Abstract
Dopamine signaling in nucleus accumbens is essential for cocaine reward. Interestingly, imaging studies have reported blunted dopamine increases in striatum (assessed as reduced binding of [11C]raclopride to D2/D3 receptors) in detoxified cocaine abusers. Here, we evaluate whether the blunted dopamine response reflected the effects of detoxification and the lack of cocaine-cues during stimulant exposure. For this purpose we studied 62 participants (43 non-detoxified cocaine abusers and 19 controls) using positron emission tomography and [11C]raclopride (radioligand sensitive to endogenous dopamine) to measure dopamine increases induced by intravenous methylphenidate and in 24 of the cocaine abusers, we also compared dopamine increases when methylphenidate was administered concomitantly with a cocaine cue-video versus a neutral-video. In controls, methylphenidate increased dopamine in dorsal (effect size 1.4; P < 0.001) and ventral striatum (location of accumbens) (effect size 0.89; P < 0.001), but in cocaine abusers methylphenidate’s effects did not differ from placebo and were similar whether cocaine-cues were present or not. In cocaine abusers despite the markedly attenuated dopaminergic effects, the methylphenidate-induced changes in ventral striatum were associated with intense drug craving. Our findings are consistent with markedly reduced signaling through D2 receptors during intoxication in active cocaine abusers regardless of cues exposure, which might contribute to compulsive drug use.
INTRODUCTION
Drugs of abuse increase extracellular dopamine (DA), which in the nucleus accumbens (NAc), is associated with their reinforcing effects.1–3 Imaging studies in healthy controls have shown that stimulant-induced increases in DA in the striatum (including the ventral striatum (VS) where the NAc is located) are associated with ‘euphoria’ and ‘high’.4–6 Paradoxically, in detoxified cocaine abusers the increases in extracellular DA in striatum (dorsal and ventral) produced by intravenous (i.v.) administration of stimulants drugs (including methylphenidate (MP), which is pharmacologically similar to cocaine) were markedly attenuated even when they triggered intense drug craving.7,8 This is surprising as the attenuated drug-induced DA increases make it difficult to understand why cocaine abusers would have such a markedly exaggerated motivation for cocaine intake. This suggests that non-pharmacological effects (that is, conditioning to cues that predict drug reward) are involved in the enhanced incentive value of drugs in addiction. Indeed, there is increasing evidence that DA encodes for a ‘reward prediction error’ rather than reward itself, and preclinical studies show that with repeated exposure to natural rewards (food), DA cells stop firing for the reward and instead fire for the cue that predicts the reward.9 Similarly exposure to cocaine-cues increases DA in NAc in rodents10 and in striatum in cocaine abusers.11,12 Thus, we hypothesized that in addicted subjects the cues that precede drug administration might contribute to the DA increases occurring during drug intoxication. Here, we test this hypothesis and predicted that in cocaine abusers concomitant cocaine-cue exposure would enhance stimulant-induced DA increases. Also as prior studies had been done in detoxified cocaine abusers (with at least 15 days of abstinence)7,8 and preclinical studies have shown attenuated DA signaling 14 days following cocaine withdrawal,13 we also wanted to assess whether blunted DA responses were present in active cocaine abusers (non-detoxified).
For this purpose, we measured changes in extracellular DA induced by MP in the brain of 62 males (43 non-detoxified cocaine abusers and 19 controls) using positron emission tomography (PET) and [11C]raclopride.14 In 24 of the cocaine abusers (cohort #1) we compared MP effects when given concomitantly with a cocaine cue-video versus when given with a neutral-video;11 and in controls and in 19 of the cocaine abusers (cohort #2) we measured the effects of MP with no stimulation (no video). We used MP since, like cocaine, it increases DA by blocking DA transporters,15 and cocaine abusers report it to have effects similar to that of cocaine.16
SUBJECTS AND METHODS
Subjects
This study included 62 male participants comprised of 43 active cocaine-addicted subjects and 19 healthy non-drug abusing controls recruited through advertisements in local newspapers. Participants (controls and cocaine abusers) were recruited specifically for this study and there is no overlap with previously published samples. Cocaine abusers fulfilled DSMIV criteria for cocaine dependence and were active users for at least the prior 6 months (at least ‘4 g’ a week). The cocaine abusers were predominant crack users and consisted of two independent cohorts: cohort #1 (N = 24; 45 ± 4 years of age; 18 ± 7 years of cocaine use; cocaine dose 3.6 ± 2.5 g per day, last day of use 7 ± 6 days; 14/24 smokers) was recruited to assess the effects of cocaine-cues on i.v. MP and cohort #2 (N = 19; 45 ± 3 years of age; 28 ± 6 years of cocaine use; cocaine dose 3.9 ± 3 g per day, last day of use 3 ± 3 days; 10/19 smokers) was recruited to assess the effects of i.v. MP without stimulation (no videos) just as for the controls (N = 19, 42 ± 4 years of age; 3/19 smokers). Exclusion criteria for participants included: current or past psychiatric disease other than cocaine dependence for the cocaine abusers, or nicotine dependence as defined by DSM-IV; past or present history of neurological, cardiovascular or endocrinological disease; history of head trauma with loss of consciousness > 30 min; and current medical illness. Cocaine abusers differed from controls in that they were on average 3 years older and had a higher percentage of tobacco smokers. Written informed consent was obtained from all subjects and the studies were reviewed and approved by the Institutional Review Board at the Stony Brook University.
