Positron emission tomography (PET) imaging is a functional medical imaging technique that provides information about how tissues and organs are working at the physiological and biochemical level. PET works by injecting a patient or animal with a radiotracer (a biologically active molecule tagged with positron-emitting radionuclide) and detecting pairs of γ rays resulting from annihilation of the positron emitted by the radiotracer. PET has been used to study, diagnose, and stage diseases in patients, and to support drug discovery programs.1 Because of its excellent imaging properties and ready availability from small-medical cyclotrons, fluorine-18 (18F) is one of the most commonly used PET radionuclides. However, working with radioactive 18F presents unique challenges to PET radiochemists. Most notably, (i) the half-life of 18F is 110 min, which means that the radionuclide needs to be made on demand and used immediately; and (ii) the high levels of radioactivity involved in patient-scale PET tracer syntheses necessitate fully automated synthesis and purification procedures (i.e., all operations are controlled by a computer and not by hand). Due to these requirements, scalable radiofluorination processes must involve the incorporation of 18F at a late stage of the tracer synthesis, with short reaction times (usually ≤30 min), and using operationally simple procedures. These constraints, in combination with limitations imposed by traditional reactions using fluorine-18, mean that certain bioactive molecules have historically proven extremely problematic to radiolabel.2

Reflecting these difficulties, the development of practical methods for the late-stage incorporation of fluorine-18 is of enormous current significance. An exciting emerging approach involves the development of transition-metal-mediated nucleophilic radiofluorinations (see ref (2), and references therein). Several such transformations have been used to radiofluorinate model arene substrates, and a few of these have been applied to the automated synthesis of radiotracers.3 However, compliance with the principles of current Good Manufacturing Practice (cGMP) is a necessary condition before these methods can be translated to the production of PET radiotracers for human clinical use. These regulations ensure proper design, monitoring, and control of manufacturing processes and facilities, and ultimately validate the identity, strength, quality, and purity of drug products. In a recent article published in Organometallics,4 Hooker, Ritter, and colleagues have addressed this hurdle to clinical translation by adapting a Ni-mediated 18F-fluorination process to comply with the cGMP regulations described in 21CFR212 and mandated by the U.S. FDA for PET radiotracer production (see 21CFR212 for more information; accessed 3-Mar-2016).
Molecules can be labeled with fluorine-18 using either electrophilic methods (with [18F]F2) or nucleophilic methods (with 18F–). However, because [18F]F2 gas must be mixed with [19F]F2 carrier gas, the 18F/19F ratio (known as specific activity) of the resulting radiotracer ends up significantly lower than that of tracers that arise from 18F–. For this reason, as well as the relative simplicity of handling aqueous fluoride over F2, nucleophilic fluorination reactions are preferred over their electrophilic counterparts. However, historically, certain radiotracers could only be prepared using electrophilic methods because of limitations in the chemistry of 18F–. As such, for decades, the PET radiochemistry community has been intrigued by new strategies for expanding the reactivity of [18F]fluoride. The use of transition metals to promote the key carbon–fluorine bond forming step with 18F– is a particularly attractive approach, as transition-metal catalysis often enables new reactivity that is challenging (or impossible) using traditional organic transformations. However, until very recently, few robust transition-metal-mediated fluorination reactions were available to bring this concept to fruition.
The recent discovery of new carbon–fluorine bond-forming reactions using high oxidation state copper(III) and palladium(IV) has dramatically changed the landscape in this area.5 A seminal 2011 report by Hooker and Ritter demonstrated the translation of a palladium(IV)-mediated 19F-fluorination to a radiofluorination of arene substrates.3a However, it quickly became apparent that this transformation was not compatible with the strenuous demands of routine clinical PET radiotracer production under cGMP. The authors have commented on such difficulties in translation,3c and these limitations have inhibited widespread adoption by the radiochemistry community.
