Abstract
Nonnative, invasive shrubs can affect human disease risk through direct and indirect effects on vector populations. Multiflora rose (Rosa multiflora) is a common invader within eastern deciduous forests where tick-borne disease (e.g. Lyme disease) rates are high. We tested whether R. multiflora invasion affects blacklegged tick (Ixodes scapularis) abundance, and at what scale. We sampled host-seeking ticks at two spatial scales: fine-scale, within R. multiflora-invaded forest fragments; and patch scale, among R. multiflora-invaded and R. multiflora-free forest fragments. At a fine scale, we trapped 2.3 times more ticks under R. multiflora compared to paired traps 25 m away from R. multiflora. At the patch scale, we trapped 3.2 times as many ticks in R. multiflora-free forests compared to R. multiflora-invaded forests. Thus, ticks are concentrated beneath R. multiflora within invaded forests, but uninvaded forests support significantly more ticks. Among all covariates tested, leaf litter volume was the best predictor of tick abundance; at the patch scale, R. multiflora-invaded forests had less leaf litter than uninvaded forests. We suggest that leaf litter availability at the patch-scale plays a greater role in constraining tick abundance than the fine-scale, positive effect of invasive shrubs.
Keywords: forest fragmentation, invasive species, Ixodes scapularis, nonnative plant, Rosa multiflora, scale
Introduction
Altered ecosystems can produce cascading effects. For example, nonnative, invasive species impact a range of abiotic and biotic ecosystem components from soil chemistry (Howard et al. 2004) to biodiversity (Wilcove et al. 1986, Coblentz 1990), and even human health (Juliano and Lounibos 2005, Allan et al. 2010, Morlando et al. 2012). On the east coast of the United States, multiflora rose (Rosa multiflora) is an ubiquitous invader in forest understories (Huebner et al. 2014), with documented ecosystem effects including accelerated litter nitrogen loss (Ashton et al. 2005) and reduced avian nest success (Borgmann and Rodewald 2004). Herein, we investigate whether R. multiflora may also affect human health by increasing tick-borne disease risk.
Blacklegged ticks (Ixodes scapularis Say) are an important vector of zoonotic pathogens, including Borrelia burgdorferi, Anaplasma phagocytophilum, Babesia microti, Borrelia miyamotoi, and Powassan virus (Centers for Disease Control and Prevention 2015). Borrelia burgdorferi causes Lyme disease, the most commonly reported vector-borne disease in North America (Bacon et al. 2008). Recent work in forest ecosystems suggests that the abundance of host-seeking I. scapularis (nymphs and adults) is positively associated with invasion by nonnative shrubs (Lubelczyk et al. 2004, Elias et al. 2006, Williams et al. 2009, Williams and Ward 2010). Ixodes scapularis abundance is a key factor in predicting human Lyme disease cases (Mather et al. 1996, Khatchikian et al. 2012); thus, nonnative plant invasion potentially increases human disease risk.
The dense understory structure created by nonnative plants can increase humidity and protection from extreme temperature fluctuations, thereby creating a more favorable microclimate for ticks (Williams et al. 2009). Dense understory structure is also used preferentially by host animals (e.g. white-tailed deer, Odocoileus virginianus) that are important to tick reproduction and survival (Allan et al. 2010). Both of these mechanisms (improved microclimate and increased host use) support a potential positive association between I. scapularis abundance and the presence of nonnative, invasive shrubs.
There is a critical need to determine whether fine-scale habitat associations between invasive shrubs and ticks create larger vector populations at broad enough scales to increase human disease risk (Allan et al. 2010). Ecological processes are often scale-dependent (Wiens 1989). Many biotic and abiotic factors affect I. scapularis density and distribution (Ostfeld et al. 1995) and likely operate at different scales. Nonnative plant invasion may increase tick abundance at a fine scale within a given forest patch, but it is unclear whether this would produce a larger vector population at a landscape scale relevant to human disease risk.
We assessed the effects of R. multiflora invasion at two spatial scales: at a fine scale within forest fragments and at a patch scale among different forest fragments. We tested the following: 1. whether I. scapularis abundance differs under R. multiflora compared to other shrub species or open understory within the same forest patch; and 2. whether I. scapularis abundance differs among R. multiflora-invaded forest fragments compared to forest fragments without R. multiflora invasion.
