Abstract
The surface of enveloped viruses can be extensively glycosylated. Unlike the glycans coating pathogens such as bacteria and fungi, glycans on viruses are added and processed by the host-cell during biosynthesis. Glycoproteins are typically subjected to α-mannosidase processing and Golgi-mediated glycosyltransferase extension to form complex-type glycans. In envelope viruses, exceptions to this default pathway are common and lead to the presence of oligomannose-type glycan structures on the virion surface. In one extreme example, HIV-1 utilizes a high density of glycans to limit host antibody recognition of protein. However, the high density limits glycan processing and the resulting oligomannose structures can be recognised by broadly neutralising antibodies isolated form HIV-1 infected patients. Here we discuss how divergence from host-cell glycosylation can be targeted for vaccine design.
Introduction
The glycan structures coating the surface of bacteria, fungi, parasites and viruses are critical for disease transmission through interaction with host receptors, in particular lectins, and in shielding pathogens from the immune system. Since the discovery that conjugation of polysaccharides to carrier proteins can lead to successful T cell dependent immune responses to carbohydrates, there has been significant success in the development of polysaccharide conjugate vaccines that protect against bacterial infections including Haemophilius influenzae type b (Hib), Neisseria meningitides and Streptococcus pneumoniae [1]. However, there are currently no carbohydrate-based vaccines that protect against viral infection. In this review we explore the scope and potential for targeting the glycan structures on viruses for vaccine design with particular reference to HIV-1 where, in some patients, glycan-binding broadly neutralizing antibodies (bnAbs) are elicited during HIV-1 infection.
Viral glycosylation
Upon entry into a mammalian cell, a virus must replicate and produce new viral particles to sustain and spread infection. Viruses hijack the protein synthesis, glycosylation machinery and folding pathway of the host cell to produce the necessary proteins and glycoproteins required for virion production. In the endoplasmic reticulum (ER) Glc3Man9GlcNAc2 is transferred to Asn residues within the glycosylation sequence Asn-X-Thr/Ser (where X can be any amino acid except Pro). Typically glycoproteins are next subjected to a very ordered pathway of glycosidase and glycosyltransferase enzymes that first see the glycan trimmed to Man5GlcNAc2. Diversification to complex-type glycans begins with addition of a β1,2-linked GlcNAc residue to Man5GlcNAc2 in the medial Golgi apparatus. Further trimming and processing in the medial and late Golgi apparatus leads to a wide array of hybrid- and complex-type glycans and these structures are often dependent on the producer cell type [2].
Challenges for developing vaccines targeting viral glycan epitopes
Generation of antibodies to glycans has several challenges [3]. Firstly, due to the inherent weakness of carbohydrate-protein interactions binding affinities must be enhanced through avidity effects. Lectins for example are able to overcome this by using multiple carbohydrate binding domains to interact with arrays of glycan ligands. Secondly, glycoproteins usually always exist as a number of different glycoforms where the same protein backbone is glycosylated with different glycan structures [4]. This microheterogeneity weakens the antigenic response to the individual glycan structures. Further, these glycans are often dynamic and multiple conformations may be presented to the immune system further weakening the response. Thirdly, as glycosylation is ubiquitous to all mammalian cells, the host may display tolerance towards these sugars. Combined, these effects result in glycans being poorly immunogenic. The major concern, and potential limitation of generating antibodies against self-glycan structures, is their potential auto-reactivity and negative selection in vivo.
Envelope glycosylation exhibits features of self and non-self
Cases in which the viral glycosylation diverges from the typical pathway may present opportunities for exploiting viral glycosylation for vaccine design. The producer cell dependence of the Golgi processing phase gives rise to the capacity for viruses to exhibit antigenic shift both during inter- and intra-species transmission and this can be pronounced in inter-species transmission of enveloped viruses. At one extreme, in the initial infection of a human host by arthropod-borne arboviruses the virus displays insect-derived glycans. These are typically dominated by paucimannose structures but shift to human complex-type glycosylation as soon as viral production is established in the new host. An illustration of this antigenic shift has been revealed by the mass spectrometric analysis of Semliki Forest virus glycans derived from mammalian and insect cells [5]. Similarly, Dengue virus (DENV) is transmitted to humans via mosquitoes and therefore DENV Env produced in insect cells contains mostly oligomannose and paucimannose structures whereas virus Env produced in primary dendritic cells contains complex sugars [6, 7]. These differences in glycan structures impact on binding to the viral entry factors DC-SIGN and L-SIGN and subsequently cell tropism [6].
A similar but subtler effect can even be detected during viral transmission between humans and derives from glycan modifications of the ABO blood group system. The carbohydrate epitopes have been detected on the surface of HIV-1 particles and anti-A and anti-B-group antibodies can infer some immunity to virus derived from A-positive and B-positive carriers, respectively [8]. It may be expected that antibodies to other non-human carbohydrate epitopes, such as alpha-Gal epitopes, may similarly impede intra-species viral transmission. The antiviral properties of naturally occurring anti-glycan antibodies may point to a universal vaccine strategy against viruses from particular non-human vectors.
