Abstract
Background
Breast cancer (BC) is one of the most common cancers and is among the main causes of death in females around the world. Although several serum biomarkers have been identified for breast cancer, due to lack of adequate sensitivity and specificity they do not adequately distinguish BC from confounding conditions. New approaches are urgently needed to improve BC detection and treatment.
Material/Methods
Eighty serum samples from 20 healthy individuals and 60 patients with BC (22 triple-negative breast cancer, TNBC; 38 non-triple-negative breast cancer, NTNBC) were included. Protein profiling of serum samples was analyzed using surface-enhanced laser desorption/ionization time-of-flight mass spectroscopy (SELDI-TOF-MS). Candidate biomarkers were purified by SDS-PAGE electrophoresis and identified by MALDI-TOF/TOF.
Results
The candidate biomarker positioned at 6447.9 m/z was significantly decreased in BC patients. Moreover, the expression intensity of the candidate biomarker was weaker in the TNBC and pre-surgery group compared with the NTNBC and post-surgery group. We ultimately identified the biomarker as apolipoprotein C-I (ApoC-I). Furthermore, we found that ApoC-I peptides inhibited proliferation of human breast cancer cells in vitro and suppressed tumor growth in vivo.
Conclusions
These results suggest that ApoC-I peptides may be a potential diagnostic biomarker and therapeutic approach for BC.
MeSH Keywords: Biomarkers, Pharmacological; Cell Proliferation; Peptides
Background
Breast cancer is one of the most common female malignant tumors and is the leading cause of cancer death among women worldwide. The clinical diagnostic methods for breast cancer were integrated, relying on physical examinations, imaging mammography and ultrasound, and histopathology [1]. Early detection and treatment would increase long-term survival of patients with breast cancer. Some proteins and peptides have been identified as breast cancer biomarkers in nipple aspirate fluid [2], breast tumor tissue [3], and serum [4]. However, none of the existing serum biomarkers, such as CA125, CA 13-5, or CEA, can be used individually for screening because they lack sufficient sensitivity and specificity to be applicable in detecting early-stage cancer in a large population [5,6]. Therefore, new sensitive and minimally invasive biomarkers are still urgently needed to improve breast cancer detection rates.
The plasma level of lipoprotein (a) was significantly increased in confirmed cases of breast cancer [7]. The human mature apolipoprotein E (ApoE), a ligand for the low-density lipoprotein receptor, is involved in lipid metabolism and is synthesized in most tissues of the human body [8]. Chen et al. demonstrated that ApoE levels were elevated in various malignancies, including breast cancer, and may be a defense mechanism against tumors [9]. Bcl-2 plays an important role in the process of cell apoptosis and survival as well as in epithelial differentiation, morphogenesis, and tumorigenesis. The Bax gene belongs to the Bcl-2 family and induces apoptosis [10]. Apoptosis-related genes Bcl-2 and Bax in breast cancer tissues are associated with the tumorigenesis and tumor progression of breast cancer [11]. The Ki67 proliferation-related antigen is detectable in cells during all phases of the cell cycle, except G0. Expression levels of Ki67 and proliferating cell nuclear antigen (PCNA) are significantly decreased in breast cancer cell lines and tumor tissues of patients with breast cancer [12,13].
The proteome is a complete map of all proteins and peptides expressed by a genome. Quantitative proteomics can be used for the identification of abnormally expressed proteins or peptides as potential candidate biomarkers for cancers [14]. A proteomics approach that combines other technologies will contribute to the identification of potential biomarkers with high sensitivity and high specificity. Nowadays, both surfaced-enhanced laser desorption/ionization time-of-flight mass spectroscopy (SELDI-TOF-MS) and matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF-MS) are used for detecting abnormal proteins in circulating serum or plasma of patients with cancer [15,16]. Serum protein or peptide profiling was conducted using a combination of SELDI-TOF-MS and ProteinChip array for identification of diagnostic biomarkers for early detection, prognostic biomarkers for estimation of disease outcome, predictive biomarkers for adjuvant treatment stratification, and surveillance biomarkers for disease monitoring and treatment response in patients [17,18].
In the present work, we discovered a new serum biomarker for BC using a SELDI-TOF-MS protein assay. We also investigated the in vitro and in vivo effects of ApoC-I peptides on cell proliferation, apoptosis, and tumor growth to assess its potential prognostic and therapeutic value.
