Abstract
Objectives: Omentin-1 is a novel adipokine that has a pivotal role in modulating the insulin sensitivity, immunity and inflammation. The current study was conducted to evaluate the serum omentin-1 level in hepatitis C virus (HCV) infected patients, with or without type 2 diabetes, and to investigate its correlation with liver function parameters and insulin resistance.
Methods: Eighty subjects were enrolled in this study divided into four groups: chronic HCV infected patients (n=20), chronic hepatitis C patients with concomitant type 2 diabetes (n=18), type 2 diabetic patients (n=22) and 20 healthy controls. Serum omentin-1 levels were assessed using enzyme-linked immunosorbent assay (ELISA). Fasting blood glucose, insulin levels, and liver parameters including aminotransferases (ALT and AST) were determined.
Results: Serum omentin-1 levels were significantly elevated in HCV infected patients compared to all other groups. Omentin-1 levels were positively correlated with AST and ALT levels (r =0.43, p< 0.001; r =0.423, p<0.001, respectively). Additionally, a significant negative correlation was found between omentin-1 and both fasting insulin levels and homeostasis model assessment of insulin resistance (HOMA-IR) (r = -0.238, p<0.05; r = -0.277, p<0.05, respectively). Furthermore, fasting blood glucose, HbA1c and HOMA-β were negatively correlated to serum omentin-1 levels however these correlations were not statistically significant.
Conclusion: Serum omentin-1 level is elevated in HCV infected patients and is positively associated with liver enzymes AST and ALT. This suggested that omentin-1 may be implicated in the pathogenesis of hepatitis C and its metabolic complications. However its role needs to be elucidated by further studies.
Keywords: Hepatitis C, insulin resistance, omentin-1, type 2 diabetes
Introduction
Chronic hepatitis C (CHC) has been considered a metabolic disease rather than just a viral disease as it is usually associated with impaired glucose tolerance, insulin resistance, steatosis and changes in lipid metabolism (Mehta et al., 2000[19]; Perlemuter et al., 2002[21]). The prevalence of insulin resistance (IR) and type 2 diabetes (T2D) has been demonstrated to be higher in chronic hepatitis C than non infected population (Torres and Harrison, 2008[28]). This finding was confirmed by studies shown that the IR in CHC patients is greatly improved after successful therapy of HCV (Simó et al., 2006[25]; Kawaguchi et al., 2007[14]). Furthermore, it has been demonstrated that IR and T2D hasten the progression of liver fibrosis and cirrhosis in CHC patients (Hui et al., 2003[12]; Fartoux et al., 2005[9]). Despite the close relationship between HCV infection and T2D, the underlying mechanisms that link both of them remain elusive.
Adipokines are diverse group of mediators secreted from the adipose tissue. They have a potential role in modulating the insulin sensitivity, immunity and inflammation (Rabe et al., 2008[22]). Adipokines have been reported to play an important role in pathogenesis of liver fibrosis and in the metabolic consequences of liver disorders, however their role is not fully elucidated (Bertolani and Marra, 2008[2]; Marra and Bertolani, 2009[17]; Yilmaz et al., 2011[34]). A study by Kukla et al. (2010[15]) has shown that serum adipokines (chemrin and leptin) were significantly higher in patients with chronic hepatitis C compared to healthy controls. Additionally, chemrin was inversely correlated with the inflammatory grade in CHC patients.
Omentin-1 (Intelectin-1) is a newly identified protein that is highly and selectively expressed in visceral adipose tissue (Schäffler et al., 2005[24]). Omentin-1 may act as an endocrine factor affecting muscles, liver and omental adipose depot to enhance insulin sensitivity and glucose metabolism (Yang et al., 2006[33]). Interestingly, circulating omentin-1 levels have been negatively correlated with obesity and insulin resistance (Pan et al., 2010[20]). Recently, a study by Yilmaz et al. (2011[34]) has demonstrated that serum omentin-1 is elevated in patients with fatty liver diseases and it represents an independent predictor for hepatocyte ballooning in those patients. Another study by Eisinger et al. (2013[7]) has reported that omentin-1 level is increased in liver cirrhosis but is not associated with complications of portal hypertension. However, the role of omentin-1 in the chronic hepatitis C and its metabolic consequences is unexplored yet. This led us to conduct the present study in which we investigated the levels of omentin-1 in sera of chronic hepatitis C patients, with and without type 2 diabetes, and compared them with its levels in healthy controls. Additionally, we evaluated the correlation of omentin-1 with IR and biochemical parameters of liver injury.