Virion assembly and secretion pathway leave an imprint on the glycome
While cell-origin can influence the glycosylation of enveloped viruses, the viral structure and assembly pathway can also have a significant impact. A number of viruses display oligomannose-type glycans on their envelope glycoprotein and the mechanisms by which these oligomannose-type glycans are retained differ depending on the viral structure and secretion pathway. For example, unlike many enveloped viruses that bud from the infected cell membrane, HCV buds from the ER and as such the E1/E2 proteins that are incorporated into the virion contain almost entirely under-processed Man8–9GlcNAc2 sugars [9]. In the above example of Semliki Forest virus, a significant population of oligomannose-type glycans were retained even when derived from mammalian cells. Similarly, the Gc glycoprotein of Uukuniemi virus (UUKV), the model virus for the phlebovirus genus, is dominated by oligomannose-type glycans. These glycans provide the ligands for the attachment receptor, DC-SIGN [10]. The abundance of complex glycans on UUKV Gn together with a trace population of N-acetylpolylactosamines is consistent with viral budding from the medial-Golgi apparatus and a steric mechanism that shields Gc glycans from α-mannosidase processing [11].
Ebola virus envelope trimer has two highly glycosylated domains; the glycan cap and the mucin like domain [12]. Due to the inherent difficulties of working with this virus the glycosylation of GP1/GP2 has only been studied using recombinant proteins. The glycans released from GP consist of oligomannose, hybrid, bi-, tri- and tetra-antennary compounds [13, 14]. The high degree of glycan processing implies that the glycans are largely accessible to glycosidase and glycosyltransferases. Similar studies with recombinant attachment glycoproteins from Nipah, Hendra and Machupo viruses (the latter being the causative agent of Bolivian haemorrhagic fever) revealed similar highly processed structures typical of secreted and cell surface glycoproteins [15–17]. In this way, the self-glycans can be seen as an immunologically silent cloak covering the viral protein.
Dense clustering of glycans on HIV-1 leads to divergence from self
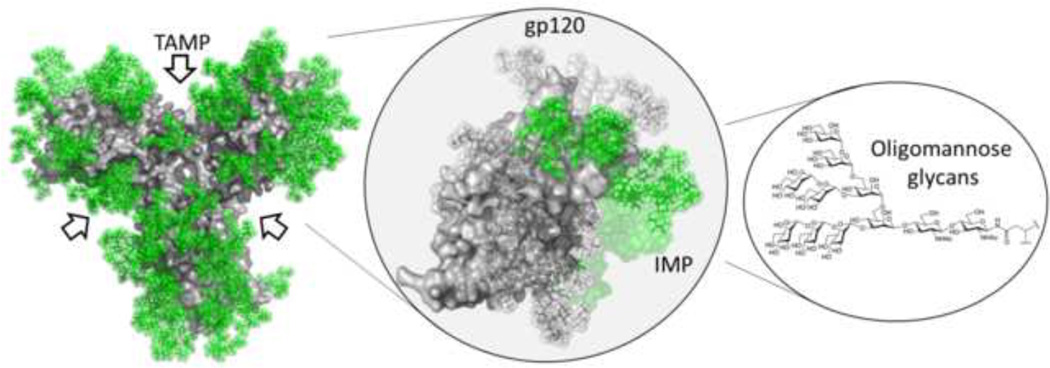
The extremely high density of glycan on the HIV-1 viral spike is unusual amongst enveloped viruses. The HIV-1 envelope glycoprotein consists of a trimer of a gp120 and gp41 heterodimer. Each gp120 subunit has a median of 25 N-linked glycosylation sites [18] and approximately 50% of its mass consists of carbohydrate making it one of the most heavily glycosylated proteins known. Although the glycans are added by the host-cell as described above, analysis of the glycans released from recombinantly expressed gp120 has revealed an unusual and conserved population of Man8–9GlcNAc2 structures [19–23]. It has been shown that the tight clustering of glycans on Env prevent the ER α-mannosidase I enzyme from accessing its substrate and glycans are trapped as Man8–9GlcNAc2 [20]. These oligomannose-type glycans form a cluster on the envelope surface, often referred to as ‘the mannose patch’ or ‘intrinsic mannose patch’ (IMP), that is present across all viral clades (Figure 1) [19, 20, 24]. The recent crystal and cryo-EM structures of a stabilised Env trimer mimic further demonstrated the close proximity of the N-linked glycans on HIV-1 [25–27]. Additional studies have shown that the abundance of oligomannose-type glycans is further increased in the context of the intact trimer [28] and on the virion surface [19, 20] leading to a ‘trimer-associated mannose-patch’ (TAMP). This increase likely arises from further restriction of the glycan processing enzymes due to additional protein-glycan and glycan-glycan interactions occurring at the interface of monomers within the trimer. Therefore although HIV-1 has used the host cell machinery for glycosylation of Env, the clustering of glycan sites creates a non-self glycan motif on gp120.
Figure 1. The HIV glycan shield.