Behavioral self-reports and scales
To study behavioral effects of MP, we assessed self-reports for ‘high’ using analog scales (rated from 1 to 10) that were obtained at 30 and 60 min after MP administration. To assess cocaine craving, we used a brief version of the cocaine craving questionnaire (CCQ),17 which evaluates current cocaine craving on a seven-point scale. The CCQ was obtained four times: before video exposure, 20 min after initiation of video (just before placebo or MP injection), at the end of video (50 min from video initiation and 30 min post MP or placebo) and at the end of study (60 min post MP or placebo).
PET scan
We used an HR+ scanner (resolution 4.5 × 4.5 × 4.5 mm full width half-maximum) with [11C]raclopride 4–8 mCi (specific activity 0.5–1.5 Ci per μm at end of bombardment) using procedures previously described.18 Briefly, 20 dynamic emission scans were obtained immediately after injection up to 54 min. Arterial sampling was used to quantify total carbon-11 and unchanged [11C]raclopride in plasma. Cocaine abusers from cohort #1 (n = 24) were scanned with [11C]raclopride three times: (1) placebo when given with a neutral-video, (2) MP when given with a cocaine cue-video and (3) MP when given with a neutral-video, over a 2-day period. The MP scans were done at least 24 h apart from each other under randomly ordered conditions, once while watching a neutral-video (non-repeating segments of nature stories) and another while watching a cocaine cues-video (non-repeating segments portraying scenes that simulated purchase, preparation and smoking of cocaine) that were previously published.11 The placebo scan was done 2 h before MP injection scan. Videos were started 20 min before [11C]raclopride injection and continued for 30 min after radiotracer injection (total of 50 min). Cocaine abusers from cohort #2 (n = 19) and controls were scanned with [11C]raclopride two different times (placebo and MP) with no stimulation and randomized over a 2-day period. For all the groups, MP (0.5 mg kg−1) and placebo (3 cc saline) were administered i.v. 2 min before [11C]raclopride injection.
PET image analysis
We analyzed the non-displaceable binding potential (BPND) images using Statistical Parametric Mapping (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK), which enabled us to make comparisons on a pixel by pixel basis.19 Specifically, we estimated for each voxel the distribution volume, which corresponds to the equilibrium measurement of the ratio of the radiotracer’s tissue concentration to that of its plasma concentration using a graphical analysis technique for reversible systems.20 These images were then spatially normalized to the stereotactic space of the Montreal Neurological Institute using a 12-parameter affine transformation as previously described.21 The intensity of the distribution volume images was normalized to that in cerebellum to obtain images of the distribution volume ratios, which correspond to BPND in each voxel.
Statistical analyses
The brain maps (BPND) were spatially smoothed in SPM8 using an 8-mm isotropic Gaussian kernel. One-way repeated analysis of variance (ANOVA) was used to assess drug effects (placebo vs MP) and to assess the effects of condition (neutral-video vs cocaine-cue video). Two-way ANOVA with group as the between factor (cocaine abusers and controls) and drug as the within factor (MP and placebo) was used to assess whether MP-induced BPND changes differed between controls and cocaine abusers (cohort #1) when tested with the cocaine cues-video and when tested with the neutral-video. A two-way ANOVA was done to compare the controls with the cocaine abusers from cohort #2 (tested with no video exposures) with group as the between factor, and drug as the within factor, and to assess the group by drug interaction. The controls were younger than cocaine abusers, so to correct for potential age confounds on MP’s effects, we used age as a covariate. Significance was set as PFWE < 0.05 corrected for multiple comparisons at the cluster level using the random field theory with a family wise error (FWE) correction.
The SPM findings were corroborated with an independent region-of-interest (ROI) analysis on preselected ROI in putamen and VS (described18) and prefrontal cortex (described22). These ROI were used to estimate effect sizes and for correlation analysis. Significance was set at P < 0.05 if it corroborated SPM findings.
To compare self-reports of ‘high’ induced by MP between groups, we used ANOVA with groups as the between factor and drug as the within factor (placebo, MP 30 min and MP 60 min) and to assess their Interaction. To assess the effects of MP on cocaine craving in cocaine abusers (cohort #1) with or without exposure to cocaine-cues, we used repeated ANOVA with two conditions (cocaine-cues and neutral-video) and time (baseline, post video, 30 min post MP). Significant findings (P < 0.05) were followed by post hoc t-test analyses. Effect sizes were estimated using Cohen’s ‘d’.23
Pearson product–moment correlations were computed between MP-induced changes in DA (BPND placebo − BPND MP) and self-reports of high (placebo − MP) for the measures taken at 30 min post MP. For the correlations with craving, we used the difference scores for CCQ (placebo − MP) obtained at the end of the video stimulations (30 min post MP), for the neutral and the cocaine-cue conditions. Correlations were considered significant at P < 0.05.