Material and Methods
Serum samples
These experiments were approved by the Ethics Review Board of the First Affiliated Hospital of Zhengzhou University. Tumor tissues and matched serum samples were collected after obtaining written informed consent from each participant. Blood samples were obtained from 60 BC patients and 20 healthy volunteers in the First Affiliated Hospital of Zhengzhou University between January 2010 and June 2014. The median age of patients was 44.6 years (age range: 22–69 years). None of the patients suffered from any other diseases that affected the serum protein content. All patients received modified radical mastectomy and standard post-surgery adjuvant therapy after being diagnosed with breast cancer. Fasting peripheral venous blood was collected from the patients. Approximately 5 mL of blood was kept at 4°C for 1–2 h and then centrifuged at 3000 rpm for 5 min, followed by another 5 min at 12 000 rpm to allow the cells to form a sediment. Serum was extracted and preserved at −80°C until use. Serum samples were only thawed once.
Serum protein profiles
Serum protein profiling was measured through SELDI-TOF-MS protein assay using the weak cation exchange (WCX2) protein chip arrays (Ciphergen® Biosystems Inc., Fremont, CA). Frozen-thawed serum samples were centrifuged at 10 000 rpm at 4°C for 5 min. Serum samples were denatured in the presence of U9 lysis buffer (9 mol/L urea, 2% CHAPS, 50 mmol/L Tris-HCl, 1% dithiothreitol, pH 9.0; Bio-Rad) and then mixed with WCX-2 (NaAC, pH 4.0) buffer. The sample was then incubated with WCX-2-pretreated magnetic beads for 1 h at room temperature. Next, the beads were gently washed twice using NaAC and eluted 3 times with 1% trifluoroacetic acid (TFA). The elution solution was lyophilized and prepared for SELDI-TOF-MS (BioRad, Hercules, CA) according to the manufacturer’s protocols. We edited the reading operating procedures as follows: the molecular weight range was 2–20 kDa, and the maximum molecular weight was 30 kDa. To acquire the analysis results of low molecular weight polypeptides or proteins, we set the range of optimized detection mass at 2–20 kDa.
Purifying and identifying specific protein peaks
Candidate protein markers were purified by SDS-PAGE electrophoresis and identified by MALDI-TOF/TOF. Blood samples from healthy individuals were subjected to SDS-PAGE electrophoresis. The protein spots were cut from gels and digested by trypsin. The purified peptides were further analyzed using MALDI-TOF/TOF. Combination of high sequence coverage and accurate molecular weight (MW) measurement using MALDI-TOF-MS provided the entire sequence of the candidate protein marker. The obtained peptide sequence was searched in SWISS PROT database with Mascot software (Matrix Science Ltd., London, UK).
Cell culture and treatment
Human breast cancer cell lines MCF-7 and MDA-MB-231 were obtained from the Cell Bank of Shanghai Institute of Cell Biology, Chinese Academy of Sciences and maintained in DMEM media (Gibco, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco, Invitrogen), 50 units/mL penicillin (Gibco, Invitrogen), and 50 μg/mL streptomycin (Gibco, Invitrogen). All cells were cultured at 37°C in a humidity-controlled atmosphere with 5% CO2.
ApoC-I peptides with or without an N-terminally conjugated fluorescein isothiocyanate (FITC) were synthesized by GL Biochem Ltd (Shanghai, China). FITC-conjugated peptides were diluted to 100 mM in PBS, and then medium (95 mL) and a peptide/PBS solution (5 mL) were gently mixed.
Fluorescence analysis
Cells were seeded on coverslips (Fisher, Pittsburgh, PA) in 24-well plates. After 24 h, cells were incubated with FITC-conjugated peptides at 37°C for 1 h. The cells were then washed twice with PBS and fixed with 4% (weight/volume) paraformaldehyde for 15 min, permeabilized with 0.5% (volume/volume) Triton-X-100 for 5 min, and then blocked in 5% goat serum (Sigma, St Louis, MO). The cells were incubated with anti-Tubulin rabbit antibody and then with goat anti-rabbit Alexa Fluor 594 secondary antibody. Nuclei were stained with 1 mg/mL of 4′, 6-diamidino-2-phenylindole (DAPI, Sigma) for 2 min. The cellular distribution of the FITC-peptides was assessed using a fluorescence microscope (Olympus, Tokyo, Japan).