Methods
Study area
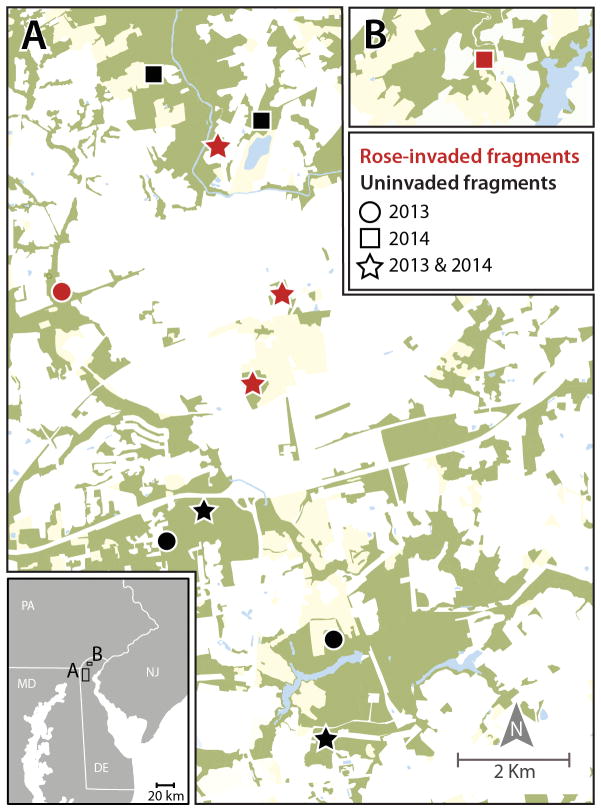
Delaware has one of the highest per capita rates of human Lyme disease cases in the United States (Bacon et al. 2008). This, combined with fragmented forests containing significant nonnative plant invasion, makes Delaware an ideal location to examine the effects of nonnative plants on I. scapularis populations. We trapped ticks in forest fragments (Figure 1) composed of mixed deciduous hardwood stands in New Castle County, DE (Rega 2012).
Figure 1.
Study area in New Castle County, Delaware. Green represents forest cover; pale yellow is agriculture; blue is water; and white is any type of human development. “Rose-invaded” and “uninvaded” fragments refer to forest fragments with and without R. multiflora invasion.
Tick trapping
In 2013 and 2014, we selected eight forest fragments ranging from 6 to 16 ha for trapping–four “uninvaded fragments” with <1% R. multiflora cover in the understory and four “invaded fragments” with >10% R. multiflora cover in the understory. We used dry-ice (CO2) baited traps (Kensinger and Allan 2011) to target host-seeking I. scapularis nymphs because they pose the greatest disease risk to humans (Centers for Disease Control and Prevention 2015). Although dragging or flagging methods are often preferred for capturing I. scapularis nymphs (Falco and Fish 1992), CO2 traps avoid sampling bias due to vegetation structure (Falco and Fish 1992, Schulze et al. 1995, 1997, Daniels et al. 2000, Kensinger and Allan 2011). Drag cloths become snagged on R. multiflora thorns, making tick habitat beneath it inaccessible for dragging or flagging.
Within invaded fragments, we deployed traps in a paired design, with each pair consisting of one trap under R. multiflora and one trap 25 meters away, not under R. multiflora. We chose four pairs (8 traps total) in each invaded fragment. Trap locations beneath R. multiflora were randomly selected within previously mapped and digitized patches of R. multiflora in ArcGIS v10 (ESRI 2012); paired trap locations not under R. multiflora were designated 25 meters away in a random cardinal direction, with the only selection criterion being that they could not be under R. multiflora. As a result, we set up these paired traps beneath a variety of understory structures and plant species. Within each uninvaded forest fragment, we randomly chose four points as trap locations using ArcGIS. Each year, we deployed 16 pairs of traps (32 total) across four R. multiflora-invaded fragments and 16 traps across four uninvaded fragments. Over the two years of the study, we used a total of 64 trap locations (Figure 1). On any given trap night, half of the traps were active. To eliminate biases associated with weather, we always deployed trap pairs together and in equal numbers of invaded and uninvaded fragments on the same nights. Each trap was baited with 1.4 kg of pelleted dry ice, and we lined the edges of the plywood base with double-sided carpet tape (3M brand). We checked traps after 24h and collected all ticks with forceps and deposited them into individual microcentrifuge tubes. Ticks were transported live, frozen at −80°C, and later identified to species and life stage using dichotomous keys (Keirans and Litwak 1989, Durden and Keirans 1996, Keirans and Durden 1998). Between April and July of 2013 and 2014, we trapped up to two nights per week, avoiding rain, for a total of 671 trap nights.
Vegetation surveys
We measured understory vegetation at several scales surrounding each trap during June and July. Within a 12.5 m radius surrounding the trap, we identified the dominant four understory plant species and estimated the percent of the ground that each covered, the percent of ground covered by R. multiflora, the percent of ground covered by coarse woody debris, and we measured understory vegetation density using 2.0 m high Nudds boards divided into four 0.5 m sections (Nudds 1977). The 12.5 m radius area was chosen to represent the approximate size of Peromyscus leucopus (an important larval tick host) home ranges (Wolff 1985) that could influence immature tick abundance, while avoiding overlap with paired traps. Within a 2.5 m radius around the trap, we estimated the percent of ground covered by R. multiflora to more closely represent the effective trapping area (Falco and Fish 1991). We identified the plant species immediately over the trap, and we quantified leaf litter volume by gathering up all litter within a 0.5m2 quadrat next to the trap and measuring its volume in a 19L bucket.
