Abstract
Lipid molecules, structural components of biomembranes, have been proposed for an important role in membrane fusion. Through various techniques based on protein-reconstituted vesicle-vesicle fusion system, we investigated the influence of several lipid molecules on different stages of yeast SNARE mediated membrane fusion process. Lipid compositions played a significant role in early stages, docking and lipid mixing, while only exhibited a minor effect on fusion pore formation and dilation phases indicated by both small and large content mixing.
In eukaryotic vesicular trafficking, soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) have been widely accepted as the central components for membrane fusion which is regulated by several other accessary proteins. Besides proteins, lipid molecules also function as important active regulators in the SNARE-mediated membrane fusion from two aspects: membrane curvature and protein assembly (van den Bogaart and Jahn, 2011). Regarding membrane curvature modulated by lipid molecules, previous studies at the lipid mixing level showed that lipid molecules, such as cholesterol (Chol) and phosphatidylethanolamine (PE) which stabilize negative curvature significantly influencing membrane fusion (Chang et al., 2009; Domanska et al., 2010; Tong et al., 2009). For the role of lipid molecules in modulating protein assembly, recent studies showed that cholesterol and phosphatidylinositol-4,5-bisphosphate (PIP2) could induce SNARE protein clustering (Murray and Tamm, 2009, 2011), which may be a critical early step for SNARE-mediated membrane fusion. It is also reported that lipid molecules might regulate trans-SNARE complex formation (Mima and Wickner, 2009).
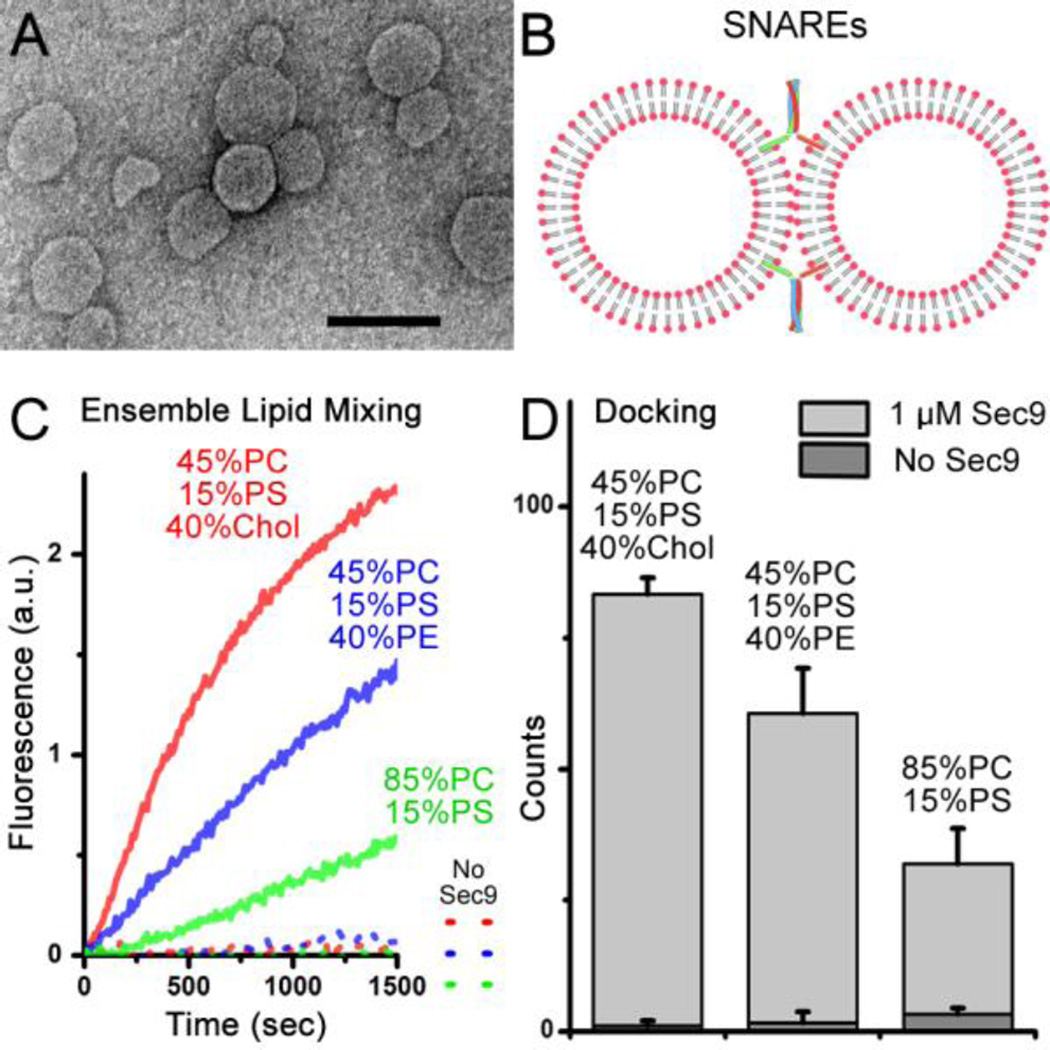