Cell proliferation assay
Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Rockville, MD) according to the manufacturer’s instructions. MCF-7 and MDA-MB-231 cells were seeded in 96-well plates at a density of 4×103 cells/well. On the next day, cells were incubated with Apo C-I peptides (0, 0.5, 1.0, 1.5, 2.0 μg/μL) at 37°C for 24, 48, and 72 h. At the end of incubation, CCK-8 mixture (10 μL) was added to each well, and the plates were incubated at 37°C for 1–1.5 h. The absorbance at 450 nm was measured in a spectrophotometer (Aquamate Plus UV-Vis, Thermo Scientific), which showed mitochondrial activity and indirectly indicated cell viability. Each experiment was conducted at least 3 times independently. Inhibition ratio=(ODControl – ODTreatment)/(ODControl – ODBlank) ×100%.
Animal models
MCF-7 cell pellets were resuspended in 0.5 mL of PBS and mixed with 0.5 mL Matrigel (BD Biosciences, San Jose, CA). MCF-7 cells (0.5×107 cells in 0.2 mL PBS) were injected subcutaneously into each flank of 4–5-week-old BALB/c female nude mice. Tumor growth rate was monitored by measuring tumor diameters every 4 days and the tumor growth curve was recorded accordingly. Tumors were allowed to grow for 2 weeks. Mice with equally sized tumors (150 mm3) were randomly divided into 2 groups: the control group (n=8) and the experimental group (n=8). Peptides dissolved in PBS were injected intravenously via the tail vein into mice every day for 5 days at the dose of 100 mg/kg. Control group mice were injected only with sterile PBS. Mice were euthanized after treatment for an additional 7 days and tumors were separated, measured, weighed, and photographed. Both maximum (L) and minimum (W) dimensions of the tumors were measured using a digital caliper, and the tumor volume was calculated as ½ LW2. All animal procedures were approved by the Institutional Animal Care and Use Committee of Zhengzhou University Health Sciences Center.
Immunohistochemistry
The specimens were embedded in paraffin and cut into 5-μm sections. The sections were deparaffinized and incubated for 10 min with PBS containing 3% H2O2 to block endogenous peroxidase activity. Heat-induced antigen heat retrieval was carried out for 10 min in a citrate buffer (pH 6.0) at 100°C. Sections were blocked for 1 h with 5% normal goat serum in PBS at room temperature and then incubated with anti-PCNA, anti-Bax, anti-Bcl-2, and anti-Ki67 antibodies overnight at 4°C. The next day, sections were incubated with their respective secondary antibodies for 1 h at room temperature. The peroxidase label was determined with diaminobenzidine hydrochloride (DAB, Sigma). Negative controls were incubated with pre-immune serum instead of the primary antibodies. Slides were counter-stained with hematoxylin and mounted with Permount.
Statistical analysis
The unpaired Student’s t-test was used to analyze differences between 2 groups. ANOVA was used to compare the means of 3 or more groups. Data are expressed as means ±SD. A value of P<0.05 was considered to indicate a statistically significant difference. All data analyses were conducted with SPSS version 17.0 (SPSS Inc., Chicago, IL).
Results
Serum protein profiles and data processing
The preprocessed serum was determined using SELDI-TOF-MS with the WCX2 protein chip. All of the MS analysis results were baseline-subtracted and normalized with overall ion current. The intensities of cluster peaks were identified by using Biomarker Wizard software. Comparisons between relative signal strength of proteins among 2 groups were performed using Wilcoxon rank sum tests. Fourteen peaks (P<0.01) were obtained after analyzing the pre-surgery and the healthy control group. Eleven of them were over-expressed in the pre-surgery group (data not shown). Six peaks (P<0.01) were obtained after analyzing the pre- and post-surgery group. Four of them were over-expressed in the pre-surgery group (data not shown). Nine peaks (P<0.01) were obtained after analyzing the TNBC and NTNBC group. Four of them were expressed at low levels in the TNBC group (data not shown). The random combination of obtained peaks with statistically significant variation assisted the support vector machine (SVM) to screen out the combined models with optimal Youden index of the predictive value, screening out a biomarker at 6447.9 m/z. The results of serum protein profiles showed that the level of 6447.9 Da protein in the breast cancer group was dramatically decreased compared with the control group (Figure 1A, 1B; Table 1). Additionally, 6447.9 Da protein level in the post-surgery group was markedly higher than that in the pre-surgery group (Figure 1C, 1D; Table 2). The 6447.9 Da protein level decreased remarkably in the TNBC group compared with the NTNBC group (Figure 1E, 1F; Table 3).