RESULTS
Comparisons with cocaine abusers from cohort #1
Behavioral effects of MP
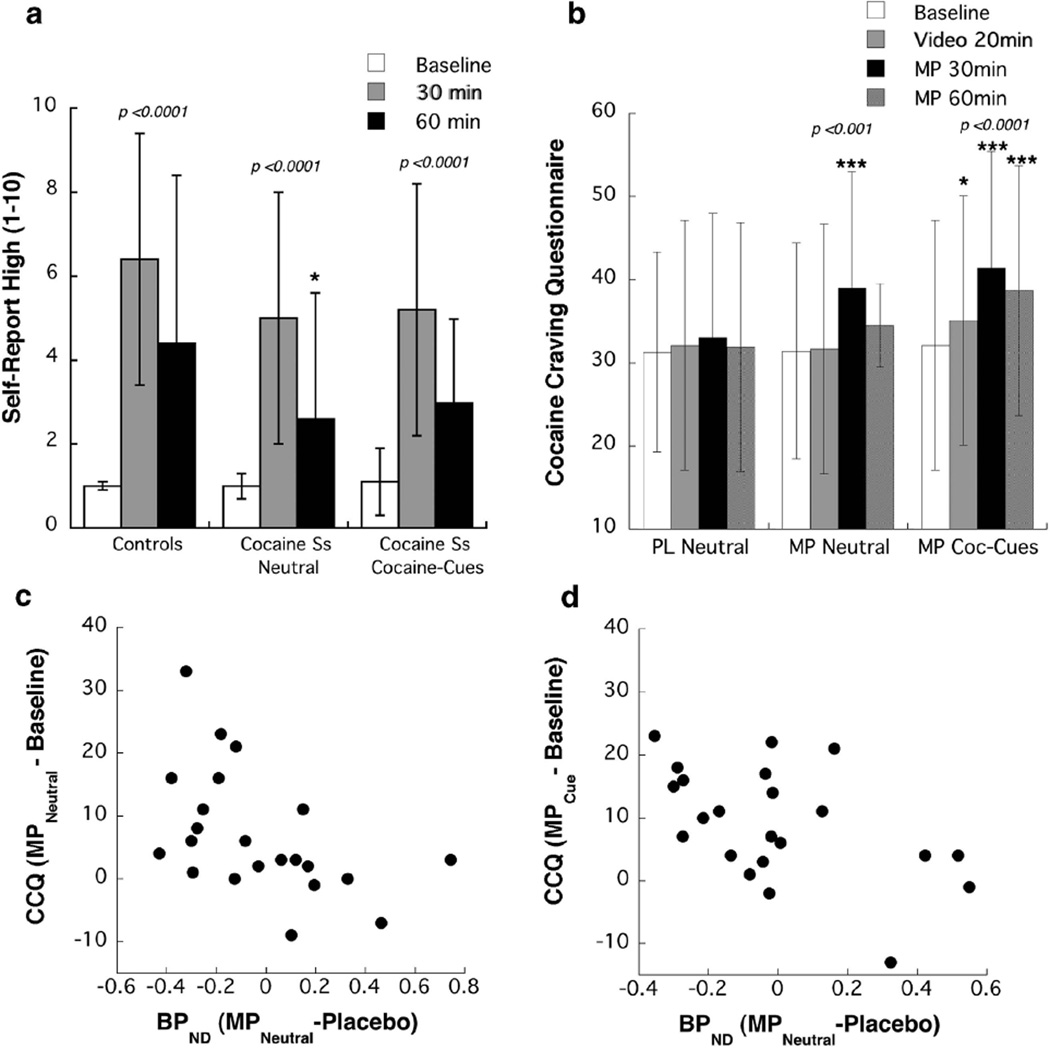
MP significantly increased self-reports of ‘high’ in controls (d = 1.49) and in cocaine abusers (d = 1.50) (ANOVA drug effect, F = 50, P = 0.0001), but the drug by group interaction effect was not significant, neither when MP was given with the neutral-video nor the cues-video (Figure 1a). Comparisons of MP-induced ‘high’ in the cocaine abusers did not differ between the cocaine-cue and the neutral-video conditions (Figure 1a).
Figure 1.
Behavioral effects of intravenous methylphenidate (MP). (a) Self-reports of ‘high’ in the controls and in cocaine abuser both when MP was given concomitant to cocaine cue-video or when it was given with the neutral-video. *Significantly different from controls (P < 0.05). (b) Scores on cocaine craving questionnaire (CCQ) in the cocaine abusers at baseline (prior to any stimulation), 20 min after exposure to the cocaine cue- or the neutral-video, 30 min after MP (end of 50 min of video stimulation) and 60 min after MP. *Significantly different from baseline measures at P < 0.05 and at ***P < 0.001. (c) Correlations between MP-induced changes in non-displaceable binding potential (BPND) in ventral striatum (VS) and the changes in craving scores (cocaine craving questionnaire or CCQ) both when it was given with the cocaine cues-video (r = − 0.51, P =0.02) and when given with the neutral-video (r = − 0.50, P=0.02).
