Abstract
Epithelial-Mesenchymal Transformation (EMT) and the subsequent invasion of epicardial and endocardial cells during cardiac development is critical to the development of the coronary vessels and heart valves. The transformed cells give rise to cardiac fibroblasts and vascular smooth muscle cells or valvular interstitial cells, respectively. The Type III Transforming Growth Factor β (TGFβR3) receptor regulates EMT and cell invasion in both cell types, but the signaling mechanisms downstream of TGFβR3 are not well understood. Here we use epicardial and endocardial cells in in vitro cell invasion assays to identify common mechanisms downstream of TGFβR3 that regulate cell invasion. Inhibition of NF-κB activity blocked cell invasion in epicardial and endocardial cells. NF-κB signaling was found to be dysregulated in Tgfbr3−/− epicardial cells which also show impaired cell invasion in response to ligand. TGFβR3-dependent cell invasion is also dependent upon Activin Receptor-Like Kinase (ALK) 2, ALK3, and ALK5 activity. A TGFβR3 mutant that contains a threonine to alanine substitution at residue 841 (TGFβR3-T841A) induces ligand-independent cell invasion in both epicardial and endocardial cells in vitro. These findings reveal a role for NF-κB signaling in the regulation of epicardial and endocardial cell invasion and identify a mutation in TGFβR3 which stimulates ligand-independent signaling.
Keywords: transforming growth factor beta, bone morphogenic protein, endocardial cell, epicardial cell, invasion, nuclear factor-kappa B
1.0 Introduction
Transforming Growth Factor Beta (TGFβ) signaling is altered in multiple human diseases including cancer, cardiovascular disease, fibrotic disease, and tissue injury (review [1]). These findings have driven efforts to explore the components of the TGFβ signaling pathway as therapeutic targets. However, experience with current TGFβ-based therapies under development for cardiovascular diseases (review [1–4]) and cancer ([5] & review [6–8]) have revealed that our current knowledge does not allow for efficiently meeting the task of drug development. The lack of detailed understanding of how TGFβ signaling is regulated and integrated impairs our ability to predict safety and efficacy; thus presenting a significant obstacle to developing therapeutic agents.
In particular, our understanding of the role of Type III TGFβ receptor (TGFβR3) in TGFβ signaling is not well understood. Targeting TGFβR3 in mice [9] and cardiac cushion explants [10] revealed a unique & non-redundant role for TGFβR3 in TGFβ signaling. Deletion of Tgfbr3 in mice is embryonic lethal due to failed coronary vessel development [9] associated with decreased proliferation and invasion of the epicardial cells required for coronary vessel development [11]. Similarly, targeting TGFβR3 in cardiac cushion explants revealed a requirement of the receptor for the endocardial cell invasion that is essential for the formation of the heart valves [10]. TGFβR3 contains a glycosylated extracellular domain and a 43 amino acid intracellular domain devoid of catalytic activity [12, 13]. TGFβR3 binds TGFβ1 & TGFβ3, is required for high affinity binding of TGFβ2 [14], and also binds and signals in response to BMP2 [15] and inhibin [16].
TGFβR3 has been reported to act as a co-receptor to augment signaling via the canonical TGFβ signaling pathway through Smads activation after presenting ligand to the Type I (TGFβR1) or Activin Receptor Like Kinase (ALK) 5 & Type II (TGFβR2) TGFβ receptors [17]. Although the cytoplasmic domain of TGFβR3 is not required for ligand presentation to TGFβR1 & TGFβR2, the regulation of migration and invasion of several cell types have been shown to require the cytoplasmic domain of TGFβR3. These include several cancer cell lines [18, 19] as well as both endocardial [20] and epicardial cells [11]. Therefore, efforts to understand TGFβR3 signaling have focused on the identification of proteins that interact with the cytoplasmic domain. The 3 C-terminal amino acids of TGFβR3, STA, serve as a Class I PDZ binding motif and bind the scaffolding protein, GIPC (GAIP-interacting protein, C terminus). GIPC stabilizes TGFβR3 at the cell surface which has been proposed to enhance TGFβ signaling [21]. The interaction between TGFβR3 and GIPC has been reported to mediate the inhibition of breast cancer cell migration in vitro and cancer progression in vivo [22]. However, in both epicardial [11] and endocardial [20] cells, ligand-stimulated cell invasion has been found to be dependent on the cytoplasmic domain of TGFβR3, specifically the 3 C-terminal amino acids that interact with GIPC. In a second, distinct region of the cytoplasmic domain, phosphorylation of Thr841 by TGFβR2 is required for βarrestin2 (βArr2) binding which leads to TGFβR3 internalization [23]. TGFβR2 is trafficked with TGFβR3 leading to the down-regulation of TGFβ signaling. Mutation of Thr841 to alanine (TGFβR3-T841A) prevents phosphorylation by TGFβR2 and renders TGFβR3 unable to interact with βArr2. The loss of βArr2 interaction with TGFβR3 resulted in enhanced TGFβ signaling as measured by TGFβ-mediated growth inhibition in keratinocytes. The interaction between TGFβR3 and βArr2 has also been suggested to regulate cell migration in cancer cell lines through βArr2-mediated activation of Cdc42 [19] and through negatively regulating NF-κB signaling [24]. Taken together, these data show a critical role for the cytoplasmic domain of TGFβR3 in the regulation of TGFβR3-dependent cell migration and invasion. Here we exploit both cultured epicardial and endocardial cells to investigate common signaling mechanisms that regulate cell invasion downstream of TGFβR3.
2.0 Material and Methods
2.1 Immortalized Epicardial Explant Culture
Multiple immortalized epicardial cell lines from Tgfbr3+/+ and Tgfbr3−/− E11.5 littermate pair mouse embryos were generated as described previously [25]. To sustain the cell’s immortalized state, they were grown at 33 °C in immorto media: 10 % fetal bovine serum (FBS), 100 U/ml Penicillin/Streptomycin (P/S), 1 X Insulin-Transferrin-Selenium (ITS; 1 µg/ml insulin, 5.5×10−4 µg/ml transferrin, 0.677 µg/ml selenium), and 10 U/ml interferon γ (INFγ). Once the cells were ready to be used in an experiment, they were transferred to standard DMEM medium (10 % FBS and 100 U/ml P/S) and cultured at 37 °C.
2.2 Growth Factors and Inhibitors
Reagents were obtained from the following sources: TGFβ1, TGFβ2, BMP2, and FGF2 were purchased from R&D Systems; SB431542 from Sigma-Aldrich; SN-50 from Enzo; BMS-345541 from Calbiochem. DMH1 was a generous gift from Dr. Charles Hong (VUMC).
2.3 Adenovirus
Adenoviruses were generated using the pAdEasy system [26]. Viruses were tittered by performing serial dilutions of the concentrated virus and counting the number of GFP-expressing HEK293 cells after 18–24 h. The following adenoviruses co-expressing GFP were used: full length TGFβR3 (FL), TGFβR3 lacking the cytoplasmic domain (CYTO), TGFβR3 lacking the last 3 amino acids (Δ3), and TGFβR3 with T841A mutation (T841A).
Epicardial cells were plated at a density of 200,000 per well in immorto media and allowed to adhere overnight at 33 °C. The following day, virus was added directly to the cells at a final concentration of 108 PFU/ml. 24 h later, the cells were plated for experiments. Equivalent levels of expression and appropriate cellular localization of receptor constructs has been previously shown under these same conditions [11].
2.4 Plasmids
Epicardial cells were plated at a density of 50,000 per well of a 6-well plate in immorto media without P/S and allowed to adhere overnight at 33 °C. The cells were transfected the following day with 2 µg of either pAdTrackCMV vector alone or expressing constitutively active (ca) ALK2, caALK3, or caALK5 or NF-κB-luciferase and cmv-β-galactosidase and 6 µl of X-treme gene DNA transfection reagent (Roche) in immorto media. The media was changed 24 h after transfection to 10 % FBS/DMEM without P/S and cells were incubated at 37 °C for the duration of the experiments. Similar percent transfection efficiency (~50%) was seen for each plasmid after 48 h (data not shown).
2.5 Invasion Assay
Invasion assays were performed using a modified Boyden chamber assay as previously described [11]. Briefly, cells were fluorescently labeled with CalceinAM (BD Biosciences) and plated at 12,000 cells per well in 0.5% FBS/DMEM on collagen gels [27] in the top chamber. Epicardial cells were allowed to adhere overnight at 37 °C. 24 h later, vehicle, growth factors, or growth factors plus inhibitors were added in 20 % FBS/DMEM to the bottom chamber. In addition, cells also received inhibitor in the top well in 0.5 % FBS/DMEM. After 24 h, the top insert was removed and placed in 0.25 % Trypsin/2.21 mM-EDTA in HBSS (CellGro). Cells that invaded the collagen and crossed the membrane detached from the membrane into the trypsin containing plate, which was then read using SpectraMax 96-well plate reader (Ex: 485, Em: 538, Cutoff: 530; sensitivity: 30).