Spurred by Hooker and Ritter’s initial work, the Sanford group developed copper(III)-mediated 19F– fluorinations to get around the toxicity and cost of palladium.6 Since then, Scott and Sanford have developed automated syntheses of PET radiotracers using these methods,3d,3g while related approaches have also been reported by Gouverneur.3e Meanwhile, Hooker and Ritter turned their attention to the [18F]fluorination of arylnickel complexes,3b optimizing the reactions to synthesize radiotracers for animal imaging studies.3f
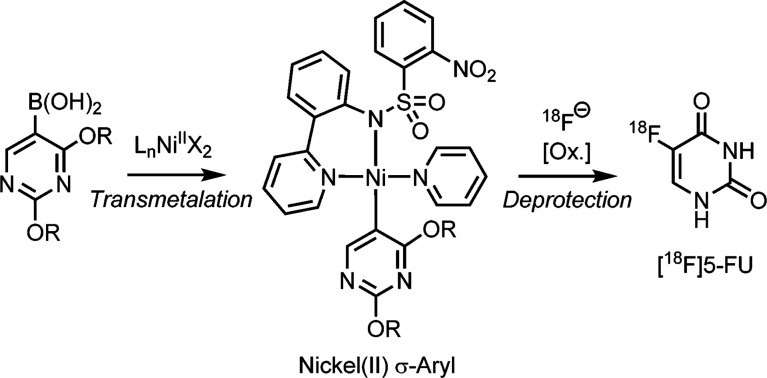
A key next step for all of these methods is to bring them into compliance with cGMP regulations so that they can be used for the synthesis of radiotracer doses for human use. Conducting cGMP validation of 18F-fluorination of nickel complexes for the synthesis of clinical doses of [18F]5-fluorouracil ([18F]5-FU, Figure 1) is the subject of the most recent paper from the Hooker and Ritter laboratories.4 In the United States, PET radiotracers for use in patients must be synthesized according to the regulations laid out in 21CFR212. While Hooker and Ritter’s report does not address all of 21CFR212’s extensive regulations (which include stipulations ranging from personnel to quality assurance, as well as how the vials or syringes containing PET radiotracers are labeled and distributed), the paper does focus on key cGMP production and process controls.
Figure 1.
Hooker and Ritter’s strategy for the synthesis of [18F]5-fluorouracil ([18F]5-FU) for human PET imaging.4
[18F]5-FU, first reported by Fowler and co-workers in 1973,7 has been used in cancer PET imaging for over
40 years. Historically it has been prepared by electrophilic fluorination
using [18F]F2, leading to only modest yields
and low specific activities. It was therefore an obvious choice with
which to challenge Hooker and Ritter’s methodology. Their team
first focused on developing an efficient and practical method for
synthesizing the key nickel precursor to be reacted with fluorine-18.
This was accomplished by converting organoboron reagents to the corresponding
nickel reagent using complex 1.
With the precursor in hand, they turned their attention to the radiofluorination reaction (Figure 1). The reaction proceeded under aqueous conditions using 18F– and an iodine(III) oxidant. While the overall yield of this reaction is modest (0.92% radiochemical yield), this represents the first synthesis of [18F]5-FU using nucleophilic [18F]fluoride. Furthermore, the amounts of product obtained are enough for clinical imaging studies. The doses prepared by this method passed all cGMP quality control testing. Most notably, residual nickel levels were within the range of acceptable residual metal impurities in pharmaceutical products (see: ICH Guideline Q3D for more information; accessed 3-Mar-2016).
Collectively, all of the transition-metal-mediated radiofluorination reactions discussed herein are exciting developments in radiochemistry that greatly expand the range of reactions that can be conducted using high specific activity nucleophilic 18F–. They should enable the synthesis of previously inaccessible PET radiotracers, and allow the community to revisit promising but underutilized radiotracers. Qualifying the first of these for human imaging is an important step toward widespread adoption by the PET radiotracer manufacturing community. It is expected that similar process validations will soon follow for many of the other new methods described herein.
