Hierarchical self-assembled structures provide a “bottom-up” method to utilize relatively simple and low cost processes to generate nanoscale patterns (usually through amphiphilic low molecular weight surfactants or block copolymers).1 These self-assembled structures from block copolymers are generally driven by a combination of repulsive and attractive interactions between the block segments and are inherently heterogeneous. Consequently, covalently bonded precursors are in high-demand to create well-defined self-assembled structures with prescribed molecular weights, low polydispersity, fixed compositions, available functional groups at chain ends, and structured topology.2,3 This month, the Cheng group reports their design for a synthetic method to prepare a series of giant surfactants with multiple precise polyhedral oligomeric silsesquioxane (POSS) nano-building blocks.4

Polymer nanocomposites based on POSS have been widely investigated due to a wide variety of available silica precursors ranging in particle size down to molecular silica. Unlike other nanofillers, POSS nanoparticles can be easily modified via organic functional substituents on their surface through suitable polymerization reactions, surface bonding, grafting, or other transformations.5,6 Thus, POSS nanoparticles have potential applications in electronic and biomedical applications.7
Early studies by the Cheng group and co-workers introduced a new giant surfactant possessing a well-defined hydrophilic head from carboxylic acid or hydroxyl-functionalized POSS and a hydrophobic polystyrene (PS) tail, called PS–POSS. The resulting self-assembled structures can be controlled to form many different arrangements from vesicles to wormlike cylinders and finally to spherical structures by increasing the degree of carboxylic acid ionization in the solution state.9
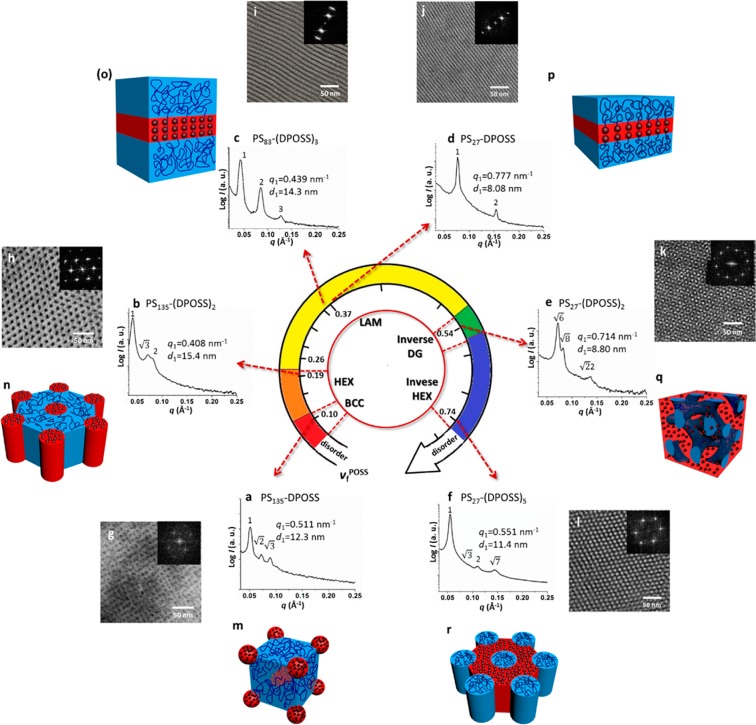
In this latest work, the authors extend their control on the system’s heterogeneity to include composition, functional groups on the POSS nanoparticle, sequence, and the topology of the primary chemical structure, which means they are also able to precisely control the self-assembled structures of these giant surfactants in the bulk state. These PS hydrophobic polymer tails end in either linear or branched configuration leading to multiple arrangements of the resulting self-assembled structures. The volume fraction of POSS nanoparticles in the PS-(POSS)n segment can be increased leading to different structures obtained compared with the linear PS–POSS as shown in Figure 1.4
Figure 1.
Self-assembled structure phase diagram of PS–POSS giant surfactant. Figure reproduced with permission from ref (4). Copyright 2016 American Chemical Society.
