Abstract

Human pluripotent stem cells (hPSCs; both embryonic and induced pluripotent) rapidly proliferate in adherent culture to maintain their undifferentiated state. However, for mammals exhibiting delayed gestation (diapause), mucin-coated embryos can remain dormant for days or months in utero, with their constituent PSCs remaining pluripotent under these conditions. Here we report cellular stasis for both hPSC colonies and preimplantation embryos immersed in a wholly synthetic thermoresponsive gel comprising poly(glycerol monomethacrylate)-poly(2-hydroxypropyl methacrylate) [PGMA55-PHPMA135] diblock copolymer worms. This hydroxyl-rich mucin-mimicking nonadherent 3D gel maintained PSC viability and pluripotency in the quiescent G0 state without passaging for at least 14 days. Similarly, gel-coated human embryos remain in a state of suspended animation (diapause) for up to 8 days. The discovery of a cryptic cell arrest mechanism for both hPSCs and embryos suggests an important connection between the cellular mechanisms that evoke embryonic diapause and pluripotency. Moreover, such synthetic worm gels offer considerable utility for the short-term (weeks) storage of either pluripotent stem cells or human embryos without cryopreservation.
Short abstract
Wholly synthetic mucin-mimicking hydroxyl-functional diblock copolymers self-assemble to form thermoresponsive aqueous hydrogels that induce stasis in human pluripotent stem cells and human embryos.
Introduction
Mucins are a family of glycoproteins that are known to play central roles in biology.1,2 Transmembrane mucins mediate important cell–cell interactions, as well as signaling events with other biomolecules such as lectins.3,4 Misregulation during mucin synthesis has been linked to inflammation and tumor development.5 Indeed, mucin-like tumor antigens have been developed for circulating cancer cells with the aim of triggering a humoral response and so inducing active immunity at a stage of disease progression for which there are few alternative therapies.6−8 More recently, synthetic mucin mimics have also been designed as microarrays9 and mucin chimeras have been assembled on living cells10 to examine the complex biological roles played by cell surface mucins.
Secreted mucins possess unusual viscoelastic properties and can provide a passive protective barrier against pathogens and other environmental toxins.11 However, there is growing evidence that secreted mucins forming the apical extracellular matrix (ECM) can influence both cell morphology and junction dynamics during embryonic development.12 These observations suggest that synthetic mucin mimics may be promising active biomaterials for regenerative medicine.
Recently, considerable attention has focused on wholly synthetic hydrogels, with the successful 2D13−16 and 3D17 culture of pluripotent stem cells (PSCs) being reported. This approach to PSC culture is appealing, because precise control over the chemical composition and purity of synthetic hydrogels addresses a number of important problems associated with biologically-derived hydrogels, such as ill-defined compositions and components, batch-to-batch variability, and the undesirable presence of xenobiotic components.18
Human PSCs (hPSCs; both embryonic and induced pluripotent) display an abbreviated cell cycle, with their pluripotency being associated with rapid proliferation in adherent cell culture.19 In contrast, preimplantation blastocysts for certain other mammals such as rats, mice, and kangaroos can exhibit an obligate (every gestation) or facultative (due to lactation/metabolic stress) diapause or developmental arrest.20 In particular, viable embryos can remain in a state of suspended animation within a mucin coating for days or even months, prior to their subsequent reactivation and gestation.20 Indeed, it has been postulated that the conditions required to induce diapause might also be relevant for the derivation and maintenance of PSCs in vitro.(21,22) Although it is not known whether embryonic diapause occurs in women, the cell-arrest mechanism(s) involved are evolutionarily conserved.23 Thus, such behavior may be characteristic of hPSCs, yet remain cryptic under standard cell culture conditions.
As well as chemical cues, cells also sense physical stimuli through complex feedback interactions involving mechanostimuli such as rigidity and contractility, which are ultimately transduced into biochemical signals.24,25 In particular, Engler and co-workers employed collagen-I/polyacrylamide hydrogels with gel moduli ranging from 0.1 to 100 kPa to show that MSCs commit to lineages specified by gel elasticity when this mimics that of natural tissues. For example, very soft gels favor brain cells, stiffer gels produce muscle, and harder gels lead to the formation of cartilage and bone.26 However, these authors did not investigate gel elasticities below 0.1 kPa. This rheological regime includes mucins, which are an important class of natural matrices.27 Depending on their composition and function, mammalian mucins can exhibit a range of elasticities (0.01–0.1 kPa).27 Of particular relevance to the present work, mucins associated with healthy female reproductive tracts are found at the lower end of this range.27
We have previously reported the design of a fully synthetic poly(glycerol monomethacrylate)-poly(2-hydroxypropyl methacrylate) (PGMA-PHPMA) diblock copolymer worm gel that undergoes reversible degelation via a worm-to-sphere order–order transition on cooling from 37 to 5 °C.28 PGMA is a hydroxyl-rich biocompatible polymer28 that is known to minimize cell adhesion.29−31 PGMA55-PHPMA135 diblock copolymer worm gels can be conveniently synthesized directly either in pure water or in physiological buffers such as phosphate-buffered saline, PBS.28 Moreover, such synthetic worm gels exhibit comparable gel moduli to natural mucins.27 Recognizing that worm gels and mucins both exhibit high degrees of hydration32 and are rich in hydroxyl functionality,33 we decided to examine the extent to which PGMA-PHPMA worm gels can mimic soft mucins (Figure 1a). In particular, we explore herein whether immersing PSC colonies within worm gels can induce long-term stasis while maintaining both pluripotency and viability. Moreover, we hypothesized that encapsulation of viable human embryos within such worm gels might lead to diapause (arrested development).
Figure 1.
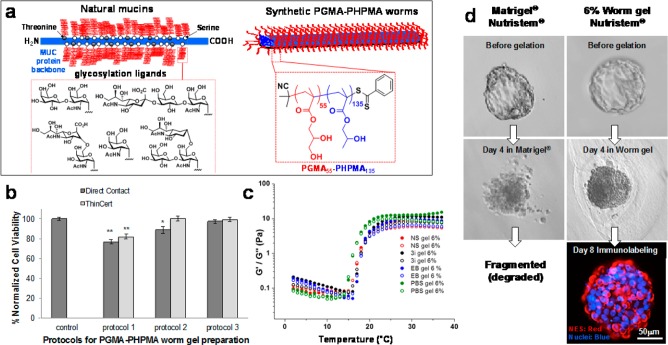
Physicochemical and biological characterization of mucin-mimicking PGMA55-PHPMA135 worm gels. (a) Schematic representation of the similar physical and chemical (hydroxyl-rich brush) structures of mucin gels and PGMA-PHPMA worm gels. (b) Temperature dependence of the storage and loss moduli (G′, G″) observed on cooling a 6% w/v worm gel reconstituted in various aqueous media from 37 to 2 °C at an applied strain of 1.0% and a fixed angular frequency of 1.0 rad s–1. (c) Three protocols were evaluated with human dermal fibroblasts: protocol 1 (20% w/v copolymer gel prepared in PBS, diluted two-fold and dialyzed for 2 days against PBS), protocol 2 (same as protocol 1, followed by dialysis against PBS for 7 days), and protocol 3 (20% w/v copolymer gel prepared in PBS, dialyzed against deionized water for 7 days, followed by freeze-drying overnight and redispersion in DMEM cell culture medium). Cell viability was evaluated by direct-contact cell monolayers and also via an indirect assay using ThinCert inserts. Experiments were conducted in triplicate; **p < 0.01, *p < 0.05. (d) Five-day-old human blastocysts immersed in either 6% w/v worm gel or Matrigel incubated at 37 °C, 5% CO2 and 5% O2. After 4 days, embryos in Matrigel undergo fragmentation, whereas embryos immersed in worm gel remained intact but became compacted. Five-day-old embryos immersed within worm gel for 8 days (i.e., up to 13 days of development) stained positive for nuclear envelope statin (NES). Localization (red, nuclear envelope statin; blue, Hoechst 33342-nuclei; scale bar = 50 μm). n = 2 embryos per condition.