We calculated landscape variables for each trap location in ArcGIS using 2007 Delaware Land Use Land Cover layer (State of Delaware 2008). We quantified landscape variables that might influence habitat suitability for ticks and/or host animals and that represent the human-dominated landscape context of the study sites (Nicholson and Mather 1996, Bunnell et al. 2003). These variables included distance to nearest stream, distance to nearest road, distance to nearest agriculture, distance to nearest human development, distance to nearest impervious surface, distance to nearest residential development, and distance to nearest forest edge from each trap location.
We included vegetation variables that characterized vegetation at the patch scale (Rega 2012). These variables included proportion of Fagus grandifolia, Acer spp, Quercus spp, Liriodendron tulipifera, or Liquidambar styraciflua as dominant canopy trees, percent of ground covered by R. multiflora, average leaf litter volume, tree basal area, average diameter at breast height (dbh) of trees, percent of ground covered by understory plants (all spp.), proportion of understory woody stems that were nonnative, year of canopy closure.
Statistical analyses
We standardized total I. scapularis nymphs captured at each trap by effort (number of trap nights) to get a daily mean tick capture rate, which we used as an index of host-seeking I. scapularis abundance (hereafter: tick abundance) at each location. Prior to analyses, we log-transformed the response, tick abundance, to satisfy parametric test assumptions. At the fine scale, we used a paired t-test to determine if tick abundance differed between paired traps under R. multiflora and not under R. multiflora. At the patch scale we used an analysis of variance (ANOVA), blocking on site, to test for a difference in tick abundance between invaded and uninvaded forest patches.
Tick abundance models
Because forest fragments differed in additional characteristics beyond presence/absence of R. multiflora invasion, we assessed which vegetation and landscape variables influenced tick abundance at the landscape scale. We used boosted regression trees (BRT) to assess relative variable importance (Friedman and Meulman 2003, Elith et al. 2008) using packages gbm (Ridgeway 2015) and dismo (Hijmans et al. 2015) to run BRTs in R v 3.1.1 (R Development Core Team 2014); we set the error distribution for the response variable as gaussian, and specified the learning rate as 0.005, bag fraction as 0.5, and tree complexity at 2. Using the top four predictor variables according to BRT rankings, we built several generalized additive models (GAM) to compare for the best fit using package mgcv (Wood 2015) in R.
Results
Tick captures
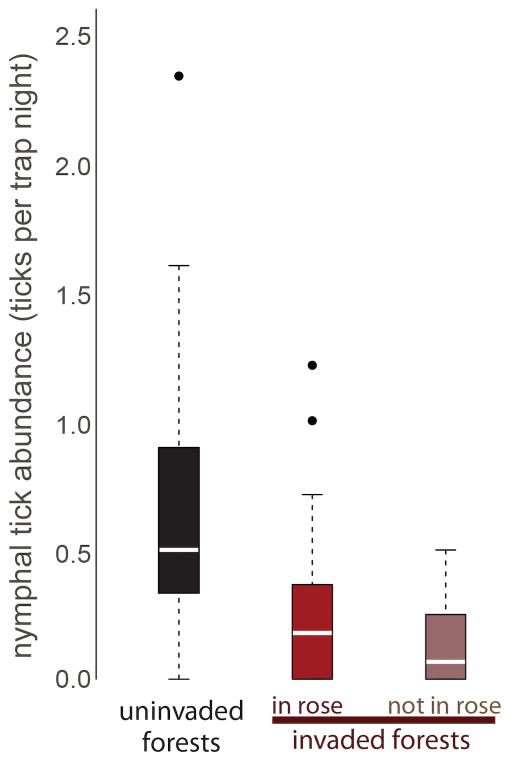
We captured 282 total I. scapularis individuals (275 nymphs, 7 adults) over 671 trap nights. At traps deployed in R. multiflora-invaded fragments, we captured a total of 79 and 33 I. scapularis nymphs in R. multiflora and not in R. multiflora, respectively. We caught a total of 163 I. scapularis nymphs in uninvaded forest fragments. Forty-seven of the 64 traps (73%) captured at least one I. scapularis nymph, and tick abundance ranged from 0 to 2.33 (nymphs/day). At the fine scale within invaded forest fragments, tick abundance under R. multiflora was 2.3 times greater than not under R. multiflora (t19=2.37, P = 0.03) (Figure 2). At the patch scale, we detected the opposite pattern; tick abundance in uninvaded forest fragments was 3.2 times greater than in R. multiflora-invaded fragments (F(1,53)=30.27, P < 0.01) (Figure 2). To verify that patch-level differences were not driven by the low capture rates in the invaded patches at the traps not under R. multiflora, we confirmed that tick abundance at traps in uninvaded patches was greater than at traps under R. multiflora (t=42=2.75, P < 0.01).