Figure 1.
Representative spectrums of SELDI-TOF-MS analysis of serum from healthy controls and breast cancer patients. (A) The expression levels of 6447.9 Da protein in serum of healthy participants (Control) and BC patients (Pre-surgery). (B) Comparison of pre-surgery and post-surgery patients. (C) Comparison of TNBC and NTNBC patients.
Table 1.
The descriptive statistics for the candidate protein marker identified between pre-surgery and control groups.
| m/z | Serum of pre-surgery (mean ±SD) | Serum of controls (mean ±SD) | P |
|---|---|---|---|
| 6447.9 | 38.0187±34.2194 | 337.5261±507.6438 | 0.0056487 |
Table 2.
The descriptive statistics for the candidate protein marker identified between pre-surgery and post-surgery groups.
| m/z | Serum of pre-surgery (mean ±SD) | Serum of post-surgery (mean ±SD) | P |
|---|---|---|---|
| 6447.9 | 38.0187±34.2194 | 294.1245±102.8634 | 0.009751 |
Table 3.
The descriptive statistics for the candidate protein marker identified between TNBC and NTNBC groups.
| m/z | Serum of TNBC group (mean ±SD) | Serum of NTNBC group (mean ±SD) | P |
|---|---|---|---|
| 6447.9 | 5.1351±3.6437 | 43.6162±18.8542 | 0.000163 |
Purifying and identifying the candidate protein biomarker
Blood samples from healthy individuals were purified to identify the candidate protein marker (6447.9 Da). Candidate biomarkers were purified by SDS-PAGE electrophoresis. After trypsin digestion, the peptide compounds were analyzed with MALDI-TOF/TOF. The obtained peptide sequence was searched in SWISS PROT database with the Mascot software. Figure 2 and Table 4 showed the MALDI-TOF-MS data of the candidate protein biomarker. The target protein biomarkers was ultimately confirmed as Apo C-I.
Figure 2.
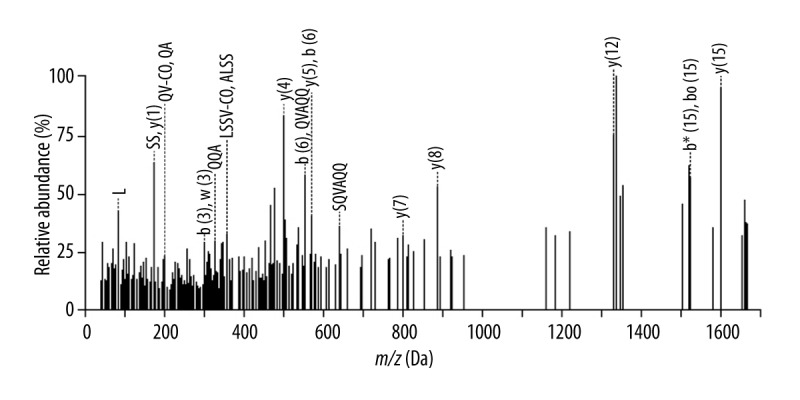
MS spectra of a peptide from the 6447.9 Da protein.
Table 4.