2.6 Cell viability Assay
Epicardial cell viability was scored using a MTS assay as previously described [11, 28], which relies on the in vivo reduction of MTS tetrazolium to a colored formazan product by NADPH in metabolically active cells. The product formed is read at 490 nm and is directly proportional to the number of living cells in culture. Briefly, cells were plated at a density of 5,000 cells/well/96-well plate in a total of 100 µl of 10 % FBS/DMEM +/− inhibitor at 37 °C. After 24 h, 20 µl of substrate (Promega: Cell Titer 96 Aqueous Solution) was added to each well and incubated for 30 min at 37 °C. Absorbance of the colorimetric reaction was read at 490 nm using SpectraMax 96-well plate reader.
2.7 Luciferase Assay
NF-κB activity was analyzed by transfecting epicardial cells with a NF-κB-luciferase reporter construct. 24 h after transfection, the cells were serum starved for 4 h and then stimulated with TGFβ1, TGFβ2, BMP2, or FGF2 for 24 h at 37 °C. Luciferase activity was normalized for transfection efficiency using a vector coding for β-galactosidase. After transfection and subsequent incubation with ligand, luciferase and β-galactosidase levels were detected using the Luciferase Assay System (Promega) and the Luminescent β-gal Detection Kit (Clontech), respectively, according to manufacturer’s protocol.
2.8 Immunohistochemistry
Epicardial cells (E11.5) were plated in 4-well collagen coated chamber slides (BD Biosciences) at a density of 50,000 cells per well. Cells were fixed with 2 % paraformaldehyde (PFA) for 30 min at room temperature and permeabilized with ice cold 1:1 methanol/acetone solution for 30 min. The cells were incubated for 1 h in 10 % FBS/PBST solution to block non-specific binding and incubated with primary antibody (Phospho c-Rel; Cell Signaling; 1:100) overnight at 4 °C. Primary antibody detection with biotinylated goat anti-rabbit IgG and Dylight 549 streptavidin was performed at RT for 1 h. Nuclei were stained with 4’, 6-diamidino-2-phenylinodole (DAPI; VectaSheild mounting media). Photomicrographs were captured with Nikon Eclipse E800 microscope and SPOT imaging software. The total number of phospho c-Rel positive cells were counted and expressed as a percentage of a given population of DAPI positive cells
2.9 Collagen Gel Assay
Atrioventricular cushions (AVC) or ventricles were excised from HH stage 16 chicken embryos. Explants were placed endocardial side down on a collagen gel previously pre-incubated with media as described by Bernanke and Markwald [29]. Explants were then incubated at 37 °C, 5 % CO2 for 48 h prior to 4 % PFA fixation.
2.10 Gene Targeting by siRNA
AVC or ventricular explants from stage 16–18 were collected in 100 µl room temperature (RT) M199 medium for each treatment condition. For transfection, first 4 µl of siPORT NeoFX (Ambion) was incubated with 96 µl M199 medium to a final volume of 100µl for 10 minutes (min) at RT. Next, the appropriate final concentration (for 300 µl total volume) of siRNA derived from 21bp RNA (target sequence) was added to a final volume of 100 µl. These two tubes were mixed and incubated for 15 min at RT to allow siRNA complexes to form. This was followed by the 200 µl mixture being added to the 100 µl containing AVC or ventricular explants, and this solution was incubated at 37 °C, 5 % CO2 for 45 min. Explants were then placed on conditioned collagen gels and incubated under the same conditions for 48 h. After 48 h, explants were fixed in 0.8 % formaldehyde, 0.05 % glutaraldehyde or 4 % PFA for 5 min at RT and washed twice with PBS. Fixed explants were scored for number of mesenchymal cells invading the gel. For control siRNA, a scrambled 21-oligonucleotide template containing the same number of the bases of the siRNA target gene that did not BLAST to any gene in the chicken genome, 5’(AGACTGT CGCGTGCTCTGTCC)3’ was used as previously described [30] and served to identify any toxicity due to the concentration of oligo or transfection reagent. siRNA targeted to TGFβR3 was used as a positive control for inhibition of EMT, 5’ (GGAAGUAAAUCUACUUGAATT) 3’. Two independent siRNA constructs against each chicken ALK (2, 3 and 5) were designed using Silencer Select custom siRNA (Ambion). For each construct targeted against a specific ALK, that sequence is lacking in other genes of interest. At the concentrations and conditions used for the siRNA’s, we have published the percent knockdown for each[11, 20, 28, 31]. All constructs show a greater than 70% knockdown in both chick and mouse. The siRNA target sequences are listed in Table 1.
Table 1.
siRNA Target Sequences
| Smad1a | 5’(GGAGUUCGCUCAGCUCUUATT)3’ |
| Smad1b | 5’(GCAGGGAGAUGAAGAAGAATT)3’ |
| Smad2a | 5’(GGAUUGAACUUCAUCUGAATT)3’ |
| Smad2b | 5’(CCACCUCCAGGAUAUAUCATT)3’ |
| Smad3a | 5’(GGAUGUAACUUGAAUAUUUTT)3’ |
| Smad3b | 5’(GGAUGUGCACGAUUCGGAUTT)3’ |
| Smad4a | 5’(CAAUGUCCAUCGCACCGAAtt)3’ |
| Smad4b | 5’(GCUAUUACCUGGAUCGGGAtt)3’ |
| Smad5a | 5’(GCAAUACAAUGAUCCCUCATT)3’ |
| Smad5b | 5’(GGUUUGCUCUCAAAUGUUATT)3’ |
| ALK2a | 5’(GCAGAUUUAUUGG ACCAUUtt)3’ |
| ALK2b | 5’(GGUUAGCAAUGGUAUAGUAtt)3’ |
| ALK3a | 5’(GAUUAACAGUGAACAAUGAtt)3’ |
| ALK3b | 5’(GGAGGAAGCUUGAAGUACAtt)3 ‘ |
| ALK5a | 5’(GCUACGACAUGAAAACAUUtt)3’ |
| ALK5b | 5’(GGAUAUUGCUGCCUUUUAAtt)3’ |
2.11 Cell Death Assay in Explants
Explants harvested from Stage 16 chick embryos were placed on collagen gels. After 48 h, explants were incubated with 2 µM lysotracker (Invitrogen) for 15 min at 37 °C while rocking, washed for 15 min with PBS, incubated with DAPI (1:1000) for 5 min, and washed a final time. Explants were fixed in 4 % PFA and followed by mounting on a slide. Lysotracker and DAPI positive cells were photographed under appropriate filter settings and corresponding photomicrographs were overlaid. The total number of lysotracker positive cells were counted and expressed as a percentage of a given population of DAPI positive cells.
2.12 Cell Proliferation in Explants
BrdU
AVC explants were excised from HH Stage 16 chick embryos and incubated on collagen gels for 48 h, incubated with 1 mM BrdU (Roche) for 1 h, and fixed with 4 % PFA. Explants were washed 3 times with PBS and permeabilized with 0.5 % tritonX-100. Antigen retrieval was accomplished with 2 M HCL. Explants were blocked with 5 % normal donkey serum and 0.05 % PBST for 1 h and incubated with monoclonal Alexafluor 594 conjugated BrdU antibody (1:50 dilution, Invitrogen) overnight. Explants were rinsed 3 times with PBS and stained with DAPI (dilution 1:1000) for 5 min. Stained explants were imaged with a fluorescent microscope. For each explant, approximately a hundred random cells were counted and scored for the presence of BrdU staining in the nucleus.
Histone H3 Phosphorylation
AVC explants where excised as previously described and fixed with 4 % PFA after 48 h incubation. Explants were washed 3 times with PBS and permeabilized with 0.5 % tritonX-100. Next, explants were blocked with 5 % normal donkey serum and 0.05 % PBST for 1 h at 4 °C. Explants were then incubated in primary antibody, mouse phospho histone-H3 (monoclonal, 1:300, Sigma), overnight at 4 °C. Explants were washed 3 times with PBST and incubated with secondary antibody, Goat anti-mouse IgG conjugated with Cy3 (1:50, Jackson Laboratories) overnight at 4°C. This was followed with explants being rinsed 3 times with PBS and stained with DAPI (1:1000) for 5 min. Stained explants were imaged with a Fluorescent scope (Nikon) and cells were digitally counted with Image J software.