To study SNARE-mediated membrane fusion, the in vitro protein-reconstituted vesicle-vesicle fusion assay using ensemble fluorescence resonance energy transfer (FRET) has been developed since the 1990’s (Weber et al., 1998). In a typical experimental scheme, v- and t-SNARE proteins are reconstituted into two independent populations of vesicles (diameter: 40~100 nm, Figure 1A) in which one population of vesicles contains both donor and acceptor (7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD)/rhodamine) fluorophore- labeled lipid molecules and the other contains no fluorophores. Upon vesicle-vesicle fusion (Figure 1B), lipid mixing results in an increase of the average distance between donors and acceptors, and the extent of fusion can be quantified from the recovery of the donor fluorescence signal.
Figure 1.
(A) Negatively stained electron microscopy (EM) image of vesicles used in the study. Scale bar: 100 nm. (B) Illustration of vesicle-vesicle fusion system. (C) Ensemble lipid-mixing measurements of vesicle-vesicle fusion with various lipid compositions: 1.5 mol% each of NBD and rhodamine were added into yeast v-SNARE Snc2-reconstituted vesicles by replacing the equal amount of PC. The fluorescence change of NBD for lipid mixing was normalized with respect to the maximum fluorescence intensity obtained by adding 0.1% Triton X-100. (D) Single-vesicle docking results of various lipid compositions. 1 µM Sec9 was used for experiments in (C) and (D).
Thus far, the ensemble vesicle-vesicle fusion assay based on lipid mixing has been widely used to study molecular mechanisms whereby SNAREs mediate membrane fusion and has achieved great successes. Here, we investigated the effect of cholesterol and that of PE as well in the fusion of vesicles composed of phosphocholine (PC) and phenylserine (PS). In traditional ensemble lipid mixing, as shown in the Figure 1C, cholesterol and PE molecules both dramatically stimulate lipid mixing.