Exposure to the cocaine-cue video significantly increased craving (CCQ) at 20 min (F = 13.6, P = 0.002; d = 0.24), whereas exposure to the neutral-video did not. Craving scores at 20 min (measure taken before MP) were significantly higher for the cue-video than the neutral-video conditions (F = 5.2, P = 0.04; d = 0.32) (Figure 1b). The CCQ scores post MP did not differ between cue-video and neutral-video conditions, which was explained by the intense craving triggered by MP (cue-video F = 22, P < 0.0001, d = 0.68; neutral-video F = 15, P = 0.001, d = 0.55) that was significantly higher than the craving triggered by the cues (before MP) (F = 15, P = 0.001, d = 0.87) (Figure 1b). The magnitude of the craving triggered by MP did not differ whether cues were present or not, which suggests that cues have little effect when the drug is in the system.
Effects of MP on [11C]raclopride
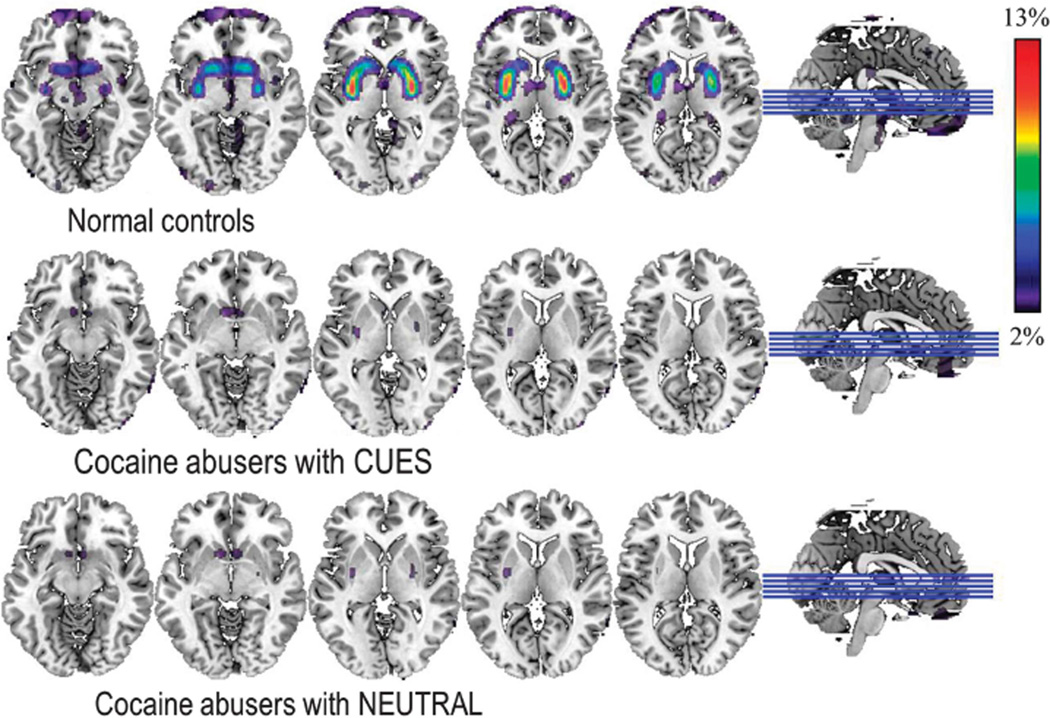
SPM revealed that in controls, MP significantly decreased BPND in striatum and medial prefrontal cortex (brodmann area (BA) 10) (Pc < 0.05) (Figure 2; Table 1) compared with placebo. In contrast, in cocaine abusers, MP’s effects were not significant (Pc < 0.05) and only achieved significance in VS when the threshold for significance was uncorrected for multiple comparisons (Pu < 0.05) (Figure 2; Table 1). MP’s effects did not differ when given with the neutral-video versus when given with the cocaine cue-video (Figure 2) and the comparisons with the controls yielded similar findings for both conditions (Figure 3). MP-related BPND decreases were significantly stronger for controls than cocaine abusers in dorsal striatum, VS and BA 10 (Table 1). Covarying for age did not alter the results.
Figure 2.
Brain maps obtained with SPM showing significant differences in non-displaceable binding potential (BPND) for [11C]raclopride between placebo and methylphenidate (MP) for the contrast PL > MP in controls and in cocaine abusers (cohort #1) when MP was given concomitant to the cocaine cues-video (CUES) and when given concomitant with the neutral-video (NEUTRAL). Significance for controls corresponds to Pu < 0.001, clusters > 100 voxels; and for the cocaine abusers to Pu < 0.05, clusters > 100 voxels.
Table 1.