HIV-1 Env glycosylation contains a population of oligomannose-type glycans that can be ascribed to the intrinsic mannose patch (IMP) and trimer-associated mannose patch (TAMP). Monomeric gp120 expressed outside of the context of the trimer only contains the IMP. The Env protein surface (grey) is derived from the crystal structure [25] and the glycans have been modelled in green (Pritchard et al., unpublished). The glycan structure is Man9GlcNAc2.
HIV-1 bnAbs target Envelope glycans
Despite the challenges described above some HIV-1 infected individuals develop antibodies capable of binding the glycans on HIV-1 Env. Approximately 10–30% of HIV-1 infected individuals develop broadly neutralizing serum after 3 years of infection [29]. Over the last 5–10 years there has been a considerable effort to identify sites of vulnerability on the HIV-1 envelope glycoprotein that can be targeted for vaccine design. Several groups have isolated bnAbs from such individuals. These bnAbs are extremely broad and potent [30–35], e.g. PGT128 can neutralize 72% of circulating HIV-1 strains with median IC50 of 0.02 µg/mL [30], and have been shown to protect against SHIV infection when passively administered to macaques at low serum concentrations [36, 37]. Therefore immunogens capable of eliciting these bnAbs would likely form an important component of an effective HIV-1 vaccine [38].
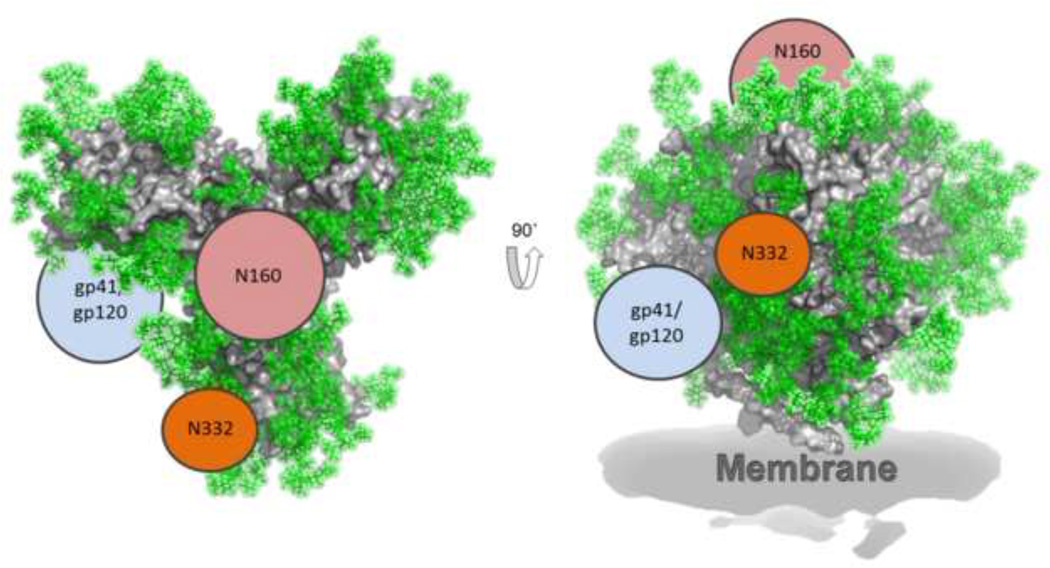
Until relatively recently, the glycosylation of HIV-1 Env has traditionally been referred to as the HIV-1 ‘glycan-shield’ or ‘silent-face’ due to the poor immunogenicity of carbohydrates and their ‘self’ nature. However, combined biochemical and structural analyses have revealed that a significant proportion of the isolated HIV-1 bnAbs bind to glycan structures on HIV-1 Env [29]. These bnAbs target three main regions on Env; i) the N332 glycan (e.g. 2G12, 10–1074, PGT121, PGT128, PGT135 [30, 39, 40]), ii) the N160 glycan (e.g. PG9, PGT145, CH04 [30, 33, 41]) and iii) the glycans at the gp120-gp41 interface (e.g. PGT151 [42] and 35O22 [43]) (Figure 2). 2G12 overcomes the weak carbohydrate-protein interactions by using a unique domain-exchanged structure in which the two variable heavy chain domains cross-over to form a multivalent binding surface [44, 45]. The remaining N332 bnAbs use long CDRH3 loops to penetrate through the glycan shield and interact with the V3/V4 loops, the N332 glycan and at least one other neighbouring glycan [25, 46, 47]. Similarly, the long CDRH3 loops of N160 binding bnAbs penetrate through the glycan shield and interact with the V1 loop, the N160 glycan and the N156 glycan [48–50]. The PGT151-Env interaction, although less characterised, appears to include either tri- and tetra-anntenary glycans on gp41 and protein residues in gp120 [42]. The common feature of the three HIV bnAb classes described is that the weak protein-carbohydrate interactions are overcome through multivalent binding to multiple glycans and protein residues within one Fab. Interestingly, crystal structures of Abs binding to other glycan antigens show whole saccharide units binding either within a pocket or groove [51, 52]. However, the majority of HIV glycan-binding bnAbs, with the exception of 2G12, bind across the face of the glycan [53]. Overall, unlike previously thought, isolation of glycan-binding bnAbs from HIV-1 infected patients strongly highlights the glycan structures on HIV-1 Env as a feasible target for HIV-1 vaccine design.