Identification of Apo C-I as a potential protein biomarker for breast cancer.
| m/z | Protein name | Sequence identified | Sequence coverage (%) | Score |
|---|---|---|---|---|
| 6447.9 | Apo C-I | LKEFGNTLEDKARELISRIKQSEL SAKMREWFSETFQKVKEK |
51.09 | 89.17 |
ApoC-I peptides inhibit proliferation of human breast cancer cells
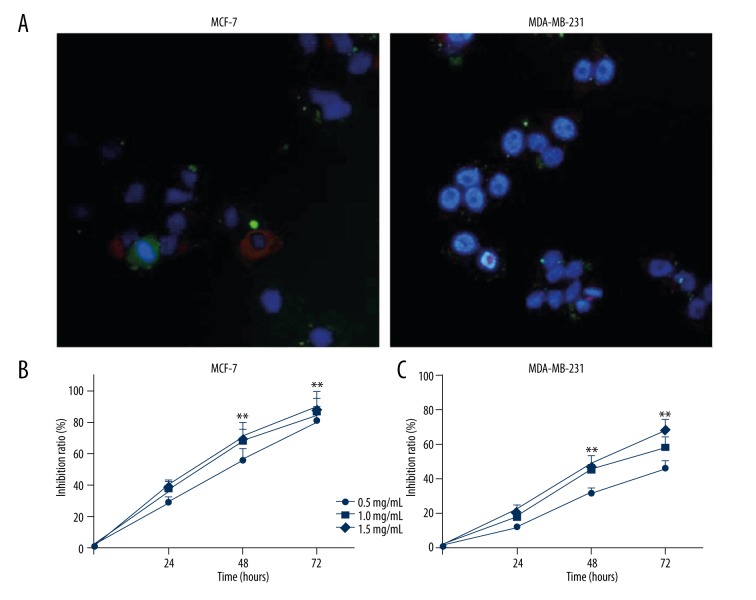
Effects of ApoC-I peptides on proliferation of human breast cancer cells were evaluated in vitro. After addition of 1 μg/μL FITC-peptides, green fluorescence was clearly detected in the cytoplasm of MCF-7 and MDA-MB-231 cells. Tubulin and nuclear were stained with tubulin tracker (red) and DAPI (blue), respectively (Figure 3A). The growth inhibition effects of ApoC-I peptides on MCF-7 and MDA-MB-231 cells in various concentrations were determined by CCK8 assay. The growth inhibition ratio increased when incubation with ApoC-I peptides was compared with the untreated control group in time- and concentration-dependent manners (Figure 3B, 3C).
Figure 3.
ApoC-I peptides inhibited proliferation of MCF-7 and MDA-MB-231cells. (A) Distribution of ApoC-I peptides (green) in MCF-7 and MDA-MB-231 cells. Tubulin (red), nuclei (blue). (Magnification, ×100). (B, C) Proliferation of MCF-7 and MDA-MB-231 cells was evaluated by CCK8 analysis after different treatments. Data are represented as means ±SD, n=4. * P<0.05; ** P<0.01.
ApoC-I peptides suppress tumor growth in nude mice
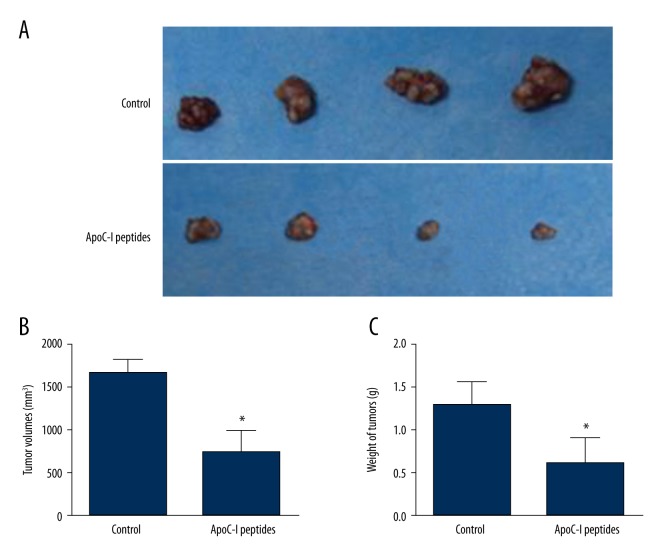
To study the effect of ApoC-I peptides on tumor growth, we established subcutaneous xenografts of MCF-7 human breast cancer cells in athymic nude mice. Two weeks after injection of MCF-7 cells, mice were randomly assigned into 2 groups and treated with PBS or ApoC-I peptides for 5 days. After an additional 7 days, mice were sacrificed; representative tumors are shown in Figure 4A. As shown in Figure 4B and C, tumor volumes and weight were decreased significantly in comparison with PBS-treated controls. No of significant body weight difference was found between the 2 groups of mice during ApoC-I peptides or PBS treatment (data not shown). To sum up, the results showed that ApoC-I peptides inhibited tumor growth in xenografted nude mice.