Figure 2.
Boxplot of tick abundance measured at traps in uninvaded forests, at traps under R. multiflora in invaded forests (“in rose”), and at traps not under R. multiflora (“not in rose”) in invaded forests. Horizontal white lines represent medians and boxes demonstrate the interquartile range.
Tick models
Several vegetation variables were correlated (coefficients ≥ 0.6), so we reduced the candidate set of local vegetation and landscape predictor variables to include only those listed in Table 1. Boosted regression trees (BRT) achieved a cross validation correlation 0.731 after fitting 1,350 trees. Of all potential predictor variables, leaf litter volume at the trap location had the highest relative influence (17% of total deviance explained) on tick abundance, followed by several variables with similar relative influence (11.7–11.9%): distance to nearest road, average tree dbh, and distance to nearest stream.
Table 1.
Summary of vegetation and landscape covariates measured at the trap and patch scale.
| Trap and patch level covariates | Uninvaded forests | Invaded forests | |
|---|---|---|---|
|
|
|||
| Trap-level covariates | traps | traps in R. multiflora | traps not in R. multiflora |
| Nudds at 0.5–1.0 m (%) | 18.0A ± 3.9 | 73.9B ± 4.5 | 53.3C ± 5.9 |
| Leaf litter volume (L/m2) | 28.0A ± 2.8 | 6.1B ± 1.4 | 6.7B ± 1.2 |
| Coarse woody debris (%) | 6.5A ± 0.9 | 3.4B ± 0.7 | 4.2B ± 0.7 |
| Distance to agriculture (m) | 288.3A ± 54.7 | 156.7B ± 24.6 | 159.6B ± 24.6 |
| Distance to edge (m) | 67.8A ± 9.1 | 39.8B ± 8.8 | 41.9B ± 8.4 |
| Distance to road (m) | 154.7 ± 16.7 | 135 ± 18.1 | 133.4 ± 15.4 |
| Distance to residential (m) | 716.9 ± 377.6 | 186.2 ± 31.5 | 174.4 ± 32.9 |
| Distance to stream (m) | 371.8A ± 62.7 | 148.4B ± 35.7 | 134.1B ± 35.6 |
| Patch-level covariates | |||
|
| |||
| Fagus grandifolia (%) | 8.5A ± 2.8 | 0.7B ± 0.2 | |
| Acer spp. (%) | 0.7A ± 0.1 | 21.2B ± 1.2 | |
| Year of canopy closure | 1916.7A ± 4.9 | 1963B ± 5.1 | |
| Nonnative stems (%) | 9.1A ± 2.7 | 40.0B ± 3.3 | |
| Liriodendron tulipifera (%) | 21.2 ± 2.4 | 22.9 ± 2.8 | |
| Average tree dbh (m) | 0.6 ± 0.0 | 0.6 ± 0.0 | |
Notes: Covariates are summarized as mean ± standard error. Letters (A,B,C) denote significant differences among groups (P < 0.05) detected using analysis of variance (ANOVA), blocking on site, followed up with Tukey post-hoc comparison when there were more than two groups. “Nudds” refers to Nudds board (Nudds 1977) measurements, and “dbh” stands for diameter at breast height.
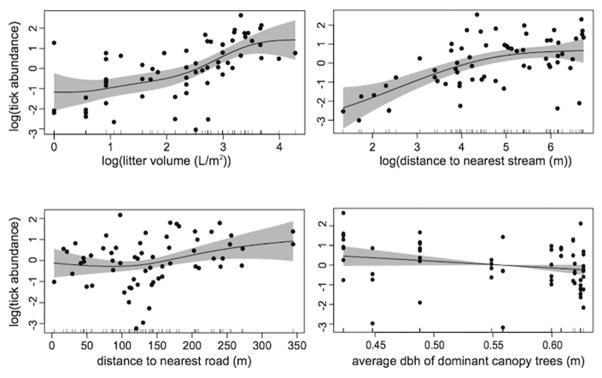
We log-transformed certain predictor variables to improve their distributions along the x-axis. Our best GAM (61% deviance explained) based on Akaike’s information criterion adjusted for small sample size (Burnham and Anderson 2002) included log-transformed litter volume, distance to nearest road, log-transformed distance to nearest stream, and average tree dbh (Figure 3, Table 2). Tick abundance responded to leaf litter volume nonlinearly. Increase in leaf litter volume at low levels (<10 L/m2) had little effect on tick abundance. Between 12 L/m2 and 20 L/m2 there was a strong positive change in tick abundance with increasing leaf litter volume, which then leveled out around 30 L/m2. We saw a similar non-linear response with the distance to stream. Close to a stream, there were very few ticks captured, but this response increased rapidly up to a certain distance from the stream, beyond which there is little effect of the stream on tick abundance. Distance to nearest road had a positive effect on tick abundance at low and high values, and average tree dbh was inversely related to tick abundance.
