Abstract
Obesity and binge-eating disorder (BED) frequently arise in adolescence, which is a critical developmental time period where self-regulatory processes are formed. Indeed, both obesity and BED are thought to arise partly due to deficits in self-regulatory processes (i.e. lack of inhibitory control to overeat or binge). Recent neuroimaging studies have implicated the frontal cortex, a brain region involved in regulating inhibitory-control, and the striatum, which is thought to be involved in food reward, satiety and pleasure, in mediating responses to food cues and feeding in normal-weight individuals as well as obese and BED subjects. Intriguingly, frontostriatal circuits have been observed to be preferentially modulated in obese adults and similar associations have been observed in obese/overweight adolescents. Furthermore, brain dopamine (DA) is selectively altered in striatum in obese relative to normal-weight individuals, and frontostriatal regions constitute a major component of DA circuitry. The aim of this review will be to present the main findings from neuroimaging studies in obese and BED adults and adolescents, as these relate to frontostriatal circuitry, and to emphasize the potential for using functional neuroimaging in both humans and animals with the scope of obtaining information on developmental and molecular contributions to obesity and BED.
Introduction
The prevalence of obesity has reached dramatic proportions and is estimated to currently affect 32% of US adults (Flegal et al., 2010). Binge-eating disorder (BED), which is currently estimated to affect 3.5% and 2% of US adult women and men respectively, is also on the rise (Hudson et al., 2007). Besides the physical health problems associated with obesity and BED, there have also been reports of psychiatric comorbidity with depression and anxiety disorders (Faith et al., 2011), which suggests that part of the behavioural phenotype of obese and BED individuals may be related to impairments in brain function. An important distinction is that this may not occur for all obese individuals, the majority of whom exhibit metabolic abnormalities. However, a subset of obese individuals are characterized by excessive, compulsive-like overeating behaviour where obesity is thought to be the manifestation of underlying psychopathology (Davis & Carter, 2009). Emerging evidence from neuroimaging experiments point to abnormalities in frontal brain circuitry in both obese and BED adults and adolescents, which are paralleled by impairments in inhibitory and cognitive control behaviour. These recent findings suggest that obesity and BED may be attributed, in part, to developmentally associated neurobiological impairments. This notion is supported by the striking increases in childhood cases of obesity (Faith et al., 2011; Ogden et al., 2010) as well as the generalized observations of BED onset during adolescence.
Neuroimaging techniques using functional magnetic resonance imaging (fMRI) offer the ability to monitor obese and BED adolescents and adults across developmental time-spans. These studies have implicated frontostriatal circuitry as being involved in obesity and BED in both adults and adolescents. However, fMRI is limited in its ability to implicate specific molecular determinants of obesity and BED. Positron emission tomography (PET) provides a way of assessing the contribution of specific molecular systems in obesity and BED, and studies in adults have implicated the neurotransmitter dopamine (DA) as heavily involved in obesity and BED within frontostriatal circuits. Nevertheless, while PET has provided insight into potential molecular determinants of obesity and BED, this method falls short of implicating similar molecular mechanisms directly in children and adolescents due to the use of radioactivity. Recent advances in neuroimaging techniques using small animals provide a way for modelling obesity and BED across distinct developmental periods and in this way provide tractable models that can be used to enhance insight into the contribution of respective developmentally regulated molecular and genetic determinants associated with each disorder. This review focuses on summarizing the recent reports of frontostriatal circuit abnormalities observed in obese and BED adolescents and adults using fMRI and PET, describes the potential involvement of DA as a potential neurobiological determinant of obesity and BED, and finally discusses the potential benefit of using animal models to study the developmental contribution of the brain DA system in obesity and BED.