Figure 4.
Effect of ApoC-I peptides on tumor growth in vivo. (A) Mice treated with ApoC-I peptides had significantly smaller tumor xenografts compared with the control group. Two weeks after MCF-7 cells injection, mice with tumors of equal size were selected and randomly divided into 2 groups (n=8) and treated with either ApoC-I peptides or PBS at a dose of 100 mg/kg daily for 5 days. After an additional 7 days, mice were sacrificed and tumor volume and weight were measured. (B, C) ApoC-I peptides significantly reduced the volume and weight of tumor xenografts. Data are expressed as means ±SD, n=8. * P<0.05.
ApoC-I peptides inhibit cell proliferation and promote apoptosis in mammary tumors
To determine whether ApoC-I peptides affect cell proliferation and apoptosis in mammary tumors of mice, the expression levels of PCNA, Ki67, Bcl-2, and Bax were detected by immunohistochemical method. Compared with the control group, the number of cells positive for PCNA, Ki67, and Bcl-2 decreased significantly but Bax increased remarkably in tumor sections (Figure 5). Taken together, the results show that ApoC-I peptides inhibit proliferation and promote apoptosis of xenografts in a nude mouse model.
Figure 5.
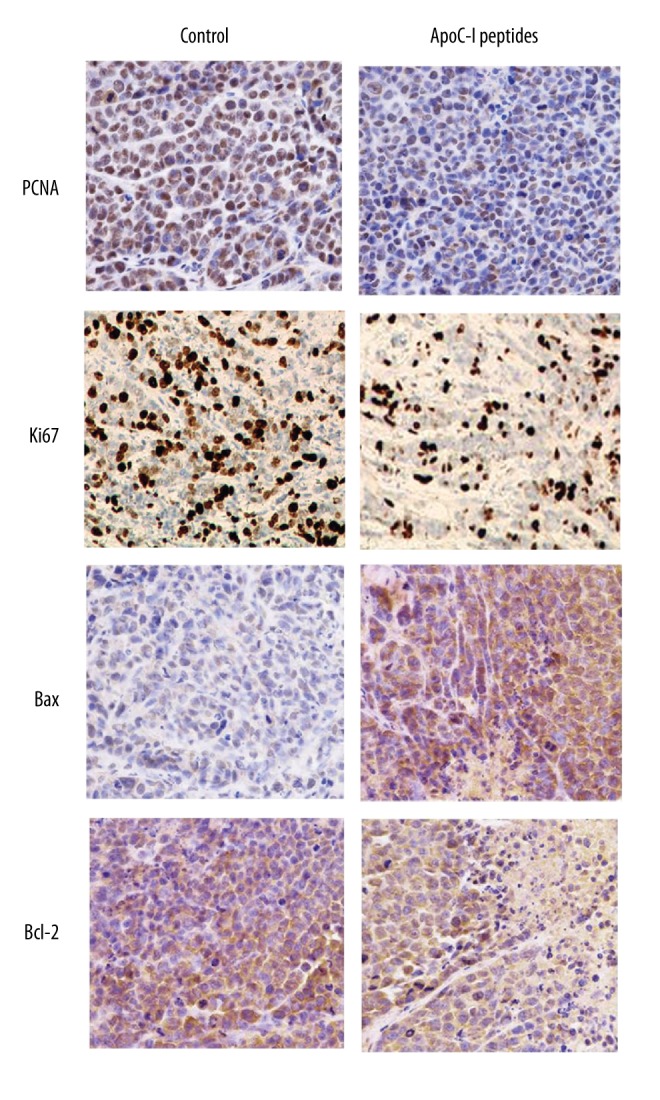
ApoC-I peptide inhibited proliferation and induced apoptosis in tumors isolated from athymic nude mice. Tumor sections were stained using specific antibodies for PCNA, Ki67, Bcl-2, and Bax. Photomicrographs (magnification, ×20) show representative images of the 2 groups.