Statistical significance for MP-induced DA increases (measured as decreases in BPND) in controls and in cocaine abusers from cohort #1 (both for neutral- and cocaine cue-videos) and for regions where controls had significantly greater increases than cocaine abusers
| Region | BA | MNI coord (mm) | PL>MP (T (P)) | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | Controls | COC | Controls>COC | ||
| Putamen | − 26 | − 6 | 0 | 7.1 (3.5 × 10 −7) | NS | 4.3 (1.7 × 10−4) | |
| VS | − 4 | 12 | − 8 | 4.6 (8.7 × 10−5) | 1.9 (0.03) | 1.9 (0.03) | |
| Putamen | 30 | − 8 | 0 | 6.4 (1.5 × 10−6) | NS | 3.8 (5.6 × 10−4) | |
| VS | 10 | 12 | − 6 | 4.0 (3.0 × 10−4) | 1.6 (0.06) | 1.7 (0.05) | |
| Prefrontal | 10 | − 30 | 64 | 10 | 3.4 (1.4 × 10−3) | NS | 2.7 (6.9 × 10−3) |
Abbreviations: BA, brodmann area; COC, cocaine; MNI, Montreal Neurological Institute; MP, methylphenidate; NS, non significant; PL, placebo; VS, ventral striatum.
The effects of MP in BPND in the cocaine abusers did not differ when given concomitantly with the cocaine cues-video or the neutral-video, so we averaged the results from both conditions for the comparisons with the controls. The locations of the clusters are based on the coordinates from the stereotactic space of the MNI in (x, y and z). The values correspond to the T-scores and in parenthesis the significance (P).
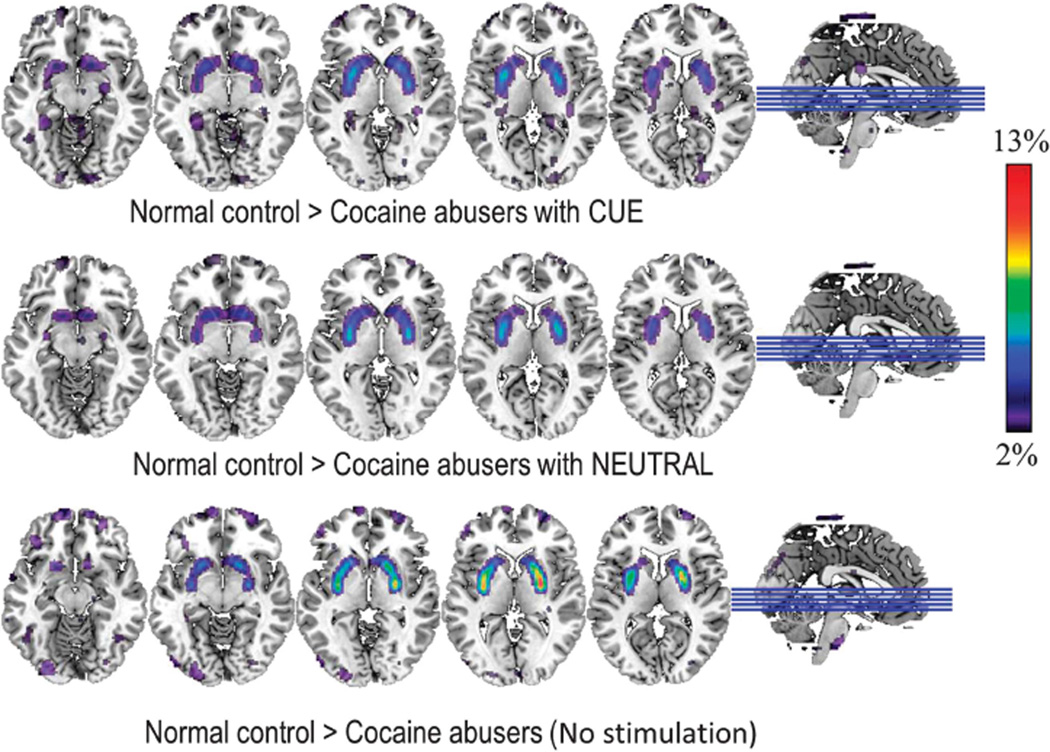
Figure 3.
Brain maps obtained with SPM showing significant differences for the comparisons of the responses (delta: PL − MP) between controls and cocaine abusers (cohort #1) when MP was given concomitantly with the cocaine cues-video (CUE) and when MP was given concomitantly with the neutral-video (NEUTRAL), and comparisons between the controls and the cocaine abusers (cohort #2) who were not exposed to videos (placebo > MP). These comparisons highlight the differences in the response to MP between controls and cocaine abusers (drug by group interaction). Significance corresponds to Pu < 0.001, clusters > 100 voxels.
The independent ROI analysis, corroborated that MP-induced decreases in BPND in dorsal and VS, and in BA 10 were significant in controls but not in cocaine abusers (Table 2). The group by drug interaction was significant and showed that MP-induced decreases in BPND were significantly larger in controls than in cocaine abusers (Table 2).
Table 2.