Figure 2. Regions of immune vulnerability on HIV-1 Env.
HIV-1 bnAbs target three main glycan sites; i) the N332 glycan (e.g. 2G12, 10–1074, PGT121, PGT128, PGT135 [30, 39, 40]), ii) the N160 glycan (e.g. PG9, PGT145, CH04 [30, 33, 41]) and iii) the glycans at the gp120-gp41 interface (e.g. PGT151 [42] and 35O22 [43]). The Env protein surface (grey) is derived from the crystal structure [25] and the glycans have been modelled in green (Pritchard et al., unpublished). Regions of vulnerability to bnAbs are indicated with only one copy per trimer shown for simplicity.
Perspective: Strategies for eliciting carbohydrate-binding antibodies
There are significant challenges to overcome in the development of vaccines that target viral glycan antigens. In the case of HIV-1 the immune system has overcome these challenges and several HIV-1 infected individuals have developed glycan-binding HIV-1 bnAbs. These bnAbs typically target the non-self cluster of oligomannose-type glycans on gp120. Attempts to elicit 2G12-like bnAbs exploiting the antigenic mimicry of yeast carbohydrates [54–56] or using synthetic derived oligomannose clusters has had very limited success [57] presumably due to the requirement of domain-exchange for neutralization and challenges associated with eliciting antibodies with this unusual structure [58]. The challenge now is to develop glycan-based immunogens that have a glycan-protein signature similar to that on HIV-1 virions [19, 20, 59] and that elicit bnAbs capable of binding multiple glycans and protein surfaces. Recent studies have revealed that some glycan-binding bnAbs, e.g. PGT121 and PGT128, must also actively avoid or accommodate additional glycans to bind potently [60, 61]. In these cases high levels of somatic hypermutation and insertions and deletions are critical for neutralization [47, 62, 63]. These findings highlight the need for immunogens to not only display the glycans important for binding but carefully positioned glycans that the antibody must evolve to avoid for potent neutralizing activity. Further, as it can take up to three years for these bnAbs to develop efforts in the HIV-1 vaccine field are also currently focussed on studying the evolution of glycan-binding bnAbs longitudinally to gain insight into how these bnAbs evolved during infection [62, 64, 65] and using this information for immunogen design.
It is clear that to generate vaccines against viral sugar antigens one must exploit their non-self features. What is not yet known is whether the glycan structures and arrangements on viruses other than HIV-1 will allow for development of potent neutralizing antibodies. Although an HCV and an influenza A neutralizing antibody (nAbs) have been identified that make minor contacts with glycans on E2 and hemagglutinin respectively [66, 67], the neutralization dependency on Env glycosylation was minimal. Lectins however have been shown to have anti-viral activity against HCV, Ebola, and DENV [68–71]. For example, cyanovirin (CVN) and scytovirin (SVN) that have specificity for oligomannose-type glycans inhibit entry of Ebola into Vero cells [68, 69]. Therefore if carbohydrate-binding antibodies could be generated against such viruses they could inhibit viral entry. Without antibody templates from which immunogens can be designed, the prospect of vaccines against the viruses discussed above might be more challenging. However with the advancements in glycan microarray technologies [72] and antibody isolation techniques [73] it may be possible to screen for such neutralizing antibodies from serum of infected patients to identify such templates.
Highlights.
Glycosylation of enveloped viruses exhibit features of self and non-self
HIV envelope displays a conserved cluster of oligomannose glycans
Divergence of HIV-1 envelope glycosylation from self forms a target for bnAbs
HIV-1 glycan-binding bnAbs are elicited during natural infection
Acknowledgments
K.J.D. is supported by an MRC Career Development Fellowship (MR/K024426/1) and M.C. is supported by the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery Grant (UM1AI100663) and the International AIDS Vaccine Initiative through the Neutralizing Antibody Consortium and Bill and Melinda Gates Center for Vaccine Discovery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Astronomo RD, Burton DR. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010;9(4):308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 3.Scanlan CN, Offer J, Zitzmann N, Dwek RA. Exploiting the defensive sugars of hiv-1 for drug and vaccine design. Nature. 2007;446(7139):1038–1045. doi: 10.1038/nature05818. [DOI] [PubMed] [Google Scholar]
- 4.Rudd PM, Dwek RA. Glycosylation: Heterogeneity and the 3d structure of proteins. Crit Rev Biochem Mol Biol. 1997;32(1):1–100. doi: 10.3109/10409239709085144. [DOI] [PubMed] [Google Scholar]
