively smoking outside of the home. Because outdoor smokers have lower dust nicotine levels than indoor smokers19, it was not surprising that we observed relatively low nicotine concentrations in homes of active smokers compared to previous California studies11, 23. However, in the one CCLS household that reported smoking inside the home, we observed nicotine concentrations which were more representative of levels found in previous California studies (i.e., 22,000 and 69,000 ng/g). Likewise, the two dust samples from this CCLS home with active indoor smoking also had concentrations of NNN (5.23 and 8.43 ng/g), NNK (21.3 and 19.4 ng/g), and nicotelline (18.2 and 42.4 ng/g) that were comparable to NNN (5.97 and 64.9 ng/g), NNK (36.9 and 131 ng/g), and nicotelline (48.4 and 297 ng/g) concentrations from two other California homes with active indoor smoking.23 One study reported higher concentrations of NNN, NNK, NAB, and NAT in vacuum dust collected from smoking and non-smoking homes in Spain compared to homes in California.10 Interestingly, in both this previous study10 and our analysis, NAB was detected more frequently than NAT in homes of active smokers, despite the fact that NAB is found at lower concentrations in mainstream cigarette smoke than NAT24. Unfortunately, we cannot meaningfully evaluate the relative concentrations of NAB and NAT in dust from homes of active smokers in our study, due to the low detection frequencies and the unequal LLOQ values for NAB (0.15 ng/g) and NAT (4.2 ng/g).
The main limitation of our analysis was our ability to characterize concentrations of tobacco constituents in only a small number of homes with a smokeless tobacco user (N = 6). Additionally, while we were able to support our findings using two separate dust samples from each household, we only identified homes with a smokeless tobacco user at the time of the second dust collection and we assumed, but did not confirm, that smokeless tobacco was also used in these homes at the time of the first dust collection. Because our analysis included only a small number of homes, our findings should be replicated in other populations.
In this analysis, we assumed that concentrations of tobacco constituents measured in dust collected from household vacuum cleaners were representative of children’s exposure to tobacco constituents; however, vacuum samples may have been misleading in homes where residents used a vacuum to clean an accidental ashtray or smokeless tobacco spill. Other differences in vacuum cleaning practices between homes (e.g., time since last bag change) as well as differences in the vacuum cleaners used to collect dust (e.g., heat produced, particle sampling efficiency) may have introduced additional variability in observed concentrations of tobacco constituents. We did not ask study participants to describe their vacuum cleaner habits with sufficient detail to evaluate these possibilities. In future studies, investigators could use a standardized dust collection protocol, such as ASTM Standard D5438, which utilizes a high volume small surface sampler rather than household vacuum cleaners. Alternatively, future studies could validate our findings using biological samples, such as urinary cotinine, to characterize exposure to tobacco constituents. In either case, future studies should involve a larger number of children living in homes occupied by smokeless tobacco users and should assess whether the health of these children is affected by exposure to tobacco constituents in settled dust.
Using dust samples collected from CCLS homes occupied by smokeless tobacco users, we demonstrated that concentrations of carcinogenic tobacco constituents, NNN and NNK, were elevated above background levels found in tobacco-free homes, and were comparable to levels in homes occupied by active smokers. We expect that young children will be exposed to these hazardous chemicals via ingestion of contaminated dust and dermal contact with contaminated dust and household surfaces. The potential health effects of a child’s exposure to smokeless tobacco contamination warrant further investigation.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the families for their participation. We also thank the clinical investigators at the following collaborating hospitals for help in recruiting patients: University of California Davis Medical Center (Dr. Jonathan Ducore), University of California San Francisco (Drs. Mignon Loh and Katherine Matthay), Children’s Hospital of Central California (Dr. Vonda Crouse), Lucile Packard Children’s Hospital (Dr. Gary Dahl), Children’s Hospital Oakland (Dr. James Feusner), Kaiser Permanente Oakland (Drs. Daniel Kronish and Stacy Month), Kaiser Permanente Roseville (Drs. Kent Jolly and Vincent Kiley), Kaiser Permanente Santa Clara (Drs. Carolyn Russo, Denah Taggart, and Alan Wong), and Kaiser Permanente San Francisco (Dr. Kenneth Leung). Finally, we acknowledge the entire California Childhood Leukemia Study staff for their effort and dedication.