Values for BPND estimated with independent ROI analysis for the regions where SPM showed that MP’s effects differed between groups for the measures taken during placebo (PL) and methylphenidate (MP), and for the difference between them (delta) for the controls (n = 19), for the cocaine abusers tested with exposure to neutral- and cocaine cue-videos (cohort #1, n = 24) and for the cocaine abusers tested with no video stimulation (cohort #2; n = 19) along with the effect size (Cohen’s d) for the within (row) and between comparisons (columns)
| Controls | COC abusers cohort #1 | Effect size | COC abusers cohort #2 | Effect size | |
|---|---|---|---|---|---|
| Putamen PL | 2.58 ± 0.24 | 2.46 ± 0.53 | 2.51 ± 0.41 | ||
| Putamen MP | 2.16 ± 0.30 | 2.36 ± 0.51 | 2.48 ± 0.50 | ||
| Delta Putamen | − 0.42 ± 0.30 | − 0.11 ± 0.28*** | 1.1 | − 0.04 ± 0.32*** | 1.22 |
| Effect size | 1.4 | NS | NS | ||
| VS PL | 2.74 ± 0.49 | 2.33 ± 0.38** | 2.15 ± 0.38*** | ||
| VS MP | 2.35 ± 0.42 | 2.33 ± 0.31 | 2.14 ± 0.40 | ||
| Delta VS | − 0.39 ± 0.44 | − 0.00 ± 0.28*** | 1.1 | − 0.01 ± 0.35** | 0.96 |
| Effect size | 0.89 | NS | NS | ||
| BA 10 PL | 1.04 ± 0.04 | 1.03 ± 0.06 | 1.04 ± 0.09 | ||
| BA 10 MP | 0.99 ± 0.10 | 1.04 ± 0.07 | 1.04 ± 0.07 | ||
| Delta BA 10 | − 0.05 ± 0.10 | 0.01 ± 0.05* | 0.75 | 0.00 ± 0.07 | 0.54 |
| Effect size | 0.65 | NS | NS |
Abbreviations: BA, brodmann area; COC, cocaine; MP, methylphenidate; NS, non significant; PL, placebo; VS, ventral striatum.
Comparisons between controls and cocaine abusers correspond to post hoc t-tests
P < 0.05;
P < 0.005 and
P < 0.001.
The within-group comparison between PL and MP were only significant in the controls for putamen (P < 0.001), VS (P < 0.002) and brodmann area 10 (P < 0.05).
Correlations between MP-induced BPND changes and behavior
In the cocaine abusers, MP-induced increases in craving (CCQ scores) were negatively correlated with changes in BPND in VS for the neutral-video (r = 0.50, P = 0.02) and the cocaine cues-video (r = 0.51, P = 0.02); such that the larger the BPND decreases the greater the CCQ increases (Figure 1c). The comparison of the strength of the correlations between both conditions was not significant.
Correlation analysis between MP-induced changes in self-reports of ‘high’ and in BPND when including controls and cocaine abusers was not significant. However, the correlation analysis when done separately on the cocaine abusers showed a negative correlation between changes in ‘high’ and BPND changes in VS for the neutral-video condition (r = 0.56, P = 0.01) but was not significant for the cue-video condition. When MP was given with the neutral-video the larger the BPND decreases in VS (reflecting DA increases) the greater the ‘high’. The correlation analysis when done separately in the controls was not significant. Comparison of the correlations between groups was not significant.
Order effects
To control for potential order in the cocaine abusers who were tested twice with MP, we compared the first and the second MP administration and showed no significant differences between them (data not shown).
Comparisons with cocaine abusers from cohort #2
Behavioral effects of MP
MP significantly increased self-reports of ‘high’ in controls (F = 32, P = 0.0001; d = 1.62) and cocaine abusers (F = 13.8, P = 0.002, d = 1.07) (ANOVA drug effect F = 46, P = 0.0001) and the interaction showed a trend of an effect (F = 2.8, P = 0.10). Post hoc t-tests showed that the ‘high’ at 30 min was lower in cocaine abusers than in controls (3.6 ± 3 vs 5.7 ± 3, P = 0.05; d = 0.63) and showed a trend at 60 min (2.1 ± 2 vs 3.6 ± 3; P = 0.07; d = 0.59).
Effects of MP on [11C]raclopride
In cocaine abusers of cohort #2, MP’s effects on BPND did not differ from placebo (Table 2). The comparison for MP-related BPND decreases between controls and cocaine abusers showed that MP-induced BPND decreases in dorsal and VS differed significantly between the groups, being significantly larger for controls than for cocaine abusers (Figure 3; Table 2).
The ROI analysis corroborated that MP-induced decreases in BPND in striatum and BA 10 were significant in controls but did not differ from placebo in cocaine abusers (Table 2). The group by drug interaction was significant and showed that striatal DA increases were significantly larger in controls than cocaine abusers (Table 2). For BA 10, the group by drug interaction only showed a trend of an effect (P = 0.08).