Results and Discussion
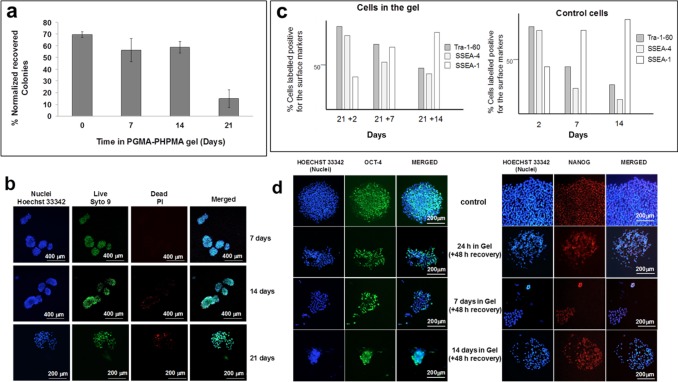
Initially, PGMA55-PHPMA135 worm gels were synthesized at 20% w/v solids in PBS followed by dialysis at 4 °C for 2 days against PBS (to remove low molecular weight impurities), and diluted to produce a 6% w/v worm gel with cell culture medium. A viability assay using human dermal fibroblasts indicated that this simple approach (protocol 1) produced cell survival rates of 75–80% (Figure 1b). Dialysis against PBS for 7 days (protocol 2) further improved cell viability. However, optimal results were achieved by preparing copolymer worms in PBS at 20% w/v, dialyzing against pure water for 7 days, followed by freeze-drying overnight to obtain a dry powder (protocol 3). This powder was reconstituted with culture medium to produce a free-standing worm gel, which retained its thermoreversible gelation behavior (Figure 1c). Varying the culture medium had only a modest effect on the worm gel rheology, with no significant differences being observed in either the gel strength (G′ ∼ 101 Pa) or critical gelation temperature (CGT ∼ 18 °C) when using EB (Millipore), ES (Nutristem, Stemgent), or 3i naive medium.34 A slightly lower CGT of 17 °C was determined in PBS (control), but the thermoreversible behavior was otherwise broadly comparable. Thus strain sweeps produced similar G′ values (9 to 13 Pa), with deviation from the linear viscoelastic region occurring at around 60% applied strain in all cases. Angular frequency sweeps were also similar (see Figure S1). We investigated human embryos cultured to zona-free blastocyst (day 5) and immersed in a 6% w/v PGMA55-PHPMA135 worm gel containing Nutristem medium or, for comparison, placed in Matrigel under similar conditions. After 4 days (day 9) embryos within the worm gel remained intact, whereas the embryos immersed in Matrigel showed clear signs of dissociation and fragmentation (Figure 1d). Two embryos remained for up to 8 days in PGMA55-PHPMA135 gel (day 13) without any signs of development or primitive streak formation. Embryos were then degelled and fixed for immunolocalization using the well-known cell stasis marker nuclear envelope statin (NES)35 (Figure 1d). This nuclear envelope protein enables rapid identification of cells that either enter or leave the cell cycle.35,36 Compacted embryos that had been immersed within the worm gel clearly expressed NES, indicating cell stasis under these conditions (Figure 1d). Given the limited availability of human embryos and the strict 14-day limit on their in vitro culture in the UK, we next investigated human ESCs (hESCs) immersed as colonies within the worm gel. No discernible change in colony size was observed over time regardless of the cell medium, suggesting little or no cell proliferation (Figure S2). Similar results were obtained for dissociated single-cell suspensions immersed in a worm gel prepared using 3i medium (Figure S3). However, in this case, some proliferative cells were recovered 24 h after degelation, but no cells remained viable after 7 days (Figure S3). Usually 3i medium supports adherent culture of single cells,34 thus this lack of success suggested that cell–cell contact was required for hESCs to retain viability. After immersion of hESC colonies within a Nutristem-prepared worm gel for 7 days, more than 90% of the cells remained viable as determined by Syto9/PI (live/dead) in situ staining (Figure 2b). After 14 days, some colonies contained dead cells (estimated to be <10% of the total area examined) but most colonies retained a majority of viable cells; some hPSC colonies still contained viable cells after 21 days (Figure 2b and Figure S4). As the passage of PSC colonies is known to compromise their viability,37 control colonies were immersed in worm gel for 10 min and recovered immediately via thermally-triggered degelation (Figure 2a, week 0). Approximately 70 ± 3% of harvested cell colonies attached to Cellstart matrix and underwent subsequent proliferation. After 7 and 14 days, 57 ± 10% and 59 ± 5% viable colonies were recovered respectively; even after 21 days, around 10% of colonies remained proliferative (Figure 2a). The pluripotency of hPSCs was assessed after their initial recovery from worm gel followed by proliferative culture of up to 14 days in standard adherent culture (Figure 2c). The majority of cells from recovered colonies expressed markers for pluripotency markers (SSEA-4, Tra-1-60), rather than markers for differentiation (SSEA-1). Significantly, even after 7 or 14 days of adherent culture, colonies recovered from worm gel (after 21 days) displayed greater levels of pluripotency markers than control cell colonies, which suggests a markedly slower resumption of differentiation (Figure 2c). Furthermore, colonies recovered from worm gels remained distinctly rounded and domed in appearance for up to 48 h after degelation, which is in marked contrast to the relatively flat morphology that is characteristic of adherent hPSC cells (Figure S5). Interestingly, hPSC colonies immersed in worm gel remained pluripotent for longer than those cultured for the same length of time under normal culture conditions. Nevertheless, optical microscopy studies indicate that hPSC colonies initially immersed in worm gel and subsequently transferred to normal culture exhibited the expected characteristic morphological differentiation into various cell types upon prolonged culture (see Figure S6).
Figure 2.
hESCs in PGMA55-PHPMA135 worm gel maintain viability and stem cell markers. (a) Proportion (%) of proliferative (hESC colonies recovered from reconstituted 6% w/v worm gels prepared using Nutristem medium for up to 21 days. (b) Syto 9 (live)/PI (dead) staining for typical cell colonies immersed in worm gel at 37 °C. Hoechst 33342 counter-stain. (c) FACS analysis of pluripotent (Tra-1-60, SSEA-4) and differentiation (SSEA-1) stem cell markers for hESCs recovered from worm gel after 21 days and then subjected to standard adherent cell culture for up to 14 days compared to control hESCs cultured in the absence of gel. Expression of pluripotent markers is equivalent or greater in cells stored in worm gel for 21 days prior to adherent culture and declines more slowly. (d) Immunofluorescent localization of Oct-4 and Nanog in hESCs after recovery from worm gel compared to a control. All experiments were performed in triplicate wells, with n = 3 independent experiments.
