Abstract
Chemoprevention has been a pivotal and effective strategy during the skin cancer treatment. Using human skin normal and tumor samples, we demonstrated that both the expression and activity levels of pyruvate kinase M2 (PKM2) were higher in skin tumor tissues than normal tissues, suggesting that PKM2, one of important metabolic enzyme, might serve as a target for skin cancer prevention and/or therapy. Shikonin, a small-molecule active chemical, has been studied as an anti-cancer drug candidate in human cancer models. However, the mechanism of action and the chemopreventive potential of shikonin are unclear. Herein, we used the skin epidermal JB6 P+ cells and demonstrated that shikonin suppressed the tumor promoter 12-O-tetradecanoylphorbol 13-acetate (TPA) induced neoplastic cell transformation and PKM2 activation in the early stage of carcinogenesis. Mitochondrial functions were inhibited by TPA treatment, as indicated by reduced mitochondrial membrane potential and mitochondrial respiration, which were restored by shikonin. We also examined the levels of lactate as a glycolysis marker, and shikonin suppressed its increase caused by tumor promoter treatment. Modulation of cell metabolism by shikonin was associated with G2–M phase accumulation, and Fra-1 (a major subunit of activator protein 1 in skin tumorigenesis) downregulation. In addition, we demonstrated that AMP-activated protein kinase (AMPK), an energy sensor, which is inactivated by TPA, shikonin could reverse AMPK activity. These results suggest that shikonin bears chemopreventive potential for human skin cancers in which PKM2 is upregulated, which might be mediated by inhibiting oncogenic activation, PKM2 activation, and mitochondrial dysfunction.
Keywords: metabolism, chemoprevention, skin tumor, mitochondrial malfunction, PKM2
INTRODUCTION
Carcinogenesis is often associated with metabolic shift. Cancer cells predominantly produce energy by a high rate of glycolysis followed by lactic acid fermentation even in the presence of oxygen, known as the “Warburg effect” [1] or aerobic glycolysis. Although glycolysis is inefficient to produce ATP compared with mitochondrial oxidative phosphorylation (OXPHOS), the metabolic intermediates produced during glycolysis can provide “building blocks” for cancer cells. Associated with this metabolic switch is the dysregulation of important metabolic enzymes. One such enzyme is the M2 splice isoform of pyruvate kinase (PKM2). Pyruvate kinase (PK) is essential for controlling glucose metabolism as the final rate-limiting step in glycolysis, and catalyzes phosphoenolpyruvate (PEP) and adenosine 5′-diphosphate (ADP) to produce pyruvate and adenosine 5′-triphosphate (ATP) [2–4]. Vertebrates have four tissue-specific isozymes of PK: an L isozyme found in the liver and kidney, an R isozyme present in erythrocytes, and the M isozymes M1 and M2, the former identified in most adult tissues and the latter in embryonic tissues and adult stem cells [5,6]. Accumulating evidence has shown that PKM2 is highly expressed in human cancers, such as colorectal cancer, lung cancer, liver cancer, breast cancer, and brain cancer [7–10]. In contrary to PKM2, PKM1, is often repressed in cancer cells [9,11]. PKM2 contributes to the metabolic shift from OXPHOS to glycolysis in cancer cells as well as diverts glycolytic intermediate to biosynthesis of amino acid, nucleotide, and lipid [12].
Since PKM2 plays an important role in cancer metabolism, it could potentially serve as a drug target for cancer therapy. Shikonin, a major active chemical component extracted from lithospermum erythrorhizon, has been shown to exert numerous pharmacological properties, including the anti-tumor activity in a number of human cancer cells [13–15]. Moreover, a clinical study indicated that shikonin was effective in treating later-stage lung cancer patients [16]. Recently, shikonin was identified as a novel inhibitor of tumor PKM2 and it can prevent cancer cell glycolysis [17]. Based on our previous study, both upregulation of PKM2 and down-regulation of mitochondrial respiration occur early in carcinogenesis [18], therefore, we hypothesize that shikonin may exert chemopreventive activity, and we will test that using the well characterized tumor promotion model, skin epidermal JB6 cells.
MATERIALS AND METHODS
Cell Lines, Reagents, and Treatment
Murine skin epidermal JB6 Cl-41 (P+) cells were used to study tumor promotion (purchased from American Type Culture Collection). Cells were grown in EMEM medium containing 4% fetal bovine serum (FBS), 2 mM L-glutamine, and 2.5 μg/ml penicillin, and 2.5 μg/ml streptomycin in a 37°C incubator under 5% CO2.
For cell culture, the levels of mycoplasma were routinely (once every three months) assessed in these cells using a MycoAlert Mycoplasma Detection Kit purchased from Lonza (Rockland, ME), and the results were negative.
Shikonin, purchased from ChromaDex (ASB-00023250), was dissolved in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO). For treatment of cells, the final concentration was 1.0 μmol except for the soft agar assay, which was 0.2 or 0.4 μmol. We have performed the caspase activity assay confirming that both 0.4 and 1 μM of shikonin did not induce apoptosis (Figure S1).
The tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA, Sigma) was also prepared in DMSO. The final concentration of TPA for the cell culture studies was 100 nM except for the soft agar assay, which was 5 nM.
Eleven skin tumor and eleven adjacent normal tissue specimens from patients with skin cancer were used. These patients had been treated at the Louisiana State University Health Sciences Center in Shreveport. These frozen tissues samples have been obtained and stored at the Tissue & Serum Repository of the Feist-Weiller Cancer Center at our institution. We did not obtain data either through intervention or interaction with living individuals, or possess identifiable private information. This study was approved by the Institutional Review Board at our institution. All tissue samples were kept in a −80°C freezer until retrieved for the study.
Preparation of Whole Cell Lysate
Collected skin cells were suspended in 150 μl of PBS containing proteinase inhibitor cocktail (Calbiochem, La Jolla, CA). Cells were sonicated on ice for two strokes (10 s per stroke) using a Fisher Sonic Dismembrator (Model 100, Scale 2). After incubating on ice for 30 min, cell lyaste was centrifuged at 14,000g for 15 min, and the supernatant was collected and designated as Whole Cell Lysate.
Western Blot Analysis
Whole Cell Lysate was used for the assay. Antibodies against pyruvate kinase M2 (PKM2, ab38237) and pyruvate kinase (PKM, ab6191) were purchased from Abcam, antibodies against Fra-1 (sc-605) and β-Actin (sc-47778) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-AMPKα (2531) was purchased from Cell Signaling Technology (Danvers, MA). AMPK (AP7045a) was purchased from Abgent (San Diego, CA).
PKM Activity Assay
PKM activities were analyzed using the lactate dehydrogenase (LDH)-coupled assay as described previously [19]. The standard assay mixture contains the following reagents in a final volume of 400 μl: 10 mmol/L Tris–acetate, pH 7.5; 10 mmol/L MgCl2; 50 mmol/L KCl; 2 mmol/L ADP; 10 mmol/L phosphoenolpyruvate; 4.4 units of LDH; and 0.12 mmmol/L NADH. The reaction was started by adding 5 μl of Whole Cell Lysate and 4 μl of 5′-AMP brings PKM2 to its maximal velocity. The baseline was measured without the addition of phosphoenolpyruvate and 5′-AMP.
Anchorage-Independent Growth Assay in Soft Agar
Soft agar-based cell transformation assay was carried out in 6-well plates. The bottom agar was made using 1.25% agar, 2× EMEM medium, 10% FBS, PBS, glutamine, and penicillin, and incubated in a 50°C water bath. The mixture (0.5% agar) was then divided and various treatment reagents were added. In each well, 2.5 ml of the 0.5% agar mix was added and allowed to harden for 30 min. The top agar mix contained two fractions of the above 0.5% agar mixtures and one fraction of 1 × 104 JB6 cells. The treatment reagents were added and 0.75 ml of the top agar mix was transferred on top of the bottom agar. The agar was allowed to solidify and incubated in a 37°C incubator under 5% CO2 for 14 days followed by staining with 0.25 mg/ml Neutral Red for 24 h.
Determination of Lactate and Pyruvate Levels
The levels of lactate and pyruvate were determined using the Lactate Assay Kit (BioVison, K607-100) and Pyruvate Assay Kit (BioVison, Mountain View, CA, K609-100) following the instructions provided by the manufacturer. Whole Cell Lysate was diluted to 2 μg/μl in PBS, and deproteinized by passing through a 10 kD cut-off membrane (VWR, 82031-348). For each sample, 50 μl of the Whole Cell Lysate filtrate was used.
Measurement of Oxygen Consumption of JB6 Cells
JB6 P+ cells (2 × 106/ml) in growth medium were suspended in a thermostated closed vessel at 37°C. Oxygen consumption was measured polarographically using a Clark-type O2 electrode (Yellow Spring Instruments, Yellow Springs, OH). The rate of mitochondrial O2 consumption was determined as the antimycin-sensitive rate after addition of anti-mycin A to the final concentration of 1 μM [20]. The rate of the oxygen consumption was calculated from each study and the data from at least three experiments were combined and plotted.
Detection of Mitochondrial Membrane Potential
Five thousand JB6 cells were seeded in 96-well plates with 150 μl growth medium. Twenty-four hours after plating, cells were treated as indicated in each experiment. After washing with PBS, cells were incubated with fresh medium containing 2 μg/ml of 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine iodide (JC-1, Molecular Probes, Eugene, Oregon) for 30 min. The medium was then removed; and cells were washed with PBS. Fluorescent intensity was measured immediately by fluorescence spectrometry (Synergy HT, BioTek, Winooski, VT). For JC-1 green, Ex = 485, Em = 528; for JC-1 red, Ex = 530, Em = 590. The fluorescent signals from the cells only (no JC-1 dye added) were subtracted from the sample values. The ratio of the red to green fluorescence of JC-1 was calculated. Experiments were repeated for three times and at least triplicate samples were included in each experiment.