Effects of smoking on MP’s effects (cohorts #1 and #2)
To assess whether cigarette smoking influenced MP’s effects on BPND, we compared cocaine abusers who were smokers and those who were not. For cohort #1, MP’s effects in striatal BPND did not differ between smokers (n = 14; −5.0 ± 7% change) and nonsmokers (n = 10; − 3.2 ± 16% change) (P = 0.68). Similarly for cohort #2, MP’s effects in striatal BPND did not differ between smokers (n = 10; − 0.3 ± 15%) and non-smokers (n = 9; +2.5 ± 10% change) (P = 0.70). This indicates that differences between controls and cocaine abusers on MP’s effects are not due to tobacco smoking.
Baseline measures of D2/D3 receptor availability (cohorts #1 and #2)
The SPM comparisons for baseline D2/D3 receptor availability between controls and cocaine abusers did not differ for cohort #1 or cohort #2 (PcFWE < 0.05). In contrast, the ROI analysis revealed that baseline D2/D3 receptor availability in VS was significantly lower in cocaine abusers than in controls for cohort #1 and cohort #2 (Table 2). The discrepancy between SPM and ROI probably reflects the high threshold of significance for SPM when correcting for multiple comparisons (corrected PcFWE < 0.05); indeed SPM differences in VS were significant when uncorrected (Pu < 0.05).
DISCUSSION
Here, we show that in non-detoxified cocaine abusers MP-induced increases in DA were profoundly attenuated, whether cocaine-cues were present or not. Moreover, in the cocaine abusers, MP’s effects did not differ from placebo (with or without cocaine-cues). These findings indicate that dopaminergic attenuation during stimulant intoxication in cocaine abusers is not due to detoxification, nor to a lack of cocaine-cues.
Effects of MP on extracellular DA
The profound attenuation of MP’s dopaminergic effects in active cocaine abusers corroborates prior findings in cocaine abusers tested at least 15 days after detoxification7,8 revealing an even larger degree of blunting (MP’s effects did not differ from placebo; whereas in prior studies they did). It is also consistent with findings in rodents showing that chronic cocaine almost abolished the stimulation of striatal D2R-expressing neurons during cocaine intoxication.24 Despite this attenuation MP induced intense craving, which indicates that the enhanced incentive value of cocaine in cocaine abusers, cannot be attributed to sensitized drug-induced DA release. However, because our PET measures identify DA changes over a 30-min period and over relatively large brain areas, we cannot exclude the possibility of short-lasting DA increases and/or dopaminergic stimulation of restricted neuronal ensembles within the NAc25 in the enhanced incentive motivation for cocaine in addiction.
Effects of MP as a function of cue exposure
In a prior study, we showed that cocaine-cues exposure in cocaine abusers was associated with DA increases in striatum,11 which led us to hypothesize that MP’s effects would be amplified by cocaine-cues as compared with when given with neutral-cues.26 Failure to observe a difference between both conditions could reflect MP’s peripheral effects, which might have acted as cues as has been documented for cocaine,27 such that the cocaine-cue video could not add much. It is also possible that the co-mingling of the two phases of reward (expectation and receipt) might reduce or eliminate the DA-signaling effects of the cues. Indeed, shorter delays between the cues and reward delivery have been shown to result in lower DA increases than longer delays.28
Baseline D2R availability in cocaine abusers
Cocaine abusers (cohorts #1 and #2) had lower baseline D2/D3 receptor availability in VS than controls, which is consistent with prior studies (reviewed29). Low striatal D2R availability has been associated with impulsivity and compulsive drug intake.30,31 Moreover, in preclinical studies, strengthening striatal D2R signaling results in resilience toward compulsive cocaine intake,32,33 and in imaging studies of non-drug-abusing controls, high striatal D2R availability is associated with aversive responses to i.v. MP.34,35 Low striatal D2R availability in cocaine abusers is associated with reduced activity in prefrontal regions involved with self-control,18 which could be a mechanism through which reduced striatal D2R signaling mediates compulsive drug intake. Indeed, in cocaine and methamphetamine abusers, reduced striatal D2R availability is associated with worse clinical outcomes.36,37
Behavioral effects of MP
Despite the markedly attenuated DA effects of MP in the cocaine abusers they still experienced a ‘high’. The greater group differences for MP-induced DA changes, which differed significantly between groups, than for the ‘high’, which while somewhat lower in cocaine abusers did not differ from controls, could reflect the fact that PET measures represent DA binding to D2R (also D3R) and D2R are not necessary for cocaine reward. In fact, stimulant drugs (including cocaine) are still rewarding when D2R are blocked,38 inhibited32 or not expressed (knockouts).39,40 However, it is possible that in cocaine abusers MP triggered a short-lasting increase in DA that could have activated D1 receptors (D1R), which are necessary for cocaine reward41 but which the limited temporal resolution of [11C]raclopride (30 min) could not detect. Alternatively other neurotransmitters (opioids)42 might have contributed to the ‘high’.