Figure 3.
Generalized additive model (GAM) partial dependence plot of top-ranked model. Lines display the best fit for the relationship between log-transformed tick abundance and predictor variables. Gray bars represent 95% confidence interval and black dots show actual data points.
Table 2.
Summary of candidate generalized additive models including number of parameters (K), AICc, differences (ΔAICc), and Akaike weights (wi).
| Predictor variable(s) | K | AICc | ΔAICc | wi |
|---|---|---|---|---|
| litter, road, stream, dbh | 6 | 209.47 | 0 | 0.59 |
| litter, dbh, stream | 5 | 211.65 | 2.18 | 0.20 |
| litter, road, stream | 5 | 212.66 | 3.19 | 0.12 |
| litter, stream | 4 | 213.26 | 3.79 | 0.09 |
| litter, road, dbh | 5 | 224.19 | 14.72 | 0.00 |
| litter, road | 4 | 231.72 | 22.25 | <0.001 |
| stream, road, dbh | 5 | 232.41 | 22.93 | <0.001 |
| litter, dbh | 4 | 233.07 | 23.60 | <0.001 |
| road, stream | 4 | 233.74 | 24.27 | <0.001 |
| stream, dbh | 4 | 237.33 | 27.86 | <0.001 |
| stream | 3 | 237.84 | 28.37 | <0.001 |
| litter | 3 | 238.47 | 28.99 | <0.001 |
| dbh | 3 | 239.15 | 29.68 | <0.001 |
| road, dbh | 4 | 239.64 | 30.17 | <0.001 |
| road | 3 | 249.95 | 40.48 | <0.001 |
| null | 2 | 258.45 | 48.98 | <0.001 |
Notes: The variable “litter” is the volume of leaf litter (L/m2) at each trap. The variables “road,” and “stream” refer to the distance between these features and the trap; “dbh” is the average diameter at breast height of dominant canopy trees in each forest fragment. The response (tick abundance) and two predictors (litter and stream) were log-transformed prior to analysis.
Discussion
At a fine scale, we trapped 2.3 times more nymphal blacklegged ticks under R. multiflora compared to areas without R. multiflora within the same forest fragments. However, we trapped over 3 times as many ticks in uninvaded forest fragments compared to R. multiflora-invaded forest fragments. Host-seeking ticks were concentrated beneath R. multiflora within invaded forests, but they were more abundant in uninvaded forests. Thus, although at fine scales R. multiflora appears to increase tick abundance, R. multiflora invasion actually suppresses tick abundance at the patch scale. Our results suggest that reduced leaf litter volume constrains tick abundance (Schulze et al. 1995, Burtis et al. 2014). The lack of leaf litter in R. multiflora-invaded forest fragments creates an inhospitable environment for ticks, from which they are rescued by invasive shrubs with dense growth forms (e.g. Williams et al. 2009).
Within invaded forests, our results are consistent with prior studies that show a positive relationship between nonnative, invasive shrubs (e.g. B. thunbergii) and questing nymphal and adult I. scapularis abundance (Williams et al. 2009, Lubelczyk et al. 2004, Elias et al. 2006). Densities of nymphal and adult lone star ticks (Amblyomma americanum) were also higher in invasive honeysuckle (Lonicera maackii)-dominated plots compared to uninvaded, adjacent plots (Allan et al. 2010). At a landscape scale, however, we found ticks were more abundant in uninvaded forest fragments than invaded fragments. We captured more than three times as many I. scapularis nymphs in uninvaded forest fragments compared to R. multiflora-invaded forest fragments. Rosa multiflora invasion was associated with habitat changes at the forest-fragment scale that create an aggregation of ticks around R. multiflora within invaded forests. However, uninvaded habitats appear to be more suitable for ticks than invaded habitats, seemingly due to the greater volume of leaf litter in uninvaded forest fragments.