Cell Cycle Assay
JB6 P+ cells were seeded at 1 × 106 cells per plate in 100 mm tissue culture plates. The cells were incubated at 37°C in a 5% CO2 incubator overnight. Cells were then starved in serum-free medium for 24 h followed by treatment for 24 h with TPA (100 nM) and Shikonin (1 μM). Cells were trypsinized, washed twice with cold PBS, and fixed with ice-cold 70% ethanol at −20°C overnight. Cells were washed twice with PBS, incubated with 0.2 mg/ml RNase A and 20 μg/ml propidium iodide in PBS at 37°C for 1 h in the dark. The cell cycle phase was determined using the BD Biosciences flow cytometer. Data were gathered using the ModFit LT software.
Statistical Analysis
One-way ANOVA followed by Newman–Keuls post-test was used for multi-group comparisons. The PKM2 activity data in human skin tissue were analyzed using the Student’s t-test. All of the experiments have been repeated at least three times. Data were reported as mean ± standard error (SEM). P < 0.05 was considered significant.
RESULTS
PKM2 Was Upregulated in Human Skin Cancer Tissue Samples
We first detected of the activity and expression levels of PKM2 in human skin cancer frozen tissue samples. The histology of these samples has been summarized in Table 1. Eleven pairs of samples (tumor + normal) have been obtained. Among the tumor samples, six were squamous cell carcinomas, three were basal cell carcinomas, one was melanoma, and one was fibroxanthoma. As shown in Figure 1A, PKM2 activity was increased by 2.5-fold in tumor samples than normal tissues, and the expression levels of PKM2 were higher in almost all of the tumor samples than their normal controls (Figure 1B). Furthermore, the levels of whole PKM protein expression remained little changed between tumor and normal skin tissues. These results suggest that PKM2 could play an important role on human skin cancer.
Table 1.
Patient Clinical Characteristics
| Patient no. | Histology |
|---|---|
| 1 | Squamous cell carcinoma |
| 2 | Basal cell carcinoma with squamoid differentiation |
| 3 | Basal cell carcinoma with follicular and squamous differentiation |
| 4 | Basal cell carcinoma |
| 5 | Atypical fibroxanthoma |
| 6 | Squamous cell carcinoma |
| 7 | Squamous cell carcinoma |
| 8 | Squamous cell carcinoma |
| 9 | Nodular melanoma |
| 10 | Squamous cell carcinoma |
| 11 | Squamous cell carcinoma |
Figure 1.
Enzymatic activity and protein expression levels of PKM2 in paired tumor and normal tissue specimens from patients with skin cancer. Whole Cell Lysate was extracted from the frozen tissues and subjected to activity and Western blot analysis to determine the enzymatic activity (A) and protein expression (B) of PKM2. β-Actin served as the loading control. *P < 0.05 compared with the normal group.
Shikonin Suppressed Neoplastic Transformation of JB6 P+ Cells Induced by the Tumor Promoter TPA
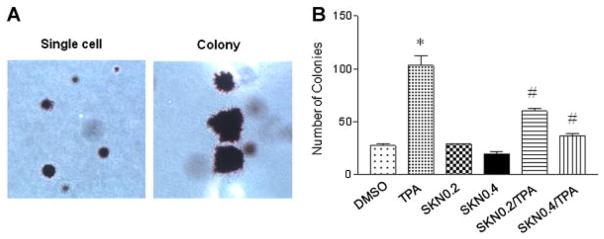
Since PKM2 is highly expressed in human skin tumor samples, we studied if shikonin, a specific inhibitor of PKM2, can block skin cell transformation using the well-characterized skin epidermal JB6 P+ cell model [21]. JB6 P+ cells were treated with shikonin (0.2 or 0.4 μM); concurrently, the cells were treated with 5 nM TPA and anchorage-independent growth of these cells was determined. As shown in Figure 2A and B, Shikonin at 0.2 and 0.4 μM caused a 36% and 58% decrease (respectively) in the number of colonies compared to the TPA group. These results suggest that shikonin prevents skin cell transformation.
Figure 2.
Shikonin (SKN) inhibited tumor promoter TPA-induced skin cell transformation. The final concentration of TPA was 5 nM. Two concentrations (0.2 and 0.4 μM) of shikonin were used. (A) Soft agar colony formation of JB6 P+ cells (light micrograph: 10× magnification). (B) Quantification of formed colonies. Cells grown in 0.33% soft agar containing 5 nM TPA and/or 0.2 or 0.4 shikonin or control (DMSO 1000× diluted). *P < 0.05 compared with the control/DMSO group; #P < 0.05 compared with the TPA group (n = 3 in each group).