Functional magnetic resonance imaging
Functional magnetic resonance imaging (fMRI) is used to assess changes in regional brain blood flow that are thought to be driven by the presentation of specific stimuli or in response to specific events. These blood flow-driven responses are based on the blood oxygen level-dependent (BOLD) contrast signal, which results from a distortion of the magnetic field as a function of changes in the ratio between oxygenated and deoxygenated haemoglobin in the tissue (Pagoto et al., 2009). These contrast measures are then superimposed onto a structural brain image, which allows for determining the approximate anatomical locations of the BOLD signal responses. The BOLD signal change is then correlated with the stimulus or event presentation to determine functional relevance of activated brain areas to the stimulus presentation or event occurrence. The physiological relevance of the BOLD signal to neuronal function centres on the notion that BOLD signal measures correlate well with local field potentials of neurons in certain brain regions via neurovascular coupling processes (Ojemann et al., 2010). That is, the greater the activity in a given brain region, the larger the increase in blood flow to the area with a concomitant excess delivery of oxygenated haemoglobin, which generates the BOLD contrast.
Positron emission tomography
Positron emission tomography (PET) relies on the administration and subsequent detection of positron emitting radiotracers that target specific molecular systems in biological tissue. PET utilizes the principles of radioactive decay where positrons emitted from the decaying radioisotope, which is tagged to a radiotracer, will collide with random electrons located in close proximity to the decay site. Once the two particles collide, an emission of gamma rays is produced which project at opposite directions (180 degrees) from the point of collision. PET scanners are equipped with specialized detectors which pick up this signal. Once the signal is detected, the information is processed and the point of annihilation can be estimated. Further information processing is used to produce a two-dimensional image rendering of radiotracer occupancy in the targeted tissue. PET imaging facilities are usually equipped with radioisotope-generating facilities (cyclotrons) and radiochemistry workstations for dedicated radiotracer synthesis. There are many different types of radiotracers that can be applied to the study of various disease states and neurobiological mechanisms. For the purposes of this review we will focus on those that have been utilized in the context of obesity and BED research. These include [15O]H2O, which has been used for measuring regional cerebral blood flow (rCBF), 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG), which has been used to measure regional brain glucose metabolism (BGluM), and the dopamine (DA) D2 receptor (D2R) antagonist [11C] raclopride, which has been used to measure D2R availability as well as synaptic DA. A limitation for PET is that it involves the administration of radiotracers, which restricts its use in children and adolescents since they are more sensitive to the adverse effects of radiation. However, with the advent of small animal imaging scanners, PET provides an avenue for assessing candidate molecular mechanisms longitudinally in the same subject, thereby allowing for disease progression during various developmental stages.
Frontostriatal abnormalities in obesity and BED in adults
Visual and chemosensory food stimulation as well as food anticipation and intake preferentially affect frontostriatal circuitry in adults. In particular, food taste increases striatal rCBF in normal-weight subjects (Gautier et al., 1999), while visual and olfactory exposure to food activates orbitofrontal cortex (OFC), and this activation correlates with hunger and desire for the presented food (Wang et al., 2004). Intriguingly, obese individuals show enhanced striatal BOLD signal responses during food anticipation (Stice et al., 2008). Obese subjects also show enhanced striatal and OFC BOLD signal responses to images of food, and these responses are associated with susceptibility to weight gain (Rothemund et al., 2007; Stoeckel et al., 2008; Stice et al., 2010a). Conversely, food intake is associated with decreased striatal BOLD signal responses in obese subjects and weight gain in these subjects is associated with further decreases in striatal BOLD signal responses (Stice et al., 2008), suggesting that overeating is associated with blunted striatal responses to food but elevated frontostriatal responses to food-related cues (Stice et al., 2011). This differential involvement of striatum and OFC in feeding and food-cues is intriguing, given similar associations in other disease states such as drug addiction where the striatum is believed to respond to reward or saliency associated with the euphoric feelings of drug intake while frontal circuits are associated with drug-related cues and craving (Goldstein & Volkow, 2011). Another important aspect underlying brain responses to food involves how the brain responds to food cues in response to the degree of hunger of the individual. A recent study showed that during a fasted state, obese individuals showed enhanced activation of frontal cortical regions in response to food cues, while after these subjects were fed a meal, frontostriatal BOLD activation was observed (Martin et al., 2010), implicating the striatum in post-ingestive processes and satiation.