It was noted that colony size affected hPSC recovery after worm gel immersion; few colonies smaller than 100 μm diameter adhered to Cellstart, and those that did rapidly differentiated (Figure S7a,b). However, much higher recovery was observed for colonies of 100–200 μm diameter, many of which exhibited the characteristic PSC morphology (Figure S7c,d). A correlation between PSC colony size and in vitro cell viability has been reported previously.38 Relatively large (≫200 μm) colonies immersed in worm gels (Figure S7e) were also compromised, since they tended to be more susceptible to differentiation once removed from the gel. Moreover, rather than spreading laterally, they became significantly denser normal to the substrate, and peripheral cells exhibited neuronal differentiation. Similar observations have been reported for high-density colonies dispersed in a liquid medium.38
To examine the extent of cell proliferation of hPSCs immersed within worm gel, colonies were recovered after 7, 14, and 21 days, and the normalized amount of DNA (relative to day 0) was determined (see Table 1). In addition to little or no change in the colony morphology, the total amount of DNA remained constant within experimental error, suggesting negligible proliferation of viable PSC colonies. This finding was confirmed by immunofluorescent localization of Ki-67 antigen (Figure S8), which is normally present for each stage of the cell cycle but is absent in G0.39 Essentially no Ki-67 expression was observed for viable colonies immersed in worm gels, suggesting that the hESCs exit the cell cycle at G0 to become dormant (Figure S8). In contrast, hESCs recovered from worm gels and immediately placed in adherent culture displayed a characteristic Ki-67 fluorescent signature after their subsequent proliferation (Figure S8).
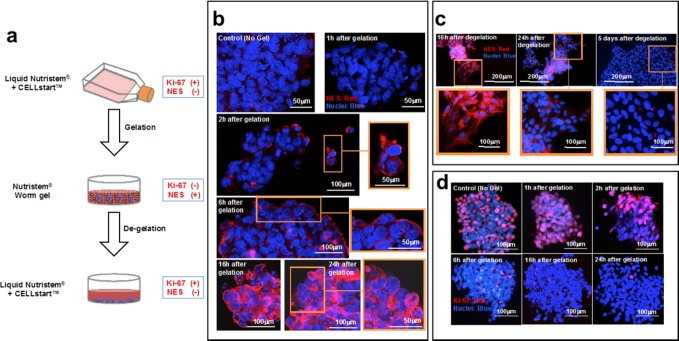
Under standard adherent culture conditions, proliferative PSCs did not express the cell stasis marker NES (Figure 3b). PSCs also remained negative for this marker after immersion in worm gel for less than 2 h, followed by degelling and immediate fixation (see Figure 3b). However, after 2 h incubation in worm gel, a few cells at the periphery of colonies were found to be NES+ (Figure 3b). This expression became more evident after 6 h, and NES+ cells were observed distributed throughout the colonies after worm gel immersion for 16–24 h (Figure 3b). This time scale is comparable to that of a single cell cycle.
Figure 3.
Changes in nuclear envelope statin (NES) and Ki-67 expression for hESCs immersed within a 6% w/v worm gel: (a) Overview of gelation/degelation process and associated changes in cell stasis markers. (b) Immunofluorescent localization of NES in hESC colonies. Control hESCs (no worm gel) did not display NES. After 2–6 h immersion, NES+ cells were only observed at the periphery of hESC colonies, but NES+ cells were distributed throughout the colony after 16–24 h. (c) Effect of degelation on NES expression. Cytosolic expression of NES after 16 h. Expression of NES+ was significantly reduced both 24 h after degelation and also after 5 days. (d) Immunofluorescent localization of Ki-67 in hESC colonies in gel. Control hESC (no gel) displayed Ki-67 expression, but this was considerably reduced after 16–24 h within the worm gel. All experiments were performed in triplicate, n = 3 independent experiments.
After thermally-triggered degelation, the recovered hESC colonies were allowed to attach to Cellstart matrix and cultured in six-well plates for 16 h, 24 h, or 5 days. Cells remained NES+ for 16–24 h but lacked this marker after 5 days (Figure 3c). Immediately after degelling, NES staining was not restricted to the nuclear envelope ring but was also distributed within the cytosol (Figure 3c). Similar cytosolic staining of other nuclear envelope components has been reported.40 This suggests reprocessing of nuclear envelope proteins, which would require membrane redistribution via cytosolic endoplasmic reticulum during degradation.40 Therefore, it seems likely that reinitialization of the cell cycle upon degelation of hESCs also triggers degradation of the NES. This would account for redistribution to the cytosol within 16–24 h, with full degradation occurring only after 5 days.
The onset of stasis was highly dependent on the genetic profile of the cells and also on the chemical composition of the synthetic hydrogel. Thus normal hPSCs underwent stasis when immersed in PGMA-PHPMA worm gels, whereas they grew to form typical embryoid bodies when immersed in a commercial thermoresponsive nonhydroxylated synthetic hydrogel (i.e., Mebiol), see Figure S9. This suggests that the mucin-like hydroxyl functionality of PGMA-PHPMA worm gels most likely plays a critical role in inducing stasis. Normal hPSCs immersed in Mebiol gel remained Ki-67+/NES– even after 14 days immersion (Figure S9). Moreover, genetically modified hPSCs adapted to feeder cells (similar to those found in teratoma41) also underwent proliferation when immersed within PGMA-PHPMA worm gels (Figure S10).
Quiescence and reactivation of stem or blast cells is a common theme across phyla for cellular regeneration and tissue homeostasis.42−44 However, as far as we are aware, this is the first report of hPSCs entering stasis or human blastocysts entering diapause during culture. Recent literature suggests that, even when not manifest, mammalian embryonic diapause is evolutionarily conserved. Thus sheep blastocysts do not naturally undergo diapause, but Ptak and co-workers23 demonstrated that this quiescent state can be temporarily induced for at least 7 days via transfer to the mouse uterus, with full developmental capacity being retained on subsequent return to primed ovine recipients. hPSCs immersed in a synthetic PGMA55-PHPMA135 worm gel in vitro revealed a similar cryptic ability: they exit the cell cycle at the G0 stage to enter dormancy or stasis, as judged by the absence of DNA synthesis and a Ki-67/NES assay. After immersion within worm gel for at least 14 days, recovered hPSC colonies maintained pluripotency markers and were able to exit stasis and resume proliferation, retaining their capacity for differentiation.