Further flexibility in shape-control arises from increasing the chain length of PS segments in the bulk state, leading to lamellae, bicontinuous gyroid, hexagonal packed cylinder, and spherical structures.10 This giant surfactant from PS–POSS is able to bridge the gap between the amphiphilic low-molecular-weight surfactants and block copolymers, and it also can self-assemble with feature scales below 10 nm in the solution, thin film, and bulk state, which is sensitive to the primary chemical structure. They also designed four different POSS nanoparticles with slightly different functional groups onto the single tetrahedral framework. Breaking the symmetry of these giant tetrahedral frameworks also results in the well-defined self-assembled structures such as lamellae, bicontinuous gyroid, hexagonal packed cylinder, spherical structures, and even Frank-Kasper A15 phase, which is similar to the metal alloys. The molecular nanoparticle for this kind of giant surfactant is not only limited to POSS, but also for [60]fullerences (C60), polyoxometalates (POM), and folded globular protein.10
Cheng and co-workers’ approach is akin to playing with Legos, the various shapes of precise nanoarchitectures could create different hierarchical self-assembled structures such as lamellae, bicontinuous gyroid, hexagonal packed cylinder, and spherical structures. This way provides promising opportunities to mediate the hierarchical self-assembled structure, not only for polymer POSS nanocomposites, but also for nano-building blocks.
References
- Qiu H.; Hudson Z. M.; Winnik M. A.; Manners I. Science 2015, 347, 1329–1332. 10.1126/science.1261816. [DOI] [PubMed] [Google Scholar]
- Klok H. A.; Lecommandoux S. Adv. Mater. 2001, 13, 1217–1219. . [DOI] [Google Scholar]
- Kuo S. W. Polym. Int. 2009, 58, 455–464. 10.1002/pi.2513. [DOI] [Google Scholar]
- Zhang W.; Huang W.; Su H.; Zhang S.; Yue K.; Dong X. H.; Li X.; Liu H.; Zhang S.; Wesdemiotis C.; Lotz B.; Zhang W. B.; Li Y.; Cheng S. Z. D. ACS Cent. Sci. 2016, 2, 48. 10.1021/acscentsci.5b00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T.; Leolukman M.; Jin S.; Goseki R.; Ishida Y.; Kakimoto M.; Hayakawa T.; Ree M.; Gopalan P. Macromolecules 2009, 42, 8835–8843. 10.1021/ma9018944. [DOI] [Google Scholar]
- Kuo S. W.; Chang F. C. Prog. Polym. Sci. 2011, 36, 1649–1696. 10.1016/j.progpolymsci.2011.05.002. [DOI] [Google Scholar]
- Zhang W.; Muller A. H. E. Prog. Polym. Sci. 2013, 38, 1121–1162. 10.1016/j.progpolymsci.2013.03.002. [DOI] [Google Scholar]
- Yu X.; Zhong S.; Li X.; Tu Y.; Yang S.; Van-Horn R. M.; Ni C.; Pochan D. J.; Quirk R. P.; Wesdemiotis C.; Zhang W. B.; Cheng S. Z. D. J. Am. Chem. Soc. 2010, 132, 16741–1674. 10.1021/ja1078305. [DOI] [PubMed] [Google Scholar]
- Yu X.; Yue K.; Hsieh I. F.; Li Y.; Dong X. H.; Liu C.; Xin Y.; Wang H. F.; Shi A. C.; Newkome G. R.; Ho R. M.; Chen E. Q.; Zhang W. B.; Cheng S. Z. D. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 10078–10083. 10.1073/pnas.1302606110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.; Hsu C. H.; Wang J.; Mei S.; Dong X. H.; Li Y.; Li M.; Liu H.; Zhang W.; Aida T.; Zhang W. B.; Yue K.; Cheng S. Z. D. Science 2015, 348, 424–428. 10.1126/science.aaa2421. [DOI] [PubMed] [Google Scholar]