- 5.Crispin M, Harvey DJ, Bitto D, Bonomelli C, Edgeworth M, Scrivens JH, Huiskonen JT, Bowden TA. Structural plasticity of the semliki forest virus glycome upon interspecies transmission. J Proteome Res. 2014;13(3):1702–1712. doi: 10.1021/pr401162k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dejnirattisai W, Webb AI, Chan V, Jumnainsong A, Davidson A, Mongkolsapaya J, Screaton G. Lectin switching during dengue virus infection. J Infect Dis. 2011;203(12):1775–1783. doi: 10.1093/infdis/jir173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hacker K, White L, de Silva AM. N-linked glycans on dengue viruses grown in mammalian and insect cells. J Gen Virol. 2009;90(Pt 9):2097–2106. doi: 10.1099/vir.0.012120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neil SJ, McKnight A, Gustafsson K, Weiss RA. Hiv-1 incorporates abo histo-blood group antigens that sensitize virions to complement-mediated inactivation. Blood. 2005;105(12):4693–4699. doi: 10.1182/blood-2004-11-4267. [DOI] [PubMed] [Google Scholar]
- 9.Vieyres G, Thomas X, Descamps V, Duverlie G, Patel AH, Dubuisson J. Characterization of the envelope glycoproteins associated with infectious hepatitis c virus. J Virol. 2010;84(19):10159–10168. doi: 10.1128/JVI.01180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozach PY, Kuhbacher A, Meier R, Mancini R, Bitto D, Bouloy M, Helenius A. Dc-sign as a receptor for phleboviruses. Cell Host Microbe. 2011;10(1):75–88. doi: 10.1016/j.chom.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Crispin M, Harvey DJ, Bitto D, Halldorsson S, Bonomelli C, Edgeworth M, Scrivens JH, Huiskonen JT, Bowden TA. Uukuniemi phlebovirus assembly and secretion leave a functional imprint on the virion glycome. J Virol. 2014;88(17):10244–10251. doi: 10.1128/JVI.01662-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. Structure of the ebola virus glycoprotein bound to an antibody from a human survivor. Nature. 2008;454(7201):177–182. doi: 10.1038/nature07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powlesland AS, Fisch T, Taylor ME, Smith DF, Tissot B, Dell A, Pohlmann S, Drickamer K. A novel mechanism for lsectin binding to ebola virus surface glycoprotein through truncated glycans. J Biol Chem. 2008;283(1):593–602. doi: 10.1074/jbc.M706292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchie G, Harvey DJ, Stroeher U, Feldmann F, Feldmann H, Wahl-Jensen V, Royle L, Dwek RA, Rudd PM. Identification of n-glycans from ebola virus glycoproteins by matrix-assisted laser desorption/ionisation time-of-flight and negative ion electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom. 2010;24(5):571–585. doi: 10.1002/rcm.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowden TA, Crispin M, Harvey DJ, Aricescu AR, Grimes JM, Jones EY, Stuart DI. Crystal structure and carbohydrate analysis of nipah virus attachment glycoprotein: A template for antiviral and vaccine design. J Virol. 2008;82(23):11628–11636. doi: 10.1128/JVI.01344-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowden TA, Crispin M, Harvey DJ, Jones EY, Stuart DI. Dimeric architecture of the hendra virus attachment glycoprotein: Evidence for a conserved mode of assembly. J Virol. 2010;84(12):6208–6217. doi: 10.1128/JVI.00317-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden TA, Crispin M, Graham SC, Harvey DJ, Grimes JM, Jones EY, Stuart DI. Unusual molecular architecture of the machupo virus attachment glycoprotein. J Virol. 2009;83(16):8259–8265. doi: 10.1128/JVI.00761-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korber B, Gaschen B, Yusim K, Thakallapally R, Kesmir C, Detours V. Evolutionary and immunological implications of contemporary hiv-1 variation. Br Med Bull. 2001;58:19–42. doi: 10.1093/bmb/58.1.19. [DOI] [PubMed] [Google Scholar]
- 19. Bonomelli C, Doores KJ, Dunlop DC, Thaney V, Dwek RA, Burton DR, Crispin M, Scanlan CN. The glycan shield of hiv is predominantly oligomannose independently of production system or viral clade. PLoS One. 2011;6(8):e23521. doi: 10.1371/journal.pone.0023521. **These papers analyze the glycan structures on Env from both pseudovirions and virions produced in CD4 cells using mass spectrometry. Analysis reveals that there are elevated levels of oligomannose on virion-associated gp120 compared to recombinantly expressed gp120. This is an important consideration when designing immunogens that mimic the glycans on HIV Env.
- 20. Doores KJ, Bonomelli C, Harvey DJ, Vasiljevic S, Dwek RA, Burton DR, Crispin M, Scanlan CN. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci U S A. 2010;107(31):13800–13805. doi: 10.1073/pnas.1006498107. **These papers analyze the glycan structures on Env from both pseudovirions and virions produced in CD4 cells using mass spectrometry. Analysis reveals that there are elevated levels of oligomannose on virion-associated gp120 compared to recombinantly expressed gp120. This is an important consideration when designing immunogens that mimic the glycans on HIV Env.