Soft synthetic substrates decorated with adhesion motifs induce quiescence in somatic stem cells.45 However, PGMA55-PHPMA135 worm gels contain no specific binding motifs to promote cell adhesion. Indeed, the multiple hydroxyl functionality on the copolymer worms most likely minimizes cell adhesion.29,30 This is confirmed by a direct contact assay (see Figure S11). Moreover, in the absence of adherence to a matrix (whether biological or synthetic), hPSCs usually undergo cell death via various anoikis/apoptosis mechanisms.46 Furthermore, individual hPSCs did not survive immersion in worm gel. Significantly, mammalian embryos that exhibit diapause are typically encapsulated within a glycoprotein/mucin coat (zona pellucida/mucin), which is both relatively benign and nonadhesive.20,47 It is emphasized that both mucin and the PGMA55-PHPMA135 worm gel comprise relatively soft, highly hydrated hydroxyl-rich polymeric gels. This suggests that both deprivation of suitable cell adhesion motifs and the lack of appropriate mechanical cues may be required to induce cellular stasis. In the Ptak study, ovine embryos collapsed their blastocoel cavity and became compacted when induced into diapause within the mouse reproductive tract, with no discernible contact with the ECM of the zona pellucida.23 In a remarkably similar fashion, human embryos immersed in worm gels also became more compact and displayed no obvious interaction with the gel matrix, in striking contrast to the fragmentation of control embryos immersed within Matrigel over the same time scale.
There is a general consensus that cellular stasis occurs in stem cells as a response to metabolic and/or environmental stress in order to preserve key functional features and therefore guarantee homeostasis and regeneration of the niche.42 Absence of Ki-67 staining revealed that hESC colonies displayed little cell division 16–24 h after gelation (Figure 3d). Moreover, expression of NES35,36 indicates a relatively rapid response to encapsulation within worm gel. Interestingly, not all cells were NES+/Ki-67– after immersion within worm gel for 24 h. Since live/dead assays indicate that the vast majority of cells remain viable, this suggests that a small proportion of cells may still proliferate, but not at a rate that is detectable by total DNA analysis.
Cell viability studies indicate that worm gels are non-toxic to PSC cells and allow proliferation of adapted PSCs. Furthermore, the culture media used in this study promoted PSC proliferation in adherent culture both prior to and after their worm gel encapsulation. Since freeze-dried worm gel was rehydrated using culture medium, it seems unlikely that induction of PSC stasis was merely due to the absence of specific growth factor(s). Thus the present work indicates that diapause (or stasis for stem cells) can be simply induced by a hydroxyl-rich environment within a very soft matrix. This sheds new light on the likely role played by mucins in triggering diapause.
In principle, the cell nuclear architecture senses the stiffness of the external environment and this information in turn directs cell differentiation and proliferation. Swift and co-workers elegantly demonstrated that matrix stiffness in tissue culture increased mesenchymal stem cell cytoskeletal tension and stabilized the nuclear envelope protein lamin-A, regulating both its own transcription and that of stress fiber genes.48 Such lamin-A expression has been linked to the maintenance of adult stem cell quiescence.49 Nuclear envelope statin may well function similarly in PSCs, leading to cell division arrest and hence stabilization of their pluripotent state. In this context, it is perhaps noteworthy that PSCs recovered from worm gel after 21 days underwent spontaneous differentiation significantly more slowly compared to control cells when placed on Cellstart for 2, 7, or 14 days. As implicated for murine-diapaused embryos,21,22 this may reflect a more naive stem cell state and clearly warrants further investigation.
Conclusions
In summary, we demonstrate that a biocompatible PGMA-PHPMA diblock copolymer worm gel is a remarkably good synthetic mimic for at least some types of natural mammalian mucins. Furthermore, these worm gels can also function as a suitable bioinert 3D matrix for PSCs. Immunolabeling assays confirm that PSC colonies immersed in such worm gels enter stasis (the quiescent G0 state in the cell cycle) within 24 h at 37 °C. This state of suspended animation is reversible upon degelation and enables survival of pluripotent human stem cells for up to 2 weeks without any passaging, with little or no differentiation. The mucin-like nature of such soft hydroxyl-functional worm gels is also apparent in preliminary experiments conducted using human embryos, for which diapause is observed for up to 4 days after worm gel immersion. The design of such new biomimetic stem cell niches suggests novel therapeutic avenues for arresting disease processes and/or promoting cell regeneration.
Materials and Methods
Consent and Donation of Embryos
Embryo manipulation was carried out under license from the Human Fertilization and Embryology Authority, HFEA, following independent ethical review. Patients donated embryos following fully informed consent. All embryos were coded to protect patient anonymity.
Embryo Culture and Gelation
Cryopreserved embryos were thawed using an embryo thawing pack (Origio, DK). Viable four-cell embryos were cultured in a 50 μL microdrop system until the late morula–early blastocyst stage (typically day 5 of development) with BlastAssist medium (Origio, DK). Embryos were monitored regularly until they naturally hatched (typically at day 6), or were aided to hatch by laser dissection with Integra 3 micromanipulator with a Saturn 5 laser (RI life sciences, UK). Blastocysts were gelled using either Matrigel (50 μL) or 6% w/v PGMA55-PHPMA135 worm gel reconstituted in Nutristem medium (50 μL). Preparations were coated with mineral oil and cultured in an incubator at 37 °C in 5% O2, 5% CO2 in N2 and were monitored daily under the microscope. After various time intervals in gel (up to day 13 of development), embryos were recovered and fixed in 4% formaldehyde in preparation for immunolabeling of the nuclear envelope statin. In the UK, the legal limit for the development of human embryos is 14 days (or before appearance of the primitive streak).
Cells
Pluripotent Stem Cell Lines Maintenance and Preparation
Human embryonic stem cell (hESC) lines, MasterShef (clinical grade) 14 and 11, were used. These were derived under license from the HFEA and deposited with the UK Stem Cell Bank. hESCs were maintained in feeder-free adherent culture using Nutristem medium (Stemgent, UK) and extracellular matrix Cellstart (Life Technologies, UK) with nonenzymatic mechanical passage every 5 days.
Human Dermal Fibroblasts
Primary human dermal fibroblasts (HDF) were obtained in batches from the ATCC, LGC standards (UK). Fibroblasts were routinely cultured in T75 flasks using standard culture medium (DMEM supplemented with 10% FCS, 2.0 mmol dm–3l-glutamine, 0.625 mg dm–3 amphotericin B, 100 IU/mL penicillin, and 100 mg dm–3 streptomycin). HDFs were used for testing between passages 4 and 9.
Preparation of PGMA55-PHMPA135 Worm Gels for Cell Culture Experiments
Materials
Glycerol monomethacrylate (GMA; 99.8%) was donated by GEO Specialty Chemicals (Hythe, UK) and used without further purification. 2-Hydroxypropyl methacrylate (HPMA) and 4,4′-azobis(4-cyanopentanoic acid) (ACVA; V-501; 99%) were purchased from Alfa Aesar (Heysham, UK). 2-Cyano-2-propyl dithiobenzoate (CPDB, 80% as judged by 1H NMR spectroscopy) was purchased from Strem Chemicals (Newton, UK). CD3OD (99.8%) was purchased from Goss Scientific (Nantwich, UK) and used as received. All solvents were of HPLC quality; they were purchased from Fisher Scientific (Loughborough, UK) and used as received.