2.13 Viral Introduction into Explants
Stage 10–12 chick embryos were harvested onto Whatman paper rings and immediately injected with adenovirus in the heart lumen. Injections were performed using 20–50 pulses, approximately 50 ms each, delivered by a picospritzer. Fast Green Dye (Sigma) was used to monitor injection of the virus. Immediately after injection, the embryos were placed on egg agar and incubated at 37 °C. After 24 h, ventricular or AVC explants were excised from the infected hearts and placed in culture as described previously [32]. After 48 h, the explants were fixed in either 0.8 % formaldehyde, 0.05 % glutaraldehyde or 4 % PFA. GFP expression by virally infected cells was observed on an inverted fluorescent microscope. The phenotype of each GFPexpressing cell was determined and scored as epithelial, activated, or transformed as described [32].
2.14 Statistical Analysis
Paired Students T-test was performed to establish significance. Data are presented as the mean of three experiments ± SEM for one littermate pair, unless otherwise specified. P-values of <0.05 were considered significant.
3.0 Results
3.1 TGFβR3-T841A promotes ligand-independent invasion in epicardial cells
Tgfbr3−/− epicardial cells have decreased invasion into a collagen matrix in response to ligand when compared to Tgfbr3+/+ cells [11]. The rescue of invasion in Tgfbr3−/− epicardial cells by TGFβR3 requires specific amino acid residues in the cytoplasmic domain that interact with GIPC [11, 28]. Here we wished to explore the role of threonine residue 841 found in the cytoplasmic domain of TGFβR3. Phosphorylation of this residue by TGFβR2 leads to a phosphorylation-dependent interaction with βArr2 that results in the internalization of TGFβR3 and downregulation of signaling [5, 7]. A point mutation introduced at position 841 substituting alanine for threonine, TGFβR3-T841A, is sufficient to prevent these events [7]. We asked if TGFβR3-T841A overexpression in epicardial cells alters known TGFβ-stimulated, TGFβR3-mediated responses. Tgfbr3−/− epicardial cells were infected with adenovirus expressing GFP alone or GFP in combination with full length TGFβR3 (TGFβR3-FL), TGFβR3 lacking the entire cytoplasmic domain (TGFRβ3-CYTO), TGFβR3 lacking the C-terminal 3 amino acids (TGFβR3-Δ3), or TGFβR3-T841A and scored for invasion. Consistent with prior results [11] the addition of TGFβR3-FL, but not TGFRβ3-CYTO or TGFβR3-Δ3, rescued invasion with the addition of ligand (Fig. 1A). Cells overexpressing TGFβR3-FL in the presence of 250 pM TGFβ2 have increased levels of invasion (150%) when compared to vehicle-incubated cells overexpressing TGFβR3-FL. Tgfbr3−/− cells expressing TGFβR3-T841A showed significantly increased levels of invasion when compared to cells expressing full length receptor (TGFβR3-FL) in the absence of TGFβ2 (Fig. 1A). The addition of ligand to Tgfbr3−/− cells expressing TGFβR3-T841A did not further increase invasion when compared to cells expressing TGFβR3-T841A in the absence of ligand (Fig. 1A). These data demonstrate that the introduction of TGFβR3-T841A into Tgfbr3−/− cells results in ligand-independent cell invasion.
Fig 1. TGFβR3-T841A caused ligand-independent epicardial cell invasion and required ALK2/3, ALK5, and NFκB.
Quantification of invasion using a modified boyden chamber assay with Tgfbr3+/+ and Tgfbr3−/− immortalized epicardial cells (E11.5). A. Tgfbr3−/− epicardial cells were infected with GFP, TGFβR3-FL, TGFβR3-CYTO, TGFβR3-Δ3, or TGFβR3-T841A and scored for ligand-independent and ligand-dependent invasion. TGFβR3-T841A stimulated ligand-independent invasion that was not increased by the addition of ligand. TGFβR3-FL stimulated invasion in the presence of TGFβ2. TGFβR3-CYTO or TGFβR3-Δ3 did not promote invasion. B. Tgfbr3−/− epicardial cells were infected with GFP, TGFβR3-FL, or TGFβR3-T841A and incubated with DMSO, TGFβ2, SB431542, DMH1, or BMS345541 during invasion. TGFβR3-T841A + DMSO, TGFβR3-FL + TGFβ2, and TGFβR3-T841A + TGFβ2 stimulated invasion which was inhibited by the presence of the small molecule inhibitors. C. Tgfbr3+/+ epicardial cells were scored for invasion in the presence of indicated ligands with DMSO, SN-50, or BMS345541. Ligands + DMSO stimulated invasion while the addition of SN-50 or BMS345541 was inhibitory. D. Tgfbr3−/− epicardial cells were transfected with GFP, caALK2, caALK3, or caALK5. Prior to scoring for invasion they were treated with either DMSO or BMS345541. Cells invaded in response to caALKs + DMSO. Invasion of cells was modestly inhibited by BMS345541. E, F. Cell viability was analyzed in Tgfbr3−/− cells incubated in the presence of small molecule inhibitors or infected with adenovirus expressing the different receptor constructs. n=3, * = p < 0.05 compared to GFP control, $ = p < 0.05 compared to DMSO.
3.2 Ligand-independent invasion stimulated by TGFβR3-T841A requires ALK activity
We next used small molecule inhibitors to specifically block effectors known to be downstream of TGFβR3 to determine if these same effectors were acting downstream of TGFβR3-T841A. Previous work by our laboratory demonstrated a requirement for ALK2, ALK3, and ALK5 for ligand-stimulated invasion in Tgfbr3+/+ epicardial cells [28]. Tgfbr3−/− epicardial cells were infected with adenovirus overexpressing GFP, GFP and TGFβR3-FL, or GFP and TGFβR3-T841A (Fig. 1B). Cells overexpressing TGFβR3-FL increased cell invasion in response to TGFβ2 whereas cells overexpressing TGFβR3-T841A increased invasion independent of ligand addition. Incubation with either 2.5 µM of the ALK5 kinase inhibitor SB431542 or 2 µM of the ALK2/ALK3 inhibitor DMH1 [33] significantly decreased both TGFβ2-dependent invasion in cells overexpressing TGFβR3-FL and TGFβ2-independent invasion in cells overexpressing TGFβR3-T841A. Neither inhibitor altered cell viability (Fig. 1E). As expected, overexpression of TGFβR3-FL rescued a modest, but significant, deficit in cell proliferation seen in Tgfbr3−/− cells when compared to Tgfbr3+/+ cells (Fig. 1F). Of note, TGFβR3-T841A, but not TGFβR3-CYTO or TGFβR3-Δ3, also rescued this deficit. These data demonstrate that TGFβR3-T841A mediated ligand-independent cell invasion requires the activation of ALK2, ALK3, and ALK5 as does TGFβR3-FL.
3.3 NF-κB activity in epicardial cells is required for ligand-dependent and ligand-independent invasion
Loss of even a single allele of Tgfbr3 results in decreased NF-κB activity in mouse embryonic fibroblasts [34] and TGFβR3 phosphorylation by TGFβR2 at position 841 has been reported to modulate NF-κB activity in breast epithelial and breast cancer cell lines [35]. Therefore, we next asked if the ligand-independent invasion seen in epicardial cells overexpressing TGFβR3-T841A was dependent on NF-κB activity. Tgfbr3−/− epicardial cells overexpressing GFP, GFP and TGFβR3-FL, or GFP and TGFβR3-T841A were incubated with 10 µM of the NF-κB inhibitor BMS345541 which selectively targets I kappa B kinase (IκB) (Fig. 1B). These data show significant inhibition of both TGFβ2-dependent invasion in cells overexpressing TGFβR3-FL and ligand-independent invasion in cells overexpressing TGFβR3-T841A. These findings suggest that NF-κB activity is required downstream of both TGFβR3-FL and TGFβR3-T841A. To further address the role of NF-κB in epicardial cell invasion, we pre-incubated Tgfbr3+/+ epicardial cells with either 10 µM BMS345541, an IκB kinase inhibitor, or 2 µM SN50, a cell permeable peptide inhibitor of NF-κB translocation to the nucleus, prior to the addition of ligands known to require TGFβR3 for stimulating cell invasion [11]. Each inhibitor blocked invasion induced by TGFβ1, TGFβ2, BMP2, or FGF2 (Fig. 1C), all ligands known to stimulate TGFβR3-dependent cell invasion [11]. Neither inhibitor nor viral vectors containing receptor constructs altered cell viability (Fig. 1D, E).