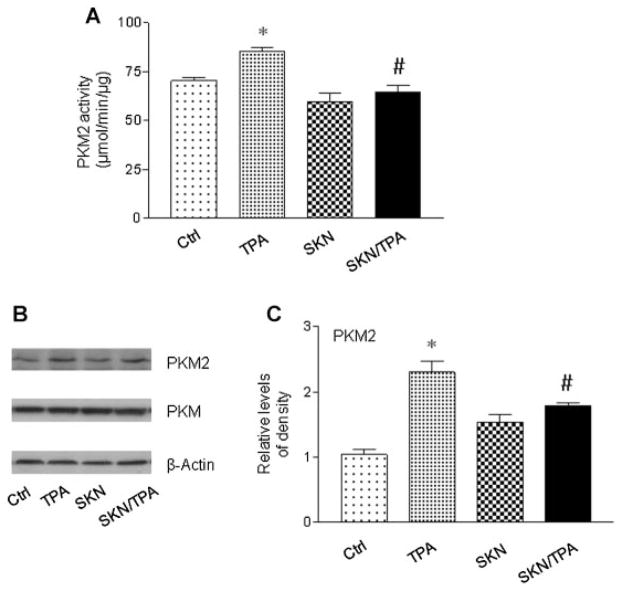
TPA Induced PKM2 Activated, Which Was Suppressed by Shikonin
Apparently, the aforementioned data shows the effect of shikonin on tumor promotion induced by the tumor promoter TPA. Next, the levels of PKM2 protein expression and its enzymatic activity were detected in JB6 P+ cells. Based on our preliminary studies, TPA (100 nM) increases the expression and activity of PKM2 at 24 h [18]. Therefore, JB6 P+ cells were treated with shikonin and TPA for 24 h. PKM2 activity was detected using a LDH-coupled assay, and it has been confirmed that LDH activity is not affected by shikonin [17]. As shown in Figure 3A, there was a reduction in the levels of PKM2 activity when shikonin treated in combination with TPA, compared with the levels of PKM2 activity upon treatment with TPA. As for the protein levels (Figure 3B and C), tumor promoter only induced the expression of PKM2, not the whole PKM. However, in the presence of shikonin, the increased levels of PKM2 expression were also reduced. Shikonin alone had no obvious effect on the expression of PKM2 or PKM. These results show that shikonin suppresses TPA-induced PKM2 activation.
Figure 3.
Shikonin (SKN) inhibited TPA-induced activation of PKM2 in JB6 P+ cells. Whole Cell Lysate was used for the assay. (A) Shikonin suppressed TPA-induced increases in PKM2 activity. Cells were treated with TPA (100 nM) and shikoin (1 μM) for 24 h. (B) and (C) Western blot analysis of PKM2 and PKM. Cells were treated with TPA (100 nM) and shikoin (1 μM) for 24 h. The level of PKM2 was normalized to that of β-actin. The experiments have been repeated more than three times and a representative result was shown. *P < 0.05 compared with the control (DMSO) treatment; #P < 0.05 compared with the TPA treatment.
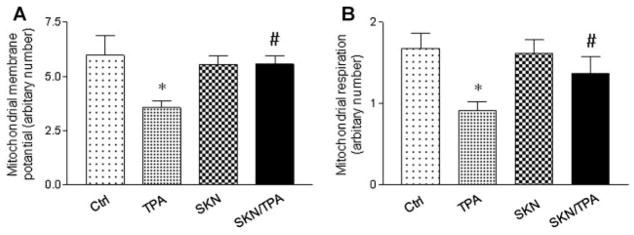
Shikonin Suppressed TPA-Induced Mitochondrial Dysfunction
To determine how mitochondrial metabolism is affected by PKM2 inhibition, mitochondrial membrane potential was first measured in JB6 P+ cells. As shown in Figure 4A, mitochondrial membrane potential was decreased upon tumor promoter TPA treatment, which was completely blocked in the presence of shikonin. Next, mitochondrial respiration was also measured in JB6 P+ cells. As shown in Figure 4B, shikonin could maintain the mitochondrial respiration which was inhibited by TPA. These results indicate that shikonin can prevent tumor promoter TPA-induced mitochondrial dysfunction.
Figure 4.
Shikonin (SKN) blocked TPA-induced mitochondrial dysfunction in JB6 P+ cells. Shikonin suppressed TPA-induced decreases in mitochondrial membrane potential (A), and mitochondrial respiration (B). Cells were treated with TPA (100 nM) and shikoin (1 μM) for 24 h. The experiments have been repeated more than three times and a representative result was shown. *P < 0.05 compared with the control (DMSO) treatment; #P < 0.05 compared with the TPA treatment.
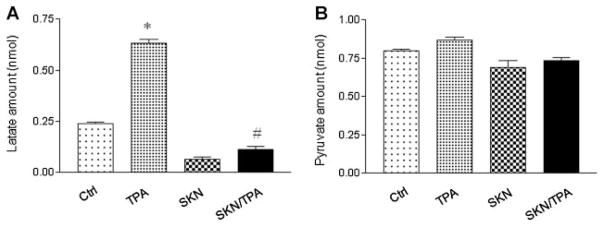
Shikonin Suppressed TPA-Induced Lactate Production in JB6 P+ Cells
Metabolism in cancer cells prefer aerobic glycolysis for ATP production, leading to increases in lactate production [22]. After examining the changes in mitochondrial functions and PKM2 expression/activity, we next detected whether shikonin could exert an inhibition influence on glycolysis in JB6 P+ cells. As shown in Figure 5A, the levels of lactate were increased after TPA treatment, which was suppressed by shikonin. In addition, we detected the levels of pyruvate, the end product of glycolysis. As shown in Figure 5B, pyruvate remained at similar levels among all the treatment groups. These results indicate that shikonin suppresses lactate production during early tumor promotion.