Of all vegetation and landscape covariates tested, leaf litter volume was the best predictor of tick abundance. Leaf litter is an important component of I. scapularis habitat (Schulze et al. 1995, 2002, Lubelczyk et al. 2004, Elias et al. 2006). In the forests investigated, leaf litter volume was negatively correlated with invasion by nonnative plants at the patch scale. We found more than four times the volume of leaf litter in R. multiflora-free forest fragments compared to R. multiflora-invaded forest fragments (P < 0.01), and there was no difference in leaf litter volume between paired points within invaded forests. The widespread relationship between leaf litter and invasive plants is ultimately driven by nonnative, invasive earthworms (Nuzzo et al. 2009). These earthworms lead the nonnative plant invasion front by devouring leaf litter and disrupting mycorrhizal networks, inhibiting native plant growth and regeneration (Lawrence et al. 2003, Suárez et al. 2006, Hale et al. 2006). This reduction in habitat quality for native plants, combined with high densities of white-tailed deer over-browsing native plants, helps nonnative plants to invade. Therefore, at a patch scale, the forest fragments with significant nonnative plant invasion are lacking the layer of leaf litter that comprises favorable tick habitat.
The presence or absence of earthworm invasion and subsequent nonnative plant invasion are determined by numerous factors including soil chemistry (Moore et al. 2013), litter composition (Belote and Jones 2009), and historical land use patterns (Beauséjour et al. 2015). With increasing disturbance and propagule pressure in human-dominated landscapes, currently uninvaded forest fragments may eventually host high densities of earthworms and nonnative plants, with implications for tick and litter arthropod survival. On the other hand, uninvaded forest fragments may possess certain characteristics, such as high soil acidity (Bernard et al. 2009), that make them more resistant to earthworm invasion. In either scenario, we suggest continued monitoring at multiple spatial scales to understand the impacts of invasion on tick population dynamics.
Conclusions and Future Directions
Our results suggest that while R. multiflora invasion concentrates host-seeking I. scapularis nymphs at a fine scale, the loss of leaf litter associated with R. multiflora invasion reduces tick habitat quality at the patch scale. We found the greatest abundance of host-seeking I. scapularis nymphs in uninvaded forests with deep litter layers. Thus, while removal of invasive plants may reduce tick abundance within certain forests, management actions toward reducing host-seeking tick abundance may have a greater impact in forests with deep litter layers.
Future work should test potential mechanisms influencing tick abundance in invaded and uninvaded forest fragments. Furthermore, we should implement broader scale studies using multiple landscapes within the temperate deciduous forest biome to test our hypotheses about invasive earthworms, leaf litter, and invasive shrub effects on host-seeking I. scapularis abundance.
Acknowledgments
SA was supported by a University of Delaware Graduate Research Fellowship. Other financial support was provided by the USDA Forest Service NRS-16 (VD), USDA McIntire Stennis (WGS), and University of Delaware (JJB). DB was supported by National Institutes of Health (AI076342 and AI097137), National Science Foundation (DEB-1354184), and the Burroughs Wellcome Fund (1012376). We thank the following individuals who helped collect field data: Z. Ladin, K. Handley, J. Nimmerichter, A. Lutto, K. Serno, J. Bondi, C. Piazza, L. Newton, and J. Curry. We thank R. Falco and B. Kensinger for valuable input on CO2 trap design and acknowledge the cooperation by land managers of sites where we conducted field work: Newark City Parks, New Castle County Parks, University of Delaware, Mt. Cuba Center, and Delaware State Parks.
Literature Cited
- Allan BF, Dutra HP, Goessling LS, Barnett K, Chase JM, Marquis RJ, Pang G, Storch GA, Thach RE, Orrock JL. Invasive honeysuckle eradication reduces tick-borne disease risk by altering host dynamics. Proceedings of the National Academy of Sciences. 2010;107:18523–18527. doi: 10.1073/pnas.1008362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton IW, Hyatt LA, Howe KM, Gurevitch J, Lerdau MT. Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest. Ecological Applications. 2005;15:1263–1272. [Google Scholar]
- Bacon R, Kugeler K, Mead P. Surveillance for Lyme disease--United States, 1992–2006. Centers for Disease Control and Prevention; USA: 2008. http://www.cdc.gov/MMWR/PREVIEW/MMWRHTML/ss5710a1.htm. [PubMed] [Google Scholar]
- Beauséjour R, I, Handa T, Lechowicz MJ, Gilbert B, Vellend M. Historical anthropogenic disturbances influence patterns of non-native earthworm and plant invasions in a temperate primary forest. Biological Invasions. 2015;17:1267–1281. [Google Scholar]
- Belote RT, Jones RH. Tree leaf litter composition and nonnative earthworms influence plant invasion in experimental forest floor mesocosms. Biological Invasions. 2009;11:1045–1052. [Google Scholar]
- Bernard MJ, Neatrour MA, McCay TS. Influence of soil buffering capacity on earthworm growth, survival, and community composition in the western Adirondacks and central New York. Northeastern Naturalist. 2009;16:269–284. [Google Scholar]
- Borgmann KL, Rodewald AD. Nest predation in an urbanizing landscape: the role of exotic shrubs. Ecological Applications. 2004;14:1757–1765. [Google Scholar]
- Bunnell JE, Price SD, Das A, Shields TM, Glass GE. Geographic information systems and spatial analysis of adult Ixodes scapularis (Acari: Ixodidae) in the Middle Atlantic Region of the U.S.A. Journal of Medical Entomology. 2003;40:570–576. doi: 10.1603/0022-2585-40.4.570. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. Springer Science & Business Media; New York, NY, USA: 2002. [Google Scholar]
- Burtis JC, Fahey TJ, Yavitt JB. Impact of invasive earthworms on Ixodes scapularis and other litter-dwelling arthropods in hardwood forests, central New York state, USA. Applied Soil Ecology. 2014;84:148–157. [Google Scholar]
- Centers for Disease Control and Prevention. Tickborne diseases of the United States. 2015 http://www.cdc.gov/ticks/diseases.