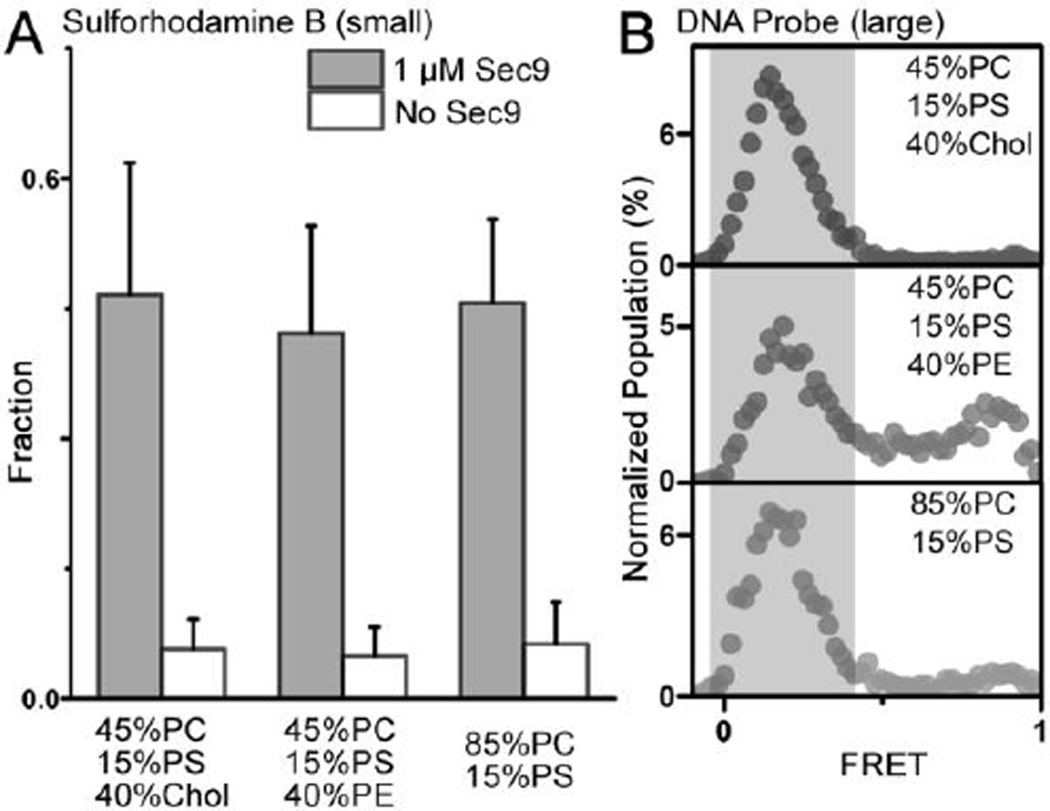
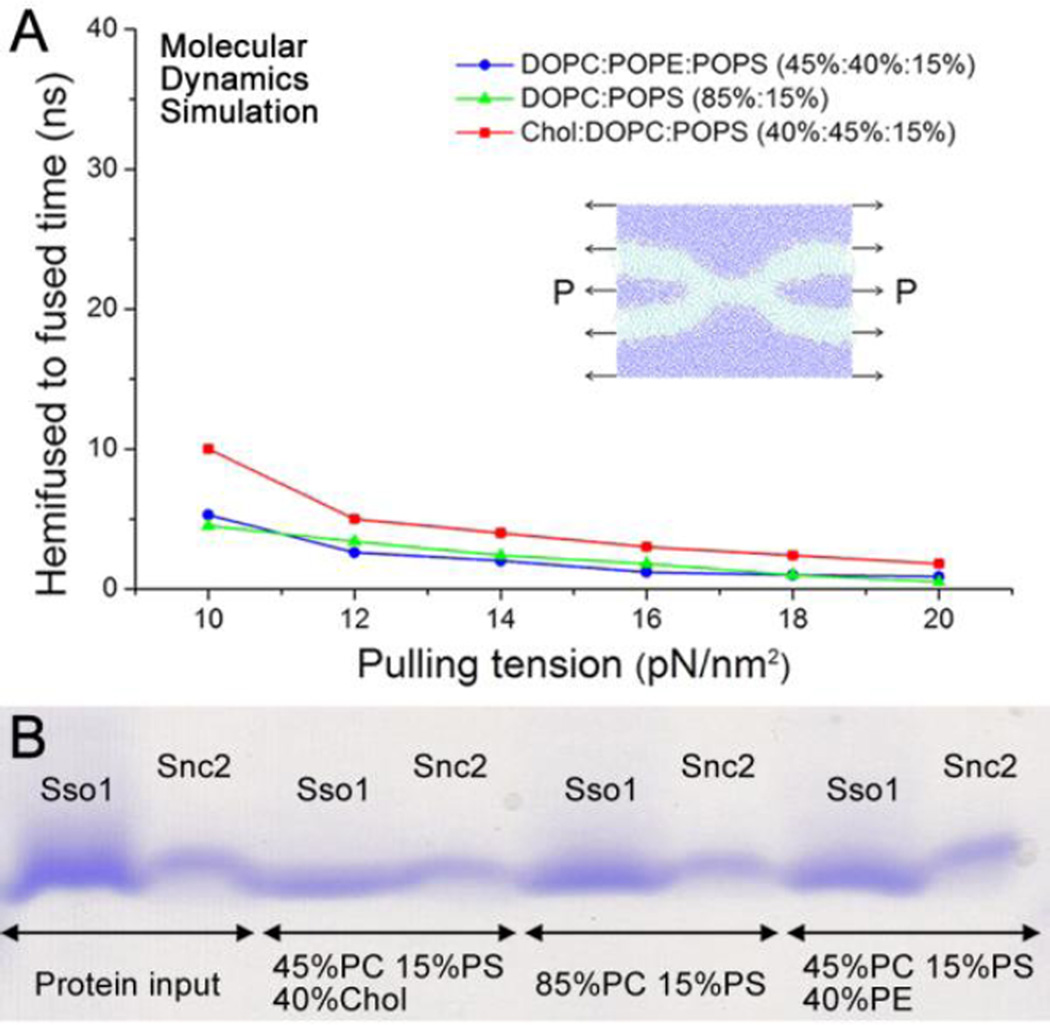
However, it has been shown that the rate of ensemble lipid mixing is largely limited by early stages of membrane fusion, such as docking (Diao et al., 2013a; Diao et al., 2012b; Diao et al., 2013b; Lai et al., 2014). On the other hand, content-mixing through the fusion pore has been considered as a gold standard to access true membrane fusion. To this end, several content mixing assays have been developed at the single-vesicle level. Recently, a single-vesicle content-mixing assay based on dequenching of a small content indicator, sulforhodamine B, has been shown to monitor opening of the nascent fusion pores in Ca2+-triggered fast fusion (Diao et al., 2012a; Diao et al., 2013c; Kyoung et al., 2011; Kyoung et al., 2013; Lai et al., 2014). Through a single vesicle assay involving mixing of small content which can delineate intermediates of the fusion pathway, we found that the effect of lipid compositions on vesicle docking (Figure 1D) is much larger than that on fusion pore opening (Figure 2A). We have also developed a single-vesicle content-mixing assay employing large content mixing indicators intended to report the expansion of fusion pores (Diao et al., 2012b; Diao et al., 2010; Lai et al., 2013). Through this new assay, we also found a minor influence of lipid composition on fusion pore expansion in our vesicle-vesicle fusion. As shown in the Figure 2B, even at the lipid composition that shows the highest lipid mixing in Figure 1C and the best docking in Figure 1D, 45 mol% PC, 40 mol% Chol, and 15 mol% PS, the deletion of cholesterol has only negligible