Figure 5.
Shikonin (SKN) suppressed lactate production, but did not affect pyruvate levels in JB6 P+ cells. Cells were treated with TPA (100 nM) and shikoin (1 μM) for 24 h. Whole Cell Lysate was collected and deproteinized. Fifty microliter lysate (2 μg/μl before deproteinization) was used for lactate (A) and pyruvate (B) measurements. The experiments have been repeated more than three times. *P < 0.05 compared with the Control (DMSO) treatment; #P < 0.05 compared with the TPA treatment.
Shikonin Suppressed TPA-Induced Cell Proliferation
As mentioned above, shikonin suppresses tumor promoter-induced mitochondrial dysfunction and glycolysis. To address whether these effects are associated with regression of cell cycle and cell proliferation, we performed cell cycle analysis. As shown in Figure 6, TPA treatment resulted in a decreased population of cells in G1 phase (Figure 6A), both shikonin alone and the combination showed a similar effect. Not surprising, TPA treatment caused an increased accumulation of cells in S phase (Figure 6B), which was blocked by shikonin. In addition, shikonin promoted G2/M phase accumulation (Figure 6C). As a member of the activator protein 1 (AP-1) family, Fra-1 expression is regulated during cell cycle, and Fra-1 knockdown causes an accumulation of cells in G2 phase [23]. Therefore, we detected the effect of shikonin on Fra-1 expression. As showed in Figure 6D, the increases in Fra-1 expression levels after TPA treatment were reduced by shikonin. These results suggest that shikonin inhibits TPA-induced S-phase accumulation and causes G2/M accumulation which may be modulated by Fra-1-involved transcript regulation.
Figure 6.
Shikonin (SKN) inhibited cell growth through causing G2–M accumulation and suppressing Fra-1 protein expression in JB6 P+ cells. (A)–(C) Cell cycle analysis. Cells were starved in serum-free EMEM medium for 24 h and then treated with TPA (100 nM) or shikonin (1 μM) for 24 h. Cell cycle analysis was performed by flow cytometry. (D) Western blot to detect Fra-1 expression. Cells were treated with TPA (100 nM) or shikonin (1 μM) for 24 h. The level of Fra-1 was normalized to that of β-actin. *P < 0.05 compared with the control (DMSO) treatment; #P < 0.05 compared with the TPA treatment.
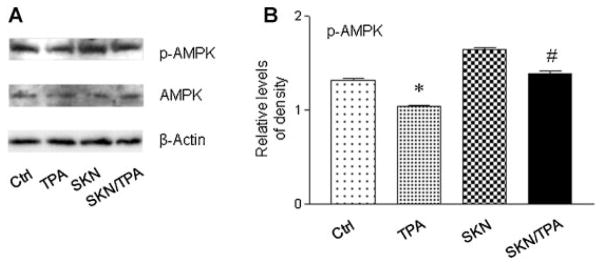
Shikonin Restored TPA-Induced Inactivation of AMP-activated Protein Kinase (AMPK)
Our previous studies have demonstrated that shikonin inhibites tumor promoter-induced cell proliferation, tumor promotion and mitochondrial metabolism. As a sensor of cellular energy status, AMPK plays a key role in tumorigenesis [24]. We next detected the protein level of AMPK. As shown in Figure 7, shikonin markedly blocked TPA-induced AMPK inactivation. These results indicate that AMPK can be an important mediator of shikonin’s chemopreventive activity.
Figure 7.
Shikonin blocked TPA-induced inactivation of AMPK. (A) Western blot analysis to detect AMPK. Cells were treated with TPA (100 nM) or shikonin (1 μM) for 4 h. (B) Quantification of the protein levels of p-AMPK. The level of p-AMPK was normalized to that of β-actin. *P < 0.05 compared with the control (DMSO) treatment; #P < 0.05 compared with the TPA treatment.
DISCUSSION
How to target cancer metabolism for cancer prevention and therapy is an emerging field in drug discovery. In human skin cancer tissue samples, our results indicate that PKM2 is upregulated, making PKM2 a potential anti-cancer target. Shikonin, a natural product isolated from the dried root of lithospermum erythrorhizon [25], is a newly identified specific inhibitor of PKM2 activity [17]. As a matter of fact, the anti-tumor effects of shikonin have been studied. In summary, shikonin induces apoptosis through the activation of caspase-3 in T24 human bladder cancer cells [26]; shikonin functions as a selective estrogen enzyme regulator by downregulating the expression of steroid sulfatase in breast cancer cells [27]; shikonin induces apoptosis in hepatocellular carcinoma cells by the reactive oxygen species (ROS)/Akt and RIP1/NF-κB pathways [28], etc. However, how shikonin affects cellular metabolism and whether targeting PKM2 using shikonin can serve as a chemopreventive approach, are largely unknown.