- Coblentz BE. Exotic organisms: a dilemma for conservation biology. Conservation Biology. 1990;4:261–265. [Google Scholar]
- Daniels TJ, Falco RC, Fish D. Estimating population size and drag sampling efficiency for the blacklegged tick (Acari: Ixodidae) Journal of Medical Entomology. 2000;37:357–363. doi: 10.1603/0022-2585(2000)037[0357:EPSADS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Durden LA, Keirans JE. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, identification key, distribution, hosts, and medical/veterinary importance. Thomas Say Publications in Entomology: Monographs. 1996:1–50. [Google Scholar]
- Elias SP, Lubelczyk CB, Rand PW, Lacombe EH, Holman MS, Smith RP. Deer browse resistant exotic-invasive understory: An indicator of elevated human risk of exposure to Ixodes scapularis (Acari: Ixodidae) in southern coastal Maine woodlands. Journal of Medical Entomology. 2006;43:1142–1152. doi: 10.1603/0022-2585(2006)43[1142:dbreua]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. Journal of Animal Ecology. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- ESRI. ArcMap v 10.1. ESRI; Redlands, CA: 2012. [Google Scholar]
- Falco RC, Fish D. Horizontal movement of adult Ixodes dammini (Acari: Ixodidae) attracted to CO2-baited traps. Journal of Medical Entomology. 1991;28:726–729. doi: 10.1093/jmedent/28.5.726. [DOI] [PubMed] [Google Scholar]
- Falco RC, Fish D. A comparison of methods for sampling the deer tick, Ixodes dammini, in a Lyme disease endemic area. Experimental & Applied Acarology. 1992;14:165–173. doi: 10.1007/BF01219108. [DOI] [PubMed] [Google Scholar]
- Friedman JH, Meulman JJ. Multiple additive regression trees with application in epidemiology. Statistics in Medicine. 2003;22:1365–1381. doi: 10.1002/sim.1501. [DOI] [PubMed] [Google Scholar]
- Hale CM, Frelich LE, Reich PB. Changes in hardwood forest understory plant communities in response to european earthworm invasions. Ecology. 2006;87:1637–1649. doi: 10.1890/0012-9658(2006)87[1637:cihfup]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Phillips S, Leathwick J, Elith J. Species distribution modeling: package “dismo”. CRAN 2015 [Google Scholar]
- Howard TG, Gurevitch J, Hyatt L, Carreiro M, Lerdau M. Forest invasibility in communities in southeastern New York. Biological Invasions. 2004;6:393–410. [Google Scholar]
- Huebner CD, Steinman J, Hutchinson TF, Ristau TE, Royo AA. The distribution of a non-native (Rosa multiflora) and native (Kalmia latifolia) shrub in mature closed-canopy forests across soil fertility gradients. Plant and Soil. 2014;377:259–276. [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health: Invasive mosquitoes. Ecology Letters. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Durden LA. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. Journal of Medical Entomology. 1998;35:489–495. doi: 10.1093/jmedent/35.4.489. [DOI] [PubMed] [Google Scholar]
- Keirans JE, Litwak TR. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. Journal of Medical Entomology. 1989;26:435–448. doi: 10.1093/jmedent/26.5.435. [DOI] [PubMed] [Google Scholar]
- Kensinger BJ, Allan BF. Efficacy of dry ice-baited traps for sampling Amblyomma americanum (Acari: Ixodidae) varies with life stage but not habitat. Journal of Medical Entomology. 2011;48:708–711. doi: 10.1603/me10275. [DOI] [PubMed] [Google Scholar]
- Khatchikian CE, Prusinski M, Stone M, Backenson PB, Wang IN, Levy MZ, Brisson D. Geographical and environmental factors driving the increase in the Lyme disease vector Ixodes scapularis. Ecosphere. 2012;3:art85. doi: 10.1890/ES12-00134.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence B, Fisk MC, Fahey TJ, Suárez ER. Influence of nonnative earthworms on mycorrhizal colonization of sugar maple (Acer saccharum) New Phytologist. 2003;157:145–153. doi: 10.1046/j.1469-8137.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- Lubelczyk CB, Elias SP, Rand PW, Holman MS, Lacombe EH, Smith RP. Habitat Associations of Ixodes scapularis (Acari: Ixodidae) in Maine. Environmental Entomology. 2004;33:900–906. [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT. Entomologic index for human risk of Lyme disease. American Journal of Epidemiology. 1996;144:1066–1069. doi: 10.1093/oxfordjournals.aje.a008879. [DOI] [PubMed] [Google Scholar]