Synthesis of PGMA55 Macro-CTA Using 2-Cyanopropyl Dithiobenzoate (CPDB)
CPDB (0.80 g, 3.6 mmol) and glycerol monomethacrylate (GMA, 40.59 g, 0.25 mol) were weighed into a 250 mL round-bottom flask and purged with N2 for 20 min. ACVA (202.9 mg, 0.72 mmol) was added, and the solution was degassed for a further 5 min. Degassed anhydrous ethanol (61 mL, 1.04 mol) was added, and the solution was again degassed for a further 5 min prior to immersion in an oil bath set at 70 °C. After 2 h, a 1H NMR spectrum recorded in CD3OD indicated approximately 80% GMA monomer conversion. The crude polymer was purified by precipitating twice into excess dichloromethane from methanol to remove unreacted monomer. After the second precipitation, the polymer was isolated via filtration and the resulting solid was dissolved in water (200 mL). Residual dichloromethane was evaporated at 30 °C using a rotary evaporator. Once all traces of solvent were removed, the aqueous solution was freeze-dried overnight to afford a pink powder. 1H NMR spectroscopy studies of the purified polymer dissolved in CD3OD indicated a mean degree of polymerization of 55. DMF GPC analysis indicated an Mn of 14,100 g mol–1 and an Mw/Mn of 1.09 (obtained using refractive index detection calibrated with series of near-monodisperse poly(methyl methacrylate) standards for calibration).
Synthesis of PGMA55–PHPMA135 Diblock Copolymer Worms in 0.15 M PBS at 20% w/v Solids
PGMA55 (3.023 g, 0.33 mmol) and HPMA (6.240 g, 41.62 mmol) were weighed in turn into a 100 mL round-bottom flask and purged with N2 for 20 min. ACVA (31.0 mg, 0.11 mmol) was added, and the flask was degassed for a further 5 min. Phosphate-buffered saline (PBS) solution (Dulbecco A, Oxoid, Basingstoke, 37 mL, 150 mM, previously purged with N2 for 30 min) was then added, and the solution was degassed for a further 5 min prior to immersion of the reaction flask in an oil bath set at 70 °C for 2 h, after which 1H NMR spectra recorded in CD3OD indicated almost 100% HPMA conversion (as judged by integration of the attenuated vinyl signals at 5.6 and 6.2 ppm). DMF GPC analysis using the same experimental setup as described above gave an Mn of 35,900 g mol–1 and an Mw/Mn = 1.10.
For cell biology studies, three protocols were evaluated for purification and preparation of PGMA55-PHPMA135 worm gels, as described below.
Protocol 1
The as-synthesized 20% w/v aqueous PGMA55-PHPMA135 worm gel was dialyzed against PBS for 2 days at 4 °C with dialysate (PBS) changes every 12 h (MWCO = 1,000). The resulting cold free-flowing copolymer dispersion was diluted in the appropriate cell medium (DMEM, or Nutristem; precooled to 4 °C prior to mixing) to the desired concentration (typically either 6% w/v or 10% w/v copolymer) and filter-sterilized at this temperature prior to use, as described below.
Protocol 2
The as-synthesized 20% w/v PGMA55-PHPMA135 gel was dialyzed against PBS for 7 days at 4 °C with dialysate changes every 12 h (MWCO = 1,000). The resulting cold free-flowing dispersion was diluted in the appropriate cell medium (DMEM, or Nutristem; precooled to 4 °C prior to mixing) to the desired concentration (typically either 6% w/v or 10% w/v copolymer) and filter-sterilized at this temperature prior to use, as described below.
Protocol 3
The as-synthesized 20% w/v PGMA55-PHPMA135 gel was dialyzed against pure water for 7 days at 4 °C with dialysate changes every 12 h (MWCO = 1,000). The resulting gel was freeze-dried to yield a fine pink powder, which was redispersed in the appropriate cell culture medium (DMEM, EB (Embryoid body medium), or Nutristem; precooled to 4 °C prior to mixing) to afford either a 6% w/v or 10% w/v copolymer dispersion. The temperature was maintained at approximately 4 °C using an ice bath, and magnetic stirring was continued for at least 20 min until full dispersion was achieved. The resulting liquid was filter-sterilized prior to use, as described below.
Sterilization Protocol
A 6% w/v PGMA55-PHPMA135 worm gel dispersed in the desired cell culture medium was cooled to 4 °C to induce the worm-to-sphere transition, and hence underwent degelation to afford a free-flowing dispersion of copolymer spheres. This cold low-viscosity fluid was then ultrafiltered using a sterile 0.20 μm syringe filter into a sterile vessel within a laminar flow cabinet. Syringes and filters were stored at −20 °C for at least 1 h prior to ultrafiltration to prevent gelation on contact. The resulting sterilized copolymer dispersion was then used immediately for cell colony encapsulation experiments, or stored at either 4 °C or −20 °C for future use (depending on the specifications of the cell medium).
Cell Viability in Direct Contact with PGMA55-PHPMA135 Worm Gels
Cell Viability Assays on Human Dermal Fibroblasts (HDFs) Using MTT Assay.
HDFs were seeded in 24-well plates at a density of 3 × 104 cells per well and grown until 80% confluence (typically 48 h). Gels were evaluated both in direct contact with the cells and also in nondirect contact (basket method). A noncontact ThinCert (Greiner Bio-One, UK) setup was used to identify any toxic low molecular weight compounds that might be present in the worm gels (e.g., unreacted HPMA monomer). ThinCert comprises small basket-like structures of tissue culture plastic with a polycarbonate membrane bottom that fits over a 24-well plate. Thus, cells are exposed to the gel through the cell medium in the 24-well plates, but not by direct contact. This setup discriminates between the effect of direct contact of the worm gel on the cells and the effect of residual small molecule impurities.
For the indirect contact setup, 250 μL of the 10% w/v copolymer gel was added to each ThinCert basket. Cells were placed below each basket and immersed in the appropriate cell culture medium (500 μL). For the direct contact setup, the cell medium was removed from the wells and the gel (typically 500 μL) was applied directly onto the cell monolayers. Gel batches were tested on 80% confluent HDF cells over 24 h. Cell viabilities were then assessed via an MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (Sigma-Aldrich, St Louis, MO). Briefly, cells were washed at 4 °C with cold PBS, then incubated with MTT solution (0.50 g/L MTT in PBS at 20 °C, 1 mL per well of a 24-well plate) for 1 h at 37 °C in a humidified incubator (5% CO2/95% air). In healthy viable cells, MTT is reduced to a purple formazan salt by the mitochondrial enzyme, succinyl dehydrogenase, which allows spectrophotometric quantification of cell viability. After 1 h, the solution was aspirated and the insoluble intracellular formazan product was solubilized and removed from cells by adding acidified isopropanol (0.30 mL per well of a 24-well plate), followed by incubation for 10 min. The absorbance at 540 nm was then determined using a plate reading visible absorption spectrophotometer, with the absorbance at 630 nm being used as a reference. Mean viability data and SEM were normalized using a negative control (no treatment, 100% viability) and expressed as a percentage viability ± SEM. Experiments were performed in duplicate well samples with n = 3 independent experiments. For statistical analysis, the Student’s paired t test was used in the raw data to assess the significance of differences between the samples and the control group.