effect on pore expansion; while the addition of PE molecules only slightly reduces content mixing, which is not significant. Through molecular dynamics (MD) simulation, we also confirmed that all three different lipid compositions have similar transition times from the hemifusion state to the full- fused state (Figure 3A). We also noticed an increase in fusion time for cholesterol-containing membrane. This could be explained by the faster formation of syntaxin clusters and SNARE complexes in the cholesterol-containing membrane (Murray and Tamm, 2009, 2011) resulting in a stronger pulling tension generated by the SNAREs. Meanwhile, SNARE-reconstitution efficiency was not dependent upon lipid compositions as confirmed by the sodium dodecyl sulfate (SDS) Page analysis (Figure 3B), which excludes the possible influence of the variation of the protein density in vesicles.
Figure 2.
Results of the single vesicle fusion assay for various lipid compositions; (A) mixing of sulforhodamine B after 1 min of the reaction at room temperature and (B) mixing of the larger DNA probe after 30 min of the reaction at 37 °C. The gray areas in (B) highlight the peaks representing content mixing. For yeast t-SNAREs, Syntaxin 1a-analog Sso1p was reconstituted into vesicles but SNAP-25-analog Sec9 was directly injected into the solution. 1 µM Sec9 was used for all single vesicle experiments in (A) and (B) to initiate the fusion reaction.
Figure 3.
(A) The times it takes to go from the hemifused state to the fully- fused state (Supplementary Figure S1) for various lipid compositions and with various lateral pulling tensions to mimic SNARE-pulling in MD simulations. (B) Sodium dodecyl sulfate (SDS) gel image of Sso1p and Snc2 proteins before and after reconstitution at different lipid compositions.
