“There’s plenty of room at the bottom.” These famous words from Richard Feynman are most commonly associated with the special properties of nanoscale objects, but they might as easily be applied to further taking apart these materials into their simplest units. For example, carbon nanotubes may be conceptually simplified to layers of interlocked conjugated rings. In practice, synthesizing these rings can actually be more challenging than synthesizing their tubular counterparts (nanotubes). The strain associated with functionally curving nanowires contributes much to the challenge of synthesizing these macrocycles, but also provides the basis for their unusual properties. In addition, synthesizing these macrocycles as chemically defined small molecules allows for more precise evaluation of their unique properties.
Three related papers1−3 published recently describe methods to install donor/acceptor (DA) motifs into conjugated, macrocyclic molecules (their chemical structures are shown in Figure 1). The common goal in each report is to use the DA motif as a means to adjust the energetics of the HOMO/LUMO levels. Installation of donor and acceptor moieties is a proven strategy to tune the optoelectronic properties of linear, conjugated oligomers and polymers4 and has been extensively studied in material science, and it is specifically efficacious in organic photovoltaics. However, until now, DA systems did not exist in conjugated macrocycles. These three studies utilize very different synthetic chemistry to construct the strained, cyclic molecules, and each has a unique method to introduce the DA motif.
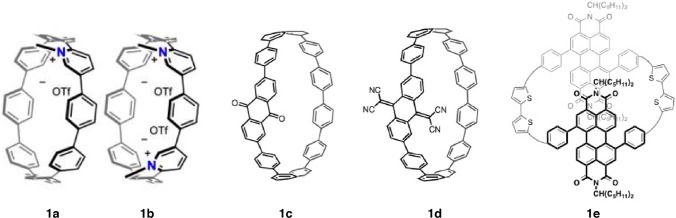
Figure 1.
Conjugated DA macrocycles from Darzi et al.1 (1a and 1b), Kuwabara et al.2 (1c and 1d), and Ball et al.3 (1e). Reprinted with permission from refs (1−3). Copyright 2015 American Chemical Society1,3 and Wiley.2
The DA strategy used by Darzi et al.1 swaps the aromatic rings of cycloparaphenylenes (CPPs) with pyridine rings. Upon alkylation, the pyridine ring(s) serves as the acceptor and the oligophenylene chain serves as the donor. The strain in these systems makes them challenging synthetic targets. The key to the synthesis is to create a U-shaped subunit whose curvature is introduced by hinge pieces resulting from addition to both carbonyls of 1,4-benzoquinone. Subsequent macrocyclization is achieved by bridging the ends of the U-shaped piece with a pyridyl unit. Aromatization and alkylation of the arylated quinone hinge moieties affords conjugated, strained cycles (1a and 1b). The exciting finding is that the absorbance is bathochromically shifted into the visible region and the molecules have a more accessible LUMO level compared to [8]CPP.
Kuwabara et al.2 also utilize an oligophenylene backbone as the donor, but instead install an anthraquinone as the acceptor (1c). Their synthesis also involves bridging a U-shaped intermediate, but here, the curvature results from 1,4-diarylated cyclohexane subunits. After aromatization to form the conjugated macrocycle, the electron accepting ability of the anthraquinone moiety (1c) is improved through conversion to the tetracyanoanthraquinone (1d). Evidence to the profound effect of the installation of the electron acceptors in 1c and 1d includes the UV–vis and fluorescence that show a unique a solvatofluorochromism effect.
The third DA strategy, used by Ball et al.,3 is the direct, cyclic analogue for how DA strategies are employed in linear conjugated polymers. The donor is a 2,2′-substituted bithiophene, and the acceptor is a 1,7-diaryl substituted perylene diimide (PDI). They are linked together in an alternating D–A–D–A pattern (1e). The synthetic approach utilizes a strategy from Baüerle for cyclic thiophenes5 and from Yamago6 for CPPs where macrocycles are formed from tetranucluear, square-planar platinum intermediates. Reductive elimination forms the strained and conjugated macrocycle. The macrocycle 1e has an unusual dynamic stereoisomerism due to the topology of the macrocycle. Absorption spectroscopy reveals that these black-hued materials possess broad absorbance with an onset at ∼700 nm. Density functional calculations show that this low-energy absorption corresponds to an intramolecular charge transfer from donor bithiophene to acceptor PDI.
Collectively, these three studies are exciting and chart a clear path to further exploration and creation of structurally interesting and functional materials using conjugated cyclic molecules with DA motifs. Important first steps are using the engineered orbital levels of these DA systems to absorb light of wavelengths to be useful in photovoltaics or to allow efficient charge transport in organic field effect transistors. An added benefit of cyclic, conjugated molecules that might be exploited is their ability to complex guests in their cavity. Host–guest chemistry can be used to tune the properties of the materials or to create sensors and switches. These three studies are the beginning of a brave new world for cyclic conjugated molecules where their syntheses are used not to generate chemical curiosities, but as means to create functional materials.
References
- Darzi E.; Hirst E.; Weber C.; Zakharov L.; Lonergan M.; Jasti R. Synthesis, properties, and design principles of donor–acceptor nanohoops. ACS Cent. Sci. 2015, 1, 335. 10.1021/acscentsci.5b00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T.; Orii J.; Segawa Y.; Itami K. Curved Oligophenylenes as Donors in Shape-Persistent Donor–Acceptor Macrocycles with Solvatofluorochromic Properties. Angew. Chem., Int. Ed. 2015, 54, 9646–9649. 10.1002/anie.201503397. [DOI] [PubMed] [Google Scholar]
- Ball M.; Fowler B.; Li P.; Joyce L. A.; Li F.; Liu T.; Paley D.; Zhong Y.; Li H.; Xiao S.; Ng F.; Steigerwald M. L.; Nuckolls C. Chiral Conjugated Corrals. J. Am. Chem. Soc. 2015, 137, 9982–9987. 10.1021/jacs.5b05698. [DOI] [PubMed] [Google Scholar]
- Havinga E. E.; ten Hoeve W.; Wynberg H. A new class of small band gap organic polymer conductors. Polym. Bull. 1992, 29, 119–126. 10.1007/BF00558045. [DOI] [Google Scholar]
- Zhang F.; Götz G.; Winkler H. D. F.; Schalley C. A.; Baüerle P. Giant cyclo[n]thiophenes with extended pi conjugation. Angew. Chem., Int. Ed. 2009, 48, 6632–6635. 10.1002/anie.200900101. [DOI] [PubMed] [Google Scholar]
- Iwamoto T.; Watanabe Y.; Sakamoto Y.; Suzuki T.; Yamago S. Selective and random syntheses of [n]cycloparaphenylenes (n = 8–13) and size dependence of their electronic properties. J. Am. Chem. Soc. 2011, 133, 8354–8361. 10.1021/ja2020668. [DOI] [PubMed] [Google Scholar]