FUNDING INFORMATION
This work was supported by the National Institutes of Health, National Institute of Environmental Health Sciences (Grant Nos. R01ES009137, R01ES015899, and P42ES0470518), the National Institute on Drug Abuse (Grant No. P30 DA012393), the National Center for Research Resources (Grant No. S10 RR026437), and the California Consortium on Thirdhand Smoke, California Tobacco Related Disease Research Program (Grant No. 20PT-0184). These funding agencies had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the paper for publication.
ABBREVIATIONS
- CCLS
California Childhood Leukemia Study
- IQR
interquartile range
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LLOQ
lower limit of quantitation
- NAB
N′-nitrosoanabasine
- NAT
N′-nitrosoanatabine
- NNA
4-(methylnitrosamino)-4-(3-pyridyl)butanal
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNN
N′-nitrosonornicotine
Footnotes
SUPPORTING INFORMATION AVAILABLE
Supporting Information includes a detailed dust sample preparation protocol, representative LC-MS/MS chromatograms, a description of the analytical limits of detection, accuracy, and precision, complete summary statistics of tobacco constituents in vacuum dust collected from participating homes, results from generalized estimating equations derived from marginal models, and summary statistics of nicotelline-to-nicotine ratios by tobacco-use category. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).International Agency for Research on Cancer . Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. Vol. 89. World Health Organization; Lyons, France: 2007. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [PMC free article] [PubMed] [Google Scholar]
- (2).Capehart TC. Market and Trade Economics Division, Economic Research Service. United States Department of Agriculture; Washington, D.C.: 2006. Tobacco situation and outlook yearbook. [Google Scholar]
- (3).Centers for Disease Control and Prevention State-specific prevalence and trends in adult cigarette smoking--United States, 1998-2007. MMWR.Morbidity and mortality weekly report. 2009;58:221–226. [PubMed] [Google Scholar]
- (4).Substance Abuse and Mental Health Services Administration . Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2012. [Google Scholar]
- (5).Cohen Hubal EA, Sheldon LS, Burke JM, McCurdy TR, Berry MR, Rigas ML, Zartarian VG, Freeman NC. Children's exposure assessment: a review of factors influencing children's exposure, and the data available to characterize and assess that exposure. Environ. Health Perspect. 2000;108:475–486. doi: 10.1289/ehp.108-1638158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Whitehead TP, Metayer C, Park JS, Does M, Buffler PA, Rappaport SM. Levels of nicotine in dust from homes of smokeless tobacco users. Nicotine Tob. Res. 2013;15:2045–2052. doi: 10.1093/ntr/ntt096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Jacob P, Wu S, Yu L, Benowitz NL. Simultaneous determination of mecamylamine, nicotine, and cotinine in plasma by gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. (3rd) 2000;23:653–661. doi: 10.1016/s0731-7085(00)00343-5. [DOI] [PubMed] [Google Scholar]
- (8).Hang B, Sarker AH, Havel C, Saha S, Hazra TK, Schick S, Jacob P, Rehan VK, Chenna A, Sharan D, Sleiman M, Destaillats H, Gundel LA. Thirdhand smoke causes DNA damage in human cells. Mutagenesis. (3rd) 2013;28:381–391. doi: 10.1093/mutage/get013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Whitehead T, Metayer C, Ward MH, Nishioka MG, Gunier R, Colt JS, Reynolds P, Selvin S, Buffler P, Rappaport SM. Is house-dust nicotine a good surrogate for household smoking? Am. J. Epidemiol. 2009;169:1113–1123. doi: 10.1093/aje/kwp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ramirez N, Ozel MZ, Lewis AC, Marce RM, Borrull F, Hamilton JF. Exposure to nitrosamines in thirdhand tobacco smoke increases cancer risk in non-smokers. Environ. Int. 2014;71:139–147. doi: 10.1016/j.envint.2014.06.012. [DOI] [PubMed] [Google Scholar]