We knew that in neuronal SNARE mediated membrane fusion system, other than SNARE proteins, many other factors like C2AB and BAR domain (Diao et al., 2009; Peter et al., 2004; Sutton et al., 1995) could affect membrane curvature and therefor affect the fusion process. Also in neuronal SNARE system, it was reported that different lipid components could affect syntaxin-1A oligomerization and SNARE assembly (Lam et al., 2008; van den Bogaart et al., 2011), thereby regulate vesicle docking and lipid mixing. Here, we also have shown that PE, a lipid molecule with a small head group favoring the negatively curved monolayer, and cholesterol with the intrinsic negative curvature, promote ensemble lipid mixing substantially. Interestingly, however, we found, using single vesicle assays that vesicle docking is affected significantly by the lipid composition. These results might suggest that vesicle docking, which results from yeast SNARE complex formation, is strongly coupled with lipid mixing that accompanies bilayer remodeling involving curvature formation. Thus, our results suggest that trans-SNARE assembly occurs concurrently or in the similar time frame with some lipid mixing, perhaps hemifusion as has been previously proposed (Yang et al PNAS 2009).
On the other hand, it was reported that fusion pore opening and expansion in neuronal SNARE mediated membrane fusion required specific lipid molecules like cholesterol and acidic lipid PIP2 (Cho et al., 2007; Lewis et al., 2014; Shin et al., 2010). This could be caused by certain protein regulators, such as C2AB which could significantly change membrane curvature upon binding to specific lipid molecules (Hui et al., 2009; Martens et al., 2007). In stark contrast, we found that fusion pore opening and expansion are less affected by the lipid composition in a simplified yeast system. For late fusion steps after hemifusion or outer leaflet mixing, transmembrane domains (TMDs) of both Q- and R- SNAREs are co-located in the same membrane to be able to interact with each other, which might ease curvature stress and line tension for the small nascent fusion pore (Risselada et al., 2011). Alternatively, the highly curved membrane in the vesicle-vesicle (100 nm in diameter) fusion system might experience less energy barrier towards full fusion. Therefore, it is reasonable to expect a mild effect of the lipid composition on the later steps of membrane fusion, warranting further investigation.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants (R01 GM051290), 973-Program (2015CB856304), and the Fundamental Research Funds for the Central Universities.
References
- Chang JY, Kim SA, Lu XB, Su ZL, Kim SK, Shin YK. Fusion Step-Specific Influence of Cholesterol on SNARE-Mediated Membrane Fusion. Biophys J. 2009;96:1839–1846. doi: 10.1016/j.bpj.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WJ, Jeremic A, Jin H, Ren G, Jena BP. Neuronal fusion pore assembly requires membrane cholesterol. Cell Biol Int. 2007;31:1301–1308. doi: 10.1016/j.cellbi.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J, Burre J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Sudhof TC, Brunger AT. Native alpha-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife. 2013a;2:e00592. doi: 10.7554/eLife.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J, Grob P, Cipriano DJ, Kyoung M, Zhang Y, Shah S, Nguyen A, Padolina M, Srivastava A, Vrljic M, et al. Synaptic proteins promote calcium-triggered fast transition from point contact to full fusion. eLife. 2012a;1:e00109. doi: 10.7554/eLife.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J, Ishitsuka Y, Lee H, Joo C, Su ZL, Syed S, Shin YK, Yoon TY, Ha T. A single vesicle-vesicle fusion assay for in vitro studies of SNAREs and accessory proteins. Nature Protocols. 2012b;7:921–934. doi: 10.1038/nprot.2012.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J, Su ZL, Ishitsuka Y, Lu B, Lee KS, Lai Y, Shin YK, Ha T. A single-vesicle content mixing assay for SNARE-mediated membrane fusion. Nat Commun. 2010;1:54. doi: 10.1038/ncomms1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J, Yoon TY, Su ZL, Shin YK, Ha T. C2AB: A Molecular Glue for Lipid Vesicles with a Negatively Charged Surface. Langmuir. 