Materials and Methods
Study population
Eighty subjects (65 male and 15 female) were enrolled in this study. They were classified into four groups: group 1 (HCV) comprised of patients with chronic HCV infection (n=20), group 2 (HCV/DM) patients suffering from both HCV and type 2 diabetes (n=18), group 3 (DM) patients with type 2 diabetes (n=22), and group 4 healthy controls (n=20). Patients of groups 2 and 3 were recruited from the National Institute of Diabetes and Endocrinology (NIDE). However, group 1 patients were recruited from the National Institute of Endemic Diseases and Liver, El Kasr El-Einy, Cairo.
Type 2 diabetes was diagnosed according to WHO criteria (WHO, 2006[31]). The duration of diabetes was not more than 4 years in order to exclude any diabetic complications. On the other hand, chronic hepatitis C patients, with or without diabetes, showed abnormal serum aminotransferases for more than 6 months duration and showed positive results when tested for serum anti-HCV antibodies.
The following exclusion criteria were used for all subjects: hypertension, cardiopulmonary disease, renal disease, thyroid dysfunction, malignancy, smokers, alcoholics, other causes of chronic liver disease as chronic hepatitis B, autoimmune hepatitis, acute hepatitis, haemochromatosis, hepatocellular carcinoma, biliary disorders. Patients have previous interferon treatment or recently received any anti-inflammatory drugs were also excluded.
The study was approved by the Research Ethics Committee of the General Organization for Teaching Hospitals and Institutes and the National Research Centre (Approval No. IDEG0147, Date 25/6/2012). All subjects gave written informed consent prior to participation in the study. The study was carried out in accordance with the regulations and recommendations of the Declaration of Helsinki.
A detailed patient's history was obtained; body mass index (BMI) was calculated as an index of the weight (in kilograms) divided by the square of the height (in meters) measured on the same day of sample withdrawal.
Overnight fasting blood samples were withdrawn from the antecubital vein. Blood samples were divided into three parts: the first part was collected into 0.5 M EDTA-containing tubes for determination of glycated heamoglobin (HbA1C), the second part was collected into fluoride-containing tubes for determination of FBG and the last part (6 ml) was collected into serum separating tubes for determination of lipid profile, liver function, serological testing (Anti HCV, HBsAG ), insulin and omentin-1 assay. Sera were separated by centrifugation at 3000 rpm for 10 min. Each serum sample was divided into several aliquots and kept at -80 °C until analysis.
Laboratory assessments
Serological testing: to exclude HBV infection, hepatitis B surface antigen (HBsAg) was tested in serum by chromatographic immunoassay using rapid test strip (Acon® Diagnostics, USA). Anti-HCV antibodies were tested qualitatively by chromatographic immunoassay using rapid test strips provided by Acon® Diagnostics, USA. Serum aminotransferase (AST, ALT) were assayed colorimetrically according to Reitman and Frankel Method (Reitman and Frankel, 1957[23]) using kits from Spectrum Diagnostics, Egypt. Fasting blood glucose was determined by colorimetric enzymatic hexokinase method (Siemens Healthcare Diagnostic, USA). Total cholesterol and triacylglycerol were determined by enzymatic colorimetric end point methods (Allain et al., 1974[1]; Fossati and Prencipe, 1982[10]). LDL cholesterol was determined according to heparin/citrate precipitation method and HDL cholesterol was measured according to phosphotungestic acid precipitation method (Burstein et al., 1970[3]; Castelli et al., 1977[5]). All of lipid profile parameters were measured using kits provided by Spectrum Diagnostics, Egypt. HbA1C was determined using HPLC fully automated system with Bio-Rad D-10 Hemoglobin testing system (Jeppsson et al., 1986[13]). All colorimetric and spectrophotometric assays were determined using appropriate semiautomated photometer (JASCO®V-630UV-Vis Spectrophotometer, USA).