- (11).Matt GE, Quintana PJ, Zakarian JM, Fortmann AL, Chatfield DA, Hoh E, Uribe AM, Hovell MF. When smokers move out and non-smokers move in: residential thirdhand smoke pollution and exposure. Tob. Control. 2011;20:e1. doi: 10.1136/tc.2010.037382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Da Silva FR, Da Silva J, Nunes E, Benedetti D, Kahl V, Rohr P, Abreu MB, Thiesen FV, Kvitko K. Application of the buccal micronucleus cytome assay and analysis of PON1Gln192Arg and CYP2A6*9(-48T>G) polymorphisms in tobacco farmers. Environ. Mol. Mutagen. 2012;53:525–534. doi: 10.1002/em.21713. [DOI] [PubMed] [Google Scholar]
- (13).Hoh E, Hunt RN, Quintana PJ, Zakarian JM, Chatfield DA, Wittry BC, Rodriguez E, Matt GE. Environmental tobacco smoke as a source of polycyclic aromatic hydrocarbons in settled household dust. Environ. Sci. Technol. 2012;46:4174–4183. doi: 10.1021/es300267g. [DOI] [PubMed] [Google Scholar]
- (14).Weschler CJ, Nazaroff WW. SVOC exposure indoors: fresh look at dermal pathways. Indoor Air. 2012;22:356–377. doi: 10.1111/j.1600-0668.2012.00772.x. [DOI] [PubMed] [Google Scholar]
- (15).Piade JJ, Hoffman D. Chemical studies on tobacco smoke LXVII. Quantitative determination of alkaloids in tobacco by liquid chromatography. J. Liq. Chromatogr. 1980;3:1505–1515. [Google Scholar]
- (16).Chen PX, Qian N, Burton HR, Moldoveanu SC. Analysis of minor alkaloids in tobacco: A collaborative study. Beitr. Tabakforsch. Int. 2005;21:369–379. [Google Scholar]
- (17).Bodnar JE, Schumacher JN. Sidestream smoke composition comparison of alpha TM-6 cigarettes and 1R4F reference cigarettes using capillary gas chromatography and mass selective detection. R.J. Reynolds, New Product Technologies Department, Report No . 42. Available from the Legacy Tobacco Document Library; Unviersity of California, San Francisco: 1988. [Google Scholar]
- (18).Schmeltz I, Wenger A, Hoffman D, Tso TC. Chemical studies on tobacco smoke. 63. On the fate of nicotine during pyrolysis and in a burning cigarette. J. Agric. Food Chem. 1979;27:602–608. [Google Scholar]
- (19).Matt GE, Quintana PJ, Hovell MF, Bernert JT, Song S, Novianti N, Juarez T, Floro J, Gehrman C, Garcia M, Larson S. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob. Control. 2004;13:29–37. doi: 10.1136/tc.2003.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Benner CL, Bayona JM, Caka FM, Tang H, Lewis L, Crawford J, Lamb JD, Lee ML, Lewis EA. Chemical composition of environmental tobacco smoke. 2. Particulate-phase compounds. Environ. Sci. Technol. 1989;23:688–699. [Google Scholar]
- (21).Eatough DJ, Benner CL, Bayona JM, Richards G, Lamb JD, Lee ML, Lewis EA, Hansen LD. Chemical composition of environmental tobacco smoke. 1. Gas-phase acids and bases. Environ. Sci. Technol. 1989;23:679–687. [Google Scholar]
- (22).Petrick LM, Sleiman M, Dubowski Y, Gundel LA, Destaillats H. Tobacco smoke aging in the presence of ozone: A room-sized chamber study. Atmos. Environ. 2011;45:4959–4965. [Google Scholar]
- (23).Jacob P, Goniewicz ML, Havel CM, Schick SF, Benowitz NL. Nicotelline: a proposed biomarker and environmental tracer for particulate matter derived from tobacco smoke. Chem. Res. Toxicol. (3rd) 2013;26:1615–1631. doi: 10.1021/tx400094y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul. Toxicol. Pharmacol. 2005;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.