3.4 NF-κB activity is not required for ALK-mediated cell invasion in epicardial cells
We have previously reported that constitutive active (ca) constructs of ALK2, ALK3, or ALK5 induces invasion independent of ligand in Tgfbr3+/+ or Tgfbr3−/− epicardial cells [28]. Since NF-κB activity is required for invasion, we next asked if NF-κB activity is downstream of ALK activation. Tgfbr3−/− were transfected with constructs expressing GFP alone, GFP and caALK2, GFP and caALK3, or GFP and caALK5, incubated with either vehicle or 10 µM BMS345541, and scored for invasion. Overexpression of GFP or caALKs had no measurable effect on cell viability (Fig. 1F). BMS345541 inhibited invasion of Tgfbr3−/− epicardial cells overexpressing caALKs by 10% (Fig. 1D). The modest inhibition seen suggests that caALKs do not require NF-κB activity to stimulate cell invasion.
3.5 NF-κB activity is altered by the loss of TGFβR3
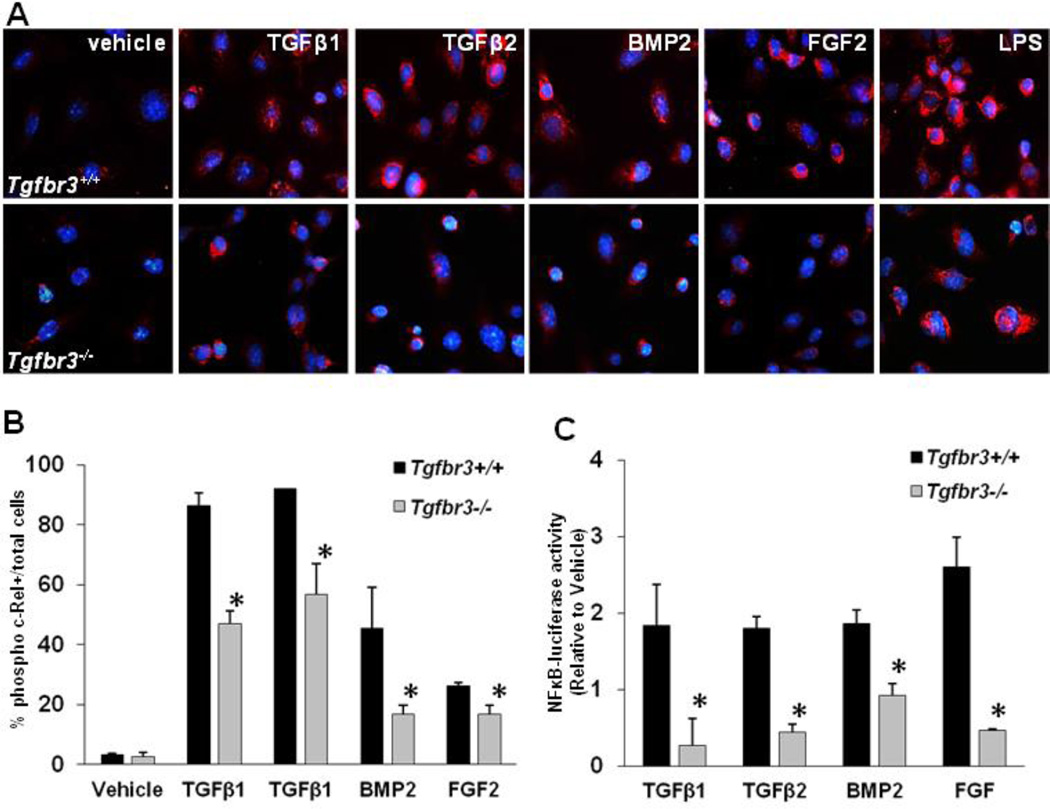
Given that Tgfbr3−/− epicardial cells have decreased invasion when compared to Tgfbr3+/+ epicardial cells and that invasion stimulated by TGFβR3-T841A overexpression was blocked by the NF-κB inhibitor BMS345541, we hypothesized that NF-κB is activated downstream of TGFβR3 during epicardial cell invasion in vitro. Therefore, we asked if Tgfbr3+/+ and Tgfbr3−/− epicardial cells activate NF-κB in response to ligand. We incubated Tgfbr3+/+ or Tgfbr3−/− epicardial cells with ligands known to induce epicardial cell invasion and scored for nuclear phospho c-Rel by immunostaining (Fig. 2A, B). Cells of both genotypes incubated with ligands known to stimulate cell invasion (either 250 pM TGFβ1 or TGFβ2, 5nM BMP2, or 10 ng/ml FGF2) demonstrated an increase in the number of phospho c-Rel-positive nuclei when compared to vehicle-incubated cells. Analysis of the percent of total nuclei positive for c-Rel immunostaining revealed that Tgfbr3+/+ epicardial cells have a larger percentage of positive nuclei after growth factor addition than Tgfbr3−/− epicardial cells (Fig. 2B). The addition of LPS, an inducer of I kappa B alpha phosphorylation and NF-kappa B activation, causes c-rel phosphorylation and nuclear translocation (Fig 2A, last row of panels) as indicated by immunostaining with the antibody. This important control demonstrates that there is sufficient expression of all the necessary components of the NF-kappa B pathway to support phospho c-rel nuclear translocation in Tgfbr3−/− cells. This apparent difference in NF-κB activation in Tgfbr3+/+ cells when compared to Tgfbr3−/− cells was confirmed by measuring the activity downstream of an NF-κB reporter (Fig. 2B). Cells were co-transfected with a NF-κB-luciferase reporter construct and CMV-β-galactosidase. After 24 h, cells were incubated for 24 h with TGFβ1, TGFβ2, BMP2, or FGF2, ligands known to require TGFβR3 to stimulate invasion. The presence of luciferase in the media was analyzed and quantified. Our results demonstrate that Tgfbr3+/+ epicardial cells activate NF-κB activity approximately 2-fold greater in response to TGFβ1, TGFβ2, BMP2, or FGF2 than do Tgfbr3−/− cells. Taken together, these data show that loss of TGFβR3 is associated with the loss of ligand-stimulated NF-κB activity coincident with the loss of ligand-dependent invasion in Tgfbr3−/− epicardial cells.
Fig. 2. Loss of TGFβR3 in epicardial cells resulted in decreased NFκB activation.
Tgfbr3+/+ and Tgfbr3−/− immortalized epicardial cells (E11.5) were incubated with vehicle, TGFβ1, TGFβ2, BMP2, or FGF2 and scored for NFκB activity. A. Cells were stained with phospho c-Rel (cell signaling) following 24 h incubation with ligands. B. Cell were counted and the percent of phospho c-Rel + cells / total Dapi + cells was quantified. C. Cells were transfected with NFκB-luciferase reporter plasmid and incubated with ligands for 24 h. Luciferase luminescence was measured and graphed relative to cells incubated in vehicle. Tgfbr3+/+ epicardial cells demonstrated up to a 2-fold increase in activation of NFκB signaling over Tgfbr3−/− cells. Data plotted as the mean percent ± SE of three independent experiments (*=p<0.05)
3.6 Invasion of endocardial cells requires ALK activity
Given that endocardial cell EMT represents a second system where TGFβR3 is required for ligand-stimulated cell invasion in vitro [36], we next examined TGFβR3 signaling mechanisms in this cell type in order to determine if the results seen in epicardial cells were shared between these two cell types. Experiments examining TGFβR3-dependent endocardial cell invasion in the chick atrioventricular cushion explant assay have revealed several similarities with this process in epicardial cells. Targeting TGFβR3 in cardiac cushion explants revealed a requirement of the receptor for endocardial cell invasion in vitro [10] which is dependent on the 3 C-terminal amino acids of TGFβR3 and interaction with GIPC [20]. Experiments in both endocardial cells and epicardial cells have also revealed a role for the Par6/Smurf/Rho pathway and multiple ALKs in cell invasion [20, 28, 31, 37, 38]. Here, incubation of atrioventricular cushion explants, which include endocardial cells that express TGFβR3 that undergo EMT and cell invasion in response to TGFβ released from associated cardiac myocytes, with ALK-specific small molecule inhibitors (2µM DMH1; ALK2 & ALK3 or 2.5µµ SB431542; ALK5) resulted in a 60% decrease in endocardial cell invasion when compared to vehicle incubated explants (Fig. 3A). To exclude toxicity of small molecule inhibitors, we measured cell death and proliferation after the explants were incubated in the presence of vehicle, SB431542 or DMH1. There was no significant change in cell death or proliferation between groups (Fig. 3B, C). We next asked directly if ALK2 and ALK3 were required downstream of the TGFβR3 by overexpressing GFP or GFP and TGFβR3-FL in ventricular explants, which include endocardial cells that lack TGFβR3 expression, and EMT was measured by scoring the percentage of total GFP-positive cells as epithelial, activated or transformed (Fig. 3D) as described previously [15, 20, 37, 38]. Ventricular endocardial cells overexpressing GFP alone show a small percentage of cells that undergo EMT as measured by the percentage of epithelial cells, activated cells (elongated cells on surface of gel), and transformed cells (cells that have entered the gel). However, the overexpression of TGFβR3-FL in the presence of TGFβ2 results in a significant increase in EMT and the number of cells that invade the gel. This increase in invasion was decreased by 18% in the presence of the ALK2/ALK3 inhibitor DMH1. These data are consistent with prior studies using siRNA that demonstrated a requirement for ALK2 and ALK3 in endocardial cell invasion [20] and they further support the hypothesis that ALK2 and ALK3 are required for TGFβR3-dependent EMT and cell invasion.