Serum insulin was estimated using insulin enzyme-linked immunosorbant assay (ELISA) kit (DRG Insulin, DRG International Inc., USA) according to the manufacturer's protocol. Insulin resistance was determined by the homeostasis model of assessment (HOMA-IR) (Matthews et al., 1985[18]) using the formula: [fasting blood glucose (mg/dL) X fasting insulin (μIU/mL)]/405. Beta cell function was determined by (HOMA-β) using the formula:
[360×fasting insulin (μIU/mL)] / [fasting blood glucose (mg/dL) - 63] %.
Serum omentin-1 concentrations were determined by enzyme-linked immunosorbant assay (ELISA) kit (CUSABIO Biotech Co., USA) according to the manufacturer's instructions using ELx808™ Absorbance Microplate Reader, Bio-tek instruments, USA.
Statistical analysis
GraphPad Instat™ statistics software (1992-2000 Graph software Inc., V 3.05, Ralf Stahlman, Purdue Univ.931897 S) was used for data analysis. Results were expressed as mean ± SEM. To analyze more than two sets of data, ordinary one way analysis of variance (ANOVA) for parametric data was tried first, followed by Tukey-Kramer multiple comparisons test, when Bartlett's test for homogeneity of variance was not-significant. If Bartlett's test was significant, logarithmic transformation of the individual data was performed before testing ANOVA again, and then followed by Tukey-Kramer, when Bartlett's test became not-significant. If logarithmic transformation did not reduce variability between individual values shown as significant difference of Bartlett's test, the comparison was carried out using ANOVA for non-parametric data (Kruskal Wallis test) followed by Dunn's test. The difference between groups was considered significant if the probability of error was ≤ 0.05. The correlations between variables were determined by Pearson's correlation coefficient. The analysis was repeated using multivariate analysis of covariance (MANCOVA) which was adjusted for age using SPSS software program version 14 (SPSS Inc, Chicago, IL).
Results
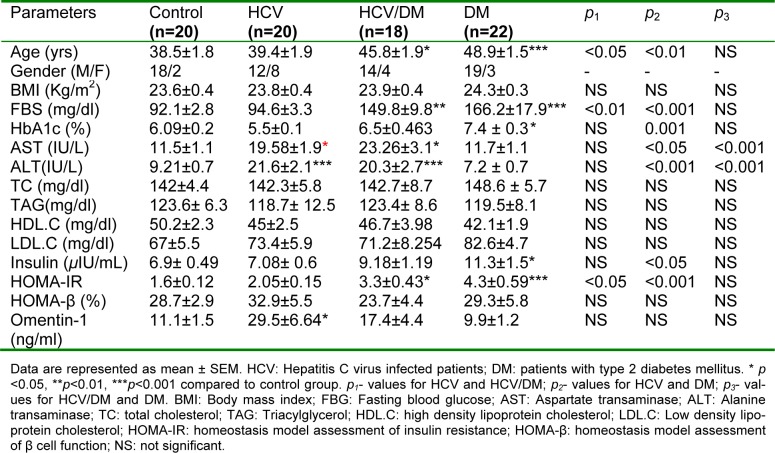
Table 1(Tab. 1) displays the demographic data and the studied biochemical parameter for all groups. No significant difference was found among the studied groups in terms of BMI, triglycerides, total cholesterol, HDL cholesterol and LDL cholesterol. However, diabetic and HCV/DM patients were characterized by slightly higher mean age (48.9±1.553 and 45.83±1.952, respectively) compared to the healthy controls and HCV group (38.5±1.8 and 39.4±1.9, respectively).
Table 1. Demographic data and laboratory characteristics of the studied groups.
Chronic hepatitis C patients, either diabetic or non-diabetic, had significantly higher AST and ALT levels compared to both control and diabetic groups as depicted in Table 1(Tab. 1). The fasting blood glucose levels were increased significantly in diabetic group and HCV/DM group (166.18±17.9 and 149.88±9.8, respectively) compared to control and HCV groups (92.1±2.8 and 94.6±3.33, respectively). On the other hand, insulin levels in diabetic and HCV/DM patients were markedly higher than its levels in HCV patients and control subjects (11.3±1.5 and 9.18±1.19 vs. 7.08±0.6 and 6.9±0.49). However, this increase was statistically significant only in the diabetic group (Table 1(Tab. 1)).