- 21.Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, Gregory TJ. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in chinese hamster ovary cells. J Biol Chem. 1990;265(18):10373–10382. [PubMed] [Google Scholar]
- 22.Zhu X, Borchers C, Bienstock RJ, Tomer KB. Mass spectrometric characterization of the glycosylation pattern of hiv-gp120 expressed in cho cells. Biochemistry. 2000;39(37):11194–11204. doi: 10.1021/bi000432m. [DOI] [PubMed] [Google Scholar]
- 23.Go EP, Liao HX, Alam SM, Hua D, Haynes BF, Desaire H. Characterization of host-cell line specific glycosylation profiles of early transmitted/founder hiv-1 gp120 envelope proteins. J Proteome Res. 2013;12(3):1223–1234. doi: 10.1021/pr300870t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexandre KB, Gray ES, Lambson BE, Moore PL, Choge IA, Mlisana K, Karim SS, McMahon J, O'Keefe B, Chikwamba R, Morris L. Mannose-rich glycosylation patterns on hiv-1 subtype c gp120 and sensitivity to the lectins, griffithsin, cyanovirin-n and scytovirin. Virology. 2010;402(1):187–196. doi: 10.1016/j.virol.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, et al. Crystal structure of a soluble cleaved hiv-1 envelope trimer. Science. 2013;342(6165):1477–1483. doi: 10.1126/science.1245625. ** This paper reported the first high resolution crystal structure of an HIV-1 envelope trimer. The structure revealed that the two key glycan epitopes on Env (N332 and N160) are larger than first thought and overlap to some extent. A map of the positioning on N-linked glycans in the context of the trimer allows a better understanding of how the bnAbs epitopes may have evolved.
- 26. Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, et al. Cryo-em structure of a fully glycosylated soluble cleaved hiv-1 envelope trimer. Science. 2013;342(6165):1484–1490. doi: 10.1126/science.1245627. ** This paper reported the first high resolution crystal structure of an HIV-1 envelope trimer. The structure revealed that the two key glycan epitopes on Env (N332 and N160) are larger than first thought and overlap to some extent. A map of the positioning on N-linked glycans in the context of the trimer allows a better understanding of how the bnAbs epitopes may have evolved.
- 27.Pancera M, Zhou T, Druz A, Georgiev IS, Soto C, Gorman J, Huang J, Acharya P, Chuang GY, Ofek G, Stewart-Jones GB, et al. Structure and immune recognition of trimeric pre-fusion hiv-1 env. Nature. 2014;514(7523):455–461. doi: 10.1038/nature13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eggink D, Melchers M, Wuhrer M, van Montfort T, Dey AK, Naaijkens BA, David KB, Le Douce V, Deelder AM, Kang K, Olson WC, et al. Lack of complex n-glycans on hiv-1 envelope glycoproteins preserves protein conformation and entry function. Virology. 2010;401(2):236–247. doi: 10.1016/j.virol.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an hiv-1 vaccine: The end of the beginning. Nat Rev Immunol. 2013;13(9):693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- 30.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, et al. Broad neutralization coverage of hiv by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to hiv-1. Science. 2010;329(5993):856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, et al. Sequence and structural convergence of broad and potent hiv antibodies that mimic cd4 binding. Science. 2011;333(6049):1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, et al. Broad and potent neutralizing antibodies from an african donor reveal a new hiv-1 vaccine target. Science. 2009;326(5950):285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, et al. Co-evolution of a broadly neutralizing hiv-1 antibody and founder virus. Nature. 2013;496(7446):469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, et al. Broad and potent neutralization of hiv-1 by a gp41-specific human antibody. Nature. 2012;491(7424):406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. Highly potent hiv-specific antibody neutralization in vitro translates into effective protection against mucosal shiv challenge in vivo. Proc Natl Acad Sci U S A. 2012;109(46):18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, Diskin R, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-hiv monoclonal antibodies block shiv infection in macaques. J Exp Med. 2014;211(10):2061–2074. doi: 10.1084/jem.20132494. * This study demonstrates the ability of glycan-binding HIV-1 bnAbs to protect macaques from SHIV infection when passively administrated at serum concentrations as low as 20 µg/mL. This data demonstrates that if glycan-binding HIV-1 bnAbs can be elicited through vaccination they would protect from infection.
- 38.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, et al. A blueprint for hiv vaccine discovery. Cell Host Microbe. 2012;12(4):396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, Schuitemaker H, et al. Complex-type n-glycan recognition by potent broadly neutralizing hiv antibodies. Proc Natl Acad Sci U S A. 2012;109(47):E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2g12 recognizes a cluster of alpha1-->2 mannose residues on the outer face of gp120. J Virol. 2002;76(14):7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, et al. Analysis of a clonal lineage of hiv-1 envelope v2/v3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85(19):9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Falkowska E, Le KM, Ramos A, Doores KJ, Lee JH, Blattner C, Ramirez A, Derking R, van Gils MJ, Liang CH, McBride R, et al. Broadly neutralizing hiv antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity. 2014;40(5):657–668. doi: 10.1016/j.immuni.2014.04.009. * This paper identifies a new site of bnAb vulnerability on HIV-1 env. This is this first bnAb shown to selectively bind cleaved trimers and to bind glycans on gp41. This bnAb represents a new target for vaccine design.