Fig 3. ALK2/3 inhibition blocked endocardial EMT.
A. DMH1, a small molecule inhibitor specific to ALK2 and ALK3, decreased endocardial EMT in AVC by 50%. Specific ALK5 inhibitor, SB431542, is used as a positive control for small molecule inhibition of EMT. Data are derived from 3 independent experiments normalized explants incubated with vehicle. Quantification of the total number of explant and total number of transformed cells and normalized median ± SEM are as follows: Vehicle (n=31, total transformed cells = 9913; normalized median to 100% ± 22.1), SB431542 (n=31, total transformed cells = 4528; normalized median = 40.2 ±14.1), DMH1 (n=31, total transformed cells = 4472; normalized median = 47.2 ± 9.9). B. Cell death is not significantly changed between explants incubated with small molecule inhibitors targeted to ALK 2, 3 or 5 compared to those incubated with vehicle alone. In vehicle incubated explants and average of 2.2% of total cells were lysotracker-positive. Incubation with SB431542 and DMH1 resulted in no significant change in endocardial EMT. The total number of AVC explants examined (n), total number of DAPI and lysotracker positive cells, and average percent positive lysotracker cells ± SEM are as follows: Vehicle (n = 56; total DAPI positive cells 11525; lysotracker positive cells = 256; average percent positive lysotracker = 2.2 ±1.9), SB431542 (n = 45; total DAPI positive cells 6882; lysotracker positive cells = 210; average percent positive lysotracker = 2.9 ±2.1), and DMH1 (n=35; total DAPI positive cells = 4544; total lysotracker positive cells = 95; average percent positive lysotracker = 2.2 ±.5). C. The percentage of cells that stain positive for phosphorylated Histone 3 (P-His3) is unchanged in explants incubated with either vehicle or ALK 2, 3 or 5 small molecule inhibitors. The number of AVC explants examined (n), total number of DAPI and P-His positive cells, and average percent positive P-His cells ± SEM are listed. Vehicle (n = 38; total number of DAPI positive cells = 5130; P-His positive cells = 70; Average percent positive P-His = 1.1 ± 0.3); SB431542 (n = 48; total number of DAPI positive cells = 9636; P-His positive cells = 87; Average percent positive P-His = 0.9 ± 0.1) DMH1 (n = 33; total number of DAPI positive cells = 9548; P-His positive cells = 161; Average percent positive P-His = 1.7 ± 0.5). D. The effect of DMH1 on ventricular endocardial cell EMT. The average percent of total GFP-expressing cells are scored as epithelial, activated or transformed. Median percent ± SEM are depicted (n=3). Overexpression of full length TGFβR3 in ventricular endocardial cells along with the addition of 250pM TGFβ2 was used as a positive control for endocardial cell EMT. Expression of TGFβR3 in ventricular cells induced a statistically significant increase (50%) in transformed cells and a concomitant decrease in epithelial cells (44%) when compared to ventricular endocardial cells expressing GFP alone. The addition if the ALK2/3 inhibitor, DMH1, resulted in a significant decrease of 18% in the number of transformed cells. * = p<0.05. The total number of GFP positive cells and SEM are listed in table 2.
3.7 NF-κB activation is required for endocardial cell invasion
We previously identified NF-κB activity as a regulator of endocardial cell invasion [39]. Here we asked if NF-κB signaling was downstream of TGFβR3. Incubation of atrioventricular cushion explants with either 10µM BMS345541 or 2µM SN50 decreased cell invasion by 89% and 51% respectively when compared to vehicle-incubated explants (Fig. 4A). Incubation with 2.5 µM of the ALK5 kinase inhibitor SB431542 was used as a positive control and decreased the number of invaded cells by over 60%. Since NF-κB has a well-defined role in regulating cell proliferation [40–43] and apoptosis [43, 44] we examined cushion explants incubated with BMS or SN50 and measured changes in proliferation and cell death. There was a small, but significant change in cell death between vehicle and inhibitor incubated groups (Fig. 4B), but the modest increase noted (1% and 3%, respectively) cannot account for the magnitude of the decrease in cell invasion. No significant change in cell proliferation was identified (Fig. 4C). To determine if NFκB activity acts downstream of TGFβR3, TGFβR3-FL was co-expressed with GFP in ventricular endocardial cells and scored as described. Overexpression of TGFβR3-FL in ventricular endocardial cells in the presence of 250pM TGFβ2 resulted in increased invasion to 47% when compared to ventricular endocardial cells overexpressing GFP alone (Fig. 4D). Ventricular explants expressing TGFβR3-FL with the addition of TGFβ2 and either 10µM BMS345541 or 2µM SN50 resulted in a significant decrease in the number of invaded cells when compared to the addition of ligand alone, 32% and 35% respectively. These data show that NF-κB activity is required for TGFβR3-mediated cell invasion.
Fig. 4. NF-κB is required for TGFβR3–mediated endocardial EMT.
A: Incubation of AV cushion explants with two structurally different small molecule NFκB inhibitors, BMS-345541 (BMS) and SN50, decreased endocardial EMT in AVC by 89% and 51% respectively compared to vehicle. Specific ALK5 inhibitor, SB431542, is used as a positive control for small molecule inhibition of EMT. Data are derived from three independent experiments normalized explants incubated with vehicle. The number of AVC explants examined (n), total number of cells, and median number of transformed cells ± SEM (compared to cells incubated with vehicle) are listed. Vehicle (n=32, total transformed cells = 7538; normalized median to 100 ± 26%), SB431542 (n=37, total transformed cells = 3163; normalized median = 31.4 ±13%), BMS (n=34, total transformed cells = 748; normalized median = 10.8 ± 3.4%) SN50 (n=36, total transformed cells = 3978; normalized median = 48.5 ± 11.4%). B: Cell death is not significantly changed between explants incubated with small molecule inhibitors targeted to NF-κB pathway compared to those incubated with vehicle alone. In vehicle incubated explants and average of 4% of total cells were lysotracker-positive. Incubation with SB431542 resulted in no significant change in endocardial EMT. However, incubation with BMS or SN50 resulted in a 1.4% and 3% increase. The total number of AVC explants examined (n), total number of DAPI and lysotracker positive cells, and median percent positive lysotracker cells ± SEM are as follows: Vehicle (n = 33; total DAPI positive cells 6701; lysotracker positive cells = 250; median percent positive lysotracker = 3.4 ±0.6), SB431542 (n = 35; total DAPI positive cells 5098; lysotracker positive cells = 225; median percent positive lysotracker = 3.8 ±1.2), and BMS (n=28; total DAPI positive cells = 3258; total lysotracker positive cells = 132; median percent positive lysotracker = 4.8 ±0.9) SN50 (n=34; total DAPI positive cells = 4568; total lysotracker positive cells = 317; average percent positive lysotracker = 6.5 ±1.4). C: The percentage of cells that stain positive for phosphorylated Histone 3 (P-Histone 3) is unchanged between explants incubated with either vehicle or small molecule inhibitors targeted to ALK5 or the NFκB pathway. The number of AVC explants examined (n), total number of DAPI and P-His positive cells, and average percent positive P-His cells ± SEM are listed. Vehicle (n = 25; total number of DAPI positive cells = 7845; P-His positive cells = 152; Average percent positive P-His = 2.4.1 ± 0.5); SB431542 (n = 32; total number of DAPI positive cells = 8372; P-His positive cells = 216, Average percent positive P-His = 2.5 ± 0.6), BMS (n = 31; total number of DAPI positive cells = 5991; P-His positive cells = 177; Average percent positive P-His = 2.8 ± 0.5), SN50 (n=31; total DAPI positive cells = 8599; total lysotracker positive cells = 195; average percent positive lysotracker = 2.3 ±1). D: The effect of NF-κB inhibitors on ventricular endocardial cell EMT. The average percent of total GFP-expressing cells are scored as epithelial, activated or transformed. Median percent ± SEM are depicted (n=3). Overexpression of full length TGFβR3 in ventricular endocardial cells along with the addition of 250pM TGFβ2 was used as a positive control for endocardial cell EMT. Expression of TGFβR3 in ventricular cells induced a statistically significant increase 47±6% in transformed cells and a concomitant decrease in epithelial cells (44%) when compared to ventricular endocardial cells expressing GFP alone. The addition if the NFκB inhibitors, BMS and SN50, resulted in a significant decrease of 32±7% and 35±6% respectively of transformed cells. * = p<0.05.