Furthermore, diabetic and HCV/DM groups demonstrated significantly higher HOMA-IR values than the control group (p<0.001 and p<0.05, respectively) and HCV group (p <0.001, p < 0.05, respectively). Yet, no significant difference was detected among groups concerning the HOMA-β mean values.
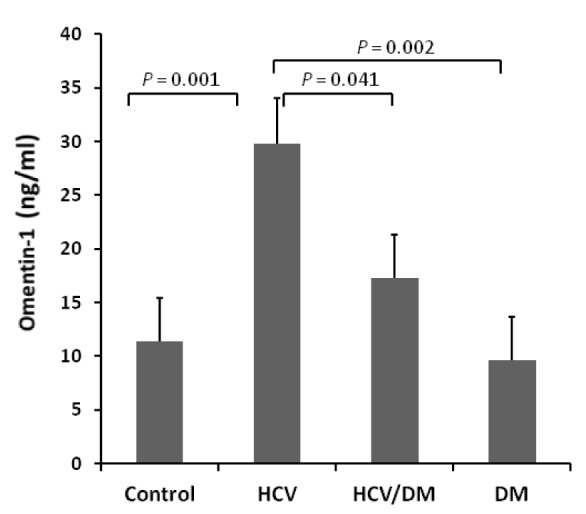
The levels of omentin-1 in all studied groups are depicted in Table 1(Tab. 1). The HCV group demonstrated significantly higher omentin-1 values compared to the control group (p<0.05). Whereas, after omentin-1 values were adjusted for age, HCV group showed significantly higher omentin-1 values than all other studied groups (Figure 1(Fig. 1)).
Figure 1. Age adjusted serum omentin-1 values in all studied groups. Data are expressed as mean ± SEM.
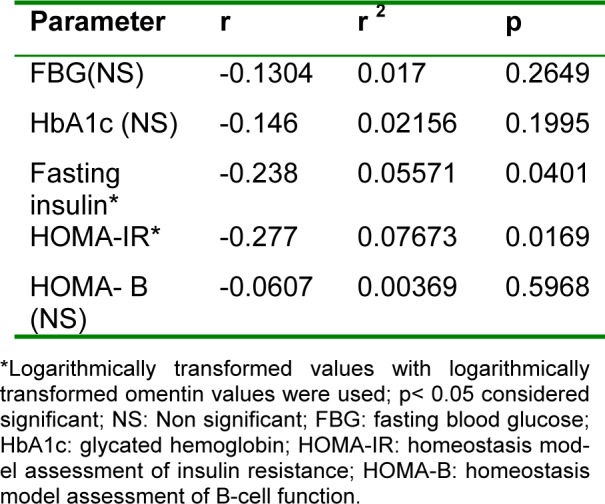
Then we evaluated the correlation coefficients of serum omentin-1 with all the studied parameters. Serum omentin-1 levels demonstrated significant positive correlations with AST and ALT levels (r =0.4, p< 0.001; r =0.423, p<0.001, respectively) (Figure 2(Fig. 2)). On the other hand, omentin-1 levels revealed significant negative correlation to fasting insulin levels and HOMA-IR (r = - 0.238, p<0.05; r = - 0.277, p<0.05, respectively). Furthermore, fasting blood glucose, HbA1c and HOMA-β were negatively correlated to serum omentin-1 levels however those correlations did not reach statistical significance (Table 2(Tab. 2)).
Figure 2. Correlation of serum omentin-1 with ALT (A) and AST (B).