Discussion
Proteins secreted by tumors, tissue- and plasma-protein digested by proteases secreted by tumors, and other proteins generated by responses to tumors are transported into circulating blood during tumor progression. Multiple methods and instruments were used to compare and integrate mass spectrometry data from aliquots of pooled serum and plasma from patients with cancer and healthy controls [19,20]. However, due to lack of high sensitivity and specificity, several biomarkers that were identified are not ideal for breast cancer screening and early detection. Here, we performed a more rigorous study to find a new approach for diagnosis and treatment of breast cancer.
With the development of mass spectrometry, this technology is becoming a powerful approach to explore the mechanisms of cancer and identify potential biomarkers for early diagnosis of diseases [15,21]. SELDI-TOF-MS analysis has been shown to be an efficient strategy for detecting small cancer-related proteins [22]. The difficulty in analyzing data of SELDI-TOF-MS is to decrease the error protein peaks [23]. In the process of data handling in our study, we reduced noise by discontinuous wavelength, identified mass-charge peaks of samples by the local extremum, and clustered mass-charge peaks through selecting 10% as the minimum threshold. We ultimately identified the peak at m/z 6447.9 as showing remarkable change in pre-surgery and the control serum. In addition, the peak with an m/z value of 6447.9 showed significant differential expression, not only in the pre- and post-surgery groups, but also in the TNBC and NTNBC groups.
Apolipoproteins are lipid carriers that play an important role in lipoprotein metabolism. In recent years, apolipoproteins have been shown to be involved in multiple cellular functions such as promotion of growth factor-mediated cell survival and inhibition [24–26]. ApoC-I is a 57 amino acid residue polypeptide primarily synthesized in the liver [27]. In the circulation, ApoC-I is a constituent of very low-density lipoprotein, intermediate-density lipoprotein, chylomicrons, and high-density lipoprotein [28]. Previous studies have shown that ApoC-I may impair very low-density lipoprotein clearance from the blood by inhibiting the uptake of very low-density lipoprotein particles by the liver [29]. Here, we found that the level of ApoC-I was decreased in serum of patients with BC, suggesting that ApoC-I is associated with BC. In addition, we investigated the anticancer function of ApoC-I peptides in breast cancer cells and an athymic nude mouse model. The protein expression levels of PCNA, Ki67, and Bcl-2 decreased significantly and Bax increased remarkably in xenografts of nude mice. The results show that ApoC-I peptides inhibited cell proliferation in vitro and suppressed tumor growth in vivo. However, the mechanism of ApoC-I in breast cancer is not yet elucidated, and further efforts should be made to clarify why ApoC-I is degenerated in patients with breast cancer.
Conclusions
ApoC-I was identified as a potential serum biomarker for breast cancer. ApoC-I peptides had a suppressive effect on tumor progression of breast cancer in a nude mouse model, suggesting that ApoC-I peptides may serve as a novel therapeutic strategy for treatment of breast cancer. However, various difficulties still exist in developing practical clinical applications from our findings. Further replicated experiments with many more samples are necessary to verify this possible protein biomarker.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Stephens PJ, Tarpey PS, Davies H, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–4. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oda M, Makita M, Iwaya K, et al. High levels of DJ 1 protein in nipple fluid of patients with breast cancer. Cancer Sci. 2012;103:1172–76. doi: 10.1111/j.1349-7006.2012.02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somlo G, Lau SK, Frankel P, et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res Treat. 2011;128:155–63. doi: 10.1007/s10549-011-1508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng E, Li R, Shin VY, et al. Circulating microRNAs as specific biomarkers for breast cancer detection. PLoS One. 2013;8:e53141. doi: 10.1371/journal.pone.0053141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy MJ, Evoy D, McDermott EW. CA 15-3: Uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411:1869–74. doi: 10.1016/j.cca.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A, Goswami B, Gupta N, et al. Lipoprotein (a) plasma levels and risk of breast cancer. Hell J Surg. 2015;87:298–302. [Google Scholar]