3.8 TGFβR3-T841A stimulates ligand-independent invasion of endocardial cells
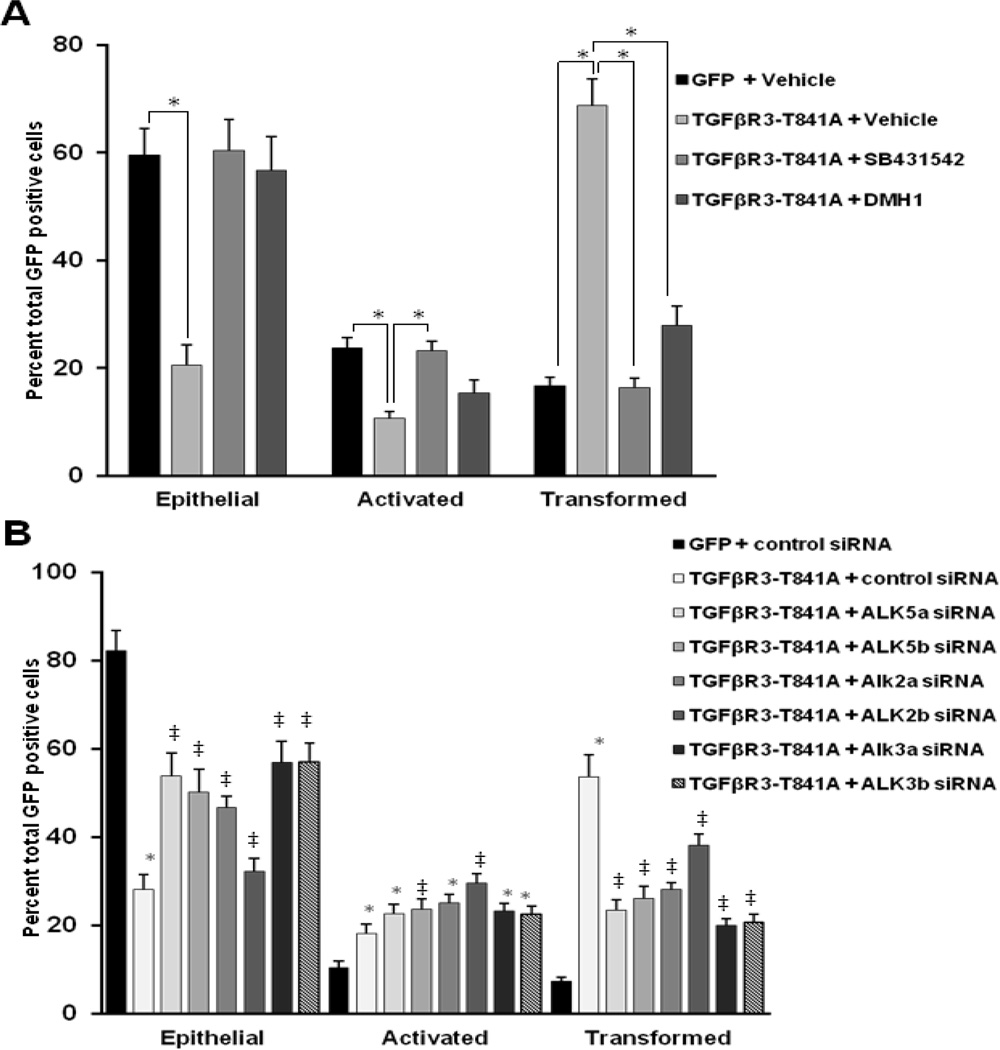
To determine if TGFβR3-T841A-stimulated ligand-independent cell invasion in endocardial cells, TGFβR3-T841A was co-overexpressed with GFP in ventricular endocardial cells and EMT was measured as described above. As seen in epicardial cells, expression of TGFβR3-T841A results in ligand-independent cell invasion of endocardial cells contained in ventricular explants. To determine if the ligand-independent, TGFβR3-T841A-stimulated cell invasion requires ALKs, we targeted ALK2, ALK3, or ALK5 with either small molecule kinase inhibitors or specific siRNA. The number of transformed or invaded endocardial cells expressing TGFβR3-T841A from explants incubated with the ALK5 kinase inhibitor, SB431542 (2.5µM), decreased by 50% compared to explants incubated with vehicle alone (Fig. 5A). Ventricular endocardial cells that overexpress TGFβR3-T841A incubated with 2µM DMH1 showed a 41% decrease in the number of transformed or invaded cells when compared to explants incubated with vehicle alone. As a second, independent approach to explore a role for ALK activity in ligand-independent cell transformation we used siRNA to target ALK2, ALK3, or ALK5 [20]. Ventricular explants were infected with adenovirus that overexpressed TGFβR3-T841A and scored as described [32]. The targeting of ALK5 resulted in an average decrease of 29% in transformed or invaded cells compared to endocardial cells overexpressing TGFβR3-T841A (Fig. 5B). Targeting of ALK2 or ALK3 with 2 independent siRNA constructs resulted in an average decrease of 21% and 34%, respectively when compared to control siRNA. These results support our findings above with DMH1 and our prior report that both ALK2 and ALK3 are required downstream of TGFβR3 [20]. Furthermore, since siRNA is specific for ALK2 or ALK3 whereas DMH1 inhibits both, these results demonstrate that both ALK2 and ALK3 are required for endocardial cell invasion.
Fig. 5. Ligand-independent receptor action requires ALK2 and ALK3 in endocardial cells.
The average percent of total GFP-expressing cells scored as epithelial, activated or transformed. Medians percent ± SEM are depicted (n=3). A. The effect of DMH1 on in ventricular endocardial cell EMT. Overexpression of TGFβR3-T841A in ventricular endocardial cells without the addition of TGFβ2 is used as a positive control for endocardial cell EMT. Expression of TGFβR3-T841A in ventricular cells induced a statistically significant increase (52 ± 3.5%) in transformed cells and a concomitant decrease in epithelial cells (50 ± 1.2%) when compared to ventricular endocardial cells expressing GFP alone. The addition of the ALK2/3 inhibitor, DMH1, resulted in a significant decrease of 41% of transformed cells. * = p<0.05. The total number of GFP positive cells and SEM are listed in table 1. B. ALK2 and ALK3 are required downstream of TGFβR3-T841A for endocardial EMT. Targeting of ALK5 by two independent constructs of siRNA was used as a positive control for siRNA-mediated inhibition of EMT Two independent siRNA’s separately targeting ALK2 or ALK3 resulted in of 21% and 34% decrease, respectively, in transformed cells when compared to ventricular explants incubated with GC content-matched control siRNA. The total number of GFP positive cells and SEM are listed in table 2. (* = p<.05 to GFP, ‡ = p <.05 to GFP and TGFβR3-T841 + control siRNA)
4.0 Discussion
Studies in cancer cell lines as well as epicardial and endocardial cells have identified TGFβR3 as a critical regulator of cell migration and invasion [10, 11, 45]. The cytoplasmic domain of TGFβR3 is particularly important in regulating these cell behaviors. The 3 C-terminal amino acids of TGFβR3 interact with GIPC; a scaffolding protein that stabilizes TGFβR3 at the cell surface to enhance TGFβ signaling [21]. Our previous studies have demonstrated that both the 3 C-terminal amino acids of TGFβR3 and GIPC are required for the invasion of epicardial and endocardial cells in vitro [11, 20]. A second protein-protein interaction on the cytoplasmic domain of TGFβR3 includes Thr841 which is phosphorylated by TGFβR2 to allow for βArr2 binding and the subsequent internalization of TGFβR3 [23]. The interaction of TGFβR3 and βArr2 has also been associated with two distinct activities: the activation of Cdc42 to regulate cell migration [19] and the inhibition of NF-κB activity [24]. Prior studies that interrogated the role of Cdc42 in epicardial [31] and endocardial [38] cell invasion failed to identify an effect of altered Cdc42 levels or activity on cell invasion. However, an examination of Tgfbr3−/− and Tgfbr3+/− mouse embryonic fibroblasts showed decreased NF-κB activity associated with Tgfbr3 loss when compared to Tgfbr3+/+ cells [34]. Since Tgfbr3−/− epicardial cells are impaired in the ability to invade a collagen matrix in vitro, we hypothesized that the loss of NF-κB activity underlies the deficit in cell invasive activity. Here we used both epicardial and endocardial cell invasion as tool to identify shared pathways downstream of TGFβR3.