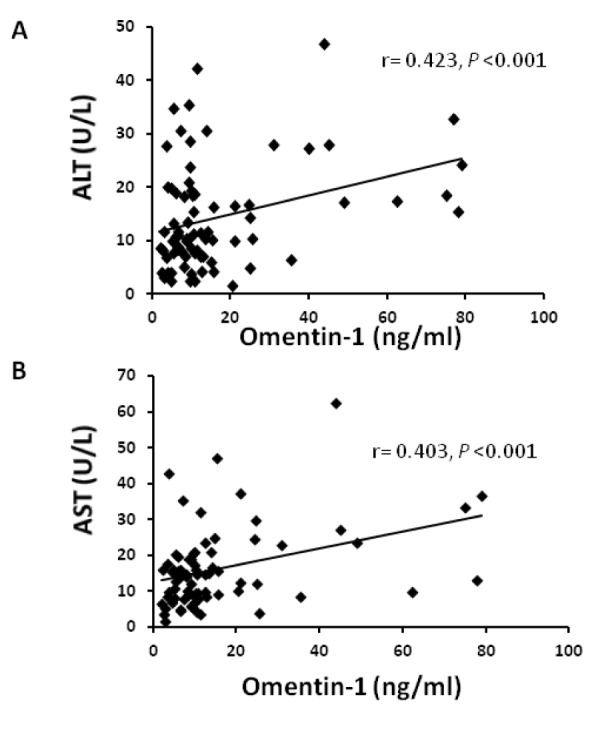
Table 2. Correlations between omentin-1 levels and other studied parameters.
Discussion
Insulin resistance (IR) and type 2 diabetes (T2D) have been demonstrated to be more prevalent among chronic hepatitis C than non infected population (Torres and Harrison, 2008[28]). Despite the close relationship between HCV infection and T2D, the underlying mechanisms of HCV-associated IR and diabetes remain elusive. Adipokines, chemrin and vaspin, have been reported to play a role in the pathogenesis of inflammatory process of CHC and IR caused by HCV (Kukla et al., 2010[15]). However, this role is not fully understood.
Omentin-1 (intelectin-1) is a newly identified adipokine that is highly and selectively expressed in visceral adipose tissue (Schäffler et al., 2005[24]). Omentin-1 enhances insulin sensitivity and glucose metabolism (Yang et al., 2006[33]). Circulating omentin-1 level is elevated in non alcoholic fatty liver disease and is positively correlated with hepatocyte ballooning (Yilmaz et al., 2011[34]). Yet, the role of omentin-1 in liver disorders and their metabolic complications is still ambiguous.
To our knowledge, the current study is the first study exploring the serum omentin-1 levels in chronic hepatitis C patients with or without type 2 diabetes. A salient finding of our study is that serum omentin-1 levels were significantly elevated in HCV patients compared to healthy controls. So it seems that omentin-1 has a role in the pathogenesis of hepatitis. This finding is in agreement with a recent study by Eisinger et al. (2013[7]) showing that omentin-1 is elevated in patients with liver cirrhosis. Another study by Yilmaz and his coworkers (2011[34]), has reported that omentin-1 levels were significantly higher in patients with non alcoholic fatty liver disease than in healthy controls. This finding may be explained, at least partially, by the implication of omentin-1 in immune and inflammatory response. According to growing body of literature, omentin-1 (intelectin-1) is increased during bacterial infection (Tsuji et al., 2009[29]), asthma (Kuperman et al., 2005[16]), and mesothelioma (Wali et al., 2005[30]). So it is thought that omentin-1 may play a pivotal role in innate immunity and inflammation. However its exact role is not fully elucidated.
Furthermore, our results demonstrated that age adjusted omentin-1 levels in HCV /DM patients were significantly decreased compared to HCV patients. This may be explained by high glucose and insulin levels in blood of HCV/DM patients compared to HCV patients without diabetes. It has been reported that plasma omentin-1 was declined after prolonged insulin-glucose infusion in healthy individuals. Additionally, treating the human omental tissue explants with glucose and insulin caused down-regulation of omentin-1 expression (Tan et al., 2008[26]). Another study by Gürsoy et al. (2010[11]) has reported that glucose and insulin levels may have a repressive effect on omentin-1 levels.
In the last few years, several studies have reported that omentin-1 levels in type 2 diabetic patients are decreased compared to healthy control (Tan et al., 2008[27]; Pan et al., 2010[20]; Gürsoy et al., 2010[11]). In the current study, the diabetic group of patients showed decreased omentin-1 level than healthy controls. Yet, this difference was not statistically significant. This finding is in accordance with a study by El-Mesallamy et al. (2011[8]) reporting that no significant difference was observed in omentin-1 serum levels between the non-obese group with type 2 diabetes, and the non-obese healthy control group (BMI < 30 kg/m2). Notably, all subjects enrolled in our study had normal BMI to avoid the variation in plasma omentin-1 level due to this factor which is one of the major determinants of omentin-1 level as shown by several studies (De Souza Batista et al., 2007[6]; Gürsoy et al., 2010[11]; El-Mesallamy et al., 2011[8]).