Aside from differences in frontostriatal activation to food and food cues between obese and normal-weight subjects, a gender-related component to frontostriatal brain activation by food has also been reported. A recent study reported enhanced frontal cortical activation in women compared to men in response to images of high-calorie foods (Killgore & Yurgelun-Todd, 2010b). In another study, male subjects exhibited an enhanced ability over females to suppress OFC activation in response to food presentation (Wang et al., 2009). Importantly, the enhanced OFC suppression in males was paralleled by increases in cognitive inhibition of food-related craving (Wang et al., 2009). These observations are intriguing, given the gender disparity in BED prevalence, with women showing increased prevalence of BED as compared to men (Hudson et al., 2007), and the well-described role of frontostriatal circuitry in disease states associated with impaired inhibitory and cognitive control behaviours (Blair, 2010) (BED is generally regarded as a disease characterized by abnormalities in self-control and inability to exert inhibitory behaviours) (Harrison et al., 2010).
Abnormalities in OFC have also been observed in obsessive compulsive (Aouizerate et al., 2004) and hoarding disorders (Tolin et al., 2009), which may have root in primeval ‘hard-wired’ behaviours important for feeding and survival. Indeed, OFC and striatum show enhanced activation in response to post-ingestive physiological processes as reviewed extensively in Mayer et al. (2009). This view is supported by a recent neuroimaging study that implicated frontostriatal circuitry in gastrointestinal interoceptive mechanisms (i.e. afferent signals originating in the gut during digestion) (Wang et al., 2006). In this study, obese subjects with implanted gastric stimulation (IGS) devices (a clinical obesity intervention that relies on curbing feelings of hunger via the modulation of gastric distention (Cigaina, 2004)), were scanned using [18F]FDG while the IGS device was either on or off. IGS resulted in increased brain metabolism in striatum and OFC in these subjects (Wang et al., 2006) and these increases were paralleled by decreases in ‘emotional eating’ scores. This latter observation sheds light onto another intriguing association, namely, the effect of emotional disturbances in modulating frontostriatal circuitry and subsequently obesity and BED. Emotional circuits are thought to be intimately involved in obesity (Berridge et al., 2010) and BED, given the significant effect these circuits exert on motivation and behaviour (Munsch et al., 2012). Indeed, a recent neuroimaging study using resting functional connectivity (method that uses fMRI to assess the functional connectivity between different brain regions during resting conditions) reported impairments in amygdala modulation of OFC–ventral striatal connectivity in obese compared to lean women (Stoeckel et al., 2009). Amygdala function is strongly implicated in emotional regulation, particularly via its effects on assigning emotional value to cues associated with a given stimulus (Murray, 2007). Such mechanisms are evolutionarily hard-wired for survival purposes which would also explain their possible involvement in BED, which is characterized by uncontrollable, compulsive eating often associated with emotional disturbances (Munsch et al., 2012). In sum, these findings highlight a role for frontostriatal circuitry in food perception, ingestion, and post-ingestive physiological mechanisms involved in feeding and possibly satiety, and suggest that imbalances in this circuitry may mediate obesity and BED.
Frontostriatal abnormalities in obesity in adolescence
Adolescence is generally viewed as a critical transitional time period during development, where children begin to learn how to develop self-regulatory control behaviours that advance their ability to organize thoughts, ideas and emotions which are crucial for their success later in life (Posner & Rothbart, 2009). Neuroimaging studies in children and adolescents are challenging primarily due to parental safety concerns, children’s apprehension towards experimental equipment, impaired ability of children in adhering to study guidelines, as well as difficulty in discerning child brain anatomy (Ketonen et al., 2008). For these reasons, neuroimaging studies in children and adolescents have not been conducted to the degree done in adults. Nevertheless, the few neuroimaging studies that have been conducted to date have reported striking similarities in brain activation to food and food cues in adults and adolescents.