Our findings in epicardial cells reveal that TGFβR3-dependent cell invasion is associated with activation of the NF-κB signaling pathway. Two distinct inhibitors of NF-κB signaling suppress cell invasion in either Tgfbr3−/− epicardial cells that overexpress TGFβR3-FL to rescue ligand-dependent invasion or Tgfbr3+/+ epicardial cells. Inhibition by NF-κB inhibitors was seen in response to not only TGFβ1 and TGFβ2 but also BMP2 and FGF2, ligands known to interact with TGFβR3 and signal TGFβR3-dependent invasion of epicardial cells [11, 28]. Each of these ligands was also found to activate NF-κB signaling as assessed by both phospho c-Rel translocation and NF-κB-luciferase activity. To further probe the mechanisms by which TGFβR3 signals cell invasion, we examined the effect of a single amino acid substitution in the cytoplasmic domain of TGFβR3, TGFβR3-T841A, which prevents βArr2 binding. TGFβR3-dependent regulation of NF-κB signaling has been shown to require βArr2 binding [34]. We hypothesized that since TGFβR3-dependent cell invasion requires NF-κB signaling, TGFβR3-T841A might stimulate ligand-independent epicardial cell invasion associated with NF-κB activation. Our results show that TGFβR3-T841A stimulated ligand-independent cell invasion is sensitive to NF-κB inhibitors. The finding that NF-κB activity regulates epicardial cell invasive behavior prompted us to ask if NF-κB activity is downstream of the ALKs known to regulate TGFβR3-dependent invasion in epicardial cells. Although we saw a modest effect from the NF-κB inhibitor, BMS345541, on invasion induced by caALK2, caALK3, or caALK5 activity, our data are most consistent with the conclusion that NF-κB activity is not required downstream of caALKs to induce invasion. To further probe the role of the interaction between ALK and NF-κB signaling in the regulation of cell invasion, we took advantage of our finding that TGFβR3-T841A confers ligand-independent invasion. We used small molecule inhibitors that target and confirm that ALK2, ALK3, and ALK5, known mediators downstream of TGFβR3, are required for the ligand-independent invasion associated with TGFβR3-T841A in epicardial cells.
We next used endocardial cell invasion as a second system in order to determine if the observations made in epicardial cells may be generalized to other cell types. We had previously used gene regulatory network analysis to identify and validate NF-κB signaling as a regulator of endocardial cell invasion [39]. Here we confirmed that ligand-dependent endocardial cell invasion is impaired in the presence of NF-κB inhibitors. These data indicate that this mechanism is operative in at least two cell types which provides broad support for a role for NF-κB signaling downstream of TGFβR3 in stimulating cell invasion. In endocardial cells, as in epicardial cells, TGFβR3-T841A stimulates ligand-independent cell invasion. We used a combination of small molecule inhibitors and siRNA to confirm that ALK2, ALK3, and ALK5, known mediators downstream of TGFβR3 [37], are required for ligand-independent invasion in endocardial cells. These results suggest that TGFβR3 activation results in a coordinated and concomitant stimulation of multiple ALKs to regulate cell invasion in both epicardial and endocardial cells. There is precedence for such a model of ALK activation. Endoglin, a TGFβ co-receptor with structure similar to TGFβR3, has been demonstrated to result in the coordinate activation of ALK5 and ALK1 to regulate endothelial cell proliferation and migration [46].
The mechanisms by which TGFβR3 might activate the NF-κB signaling pathway are not well understood. Our data using NF-κB inhibitors indicates that increased NF-κB activity is required for TGFβR3-dependent invasion in both epicardial and endocardial cells. Further, the introduction of TGFβR3-T841A into Tgfbr3−/− epicardial cells results in ligand-independent invasion that is dependent on NF-κB activity. Therefore, these results are consistent with a model where TGFβR3-T841A activates or allows NF-κB activity to increase which results in invasion. Recent studies have shown that TGFβR3-dependent epicardial cell invasion stimulated by HMW-HA or the overexpression of TGFβR3-T841A is dependent on Src activation [47] which provides a well-described mechanism for the alteration of NF-κB activity [reviewed in [48]] that may support cell invasion.
In summary, our studies sought to identify the common mechanisms of TGFβR3-dependent cell invasion in epicardial and endocardial cells. The introduction of TGFβR3-T841A into either cell type results in ligand independent cell invasion. Consistent with prior reports for ligand-stimulated, TGFβR3-dependent cell invasion, ligand-independent cell invasion associated with TGFβR3-T841A is also requires the activity of several ALKs. TGFβR3-dependent cell invasion is similarly dependent on NF-κB activity in both cell types. Overall, these data demonstrate that TGFβR3 in epicardial and endocardial cells activates ALKs and NF-κB signaling to initiate cell invasion. The identification of a mutant form of TGFβR3 that supports ligand independent cell invasion provides a useful tool for probing receptor function.
5.0 Conclusions
TGFβR3-mediated invasion in epicardial and endocardial cells shares a common signaling mechanism. Inhibition of NFκB or ALKs impairs ligand-dependent invasion in both epicardial and endocardial cells. A threonine to alanine point mutation at residue 841 in TGFβR3 resulted in ligand independent cell invasion in both cell types.
TGFβR3-T841A mediated, ligand-independent invasion of epicardial and endocardial cells required activation of ALK 2, 3, and 5.
Fig. 6. A model for the common mechanisms downstream of TGFβR3-mediated epicardial and endocardial cell invasion.
Highlights.
A TGFβR3 mutant (TGFβR3-T841A) initiates ligand-independent invasion
Epicardial and endocardial cell invasion requires activation of the NF-κB pathway
ALKs mediate both ligand-dependent and ligand-independent invasion
NFκB and ALK pathways may interact but both are independently required for invasion
Acknowledgments
The authors thank members of the Barnett laboratory and Dr. Chris Brown for helpful discussions and comments. Overall project support by National Institutes of Health, HL085708 (CRH, NSS, JVB), an initiative of the Roadmap for Medical Research/Common Fund, Systems-based Consortium for Organ Design and Engineering, NIH U54 092551 (JTR, TAT, JVB), and an American Heart Association Grant in Aid, AHA16690001 (DZT, JVB). J.A.A. was supported by R25 HL96223 short term training for minority students. JYR was supported by GM062459. J.V.B. acknowledges the support of the Vanderbilt-Ingram Cancer Center.
ABBREVIATIONS
- βArr2
Beta-arrestin2
- ALK
Activin Receptor-Like Kinases
- AV
Atrioventricular
- BMP
Bone Morphogenetic Protein
- ca
constitutively active
- EMT
Epithelial-Mesenchymal Transformation
- FGF
Fibroblast Growth Factor
- GFP
Green Fluorescent Protein
- GIPC
GAIP-interacting protein, C terminus
- IκB
Inhibitor of kappa B protein
- IKK
I kappa B kinase
- NF-κB
Nuclear Factor- kappa B
- SMαA
Smooth Muscle alpha Actin
- TGFβ
Transforming Growth Factor Beta
- TGFβR1
Type I TGFβ receptor
- TGFβR2
Type II TGFβ receptor
- TGFβR3
Type III TGFβ receptor
- TGFβR3-FL
Type III TGFβ receptor-full length
- TGFβR3-CYTO
Type III TGFβ receptor-lacking the entire cytoplasmic domain
- TGFβR3- Δ3
Type III TGFβ receptor-lacking the 3 C-terminal amino acids
- TGFβR3-T841A
Type III TGFβ receptor, threonine to alanine point mutation at residue 841
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cynthia R. Clark, Email: cynthia.r.allison@vanderbilt.edu.
Jamille Y. Robinson, Email: jamille.robinson@vanderbilt.edu.
Nora S. Sanchez, Email: NSSanchez@mdanderson.org.
Todd A. Townsend, Email: ttownsend@genetics.utah.edu.
Julian A. Arrieta, Email: julian.a.arrieta@Vanderbilt.edu.
W. David Merryman, Email: david.merryman@vanderbilt.edu.
David Z. Trykall, Email: david.trykall@vanderbilt.edu.
Harold E. Olivey, Email: holivey@iun.edu.
Charles C. Hong, Email: charles.c.hong@vanderbilt.edu.