In accordance with previous studies, our results demonstrated that serum omentin-1 level was negatively correlated with fasting insulin and HOMA-IR (De Souza Batista et al., 2007[6]; Gürsoy et al., 2010[11]; Yan et al., 2011[32]). Furthermore, in agreement to what was reported by De Souza Batista et al. (2007[6]) the correlation of omentin-1 level with fasting blood glucose was not significant in the present study. This finding is in opposition to others (Gürsoy et al., 2010[11]; El-Mesallamy et al., 2011[8]).
On the other hand, omentin-1 level showed significant positive correlation with liver enzymes AST and ALT. This declares that, omentin-1 level is correlated to the hepatocyte degeneration. This finding is in agreement with a study by Yilmaz et al. (2011[34]) reporting that serum omentin-1 level is positively correlated with C reactive protein and hepatocyte ballooning suggesting an association between omentin-1 and hepatocyte death (Caldwell et al., 2010[4]; Yilmaz et al., 2011[34]). In aforementioned study, they did not correlate the serum omentin-1 level to AST and ALT levels. Additionally, it has been recently reported that omentin-1 induces the apoptosis of hepatocytes by increasing the stability of p53 (Zhang and Zhou, 2013[35]). On the other hand, a recent study by Eisinger et al. (2013[7]) has reported that omentin-1 is not associated with liver function in patients with liver cirrhosis. We have to point out that their study was conducted on liver cirrhosis caused by different etiologies which is different than the current study in which all patients were HCV infected patients.
It is noteworthy that, information about serum omentin-1 levels in chronic hepatitis C patients are scanty and additional larger studies are required to confirm the findings of this study and to identify mechanisms for the paradoxical increase of omentin-1 levels in HCV patients. In conclusion, the salient finding of our study is the increased serum omentin-1 levels in HCV patients compared to the healthy subjects. Furthermore, serum omentin-1 showed significant positive correlation with liver enzymes AST and ALT. Age adjusted omentin-1 values in HCV patients with type 2 diabetes were significantly decreased compared to HCV patients not suffering from diabetes. This decline in omentin-1 levels may be attributed to increased glucose and insulin levels in sera of diabetic patients which have suppressive effect of omentin-1 expression. This indicates that omentin-1 may be implicated in the pathogenesis of hepatitis and its metabolic complications however its role needs to be elucidated by future studies.
Acknowledgement
The authors would like to express their deep appreciation to Prof. Ehsan Hassan, and the staff of National Institute of Endemic Diseases and Liver, Cairo University, for facilitating sample collection. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare that they have no competing interests to disclose.
References
- 1.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 2.Bertolani C, Marra F. The role of adipokines in liver fibrosis. Pathophysiology. 2008;15:91–101. doi: 10.1016/j.pathophys.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583–95. [PubMed] [Google Scholar]
- 4.Caldwell S, Ikura Y, Dias D, Isomoto K, Yabu A, Moskaluk C, et al. Hepatocellular ballooning in NASH. J Hepatol. 2010;53:719–23. doi: 10.1016/j.jhep.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, et al. Alcohol and blood lipids. The cooperative lipoprotein phenotyping study. Lancet. 1977;2:153–5. doi: 10.1016/s0140-6736(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 6.De Souza Batista CM, Yang R-Z, Lee M-J, Glynn NM, Yu D-Z, Pray J, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–61. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 7.Eisinger K, Krautbauer S, Wiest R, Karrasch T, Hader Y, Scherer MN, et al. Portal vein omentin is increased in patients with liver cirrhosis but is not associated with complications of portal hypertension. Eur J Clin Invest. 2013;43:926–932. doi: 10.1111/eci.12122. [DOI] [PubMed] [Google Scholar]
- 8.El-Mesallamy HO, El-Derany MO, Hamdy NM. Serum omentin-1 and chemerin levels are interrelated in patients with Type 2 diabetes mellitus with or without ischaemic heart disease. Diabet Med. 2011;28:1194–200. doi: 10.1111/j.1464-5491.2011.03353.x. [DOI] [PubMed] [Google Scholar]