Neoplastic transformation of a normal cell requires many gene changes, mainly in two categories: activation of oncogenes and/or inactivation of tumor suppressor genes. Since neoplastic transformation occurs during the early stage of carcinogenesis [29], transformation inhibition could be an effective approach for chemoprevention. In our studies, we have shown that shikonin suppressed tumor promoter TPA-induced neoplastic transformation in JB6 P+ cells, suggesting that shikonin may bear chemopreventive activity. However, how PKM2 inhibition can lead to chemoprevention?
Recently, the “Warburg effect” in skin cancer and how to target it for skin cancer prevention has attracted more attention [30–33], although whether mitochondrial metabolism and biogenesis are up- or down- regulated in skin cancer remains controversial and further efforts are required. In our study, the anti-tumor promotion activity of shikonin is accompanied by preventing TPA-induced glycolysis and mitochondrial malfunction. This result suggests that dysfunction of mitochondrial occurs early in carcinogenesis, and upregulation of PKM2 is an important event during this metabolic switch. PKM2 can exist in either active protein kinase (dimer) or pyruvate kinase (tetramer) [34], the dual kinase activity can connect metabolism switch and gene regulation during tumor transformation and progression. PKM2 could be allosterically activated by D-FBP, which modulates PKM2 tetrameric–dimeric oscillation [35]. Meanwhile, other metabolic sources, such as serine, could activate PKM2 whereas alanine, proline inhibit PKM2 [36]. Our previous study suggests that mitochondrial respiration substrates malate/pyruvate and succinate may promote PKM2 inhibition and reduce tumor promoter-induced skin cell neoplastic transformation. In current study, shi-konin restores TPA-induced mitochondrial respiration reduction and suppresses PKM2 activity, which could be due to shikonin’s effect on mitochondrial respiration substrates; on the other hand, we demonstrate that PKM2 activation not only is embedded in human skin cancers, but also occurs during the early stage of skin tumorigenensis [18]. Moreover, mutant PKM2 transfected lung cancer cells show oxidative stress sensitivity [37]. We have known that mitochondria are the major source of ROS [38]; in our study, shikonin could preserve mitochondrial function and decrease the levels of ROS, leading to blocking PKM2 activation. Therefore, maintaining mitochondrial function and inhibiting PKM2 could be effective targets for prevention of skin cancer.
Higher glycolysis level could be accompanied by increased cellular glucose consumption and lactate production. As a rate-limiting enzyme in glycolysis, tumor PKM2 increases in cells that produce more lactate and consume less oxygen [39]. Our results demonstrate that shikonin inhibits tumor promoter-induced glycolysis and reduction of oxygen consumption, suggesting glycolysis and mitochondrial respiration indeed counteract in this tumor promotion model.
As a key energy-sensing enzyme, AMP-activated protein kinase (AMPK) monitors cellular energy status, and promotes growth inhibition [40]. Therefore, the activation of AMPK by natural products has potential for chemotheraputic and prevention, which would be a potent target of tumorigenesis. In addition, glycolytic inhibitors are able to activate AMPK [41]. Our data shows an interesting result that shikonin blocked tumor promoter-induced AMPK inactivation. Whether and how AMPK regulates PKM2 activity needs to be studied in future experiments.
During tumor development, the activation of oncogenes and the inhibition of tumor suppressors associate with not only the increase in glycolytic metabolism [42], but also neoplastic transformation. The balance between proliferation and cell death is pivotal for carcinogenesis, the unrestrained and rapid proliferation of cells could be a characteristic of benign tumors. As a tumor promoter, TPA stimulates cell proliferation [43,44]. Our data shows that shikonin inhibits cell proliferation via suppressing TPA-induced S phase accumulation and causing G2–M accumulation. Our results show that shikonin prevents mitochondrial malfunctions. Both TPA and damaged mitochondria can generate ROS, and ROS can induce G2–M arrest [45]. AP-1 is important in the processes of cell proliferation and transformation [46,47]. As an AP-1 family member, Fra-1 is also a key factor for cell transformation and upregulated in several tumors, such as squamous cell carcinomas [48], breast [49], and thyroid tumors [19]. Tumor promoter TPA induces Fra-1 expression, which regulates cyclin expression and cell cycle progression [23]. Based on our study, shikonin may also inhibit TPA-induced tumor promotion through the inhibition of the AP-1 transcription factor.
In summary, our studies demonstrate that shikonin, as a specific PKM2 inhibitor, suppresses skin cell transformation. Inhibition of glycolysis and maintaining mitochondrial function connect suppression of cell proliferation. This study highlights the possibility of targeting PKM2 for cancer prevention.
Acknowledgments
This study was supported by NIH Grant Number 1R03CA167689. The Authors wish to thank Dr. Patrick A. Adegboyega and Mylinh Smith at the Tissue & Serum Repository of the Feist-Weiller Cancer Center at LSU Health Sciences Center in Shreveport for technical support.