Similar to adults, food images in hungry children and adolescents preferentially activate frontal cortical regions, including OFC (Holsen et al., 2005). In fact, a study that directly compared brain responses to food images in adolescents and adults found significant activation in OFC and striatum in both groups, and intriguingly, adolescents showed enhanced activation in frontostriatal regions when exposed to high-calorie food images and preferential OFC activation when exposed to low-calorie food images (Killgore & Yurgelun-Todd, 2005). This differential pattern of activation highlights developmentally mediated regulation of brain activation to food that may occur in the transition from adolescence to adulthood and suggests that impairments in development-specific mechanisms may underlie psychopathologies that arise during this transition.
Neuroimaging studies conducted in obese/overweight and normal-weight children assessed BOLD activation during the viewing of food images and found enhanced activation in frontal cortical regions and decreased activation in striatum in obese subjects (Davids et al., 2010). Another study examined pre- and post-meal brain activation during exposure to food images in obese and normal-weight children and found that obese children had enhanced activation in frontal cortical regions at both pre- and post-meal scanning sessions compared to normal-weight children (Bruce et al., 2010). Interestingly, enhanced striatal activity was observed in normal-weight but not in obese children during pre-meal sessions compared to post-meal sessions. This is an intriguing observation, given the aforementioned reports in adults of striatal activation and its association to post-ingestive mechanisms and satiety. This differential striatal activation before and after meal consumption may underlie decreased sensitivity in obese children to satiety-specific post-ingestive mechanisms and requires further investigation. Finally, a more recent study showed that adolescents at high risk for obesity showed enhanced activation in frontostriatal regions in response to both food intake and monetary reward (Stice et al., 2011). This study highlights another important aspect of frontostriatal circuitry, namely that imbalances in this circuitry are not specific to food but can also affect other types of ‘rewarding’ stimuli. This observation is particularly important because it highlights that frontostriatal activation in adolescents, like adults, is associated with food as well as non-food stimuli and that impairments in frontostriatal circuitry observed in adults with obesity and BED may arise in childhood/adolescence, or perhaps earlier as part of a genetic predisposition to such imbalances. Indeed, frontostriatal circuit imbalances have been observed in multiple psychopathologies in children (Tourette’s, ADHD, obsessive–compulsive disorder, anorexia and bulimia nervosa) where inhibitory control-related behaviours are central to the psychopathology. These studies are reviewed extensively in Marsh et al. (2009) and therefore are beyond the scope of this review.
In sum, when evaluating brain responses to food and food cues in obese adults and adolescents, striking similarities and important contrasts emerge. Obese adults show increased frontal and striatal responses to food cues but decreased striatal responses in response to feeding. Like adults, obese adolescents show decreased striatal responses to food cues after feeding. However, unlike adults, obese adolescents show increased responses to food cues in frontal but not striatal regions. This contrast highlights frontostriatal circuitry as potential mediators of obesity vulnerability that can arise early in childhood and adolescence, and specifically the striatum as a potential predictor of obesity development into adulthood. This view agrees with the well-documented role of the striatum in mediating habitual reinforcement behaviour via changes in synaptic plasticity of its medium spiny neurons and suggests that this region serves a decisive role in escalation of overweight and obesity during the transition from adolescence to adulthood. An important caveat to this interpretation is the difficulty of making valid comparisons between studies assessing brain responses to food in adults and adolescents, since ideal comparisons would require using the same imaging paradigms and experimental conditions. This highlights the importance of using the same experimental design, and future studies should take such considerations into account so that these comparisons can be objectively tested.