- 8.Uen Y-H, Liao C-C, Lin J-C, et al. Analysis of differentially expressed novel post-translational modifications of plasma apolipoprotein E in Taiwanese females with breast cancer. J Proteomics. 2015;126:252–62. doi: 10.1016/j.jprot.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y-C, Pohl G, Wang T-L, et al. Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer Res. 2005;65:331–37. [PubMed] [Google Scholar]
- 10.Duo J, Ying G-G, Wang G-W, et al. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep. 2012;5:1453–56. doi: 10.3892/mmr.2012.845. [DOI] [PubMed] [Google Scholar]
- 11.Yao Q, Chen J, Lv Y, et al. The significance of expression of autophagy-related gene Beclin, Bcl-2, and Bax in breast cancer tissues. Tumor Biol. 2011;32:1163–71. doi: 10.1007/s13277-011-0219-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Wahl R. Abundance of thymidine kinase 1 (TK1) message in breast cancer cell lines is better correlated with their doubling times than Ki67 or PCNA message levels. J Nucl Med. 2014;55(Suppl 1):1425. [Google Scholar]
- 13.Morimoto Y, Killeen J, Hernandez BY, et al. Parity and expression of epithelial histopathologic markers in breast tissue. Eur J Cancer Prev. 2013;22:404–8. doi: 10.1097/CEJ.0b013e32835c7fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu CJ, Wang CI, Chien KY, et al. Quantitative proteomics reveals regulation of KPNA2 and its potential novel cargo proteins in non-small cell lung cancer. FASEB J. 2013;27:812. doi: 10.1074/mcp.M111.016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C. The application of SELDI-TOF-MS in clinical diagnosis of cancers. Biomed Res Int. 2011;2011:245821. doi: 10.1155/2011/245821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigo MAM, Zitka O, Krizkova S, et al. MALDI-TOF MS as evolving cancer diagnostic tool: a review. J Pharm Biomed Anal. 2014;95:245–55. doi: 10.1016/j.jpba.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Lin LL, Huang HC, Juan HF. Discovery of biomarkers for gastric cancer: a proteomics approach. J Proteomics. 2012;75:3081–97. doi: 10.1016/j.jprot.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 18.Cho WC. Proteomics in translational cancer research: biomarker discovery for clinical applications. Expert Rev Proteomics. 2014;11:131–33. doi: 10.1586/14789450.2014.899908. [DOI] [PubMed] [Google Scholar]
- 19.Hanash SM, Pitteri SJ, Faca VM. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452:571–79. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- 20.Kozak KR, Su F, Whitelegge JP, et al. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics. 2005;5:4589–96. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- 21.Simsek C, Sonmez O, Yurdakul AS, et al. Importance of serum SELDI-TOF-MS analysis in the diagnosis of early lung cancer. Asian Pac J Cancer Prev. 2013;14:2037–42. doi: 10.7314/apjcp.2013.14.3.2037. [DOI] [PubMed] [Google Scholar]
- 22.Fan Y, Wang J, Yang Y, et al. Detection and identification of potential biomarkers of breast cancer. J Cancer Res Clin Oncol. 2010;136:1243–54. doi: 10.1007/s00432-010-0775-1. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Zhao S, Fan Y, et al. Detection and identification of potential biomarkers of non-small cell lung cancer. Technol Cancer Res Treat. 2009;8:455–65. doi: 10.1177/153303460900800607. [DOI] [PubMed] [Google Scholar]
- 24.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson M, Vorrsjö E, Talmud P, et al. Apolipoproteins CI and C-III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J Biol Chem. 2013;288:33997–4008. doi: 10.1074/jbc.M113.495366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cash JG, Kuhel DG, Basford JE, et al. Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J Biol Chem. 2012;287:27876–84. doi: 10.1074/jbc.M112.377549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berbée JF, van der Hoogt CC, Kleemann R, et al. Apolipoprotein CI stimulates the response to lipopolysaccharide and reduces mortality in gram-negative sepsis. FASEB J. 2006;20:2162–64. doi: 10.1096/fj.05-5639fje. [DOI] [PubMed] [Google Scholar]
- 28.Jong MC, Hofker MH, Havekes LM. Role of ApoCs in lipoprotein metabolism functional differences between ApoC1, ApoC2, and ApoC3. Arterioscl Thromb Vasc Biol. 1999;19:472–84. doi: 10.1161/01.atv.19.3.472. [DOI] [PubMed] [Google Scholar]
- 29.Huang S, Qiao J, Li R, et al. Can serum apolipoprotein CI demonstrate metabolic abnormality early in women with polycystic ovary syndrome? Fertil Steril. 2010;94:205–10. doi: 10.1016/j.fertnstert.2009.03.005. [DOI] [PubMed] [Google Scholar]