Notes
Updated March 30, 2016, to add applicable hyperlinks for cGMP regulations.
References
- For a general review of PET imaging, see:Ametamey S. M.; Honer M.; Schubiger P. A. Molecular Imaging with PET. Chem. Rev. 2008, 108, 1501–1516. 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- Brooks A. F.; Topczewski J. J.; Ichiishi N.; Sanford M. S.; Scott P. J. H. Late-stage [18F]Fluorination: New Solutions to Old Problems. Chem. Sci. 2014, 5, 4545–4553. 10.1039/C4SC02099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lee E.; Kamlet A. S.; Powers D. C.; Neumann C. N.; Boursalian G. B.; Furuya T.; Choi D. C.; Hooker J. M.; Ritter T. A Fluoride-Derived Electrophilic Late-Stage Fluorination Reagent for PET Imaging. Science 2011, 334, 639–642. 10.1126/science.1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lee E.; Hooker J. M.; Ritter T. Nickel-Mediated Oxidative Fluorination for PET with Aqueous [18F] Fluoride. J. Am. Chem. Soc. 2012, 134, 17456–17458. 10.1021/ja3084797. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Kamlet A. S.; Neumann C. N.; Lee E.; Carlin S. M.; Moseley C. K.; Stephenson N.; Hooker J. M.; Ritter T. Application of Palladium-Mediated 18F-Fluorination to PET Radiotracer Development: Overcoming Hurdles to Translation. PLoS One 2013, 8, e59187. 10.1371/journal.pone.0059187. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ichiishi N.; Brooks A. F.; Topczewski J. J.; Rodnick M. E.; Sanford M. S.; Scott P. J. H. Copper-Catalyzed [18F]Fluorination of (Mesityl) (aryl)iodonium Salts. Org. Lett. 2014, 16, 3224–3227. 10.1021/ol501243g. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Tredwell M.; Preshlock S. M.; Taylor N. J.; Gruber S.; Huiban M.; Passchier J.; Mercier J.; Geńicot C.; Gouverneur V. A General Copper-Mediated Nucleophilic 18F Fluorination of Arenes. Angew. Chem., Int. Ed. 2014, 53, 7751–7755. 10.1002/anie.201404436. [DOI] [PubMed] [Google Scholar]; f Ren H.; Wey H.-Y.; Strebl M.; Neelamegam R.; Ritter T.; Hooker J. M. Synthesis and Imaging Validation of [18F]MDL100907 Enabled by Ni-Mediated Fluorination. ACS Chem. Neurosci. 2014, 5, 611–615. 10.1021/cn500078e. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Mossine A. V.; Brooks A. F.; Makaravage K. J.; Miller J. M.; Ichiishi N.; Sanford M. S.; Scott P. J. H. Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org. Lett. 2015, 17, 5780–5783. 10.1021/acs.orglett.5b02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover A. J.; Lazari M.; Ren H.; Narayanam M. K.; Murphy J. M.; Van Dam R. M.; Hooker J. M.; Ritter T. A Transmetalation Reaction Enables the Synthesis of [18F]5-Fluorouracil from [18F]Fluoride for Human PET Imaging. Organometallics 2016, 10.1021/acs.organomet.6b00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman A. J.; Sanford M. S. High-valent Organometallic Copper and Palladium in Catalysis. Nature 2012, 484, 177–185. 10.1038/nature11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiishi N.; Canty A. J.; Yates B. F.; Sanford M. S. Cu-Catalyzed Fluorination of Diaryliodonium Salts with KF. Org. Lett. 2013, 15, 5134–5137. 10.1021/ol4025716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J. S.; Finn R. D.; Lambrecht R. M.; Wolf A. P. The Synthesis of 18F-5-Fluorouracil. J. Nucl. Med. 1973, 14, 63–64. [PubMed] [Google Scholar]