Dopamine involvement in obesity and BED
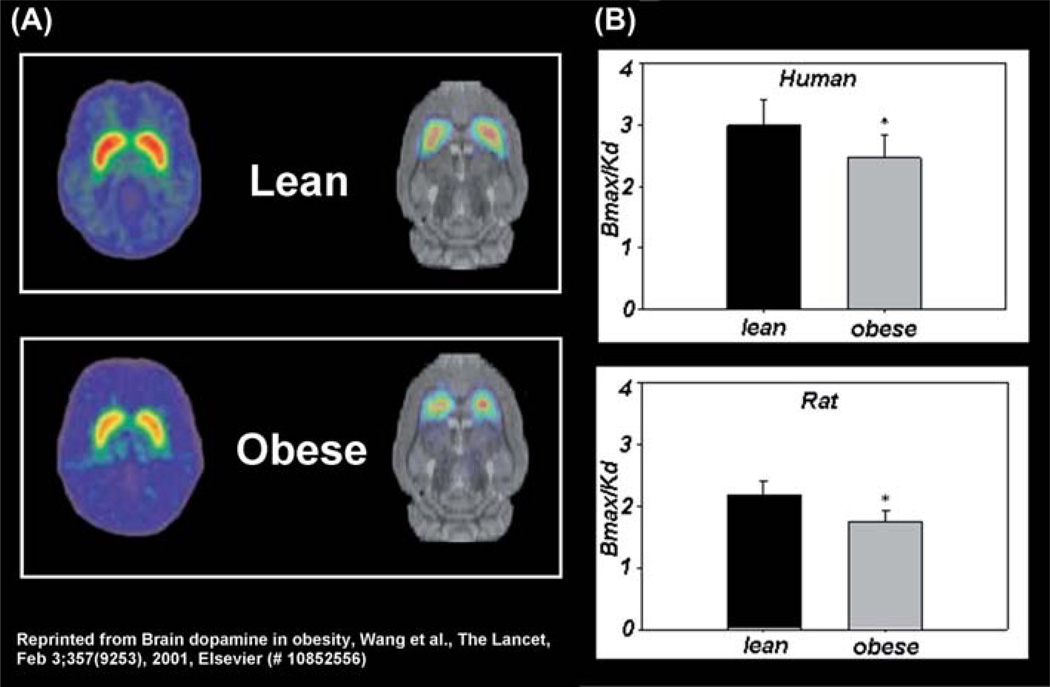
The catecholamine DA is a highly conserved neurotransmitter that has been implicated in feeding and obesity (Volkow et al., 2011). In the brain, DA is produced in specific neurons, which originate in the midbrain and project to the striatum as well as frontal cortex. These anatomical features of DA circuits make this neurotransmitter a very attractive target for studies that focus on elucidating involvement of frontostriatal circuitry in obesity and BED. In particular, brain DA function has been implicated in the regulation of cognitive processes (attention, memory, motivation, reward), behaviour, physiology as well as metabolism and this diverse functional profile is attributed in part to the selective regional distribution of its receptors. The D2R is of particular interest to obesity and BED research since it is densely expressed in the striatum, drugs that block D2R induce weight gain (Baptista, 1999) and D2Rs have been implicated in both obesity and BED (de Weijer et al., 2011; Wang et al., 2001, 2011). In particular, morbidly obese individuals are characterized by significantly lower striatal D2R (de Weijer et al., 2011; Wang et al., 2001) (Fig. 1), and food presentation elicits greater increases in striatal DA in obese BED subjects compared to non-BED obese controls (Wang et al., 2011). Intriguingly, the increases in striatal DA in obese BED subjects were found to be significantly correlated with binge-eating scores (Wang et al., 2011). In fact, similar correlations have been observed in normal subjects as well, where increases in striatal DA correlated with self-reports of hunger and desire to consume food (Volkow et al., 2002). Finally, subjects with genetic D2R mutations associated with reduced D2R signalling showed decreased BOLD signal responses in striatum and OFC in response to imagined intake of food stimuli, and these responses were associated with future weight gain (Stice et al., 2010).
Figure 1.
Obesity-related deficits in striatal dopamine D2 receptors (D2R) are conserved across species: (A) representative positron emission tomography brain scans and (B) Bmax/Kd values of D2R binding in lean and obese humans (Wang et al., 2001) and rats (Thanos et al., 2008).