- 43.Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Georgiev IS, Chuang GY, Druz A, Doria-Rose NA, Laub L, et al. Broad and potent hiv-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature. 2014;515(7525):138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300(5628):2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 45.Murin CD, Julien JP, Sok D, Stanfield RL, Khayat R, Cupo A, Moore JP, Burton DR, Wilson IA, Ward AB. Structure of 2g12 fab2 in complex with soluble and fully glycosylated hiv-1 env by negative-stain single-particle electron microscopy. J Virol. 2014;88(17):10177–10188. doi: 10.1128/JVI.01229-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kong L, Lee JH, Doores KJ, Murin CD, Julien JP, McBride R, Liu Y, Marozsan A, Cupo A, Klasse PJ, Hoffenberg S, et al. Supersite of immune vulnerability on the glycosylated face of hiv-1 envelope glycoprotein gp120. Nat Struct Mol Biol. 2013;20(7):796–803. doi: 10.1038/nsmb.2594. ** This manusript reports the crystal structure of ‘mannose-patch’ binding HIV-1 bnAb PGT135 in complex with gp120. Comparison of the crystal structure with other bnAbs binding the mannose-patch revealed that this antigenic region is highly accessible allowing for multiple binding modes and varied angles of approach. This manuscript first highlighted the mannose-patch as a ‘super-site’ for HIV-1 vaccine design.
- 47.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, Depetris R, et al. A potent and broad neutralizing antibody recognizes and penetrates the hiv glycan shield. Science. 2011;334(6059):1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Julien JP, Lee JH, Cupo A, Murin CD, Derking R, Hoffenberg S, Caulfield MJ, King CR, Marozsan AJ, Klasse PJ, Sanders RW, et al. Asymmetric recognition of the hiv-1 trimer by broadly neutralizing antibody pg9. Proc Natl Acad Sci U S A. 2013;110(11):4351–4356. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pancera M, Shahzad-Ul-Hussan S, Doria-Rose NA, McLellan JS, Bailer RT, Dai K, Loesgen S, Louder MK, Staupe RP, Yang Y, Zhang B, et al. Structural basis for diverse n-glycan recognition by hiv-1-neutralizing v1-v2-directed antibody pg16. Nat Struct Mol Biol. 2013;20(7):804–813. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, et al. Structure of hiv-1 gp120 v1/v2 domain with broadly neutralizing antibody pg9. Nature. 2011;480(7377):336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haji-Ghassemi O, Muller-Loennies S, Saldova R, Muniyappa M, Brade L, Rudd PM, Harvey DJ, Kosma P, Brade H, Evans SV. Groove-type recognition of chlamydiaceae-specific lipopolysaccharide antigen by a family of antibodies possessing an unusual variable heavy chain n-linked glycan. J Biol Chem. 2014;289(24):16644–16661. doi: 10.1074/jbc.M113.528224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brooks CL, Schietinger A, Borisova SN, Kufer P, Okon M, Hirama T, Mackenzie CR, Wang LX, Schreiber H, Evans SV. Antibody recognition of a unique tumor-specific glycopeptide antigen. Proc Natl Acad Sci U S A. 2010;107(22):10056–10061. doi: 10.1073/pnas.0915176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crispin M, Bowden TA. Antibodies expose multiple weaknesses in the glycan shield of hiv. Nat Struct Mol Biol. 2013;20(7):771–772. doi: 10.1038/nsmb.2627. [DOI] [PubMed] [Google Scholar]
- 54.Dunlop DC, Bonomelli C, Mansab F, Vasiljevic S, Doores KJ, Wormald MR, Palma AS, Feizi T, Harvey DJ, Dwek RA, Crispin M, et al. Polysaccharide mimicry of the epitope of the broadly neutralizing anti-hiv antibody, 2g12, induces enhanced antibody responses to self oligomannose glycans. Glycobiology. 2010;20(7):812–823. doi: 10.1093/glycob/cwq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunlop DC, Ulrich A, Appelmelk BJ, Burton DR, Dwek RA, Zitzmann N, Scanlan CN. Antigenic mimicry of the hiv envelope by aids-associated pathogens. Aids. 2008;22(16):2214–2217. doi: 10.1097/QAD.0b013e328314b5df. [DOI] [PubMed] [Google Scholar]
- 56.Agrawal-Gamse C, Luallen RJ, Liu B, Fu H, Lee FH, Geng Y, Doms RW. Yeast-elicited cross-reactive antibodies to hiv env glycans efficiently neutralize virions expressing exclusively high-mannose n-linked glycans. J Virol. 2011;85(1):470–480. doi: 10.1128/JVI.01349-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang LX. Synthetic carbohydrate antigens for hiv vaccine design. Curr Opin Chem Biol. 2013;17(6):997–1005. doi: 10.1016/j.cbpa.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doores KJ, Fulton Z, Huber M, Wilson IA, Burton DR. Antibody 2g12 recognizes di-mannose equivalently in domain- and nondomain-exchanged forms but only binds the hiv-1 glycan shield if domain exchanged. J Virol. 