Abbreviations
- PKM2
pyruvate kinase isoform M2
- TPA
12-O-tetradecanoylphorbol 13-acetate
- LDH
lactate dehydrogenase
- AP-1
activator protein-1
- AMP
activated Protein Kinase (AMPK)
- ROS
reactive oxygen species
Footnotes
Additional supporting information may be found in the online version of this article.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Hathurusinghe HR, Goonetilleke KS, Siriwardena AK. Current status of tumor M2 pyruvate kinase (tumor M2-PK) as a biomarker of gastrointestinal malignancy. Ann Surg Oncol. 2007;14:2714–2720. doi: 10.1245/s10434-007-9481-x. [DOI] [PubMed] [Google Scholar]
- 3.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Mazurek S, Grimm H, Boschek CB, Vaupel P, Eigenbrodt E. Pyruvate kinase type M2: A crossroad in the tumor metabolome. Br J Nutr. 2002;87:S23–S29. [PubMed] [Google Scholar]
- 5.Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986;261:13807–13812. [PubMed] [Google Scholar]
- 6.Noguchi T, Yamada K, Inoue H, Matsuda T, Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem. 1987;262:14366–14371. [PubMed] [Google Scholar]
- 7.Benesch C, Schneider C, Voelker HU, et al. The clinicopathological and prognostic relevance of pyruvate kinase M2 and pAkt expression in breast cancer. Anticancer Res. 2010;30:1689–1694. [PubMed] [Google Scholar]
- 8.de Jong A, Pena-Cruz V, Cheng TY, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nat Immunol. 2010;11:1102–1109. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazurek S, Zwerschke W, Jansen-Durr P, Eigenbrodt E. Metabolic cooperation between different oncogenes during cell transformation: Interaction between activated ras and HPV-16 E7. Oncogene. 2001;20:6891–6898. doi: 10.1038/sj.onc.1204792. [DOI] [PubMed] [Google Scholar]
- 10.Shi HS, Li D, Zhang J, et al. Silencing of pkm2 increases the efficacy of docetaxel in human lung cancer xenografts in mice. Cancer Sci. 2010;101:1447–1453. doi: 10.1111/j.1349-7006.2010.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clower CV, Chatterjee D, Wang Z, Cantley LC, Vander Heiden MG, Krainer AR. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci USA. 2010;107:1894–1899. doi: 10.1073/pnas.0914845107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han W, Li L, Qiu S, et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther. 2007;6:1641–1649. doi: 10.1158/1535-7163.MCT-06-0511. [DOI] [PubMed] [Google Scholar]
- 14.Xuan Y, Hu X. Naturally-occurring shikonin analogues–a class of necroptotic inducers that circumvent cancer drug resistance. Cancer Lett. 2009;274:233–242. doi: 10.1016/j.canlet.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Zhou P, Huang H, et al. Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. Int J Cancer. 2009;124:2450–2459. doi: 10.1002/ijc.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo XP, Zhang XY, Zhang SD. Clinical trial on the effects of shikonin mixture on later stage lung cancer. Zhong Xi Yi Jie He Za Zhi. 1991;11:598–599. 580. [PubMed] [Google Scholar]
- 17.Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011;30:4297–4306. doi: 10.1038/onc.2011.137. [DOI] [PubMed] [Google Scholar]
- 18.Wittwer JA, Robbins D, Wang F, et al. Enhancing mitochondrial respiration suppresses tumor promoter TPA-induced PKM2 expression and cell transformation in skin epidermal JB6 cells. Cancer Prev Res (Phila) 2011;4:1476–1484. doi: 10.1158/1940-6207.CAPR-11-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiappetta G, Tallini G, De Biasio MC, et al. FRA-1 expression in hyperplastic and neoplastic thyroid diseases. Clin Cancer Res. 2000;6:4300–4306. [PubMed] [Google Scholar]
- 20.Circu ML, Rodriguez C, Maloney R, Moyer MP, Aw TY. Contribution of mitochondrial GSH transport to matrix GSH status and colonic epithelial cell apoptosis. Free Radic Biol Med. 2008;44:768–778. doi: 10.1016/j.freeradbiomed.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colburn NH, Former BF, Nelson KA, Yuspa SH. Tumour promoter induces anchorage independence irreversibly. Nature. 1979;281:589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- 22.Hail N, Jr, Lotan R. Cancer chemoprevention and mitochondria: Targeting apoptosis in transformed cells via the disruption of mitochondrial bioenergetics/redox state. Mol Nutr Food Res. 2009;53:49–67. doi: 10.1002/mnfr.200700527. [DOI] [PubMed] [Google Scholar]
- 23.Casalino L, Bakiri L, Talotta F, et al. Fra-1 promotes growth and survival in RAS-transformed thyroid cells by controlling cyclin A transcription. EMBO J. 2007;26:1878–1890. doi: 10.1038/sj.emboj.7601617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang JT, Kim YM, Surh YJ, et al. Selenium regulates cyclooxygenase-2 and extracellular signal-regulated kinase signaling pathways by activating AMP-activated protein kinase in colon cancer cells. Cancer Res. 2006;66:10057–10063. doi: 10.1158/0008-5472.CAN-06-1814. [DOI] [PubMed] [Google Scholar]