These findings imply that striatal DA is involved in the motivation to seek and consume food and suggest that imbalances in striatal DA signalling may mediate susceptibility to obesity and BED. Interestingly, striatal D2R in obese subjects significantly correlated with brain metabolism in frontal cortical regions (Volkow et al., 2008), which further implicates frontostriatal circuitry in obesity and perhaps BED. Finally, striatal DA was positively correlated with restraint behaviour during food presentation (Volkow et al., 2003), a finding which further implicates DA-specific frontostriatal mechanisms in obesity and BED via its potential regulation of inhibitory control, saliency attribution and reward mechanisms.
Translational neuroimaging for assessing developmental contributions to frontostriatal impairments in obesity and BED
PET in children and adolescents is limited due to radiation exposure, and therefore PET studies to assess DA involvement in BED in children and adolescents have not been undertaken. As DA-related functions on feeding and obesity observed in humans have been shown to occur in rodents, an alternative approach to understand potential involvement of DA in obesity and BED during childhood and adolescence is the use of rodent models that partly reflect the human condition. Indeed, the recent advent of small animal fMRI and PET scanners and the development of sophisticated imaging, image processing and analysis techniques offer a valuable experimental strategy to assess the role of specific molecular mechanisms in disease and behaviour with strong experimental control and translational potential (Benveniste & Blackband, 2006). Using these imaging methodologies, neural and molecular correlates of disease and related behaviours in humans can be subsequently studied using animal models where experimental factors as well as genetic and environmental components relevant to the disease or behaviour being studied can be manipulated and controlled. This type of experimental strategy allows for the estimation of cause–effect relationships. In turn, results from animal studies can be translated to humans and eventually lead to therapeutic interventions. One particularly relevant feature of small animal imaging methods such as fMRI and PET is that these techniques are generally non-invasive, reproducible, and allow for longitudinal assessment of regional brain activity over time and during discrete developmental time periods. This makes small animal imaging a powerful research tool for elucidating neural and molecular correlates associated with development in obesity and BED animal models.
As of now fMRI experiments in rodents (Schmidt et al., 2006; Tenney et al., 2004) have not assessed the effects of food stimulation on brain regional activation in the context of obesity or BED. This is because for fMRI studies the animals need to remain motionless, which requires the use of anaesthesia, which affects the responses to stimulus presentation and thus limits the ability to study cognitive processes. Conversely, PET imaging in rodents has recapitulated some of the clinical observations made in obese and BED populations, and for certain radiotracers such as [18F]FDG (used to measure brain glucose metabolism) and [11C]raclopride (used to measure D2R binding potential) the measurements can be made in awake animals. One PET study done in genetically obese rats (leptin-receptor deficient) examined the brain metabolic responses to food stimuli in awake rats comparing metabolism in the absence and in the presence of a bacon scent (Thanos et al., 2008a). This study found that while bacon scent decreased glucose metabolism (marker of activity) in frontal cortex in both obese and lean rats, the decreases were significantly greater in the obese group. This study illustrates the potential of small animal PET for assessing the effects of exposure to food stimuli on brain activity in rodent models of obesity including those with specific genetic manipulations that are relevant to human obesity (i.e. leptin signalling). Indeed, leptin regulation of frontostriatal responses to food cues has been demonstrated in humans with fMRI (Baicy et al., 2007; Farooqi et al., 2007), providing evidence that this peptide regulates cognitive processes associated with food perception and thus individuals with leptin imbalances (obese individuals are generally leptin-resistant) may be prone to such deficits. Further evidence of the significance of small animal imaging to obesity research are recent findings reporting similar deficits in striatal D2R in obese rats as has been previously reported in humans. In particular, rodent PET experiments have replicated the decreases in striatal D2R previously observed in obese humans (Wang et al., 2001). Specifically, genetically obese rats scanned with [11C] raclopride showed lower striatal D2R levels compared to lean controls (Thanos et al., 2008b) (Fig. 1) and in a more recent study, striatal D2R levels in normal-weight rats predicted future measures of body weight (Michaelides et al., 2012). That is, rats with the lowest striatal D2R levels showed the highest body weight 2 months later, while rats with the highest D2R levels showed the lowest body weight. These recent findings suggest that striatal D2R are involved in body weight regulation and that inherent D2R deficits contribute to increased weight gain and eventually obesity. In sum, the ability to study and model human obesity and BED in animals and to study these models with PET will allow for longitudinal assessment of brain activity and striatal DA in the same animal over time and this, in turn, would allow one to study these models at selective developmental time points and importantly, obtain molecular insight into the developmental pathophysiology associated with obesity and BED.