2009;25:7177–7180. doi: 10.1021/la901676e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao JJ, Cipriano DJ, Zhao ML, Zhang YX, Shah S, Padolina MS, Pfuetzner RA, Brunger AT. Complexin-1 Enhances the On-Rate of Vesicle Docking via Simultaneous SNARE and Membrane Interactions. J Am Chem Soc. 2013b;135:15274–15277. doi: 10.1021/ja407392n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao JJ, Zhao ML, Zhang YX, Kyoung M, Brunger AT. Studying protein-reconstituted proteoliposome fusion with content indicators in vitro. Bioessays. 2013c;35:658–665. doi: 10.1002/bies.201300010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanska MK, Kiessling V, Tamm LK. Docking and Fast Fusion of Synaptobrevin Vesicles Depends on the Lipid Compositions of the Vesicle and the Acceptor SNARE Complex-Containing Target Membrane. Biophys J. 2010;99:2936–2946. doi: 10.1016/j.bpj.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui EF, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaptotagmin-Mediated Bending of the Target Membrane Is a Critical Step in Ca(2+)-Regulated Fusion. Cell. 2009;138:709–721. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoung M, Srivastava A, Zhang YX, Diao JJ, Vrljic M, Grob P, Nogales E, Chu S, Brunger AT. In vitro system capable of differentiating fast Ca(2+)-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. P Natl Acad Sci USA. 2011;108:E304–E313. doi: 10.1073/pnas.1107900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoung MJ, Zhang YX, Diao JJ, Chu S, Brunger AT. Studying calcium-triggered vesicle fusion in a single vesicle-vesicle content and lipid-mixing system. Nature Protocols. 2013;8:1–16. doi: 10.1038/nprot.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Diao JJ, Liu YX, Ishitsuka Y, Su ZL, Schulten K, Ha T, Shin YK. Fusion pore formation and expansion induced by Ca2+ and synaptotagmin 1. P Natl Acad Sci USA. 2013;110:1333–1338. doi: 10.1073/pnas.1218818110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Kim S, Varkey J, Lou X, Song JK, Diao J, Langen R, Shin YK. Nonaggregated alpha-Synuclein Influences SNARE-Dependent Vesicle Docking via Membrane Binding. Biochemistry. 2014;53:3889–3896. doi: 10.1021/bi5002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AD, Tryoen-Toth P, Tsai B, Vitale N, Stuenkel EL. SNARE-catalyzed fusion events are regulated by Syntaxin1A-lipid interactions. Mol Biol Cell. 2008;19:485–497. doi: 10.1091/mbc.E07-02-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KT, Maddipati KR, Taatjes DJ, Jena BP. Neuronal porosome lipidome. J Cell Mol Med. 2014;18:1927–1937. doi: 10.1111/jcmm.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- Mima J, Wickner W. Complex Lipid Requirements for SNARE- and SNARE Chaperone-dependent Membrane Fusion. Journal of Biological Chemistry. 2009;284:27114–27122. doi: 10.1074/jbc.M109.010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DH, Tamm LK. Clustering of Syntaxin-1A in Model Membranes Is Modulated by Phosphatidylinositol 4,5-Bisphosphate and Cholesterol. Biochemistry. 2009;48:4617–4625. doi: 10.1021/bi9003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DH, Tamm LK. Molecular Mechanism of Cholesterol- and Polyphosphoinositide-Mediated Syntaxin Clustering. Biochemistry. 2011;50:9014–9022. doi: 10.1021/bi201307u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJG, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Risselada HJ, Kutzner C, Grubmuller H. Caught in the Act: Visualization of SNARE-Mediated Fusion Events in Molecular Detail. Chembiochem. 2011;12:1049–1055. doi: 10.1002/cbic.201100020. [DOI] [PubMed] [Google Scholar]
- Shin L, Cho WJ, Cook JD, Stemmler TL, Jena BP. Membrane Lipids Influence Protein Complex Assembly-Disassembly. J Am Chem Soc. 2010;132 doi: 10.1021/ja101574d. 5596–+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Davletov BA, Berghuis AM, Sudhof TC, Sprang SR. Structure of the First C-2 Domain of Synaptotagmin .1. A Novel Ca2+/Phospholipid-Binding Fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Tong JS, Borbat PP, Freed JH, Shin YK. A scissors mechanism for stimulation of SNARE-mediated lipid mixing by cholesterol. P Natl Acad Sci USA. 2009;106:5141–5146. doi: 10.1073/pnas.0813138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaart G, Jahn R. Counting the SNAREs needed for membrane fusion. J Mol Cell Biol. 2011;3:204–205. doi: 10.1093/jmcb/mjr004. [DOI] [PubMed] [Google Scholar]
- van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, Dier M, Hell SW, Grubmuller H, Diederichsen U, et al. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 2011;479:552–555. doi: 10.1038/nature10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.