- Moore JD, Ouimet R, Bohlen PJ. Effects of liming on survival and reproduction of two potentially invasive earthworm species in a northern forest Podzol. Soil Biology & Biogeochemistry. 2013;64:174–180. [Google Scholar]
- Morlando S, Schmidt SJ, LoGiudice K. Reduction in Lyme disease risk as an economic benefit of habitat restoration. Restoration Ecology. 2012;20:498–504. [Google Scholar]
- Nicholson MC, Mather TN. Methods for evaluating Lyme disease risks using geographic information systems and geospatial analysis. Journal of Medical Entomology. 1996;33:711–720. doi: 10.1093/jmedent/33.5.711. [DOI] [PubMed] [Google Scholar]
- Nudds TD. Quantifying the vegetative structure of wildlife cover. Wildlife Society Bulletin. 1977;5:113–117. [Google Scholar]
- Nuzzo VA, Maerz JC, Blossey B. Earthworm invasion as the driving force behind plant invasion and community change in northeastern North American forests. Conservation Biology. 2009;23:966–974. doi: 10.1111/j.1523-1739.2009.01168.x. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Cepeda OM, Hazler KR, Miller MC. Ecology of Lyme Disease: Habitat associations of ticks (Ixodes scapularis) in a rural landscape. Ecological Applications. 1995;5:353. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- Rega C. MS Thesis. University of Delaware; Newark, Delaware, USA: 2012. Impacts of soil calcium availability and non-native plant invasions on an urban forest bird community. [Google Scholar]
- Ridgeway G. Generalized Boosted Regression Models: package “gbm”. CRAN 2015 [Google Scholar]
- Schulze TL, Jordan RA, Hung RW. Suppression of subadult Ixodes scapularis (Acari: Ixodidae) following removal of leaf litter. Journal of Medical Entomology. 1995;32:730–3. doi: 10.1093/jmedent/32.5.730. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Hung RW. Biases associated with several sampling methods used to estimate abundance of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) Journal of Medical Entomology. 1997;34:615–623. doi: 10.1093/jmedent/34.6.615. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Hung RW. Effects of microscale habitat physiognomy on the focal distribution of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. Environmental Entomology. 2002;31:1085–1090. [Google Scholar]
- State of Delaware. May 19 2007 Delaware Land Use and Land Cover. Office of Management and Budget, Delaware Geographic Data Committee; Dover, Delaware, USA: 2008. [Google Scholar]
- Suárez ER, Fahey TJ, Yavitt JB, Groffman PM, Bohlen PJ. Patterns of litter disappearance in a northern hardwood forest invaded by exotic earthworms. Ecological Applications. 2006;16:154–165. doi: 10.1890/04-0788. [DOI] [PubMed] [Google Scholar]
- Wilcove DS, McLellan CH, Dobson AP. Habitat fragmentation in the temperate zone. In: Soule ME, editor. Conservation biology: The science of scarcity and diversity. Sinauer Associates; Sunderland, MA, USA: 1986. [Google Scholar]
- Wiens JA. Spatial Scaling in Ecology. Functional Ecology. 1989;3:385. [Google Scholar]
- Williams SC, Ward JS, Worthley TE, Stafford KC. Managing Japanese barberry (Ranunculales: Berberidaceae) infestations reduces blacklegged tick (Acari: Ixodidae) abundance and infection prevalence with Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) Environmental Entomology. 2009;38:977–984. doi: 10.1603/022.038.0404. [DOI] [PubMed] [Google Scholar]
- Williams SC, Ward JS. Effects of Japanese barberry (Ranunculales: Berberidaceae) removal and resulting microclimatic changes on Ixodes scapularis (Acari: Ixodidae) abundances in Connecticut, USA. Environmental Entomology. 2010;39:1911–1921. doi: 10.1603/EN10131. [DOI] [PubMed] [Google Scholar]
- Wolff JO. The effects of density, food, and interspecific inference on home range size in Peromyscus leucopus and Peromyscus maniculatus. Canadian Journal of Zoology. 1985;63:2657–2662. [Google Scholar]
- Wood S. Mixed GAM Computation Vehicle with GCV/AIC/REML Smoothness Estimation: Package “mgcv”. CRAN 2015 [Google Scholar]