Conclusions
The findings and views presented in this review are consistent with the notion that obesity and BED may arise in adolescence, in response to deficits in frontostriatal circuitry and related DA mechanisms. These mechanisms are involved in mediating, among other things, reward and self-regulatory processes. Interestingly, obese subjects and subjects with BED are also characterized by uniquely altered self-regulatory capacity. These associations have recently been observed in obese/overweight adolescents, suggesting that obesity and BED onset may be due to developmental factors. In the future, technological advancements that will allow the safe and sensitive assessment of molecular profiles in children and adolescents will enable direct assessment of the contribution of DA in BED and obesity during childhood and adolescence. In addition, advanced genetic techniques will allow us to expand our understanding of how genes associated with obese and BED phenotypes in humans affect brain function throughout the various developmental time periods. Finally, small animal imaging and rodent models of obesity and BED may help to obtain an understanding of developmentally mediated factors involved in obesity and BED.
Acknowledgments
Declaration of interest: Brain imaging studies were carried out at Brookhaven National Laboratory with infrastructure support from the US Department of Energy OBER (DE-ACO2 - 76CH00016) and under support in part by the National Institutes of Health: 5T32DA007135 (M. Michaelides), Z01AA000550 (N. Volkow) and R01DA7092 (G.-J. Wang), R01DA00280 (G.-J. Wang), R01MH66961 (G.J. Wang). The authors alone are responsible for the content and writing of the paper.
References
- Aouizerate B, Guehl D, Cuny E, Rougier A, Bioulac B, Tignol J, Burbaud P. Pathophysiology of obsessive–compulsive disorder: A necessary link between phenomenology, neuropsychology, imagery and physiology. Progress in Neurobiology. 2004;72:195–221. doi: 10.1016/j.pneurobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista T. Body weight gain induced by antipsychotic drugs: Mechanisms and management. Acta Psychiatrica Scandinavica. 1999;100:3–16. doi: 10.1111/j.1600-0447.1999.tb10908.x. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Blackband SJ. Translational neuroscience and magnetic-resonance microscopy. Lancet Neurology. 2006;5:536–544. doi: 10.1016/S1474-4422(06)70472-0. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Research. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Neuroimaging of psychopathy and antisocial behavior: A targeted review. Current Psychiatry Reports. 2010;12:76–82. doi: 10.1007/s11920-009-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International Journal of Obesity. 2010;34:1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigaina V. Long-term follow-up of gastric stimulation for obesity: The Mestre 8-year experience. Obesity Surgery. 2004;14:S14–S22. doi: 10.1007/BF03342133. [DOI] [PubMed] [Google Scholar]
- Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M, Lotze M. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. International Journal of Obesity. 2010;34:94–104. doi: 10.1038/ijo.2009.193. [DOI] [PubMed] [Google Scholar]
- Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53:1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, Booij J. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. European Journal of Nuclear Medicine and Molecular Imaging Research. 2011;1:37. doi: 10.1186/2191-219X-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith MS, Butryn M, Wadden TA, Fabricatore A, Nguyen AM, Heymsfield SB. Evidence for prospective associations among depression and obesity in population-based studies. Obesity Reviews. 2011;12:e438–e453. doi: 10.1111/j.1467-789X.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Journal of the American Medical Association. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Gautier JF, Chen K, Uecker A, Bandy D, Frost J, Salbe AD, Tataranni PA. Regions of the human brain affected during a liquid-meal taste perception in the fasting state: A positron emission tomography study. American Journal of Clinical Nutrition. 1999;70:806–810. doi: 10.1093/ajcn/70.5.806. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews. Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A, O’Brien N, Lopez C, Treasure J. Sensitivity to reward and punishment in eating disorders. Psychiatry Research. 2010;177:1–11. doi: 10.1016/j.psychres.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, Savage CR. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27:669–676. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketonen LM, Valanne L. Neuroimaging of pediatric diseases. Seminars in Neurology. 2008;28:558–569. doi: 10.1055/s-0028-1083692. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Developmental changes in the functional brain responses of adolescents to images of high and low-calorie foods. Developmental Psychobiology. 2005;47:377–397. doi: 10.1002/dev.20099. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex differences in cerebral responses to images of high versus low-calorie food. Neuroreport. 2010b;21:354–358. doi: 10.1097/WNR.0b013e32833774f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. American Journal of Psychiatry. 2009;166:664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Savage CR. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, Tracey I. Brain imaging approaches to the study of functional GI disorders: A Rome working team report. Neurogastroenterology and Motility. 2009;21:579–596. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M, Thanos PK, Kim R, Cho J, Ananth M, Wang GJ, Volkow ND. PET imaging predicts future body weight and cocaine preference. Neuroimage. 2012;59:1508–1513. doi: 10.1016/j.neuroimage.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch S, Meyer AH, Quartier V, Wilhelm FH. Binge eating in binge eating disorder: A break-down of emotion regulatory process? Psychiatry Research. 2012;195:118–124. doi: 10.1016/j.psychres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. Journal of the American Medical Association. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Corina DP, Corrigan N, Schoenfield-McNeill J, Poliakov A, Zamora L, Zanos S. Neuronal correlates of functional magnetic resonance imaging in human temporal cortex. Brain. 2010;133:46–59. doi: 10.1093/brain/awp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagoto SL, Curtin C, Lemon SC, Bandini LG, Schneider KL, Bodenlos JS, Ma Y. Association between adult attention deficit/hyperactivity disorder and obesity in the US population. Obesity. 2009;17:539–544. doi: 10.1038/oby.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Toward a physical basis of attention and self regulation. Physics of Life Reviews. 2009;6:103–120. doi: 10.1016/j.plrev.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Schmidt KF, Febo M, Shen Q, Luo F, Sicard KM, Ferris CF, Duong TQ. Hemodynamic and metabolic changes induced by cocaine in anesthetized rat observed with multimodal functional MRI. Psychopharmacology. 2006;185:479–486. doi: 10.1007/s00213-006-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4. Neuroimage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010a;30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. Journal of Neuroscience. 2011;31:4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Kim J, Weller RE, Cox JE, Cook EW, III, Horwitz B. Effective connectivity of a reward network in obese women. Brain Research Bulletin. 2009;79:388–395. doi: 10.1016/j.brainresbull.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney JR, Duong TQ, King JA, Ferris CF. fMRI of brain activation in a genetic rat model of absence seizures. Epilepsia. 2004;45:576–582. doi: 10.1111/j.0013-9580.2004.39303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Gispert JD, Pascau J, Soto-Montenegro ML, Desco M, Volkow ND. Differences in response to food stimuli in a rat model of obesity: In-vivo assessment of brain glucose metabolism. International Journal of Obesity. 2008;32:1171–1179. doi: 10.1038/ijo.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Kiehl KA, Worhunsky P, Book GA, Maltby N. An exploratory study of the neural mechanisms of decision making in compulsive hoarding. Psychological Medicine. 2009;39:325–336. doi: 10.1017/S0033291708003371. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: Implications for obesity. Trends in Cognitive Sciences. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Pappas N. ‘Nonhedonic’ food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, Pappas N. Brain dopamine is associated with eating behaviors in humans. International Journal of Eating Disorders. 2003;33:136–142. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, Fowler JS. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity. 2011;19:1601–1608. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Fowler JS. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Fowler JS. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, Fowler JS. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15641–15645. doi: 10.1073/pnas.0601977103. [DOI] [PMC free article] [PubMed] [Google Scholar]