Quantification of Survival of hES Cells after Prolonged Immersion in PGMA55-PHMPA135 Worm Gels: % Colony Recovery and Live/Dead Assay
% Colony Recovery Experiments
The hES cells were typically grown under xeno-free conditions using Nutristem medium (Stemgent, UK) and t25 vessels coated with CELLstart (Life Technologies, UK), unless otherwise stated. Cell cultures were maintained at 37 °C in a humidified incubator (5% CO2/95% air), and the medium was renewed daily. When cultures achieved optimal cell density (typically 60–70% surface coverage), the cell medium was replenished and colonies were mechanically harvested. Colonies were placed onto 35 mm Petri dishes in preparation for gel seeding. Ibidi eight-well slides were placed on ice, and 500 μL of a cold 6% PGMA55-PHMPA135 copolymer dispersion (which is a free-flowing liquid at ∼4 °C) was added to each of the wells. Using a sterile plastic Pasteur pipet equipped with a “superfine” tip, individual colonies were placed on the center of each ibidi well and gently stirred to allow mixing. Gelation (Figure 3a) was immediately triggered by placing the ibidi wells in a humidified incubator (5% CO2/95% air) set at 37 °C for the desired time period (i.e., 7, 14, or 21 days) prior to harvesting. The number of colonies (typically 3–15) in each gelled well was counted microscopically. Degelation (Figure 3a) was triggered by placing each ibidi slide on ice for approximately 5 min. The resulting free-flowing copolymer containing the cell colonies was diluted 10-fold with Nutristem (5.0 mL) into a CELLstart-treated six-well plate releasing the cell colonies. Wells were inspected microscopically, and the number of colonies was recorded. The six-well plates were stored for approximately 3 h in a humidified incubator (5% CO2/95% air) to allow viable cell colonies to adhere to the matrix. Subsequently, medium was replenished daily. The % colony recovery was calculated as the average colony recovery per time point ± SEM normalized to the initial number of colonies prior to degelation. The % colony loss was similarly estimated. Controls consisted of cell colonies immersed in a worm gel for just 10 min and immediately harvested. This enabled the efficiency of the colony isolation and colony transfer protocols to be assessed. Experiments were performed in triplicate wells with n = 3 independent experiments.
Live/Dead Assay
The viabilities of hES cell colonies immersed within PGMA55-PHMPA135 worm gels were assessed using a commercial live/dead assay (Life Technologies, UK). This assay utilizes a binary mixture of a cell-permeable SYTO 9 green fluorescent nucleic acid stain (ex 480 nm, em 500 nm) and an impermeable red fluorescent nucleic acid stain, propidium iodide (PI, ex 490 nm, em 635 nm). Cells with compromised (i.e., leaky) membranes are designated as dead or dying and are stained red (PI), whereas cells with intact membranes are stained green (SYTO 9). When used alone, the latter stain generally labels all cells, but when both dyes are present the PI penetrates damaged membranes and quenches the green fluorescence due to SYTO 9, so that this signal is not detected. Briefly, the gelled colonies were cooled to around 4 °C for 5 min to trigger degelation and allowed to sediment under gravity. The free-flowing aqueous copolymer dispersion supernatant was partially removed, and colonies were washed once with cell culture medium precooled to 4 °C. The aqueous fluid was then removed, and warm (37 °C) cell culture medium was added to each well containing SYTO 9 (15 μM) and PI (60 μM). Cells were incubated in a humidified incubator (5% CO2/95% air) for 25 min in order to allow dye uptake to occur. Then cell nuclei were counterstained for a further 5 min with Hoechst 33342 (Life Technologies, UK). Finally, colonies were washed with PBS (precooled to 4 °C), and further culture medium (depending on the vessel, typically 3 mL for a six-well plate and 500 μL for ibidi imaging plates) was added prior to inspection using a Nikon A1 confocal microscope equipped with an Okolab environmental control chamber for live cell studies.
Evidence for Preservation of Stem Cell Markers on hESCs after Prolonged Immersion in PGMA55-PHPMA135 Worm Gels: Flow Cytometry (Tra-1-60, SSEA4, SSEA1) and Oct-4/Nanog Immunolabeling
Flow Cytometry (Tra-1-60, SSEA4, SSEA1)
Harvested cells were resuspended in PBS supplemented with 10% fetal calf serum. Cells (5 × 105) were incubated for 1 h with primary antibody to SSEA1, SSEA4, TRA-1-60. After washing three times in PBS, cells were labeled with FITC-conjugated secondary antibody for 1 h. This was followed by washing the cells three times with wash buffer and analyzing cell fluorescence using a CyAnADP flow cytometer equipped with O2 optics (Beckman Coulter, Brea, USA). The gate for FITC-positive cells was set using control cells incubated with a negative control immunoglobulin obtained from the parent myeloma cell line, P3X63Ag8.
Oct-4/Nanog Immunolabeling Experiments
Gel recovered colonies were allowed to attach to CELLstart coated six-well plates for up to 48 h. Colonies were then washed with PBS and fixed for 30 min using an aqueous solution of 4% formaldehyde in PBS (100 μL). All samples were then washed three times in PBS and permeabilized using a 0.1% Triton X100 PBS solution for 20 min. Colonies were then washed three times in PBS and blocked in 5% BSA–PBS for 2 h at 20 °C, prior to incubation with a primary antibody solution (1:100 rabbit anti-human Oct-4 antibody (Abcam, UK) + 1% BSA in PBS; 1:100 rabbit anti-human Nanog (Cell Signaling Technology, USA) 1% BSA in PBS) overnight at 4 °C with gentle rocking. These antibody-labeled colonies were then washed three times in PBS and then incubated with a secondary antibody solution (1:1000 goat anti-rabbit Alexa Fluor 488 IgG (Abcam) + 1% BSA in PBS; 1:1000 mouse anti-rabbit Cy3 IgG (Abcam) + 1% BSA in PBS) for 1 h at 20 °C with gentle rocking. Colonies were washed three times with PBS, and cell nuclei were counterstained for 5 min using Hoechst 33342 (Life Technologies, UK). Finally, each sample was washed three times in PBS prior to inspection using an EVOS epifluorescence imaging system.
Evidence for Stasis (Suspended Animation): DNA Extraction and Ki-67 Immunolabeling
DNA Extraction
Cell colonies were mechanically recovered from t25 flasks and placed on 35 mm Petri dishes prior to use. Each ibidi well was seeded with a fixed volume (500 μL) of cell colonies. This suspension was allowed to sediment for 5 min, and then the liquid medium was carefully removed. Then 6% PGMA55-PHPMA135 copolymer dispersion (500 μL; precooled to 4 °C) was added to each of the wells and gently stirred to allow mixing. Gelation was immediately triggered by placing the ibidi wells at 37 °C in a humidified incubator (5% CO2/95% air). Cell colonies were incubated for 7, 14, or 21 days. When required, degelation was triggered by placing each ibidi slide on ice for approximately 5 min. The contents of each well were then placed in labeled 1.5 mL Eppendorf tubes containing ice-cold PBS (1.0 mL). Samples were centrifuged twice at 4 °C for 5 min (1000 rcf). Cell pellets were incubated using 100 μL of digestion buffer (comprising TE buffer (10 mM Tris pH 8 and 1 mM EDTA) plus 0.2% SDS) for 4 h at 37 °C. Following this, a solvent mixture comprising 25:24:1 phenol:chloroform:isoamyl alcohol (100 μL) saturated with TE buffer was added to each Eppendorf tube and thoroughly mixed by vortex. The samples were centrifuged at 20 °C for 5 min (14,000 rcf). Aqueous supernatants were then decanted and mixed with ice-cold ethanol (450 μL) and 3 M sodium acetate (50 μL). DNA was sedimented via centrifugation for 5 min at 20 °C (14,000 rcf). Supernatants were carefully decanted, and DNA pellets were dried in a 37 °C oven. Dried pellets were resuspended in TE buffer (20 μL). The DNA concentration was calculated by determining the absorbance at 260 nm using a Nanodrop spectrophotometer. Data were normalized with respect to an internal control group, whereby cell colonies were gelled for approximately 10 min and then immediately harvested (day 0). Experiments were performed in duplicate wells with n = 3 independent experiments.