- 25.Mao X, Yu CR, Li WH, Li WX. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res. 2008;18:879–888. doi: 10.1038/cr.2008.86. [DOI] [PubMed] [Google Scholar]
- 26.Yeh CC, Kuo HM, Li TM, et al. Shikonin-induced apoptosis involves caspase-3 activity in a human bladder cancer cell line (T24) In Vivo. 2007;21:1011–1019. [PubMed] [Google Scholar]
- 27.Zhang Y, Qian R, Li Q, Shikonin PP. an ingredient of Lithospermum erythrorhizon, down-regulates the expression of steroid sulfatase genes in breast cancer cells. Cancer Lett. 2009;284:47–54. doi: 10.1016/j.canlet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Gong K, Li W. Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: A potential new treatment for hepatocellular carcinoma. Free Radic Biol Med. 2011;51:2259–2271. doi: 10.1016/j.freeradbiomed.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Kang NJ, Lee KW, Lee DE, et al. Cocoa procyanidins suppress transformation by inhibiting mitogen-activated protein kinase kinase. J Biol Chem. 2008;283:20664–20673. doi: 10.1074/jbc.M800263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aithal BK, Kumar MR, Rao BN, Udupa N, Rao BS. Juglone, a naphthoquinone from walnut, exerts cytotoxic and genotoxic effects against cultured melanoma tumor cells. Cell Biol Int. 2009;33:1039–1049. doi: 10.1016/j.cellbi.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Goodson AG, Cotter MA, Cassidy P, et al. Use of oral N-acetylcysteine for protection of melanocytic nevi against UV-induced oxidative stress: Towards a novel paradigm for melanoma chemoprevention. Clin Cancer Res. 2009;15:7434–7440. doi: 10.1158/1078-0432.CCR-09-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hersey P, Watts RN, Zhang XD, Hackett J. Metabolic approaches to treatment of melanoma. Clin Cancer Res. 2009;15:6490–6494. doi: 10.1158/1078-0432.CCR-09-0251. [DOI] [PubMed] [Google Scholar]
- 33.Vander Heiden MG, Christofk HR, Schuman E, et al. Identification of small molecule inhibitors of pyruvate kinase M2. Biochem Pharmacol. 2010;79:1118–1124. doi: 10.1016/j.bcp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45:598–609. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashizawa K, Willingham MC, Liang CM, Cheng SY. In vivo regulation of monomer-tetramer conversion of pyruvate kinase subtype M2 by glucose is mediated via fructose 1,6-bisphosphate. J Biol Chem. 1991;266:16842–16846. [PubMed] [Google Scholar]
- 36.Mazurek S, Boschek CB, Eigenbrodt E. The role of phospho-metabolites in cell proliferation, energy metabolism, and tumor therapy. J Bioenerg Biomembr. 1997;29:315–330. doi: 10.1023/a:1022490512705. [DOI] [PubMed] [Google Scholar]
- 37.Anastasiou D, Poulogiannis G, Asara JM, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 39.Vander Heiden MG, Locasale JW, Swanson KD, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP kinase in vascular smooth muscle. J Appl Physiol. 2005;98:296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- 42.Weinberg F, Chandel NS. Mitochondrial metabolism and cancer. Ann N Y Acad Sci. 2009;1177:66–73. doi: 10.1111/j.1749-6632.2009.05039.x. [DOI] [PubMed] [Google Scholar]
- 43.Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 44.Salo T, Turpeenniemi-Hujanen T, Tryggvason K. Tumor-promoting phorbol esters and cell proliferation stimulate secretion of basement membrane (type IV) collagen-degrading metalloproteinase by human fibroblasts. J Biol Chem. 1985;260:8526–8531. [PubMed] [Google Scholar]
- 45.Xu Z, Chen X, Zhang Q, Chen L, Wang Y, Corydalis yanhusuo WT. Wang extract inhibits MCF-7 cell proliferation by inducing cell cycle G2/M arrest. Am J Chin Med. 2011;39:579–586. doi: 10.1142/S0192415X11009044. [DOI] [PubMed] [Google Scholar]
- 46.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci USA. 1994;91:609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C, Ma WY, Young MR, Colburn N, Dong Z. Shortage of mitogen-activated protein kinase is responsible for resistance to AP-1 transactivation and transformation in mouse JB6 cells. Proc Natl Acad Sci USA. 1998;95:156–161. doi: 10.1073/pnas.95.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zajchowski DA, Bartholdi MF, Gong Y, et al. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res. 2001;61:5168–5178. [PubMed] [Google Scholar]
- 49.Belguise K, Kersual N, Galtier F, Chalbos D. FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene. 2005;24:1434–1444. doi: 10.1038/sj.onc.1208312. [DOI] [PubMed] [Google Scholar]