In cocaine abusers MP-induced cocaine craving and ‘high’ were associated with DA increases in VS (location of NAc), which is consistent with preclinical studies that identify the NAc as part of the circuitry that mediates cue-induced relapse to cocaine-seeking43 and with findings in cocaine abusers in whom purposeful inhibition of craving decreased NAc activity.44 As the NAc expresses high-D3R levels, which are upregulated in cocaine abusers,45 the relationship with craving (perhaps also with ‘high’) might be mediated by DA stimulation of D3R. Indeed, in preclinical studies, enhanced cocaine-cue reactivity following chronic cocaine was associated with upregulation of D3R in NAc.46
We recently showed that oral MP reduced brain limbic reactivity induced by cocaine-cues in cocaine abusers,47 whereas here we show that i.v. MP increased craving. This most likely reflects pharmacokinetic differences between oral and i.v. MP; the former emulating the gradual and steady DA increases associated with tonic DA firing, whereas the later emulating the fast and sharp DA increases associated with phasic DA (reviewed29).
Study limitations
The PET [11C]raclopride method cannot distinguish between D2R and D3R and has limited temporal (30 min) and spatial resolution (4 mm). Our cocaine abusers differed from controls in that they were older and had more smokers; however, this is unlikely to account for the differences as the results did not change after covarying for age and there were no differences between smokers and non-smokers. In our study, we cannot ascertain whether the attenuation of DA increases with MP in cocaine abusers might have preceded their cocaine abuse but the fact that in preclinical studies chronic cocaine markedly attenuates D2R signaling during cocaine intoxication24 suggests that they are causally linked. The mechanisms(s) that underlie the attenuated DA responses in cocaine abusers are not addressed by our study and merit investigation. Here, we also document significant decreases in BPND with MP in BA 10 in the controls that would be consistent with DA increases in prefrontal cortex and which was not present in abusers. However, the limited sensitivity of [11C]raclopride to DA changes in cortical areas lead us to interpret these as preliminary and in need of replication.
CONCLUSION
We show a profound attenuation of MP-induced DA increases in the striatum of cocaine abusers (regardless of cue exposures) although MP triggered intense drug craving. The reduced DA responses triggered by MP in the cocaine abusers could drive them to increase the doses abused and explain their tolerance to the drug’s effects.48 On the other hand the discrepancy between the expected and the actual reward (attenuated DA signals in VS) might trigger craving as a means to compensate for the deficit.
Acknowledgments
We thank Paul Vaska, Colleen Shea, Pauline Carter, Wei Zhu, Karen Apelskog and Ruben Baler for their contributions. Research supported by NIH’s Intramural Research Program (NIAAA).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 3.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 4.Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 5.Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS, et al. SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med. 1995;36:1182–1190. [PubMed] [Google Scholar]
- 6.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999;291:409–415. [PubMed] [Google Scholar]
- 7.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 9.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 10.Phillips PE, Stuber GD, Heien ML, Wightman RM, RM C. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006 Jun 14;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Schlussman SD, Rabkin J, Butelman ER, Ho A, Kreek MJ. Chronic escalating cocaine exposure, abstinence/withdrawal, and chronic re-exposure: effects on striatal dopamine and opioid systems in C57BL/6J mice. Neuropharmacology. 2013;67:259–266. doi: 10.1016/j.neuropharm.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, et al. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- 15.Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, et al. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43:181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- 16.Wang G-J, Volkow ND, Hitzemann RJ, Wong C, Angrist B, Burr G, et al. Behavioral and cardiovascular effects of intravenous methylphenidate in normal subjects and cocaine abusers. Eur Addict Res. 1997;3:49–54. [Google Scholar]
- 17.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Fowler JS, Wang GJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 19.Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 20.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 21.Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2011;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 24.Park K, Volkow ND, Pan Y, Du C. Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J Neurosci. 2013;33:15827–15836. doi: 10.1523/JNEUROSCI.1935-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, et al. "Nonhedonic" food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 27.Wakabayashi KT, Kiyatkin EA. Critical role of peripheral drug actions in experience-dependent changes in nucleus accumbens glutamate release induced by intravenous cocaine. J Neurochem. 2013;128:672–685. doi: 10.1111/jnc.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wanat MJ, Kuhnen CM, Philips PE. Delays conferred by escalating costs modulate dopamine release to rewards but not their predictors. J Neurosci. 2010;30:12020–12027. doi: 10.1523/JNEUROSCI.2691-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse. 2008;62:481–486. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- 35.Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 36.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norman AB, Tabet MR, Norman MK, Fey BK, Tsibulsky VL, Millard RW. The affinity of D2-like dopamine receptor antagonists determines the time to maximal effect on cocaine self-administration. J Pharmacol Exp Ther. 2011;338:724–728. doi: 10.1124/jpet.111.183244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, et al. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- 41.Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, et al. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Foll B, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- 47.Volkow ND, Wang GJ, Tomasi D, Telang F, Fowler JS, Pradhan K, et al. Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers. PLoS One. 2010;5:e11509. doi: 10.1371/journal.pone.0011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Small AC, Kampman KM, Plebani J, De Jesus Quinn M, Peoples L, Lynch KG. Tolerance and sensitization to the effects of cocaine use in humans: a retrospective study of long-term cocaine users in Philadelphia. Subst Use Misuse. 2009;44:1888–1898. doi: 10.3109/10826080902961179. [DOI] [PubMed] [Google Scholar]