Ki-67/Nuclear Envelope Statin Immunolabeling Experiments
Colonies were isolated from the worm gels by incubation on ice for approximately 5 min to induce degelation. Each well was then collected into 1.5 mL Eppendorf tubes containing ice-cold PBS (1 mL). Colonies were washed twice at 4 °C for 5 min (1000 rcf) and then fixed for 30 min using an aqueous solution of 4% formaldehyde in PBS (100 μL). Control colonies (not gelled) and gel-recovered colonies growing in six-well plates were also washed in cold PBS and fixed with 4% formaldehyde in PBS. All samples were then washed three times in PBS and permeabilized using a 0.1% Triton X100 PBS solution for 20 min (1 mL per well in a six-well plate and 100 μL per Eppendorf tube). Colonies were then washed three times in PBS and blocked in 5% BSA–PBS for 2 h at 20 °C, prior to incubation with a primary antibody solution (1:100 rabbit anti-human Ki-67 monoclonal antibody (Abcam) + 1% BSA in PBS; 1:20 mouse anti-human S-44 nuclear statin antibody 1% BSA in PBS) overnight at 4 °C with gentle rocking. These antibody-labeled colonies were then washed three times in PBS and then incubated with a secondary antibody solution (1:1000 goat anti-rabbit Cy3 IgG (Abcam) + 1% BSA in PBS; 1:1000 goat anti-mouse Chromeo 546 (Abcam) + 1% BSA in PBS) for 1 h at 20 °C with gentle rocking. Colonies were washed three times with PBS, and cell nuclei were counterstained for 5 min using Hoechst 33342 (Life Technologies, UK). Finally, each sample was washed three times in PBS prior to inspection using an EVOS epifluorescence imaging system or confocal microscopy using a Nikon A1R-A1 confocal microscope with dual line TIRF.
Acknowledgments
We are grateful to patients and staff of CARE Fertility and Jessop Fertility assisted conception units for the donation of embryos for research. EPSRC is acknowledged for postdoctoral support of I.C. and N.J.W. (EP/L024160/1).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscentsci.5b00370.
Rheology data, extended cell recovery data, effect of colony size in recovery, viability tests, and stasis data (PDF)
Financial support for this work was provided by EPSRC (EP/L024160/1).
The authors declare no competing financial interest.
Supplementary Material
References
- Linden S. K.; Sutton P.; Karlsson N. G.; Korolik V.; McGuckin M. A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton D. J.; Sheehan J. K. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc. Am. Thorac. Soc. 2004, 1, 54–61. 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- Rosen S. D. Endothelial ligands for L-selectin: from lymphocyte recirculation to allograft rejection. Am. J. Pathol. 1999, 155, 1013–1020. 10.1016/S0002-9440(10)65201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth M. A.; Swanson B. J. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Kufe D. W. Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sames D.; Chen X. T.; Danishefsky S. J. Convergent total synthesis of a tumour-associated mucin motif. Nature 1997, 389, 587–591. 10.1038/39292. [DOI] [PubMed] [Google Scholar]
- Glunz P. W.; Hintermann S.; Williams L. J.; Schwarz J. B.; Kuduk S. D.; Kudryashov V.; Lloyd K. O.; Danishefsky S. J. Design and synthesis of Le(y)-bearing glycopeptides that mimic cell surface Le(y) mucin glycoprotein architecture. J. Am. Chem. Soc. 2000, 122, 7273–7279. 10.1021/ja0011820. [DOI] [Google Scholar]
- Marcaurelle L. A.; Shin Y. S.; Goon S.; Bertozzi C. R. Synthesis of oxime-linked mucin mimics containing the tumor-related T-N and sialyl T-N antigens. Org. Lett. 2001, 3, 3691–3694. 10.1021/ol0166247. [DOI] [PubMed] [Google Scholar]
- Godula K.; Bertozzi C. R. Density Variant Glycan Microarray for Evaluating Cross-Linking of Mucin-like Glycoconjugates by Lectins. J. Am. Chem. Soc. 2012, 134, 15732–15742. 10.1021/ja302193u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. R.; Onoa B.; Bustamante C.; Bertozzi C. R. Chemically tunable mucin chimeras assembled on living cells. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 12574–12579. 10.1073/pnas.1516127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S. J.; Spicer A. P. Epithelial Mucin Genes. Annu. Rev. Physiol. 1995, 57, 607–634. 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- Mancuso V. P.; Parry J. M.; Storer L.; Poggioli C.; Nguyen K. C.; Hall D. H.; Sundaram M. V. Extracellular leucine-rich repeat proteins are required to organize the apical extracellular matrix and maintain epithelial junction integrity in C. elegans. Development 2012, 139, 979–990. 10.1242/dev.075135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y.; Saha K.; Bogatyrev S. R.; Yang J.; Hook A. L.; Kalcioglu Z. I.; Cho S. W.; Mitalipova M.; Pyzocha N.; Rojas F.; Van Vliet K. J.; Davies M. C.; Alexander M. R.; Langer R.; Jaenisch R.; Anderson D. G. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 2010, 9, 768–778. 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Diaz L. G.; Nandivada H.; Ding J.; Nogueira-de-Souza N. C.; Krebsbach P. H.; O’Shea K. S.; Lahann J.; Smith G. D. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol. 2010, 28, 581–583. 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.; Mjoseng H. K.; Hoeve M. A.; Bauer N. G.; Pells S.; Besseling R.; Velugotla S.; Tourniaire G.; Kishen R. E.; Tsenkina Y.; Armit C.; Duffy C. R.; Helfen M.; Edenhofer F.; de Sousa P. A.; Bradley M. A thermoresponsive and chemically defined hydrogel for long-term culture of human embryonic stem cells. Nat. Commun. 2013, 4, 1335–1344. 10.1038/ncomms2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celiz A. D.; Smith J. G.; Langer R.; Anderson D. G.; Winkler D. A.; Barrett D. A.; Davies M. C.; Young L. E.; Denning C.; Alexander M. R. Materials for stem cell factories of the future. Nat. Mater. 2014, 13, 570–579. 10.1038/nmat3972. [DOI] [PubMed] [Google Scholar]
- Saeed A.; Francini N.; White L.; Dixon J.; Gould T.; Rashidi H.; Al Ghanami R. C.; Hruschka V.; Redl H.; Saunders B. R.; Alexander C.; Shakesheff K. M. A thermoresponsive and magnetic colloid for 3D cell expansion and reconfiguration. Adv. Mater. 2015, 27, 662–668. 10.1002/adma.201403626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Diaz L. G.; Ross A. M.; Lahann J.; Krebsbach P. H. Concise review: The evolution of human pluripotent stem cell culture: from feeder cells to synthetic coatings. Stem Cells 2013, 31, 1–7. 10.1002/stem.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinas K.; Grandy R.; Ghule P.; Medina R.; Becker K.; Pardee A.; Zaidi S. K.; Lian J.; Stein J.; van Wijnen A.; Stein G. The abbreviated pluripotent cell cycle. J. Cell. Physiol. 2013, 228, 9–20. 10.1002/jcp.24104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfree M. B.; Shaw G. Embryo-endometrial interactions during early development after embryonic diapause in the marsupial tammar wallaby. Int. J. Dev. Biol. 2014, 58, 175–181. 10.1387/ijdb.140059mr. [DOI] [PubMed] [Google Scholar]