2010;84(20):10690–10699. doi: 10.1128/JVI.01110-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amin MN, McLellan JS, Huang W, Orwenyo J, Burton DR, Koff WC, Kwong PD, Wang LX. Synthetic glycopeptides reveal the glycan specificity of hiv-neutralizing antibodies. Nat Chem Biol. 2013;9(8):521–526. doi: 10.1038/nchembio.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garces F, Sok D, Kong L, McBride R, Kim HJ, Saye-Francisco KF, Julien JP, Hua Y, Cupo A, Moore JP, Paulson JC, et al. Structural evolution of glycan recognition by a family of potent hiv antibodies. Cell. 2014;159(1):69–79. doi: 10.1016/j.cell.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doores KJ, Kong L, Krumm SA, Le KM, Sok D, Laserson U, Garces F, Poignard P, Wilson IA, Burton DR. Two classes of broadly neutralizing antibodies within a single lineage directed to the high-mannose patch of hiv envelope. J Virol. 2015;89(2):1105–1118. doi: 10.1128/JVI.02905-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sok D, Laserson U, Laserson J, Liu Y, Vigneault F, Julien JP, Briney B, Ramos A, Saye KF, Le K, Mahan A, et al. The effects of somatic hypermutation on neutralization and binding in the pgt121 family of broadly neutralizing hiv antibodies. PLoS Pathog. 2013;9(11):e1003754. doi: 10.1371/journal.ppat.1003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kepler TB, Liao HX, Alam SM, Bhaskarabhatla R, Zhang R, Yandava C, Stewart S, Anasti K, Kelsoe G, Parks R, Lloyd KE, et al. Immunoglobulin gene insertions and deletions in the affinity maturation of hiv-1 broadly reactive neutralizing antibodies. Cell Host Microbe. 2014;16(3):304–313. doi: 10.1016/j.chom.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, Staupe RP, et al. Developmental pathway for potent v1v2-directed hiv-neutralizing antibodies. Nature. 2014;509(7498):55–62. doi: 10.1038/nature13036. * This paper uses longitudinal samples from an HIV-1 infected individual to study the development of N160-binding HIV-1 bnAbs. Emergence of HIV-1 bnAbs followed extensive diversification of the virus population.
- 65.Moore PL, Gray ES, Wibmer CK, Bhiman JN, Nonyane M, Sheward DJ, Hermanus T, Bajimaya S, Tumba NL, Abrahams MR, Lambson BE, et al. Evolution of an hiv glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med. 2012;18(11):1688–1692. doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iba Y, Fujii Y, Ohshima N, Sumida T, Kubota-Koketsu R, Ikeda M, Wakiyama M, Shirouzu M, Okada J, Okuno Y, Kurosawa Y, et al. Conserved neutralizing epitope at globular head of hemagglutinin in h3n2 influenza viruses. J Virol. 2014;88(13):7130–7144. doi: 10.1128/JVI.00420-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kong L, Giang E, Nieusma T, Kadam RU, Cogburn KE, Hua Y, Dai X, Stanfield RL, Burton DR, Ward AB, Wilson IA, et al. Hepatitis c virus e2 envelope glycoprotein core structure. Science. 2013;342(6162):1090–1094. doi: 10.1126/science.1243876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barrientos LG, O'Keefe BR, Bray M, Sanchez A, Gronenborn AM, Boyd MR. Cyanovirin-n binds to the viral surface glycoprotein, gp1,2 and inhibits infectivity of ebola virus. Antiviral Res. 2003;58(1):47–56. doi: 10.1016/s0166-3542(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 69. Garrison AR, Giomarelli BG, Lear-Rooney CM, Saucedo CJ, Yellayi S, Krumpe LR, Rose M, Paragas J, Bray M, Olinger GG, Jr, McMahon JB, et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against zaire ebola virus. Antiviral Res. 2014;112C:1–7. doi: 10.1016/j.antiviral.2014.09.012. * This manuscript demonstrates the anti-viral activity of a mannose binding lectin against Ebola virus. This suggests that if glycan-binding antibodies that bind the Ebola GP can be identified they may neutralize Ebola virus.
- 70.Kachko A, Loesgen S, Shahzad-Ul-Hussan S, Tan W, Zubkova I, Takeda K, Wells F, Rubin S, Bewley CA, Major ME. Inhibition of hepatitis c virus by the cyanobacterial protein microcystis viridis lectin: Mechanistic differences between the high-mannose specific lectins mvl, cv-n, and gna. Mol Pharm. 2013;10(12):4590–4602. doi: 10.1021/mp400399b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alen MM, Kaptein SJ, De Burghgraeve T, Balzarini J, Neyts J, Schols D. Antiviral activity of carbohydrate-binding agents and the role of dc-sign in dengue virus infection. Virology. 2009;387(1):67–75. doi: 10.1016/j.virol.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 72.Arthur CM, Cummings RD, Stowell SR. Using glycan microarrays to understand immunity. Curr Opin Chem Biol. 2014;18:55–61. doi: 10.1016/j.cbpa.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human b cells by single cell rt-pcr and expression vector cloning. J Immunol Methods. 2008;329(1–2):112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]