- 9.Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003–8. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–80. [PubMed] [Google Scholar]
- 11.Gürsoy G, Kırnap NG, Esbah O, Acar Y, Demirbas B, Akçayöz S, et al. The relationship between plasma omentin-1 levels and insulin resistance in newly diagnosed type 2 diabetic women. Clin Rev Opin. 2010;2:49–54. [Google Scholar]
- 12.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression. Gastroenterology. 2003;125:1695–704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 13.Jeppsson JO, Jerntorp P, Sundkvist G, Englund H, Nylund V. Measurement of hemoglobin A1c by a new liquid-chromatographic assay: methodology, clinical utility, and relation to glucose tolerance evaluated. Clin Chem. 1986;32:1867–72. [PubMed] [Google Scholar]
- 14.Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570–6. doi: 10.1111/j.1572-0241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 15.Kukla M, Zwirska-Korczala K, Gabriel A, Waluga M, Warakomska I, Szczygiel B, et al. Chemerin, vaspin and insulin resistance in chronic hepatitis C. J Viral Hepat. 2010;17:661–7. doi: 10.1111/j.1365-2893.2009.01224.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, et al. Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol. 2005;116:305–11. doi: 10.1016/j.jaci.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–69. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–9. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 20.Pan H-Y, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88:29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–94. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 22.Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–51. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Schäffler A, Neumeier M, Herfarth H, Fürst A, Schölmerich J, Büchler C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta. 2005;1732:96–102. doi: 10.1016/j.bbaexp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Simó R, Lecube A, Genescà J, Esteban JI, Hernández C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006;29:2462–6. doi: 10.2337/dc06-0456. [DOI] [PubMed] [Google Scholar]
- 26.Tan BK, Adya R, Farhatullah S, Lewandowski KC, O’Hare P, Lehnert H, et al. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57:801–8. doi: 10.2337/db07-0990. [DOI] [PubMed] [Google Scholar]
- 27.Tan BK, Pua S, Syed F, Lewandowski KC, O’Hare JP, Randeva HS. Decreased plasma omentin-1 levels in type 1 diabetes mellitus. Diabet Med. 2008;25:1254–5. doi: 10.1111/j.1464-5491.2008.02568.x. [DOI] [PubMed] [Google Scholar]
- 28.Torres DM, Harrison SA. Hepatitis C virus and insulin resistance/diabetes mellitus. Gastroenterol Hepatol. 2008;4:568–70. [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuji S, Yamashita M, Hoffman DR, Nishiyama A, Shinohara T, Ohtsu T, et al. Capture of heat-killed Mycobacterium bovis bacillus Calmette-Guérin by intelectin-1 deposited on cell surfaces. Glycobiology. 2009;19:518–26. doi: 10.1093/glycob/cwp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wali A, Morin PJ, Hough CD, Lonardo F, Seya T, Carbone M, et al. Identification of intelectin overexpression in malignant pleural mesothelioma by serial analysis of gene expression (SAGE) Lung Cancer. 2005;48:19–29. doi: 10.1016/j.lungcan.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 31.WHO, World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/international Diabetes Federation consultation. Geneva: WHO; 2006. [Google Scholar]
- 32.Yan P, Liu D, Long M, Ren Y, Pang J, Li R. Changes of serum omentin levels and relationship between omentin and adiponectin concentrations in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2011;119:257–63. doi: 10.1055/s-0030-1269912. [DOI] [PubMed] [Google Scholar]
- 33.Yang R-Z, Lee M-J, Hu H, Pray J, Wu H-B, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol. 2006;290:E1253–61. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz Y, Yonal O, Kurt R, Alahdab YO, Eren F, Ozdogan O, et al. Serum levels of omentin, chemerin and adipsin in patients with biopsy-proven nonalcoholic fatty liver disease. Scand J Gastroenterol. 2011;46:91–7. doi: 10.3109/00365521.2010.516452. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y-Y, Zhou L-M. Omentin-1, a new adipokine, promotes apoptosis through regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur J Pharmacol. 2013;698:137–44. doi: 10.1016/j.ejphar.2012.11.016. [DOI] [PubMed] [Google Scholar]