- Nichols J.; Smith A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Welling M.; Geijsen N. Uncovering the true identity of naive pluripotent stem cells. Trends Cell Biol. 2013, 23, 442–448. 10.1016/j.tcb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Ptak G. E.; Tacconi E.; Czernik M.; Toschi P.; Modlinski J. A.; Loi P. Embryonic diapause is conserved across mammals. PLoS One 2012, 7, e33027. 10.1371/journal.pone.0033027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher D. E.; Janmey P.; Wang Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Choquet D.; Felsenfeld D. P.; Sheetz M. P. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 1997, 88, 39–48. 10.1016/S0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Engler A. J.; Sen S.; Sweeney H. L.; Discher D. E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Lai S. K.; Wang Y. Y.; Wirtz D.; Hanes J. Micro- and macrorheology of mucus. Adv. Drug Delivery Rev. 2009, 61, 86–100. 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanazs A.; Verber R.; Mykhaylyk O. O.; Ryan A. J.; Heath J. Z.; Douglas C. W.; Armes S. P. Sterilizable gels from thermoresponsive block copolymer worms. J. Am. Chem. Soc. 2012, 134, 9741–9748. 10.1021/ja3024059. [DOI] [PubMed] [Google Scholar]
- Faucheux N.; Schweiss R.; Lutzow K.; Werner C.; Groth T. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials 2004, 25, 2721–2730. 10.1016/j.biomaterials.2003.09.069. [DOI] [PubMed] [Google Scholar]
- Arima Y.; Iwata H. Effects of surface functional groups on protein adsorption and subsequent cell adhesion using self-assembled monolayers. J. Mater. Chem. 2007, 17, 4079–4087. 10.1039/b708099a. [DOI] [PubMed] [Google Scholar]
- Lloyd A. W.; Faragher R. G.; Denyer S. P. Ocular biomaterials and implants. Biomaterials 2001, 22, 769–785. 10.1016/S0142-9612(00)00237-4. [DOI] [PubMed] [Google Scholar]
- Shogren R.; Gerken T. A.; Jentoft N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry 1989, 28, 5525–5536. 10.1021/bi00439a029. [DOI] [PubMed] [Google Scholar]
- Caldara M.; Friedlander R. S.; Kavanaugh N. L.; Aizenberg J.; Foster K. R.; Ribbeck K. Mucin biopolymers prevent bacterial aggregation by retaining cells in the free-swimming state. Curr. Biol. 2012, 22, 2325–2330. 10.1016/j.cub.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni O.; Weinberger L.; Mansour A. A.; Manor Y. S.; Chomsky E.; Ben-Yosef D.; Kalma Y.; Viukov S.; Maza I.; Zviran A.; Rais Y.; Shipony Z.; Mukamel Z.; Krupalnik V.; Zerbib M.; Geula S.; Caspi I.; Schneir D.; Shwartz T.; Gilad S.; Amann-Zalcenstein D.; Benjamin S.; Amit I.; Tanay A.; Massarwa R.; Novershtern N.; Hanna J. H. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013, 504, 282–286. 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- Wang E. Statin, a nonproliferation-specific protein, is associated with the nuclear envelope and is heterogeneously distributed in cells leaving quiescent state. J. Cell. Physiol. 1989, 140, 418–426. 10.1002/jcp.1041400303. [DOI] [PubMed] [Google Scholar]
- Pei X. Y.; Dai Y.; Youssefian L. E.; Chen S.; Bodie W. W.; Takabatake Y.; Felthousen J.; Almenara J. A.; Kramer L. B.; Dent P.; Grant S. Cytokinetically quiescent (G0/G1) human multiple myeloma cells are susceptible to simultaneous inhibition of Chk1 and MEK1/2. Blood 2011, 118, 5189–5200. 10.1182/blood-2011-02-339432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng B. C.; Liu H.; Ge Z.; Cao T. Mechanical dissociation of human embryonic stem cell colonies by manual scraping after collagenase treatment is much more detrimental to cellular viability than is trypsinization with gentle pipetting. Biotechnol. Appl. Biochem. 2007, 47, 33–37. 10.1042/BA20060151. [DOI] [PubMed] [Google Scholar]
- Reubinoff B. E.; Pera M. F.; Fong C. Y.; Trounson A.; Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000, 18, 399–404. 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Scholzen T.; Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. . [DOI] [PubMed] [Google Scholar]
- Antonin W.; Ellenberg J.; Dultz E. Nuclear pore complex assembly through the cell cycle: regulation and membrane organization. FEBS Lett. 2008, 582, 2004–2016. 10.1016/j.febslet.2008.02.067. [DOI] [PubMed] [Google Scholar]
- Baker D. E.; Harrison N. J.; Maltby E.; Smith K.; Moore H. D.; Shaw P. J.; Heath P. R.; Holden H.; Andrews P. W. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat. Biotechnol. 2007, 25, 207–215. 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- Cheung T. H.; Rando T. A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D.; Levi B. P.; Morrison S. J. Integrating physiological regulation with stem cell and tissue homeostasis. Neuron 2011, 70, 703–718. 10.1016/j.neuron.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga H.; Fukuyama M.; Kitazawa A.; Kontani K.; Katada T. The microRNA miR-235 couples blast-cell quiescence to the nutritional state. Nature 2013, 497, 503–506. 10.1038/nature12117. [DOI] [PubMed] [Google Scholar]
- Winer J. P.; Janmey P. A.; McCormick M. E.; Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng., Part A 2009, 15, 147–154. 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- Watanabe K.; Ueno M.; Kamiya D.; Nishiyama A.; Matsumura M.; Wataya T.; Takahashi J. B.; Nishikawa S.; Muguruma K.; Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Tyndale-Biscoe H., Renfree M.Reproductive Physiology of Marsupials; Cambridge University Press: Cambridge, 1987. [Google Scholar]
- Swift J.; Ivanovska I. L.; Buxboim A.; Harada T.; Dingal P. C.; Pinter J.; Pajerowski J. D.; Spinler K. R.; Shin J. W.; Tewari M.; Rehfeldt F.; Speicher D. W.; Discher D. E. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013, 341, 1240104. 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh G. E.; Coates P. J.; Lane E. B.; Raymond Y.; Quinlan R. A. Distinct nuclear assembly pathways for lamins A and C lead to their increase during quiescence in Swiss 3T3 cells. J. Cell Sci. 1997, 110, 2483–2493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.