Joey V. Barnett, Email: joey.barnett@vanderbilt.edu.
References
- 1.Doetschman T, et al. Transforming growth factor beta signaling in adult cardiovascular diseases and repair. Cell Tissue Res. 2012;347(1):203–223. doi: 10.1007/s00441-011-1241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106(11):1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 3.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51(4):600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29(5):196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly EC, et al. Outgrowth of drug-resistant carcinomas expressing markers of tumor aggression after long-term TbetaRI/II kinase inhibition with LY2109761. Cancer Res. 2011;71(6):2339–2349. doi: 10.1158/0008-5472.CAN-10-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-beta targeted cancer therapy. Int J Biol Sci. 2012;8(7):964–978. doi: 10.7150/ijbs.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrot CY, Javelaud D, Mauviel A. Overlapping activities of TGF-beta and Hedgehog signaling in cancer: therapeutic targets for cancer treatment. Pharmacol Ther. 2013;137(2):183–199. doi: 10.1016/j.pharmthera.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors. 2011;29(4):140–152. doi: 10.3109/08977194.2011.595411. [DOI] [PubMed] [Google Scholar]
- 9.Compton LA, et al. Coronary vessel development is dependent on the type III transforming growth factor beta receptor. Circ Res. 2007;101(8):784–791. doi: 10.1161/CIRCRESAHA.107.152082. [DOI] [PubMed] [Google Scholar]
- 10.Brown CB, et al. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283(5410):2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez NS, et al. The cytoplasmic domain of TGFbetaR3 through its interaction with the scaffolding protein, GIPC, directs epicardial cell behavior. Dev Biol. 2011;358(2):331–343. doi: 10.1016/j.ydbio.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Casillas F, et al. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67(4):785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 13.Wang XF, et al. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991;67(4):797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Casillas F, Wrana JL, Massague J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73(7):1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- 15.Kirkbride KC, et al. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem. 2008;283(12):7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- 16.Wiater E, et al. Identification of distinct inhibin and transforming growth factor beta-binding sites on betaglycan: functional separation of betaglycan co-receptor actions. The Journal of biological chemistry. 2006;281(25):17011–17022. doi: 10.1074/jbc.M601459200. [DOI] [PubMed] [Google Scholar]
- 17.Kretzschmar M, Massague J. SMADs: mediators and regulators of TGF-beta signaling. Current opinion in genetics & development. 1998;8(1):103–111. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- 18.Lee NY, et al. The transforming growth factor-beta type III receptor mediates distinct subcellular trafficking and downstream signaling of activin-like kinase (ALK)3 and ALK6 receptors. Molecular biology of the cell. 2009;20(20):4362–4370. doi: 10.1091/mbc.E09-07-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mythreye K, Blobe GC. The type III TGF-beta receptor regulates epithelial and cancer cell migration through beta-arrestin2-mediated activation of Cdc42. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(20):8221–8226. doi: 10.1073/pnas.0812879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend TA, et al. Endocardial cell epithelial-mesenchymal transformation requires Type III TGFbeta receptor interaction with GIPC. Cell Signal. 2012;24(1):247–256. doi: 10.1016/j.cellsig.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blobe GC, et al. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. The Journal of biological chemistry. 2001;276(43):39608–39617. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- 22.Lee JD, et al. The type III TGF-beta receptor suppresses breast cancer progression through GIPC-mediated inhibition of TGF-beta signaling. Carcinogenesis. 2010;31(2):175–183. doi: 10.1093/carcin/bgp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, et al. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301(5638):1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 24.You HJ, How T, Blobe GC. The type III transforming growth factor-beta receptor negatively regulates nuclear factor kappa B signaling through its interaction with beta-arrestin2. Carcinogenesis. 2009;30(8):1281–1287. doi: 10.1093/carcin/bgp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin AF, et al. Primary and immortalized mouse epicardial cells undergo differentiation in response to TGFbeta. Dev Dyn. 2008;237(2):366–376. doi: 10.1002/dvdy.21421. [DOI] [PubMed] [Google Scholar]
- 26.He TC, et al. A simplified system for generating recombinant adenoviruses. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(5):2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craig EA, et al. TGFbeta2-mediated production of hyaluronan is important for the induction of epicardial cell differentiation and invasion. Exp Cell Res. 2010;316(20):3397–3405. doi: 10.1016/j.yexcr.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill CR, et al. BMP2 signals loss of epithelial character in epicardial cells but requires the Type III TGFbeta receptor to promote invasion. Cell Signal. 2012;24(5):1012–1022. doi: 10.1016/j.cellsig.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernanke DH, Markwald RR. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Developmental biology. 1982;91(2):235–245. doi: 10.1016/0012-1606(82)90030-6. [DOI] [PubMed] [Google Scholar]
- 30.Mercado-Pimentel ME, Hubbard AD, Runyan RB. Endoglin and Alk5 regulate epithelial-mesenchymal transformation during cardiac valve formation. Developmental biology. 2007;304(1):420–432. doi: 10.1016/j.ydbio.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez NS, Barnett JV. TGFbeta and BMP-2 regulate epicardial cell invasion via TGFbetaR3 activation of the Par6/Smurf1/RhoA pathway. Cell Signal. 2012;24(2):539–548. doi: 10.1016/j.cellsig.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desgrosellier JS, et al. Activin receptor-like kinase 2 and Smad6 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2005;280(1):201–210. doi: 10.1016/j.ydbio.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Hao J, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS chemical biology. 2010;5(2):245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Criswell TL, et al. Knockdown of the transforming growth factor-beta type III receptor impairs motility and invasion of metastatic cancer cells. Cancer Res. 2008;68(18):7304–7312. doi: 10.1158/0008-5472.CAN-07-6777. [DOI] [PubMed] [Google Scholar]
- 35.Attisano L, et al. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993;75(4):671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- 36.Lencinas A, et al. Collagen gel analysis of epithelial-mesenchymal transition in the embryo heart: an in vitro model system for the analysis of tissue interaction, signal transduction, and environmental effects. Birth Defects Res C Embryo Today. 2011;93(4):298–311. doi: 10.1002/bdrc.20222. [DOI] [PubMed] [Google Scholar]
- 37.Townsend TA, et al. BMP-2 and TGFbeta2 shared pathways regulate endocardial cell transformation. Cells Tissues Organs. 2011;194(1):1–12. doi: 10.1159/000322035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Townsend TA, et al. Transforming growth factor-beta-stimulated endocardial cell transformation is dependent on Par6c regulation of RhoA. J Biol Chem. 2008;283(20):13834–13841. doi: 10.1074/jbc.M710607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeLaughter DM, et al. Spatial transcriptional profile of the chick and mouse endocardial cushions identify novel regulators of endocardial EMT in vitro. J Mol Cell Cardiol. 2013;59:196–204. doi: 10.1016/j.yjmcc.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brantley DM, et al. Nuclear factor-kappaB (NF-kappaB) regulates proliferation and branching in mouse mammary epithelium. Molecular biology of the cell. 2001;12(5):1445–1455. doi: 10.1091/mbc.12.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim NS, et al. Receptor activator of NF-kappaB ligand regulates the proliferation of mammary epithelial cells via Id2. Molecular and cellular biology. 2006;26(3):1002–1013. doi: 10.1128/MCB.26.3.1002-1013.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu A, et al. NF-kappaB negatively impacts the myogenic potential of muscle-derived stem cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20(3):661–668. doi: 10.1038/mt.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Albertini M, et al. Extracellular ATP and ADP activate transcription factor NF-kappa B and induce endothelial cell apoptosis. Biochemical and biophysical research communications. 1998;248(3):822–829. doi: 10.1006/bbrc.1998.9055. [DOI] [PubMed] [Google Scholar]
- 44.Kitajima I, et al. Induction of apoptosis in murine clonal osteoblasts expressed by human T-cell leukemia virus type I tax by NF-kappa B and TNF-alpha. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1996;11(2):200–210. doi: 10.1002/jbmr.5650110209. [DOI] [PubMed] [Google Scholar]
- 45.Mythreye K, Blobe GC. The type III TGFbeta receptor regulates directional migration: new tricks for an old dog. Cell cycle. 2009;8(19):3069–3070. doi: 10.4161/cc.8.19.9419. [DOI] [PubMed] [Google Scholar]
- 46.Lebrin F, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. The EMBO journal. 2004;23(20):4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allison P, et al. Type III TGFbeta receptor and Src direct hyaluronan-mediated invasive cell motility. Cell Signal. 2014;27(3):453–459. doi: 10.1016/j.cellsig.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korbecki J, et al. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2013;64(4):409–421. [PubMed] [Google Scholar]