Abstract
Fetal alcohol spectrum disorder (FASD) is a leading cause of neurodevelopmental disability. Some affected individuals possess distinctive craniofacial deficits, but many more lack overt facial changes. An understanding of the mechanisms underlying these deficits would inform their diagnostic utility. Our understanding of these mechanisms is challenged because ethanol lacks a single receptor when redirecting cellular activity. This review summarizes our current understanding of how ethanol alters neural crest development. Ample evidence shows that ethanol causes the “classic” fetal alcohol syndrome (FAS) face (short palpebral fissures, elongated upper lip, deficient philtrum) because it suppresses prechordal plate outgrowth, thereby reducing neuroectoderm and neural crest induction and causing holoprosencephaly. Prenatal alcohol exposure (PAE) at premigratory stages elicits a different facial appearance, indicating FASD may represent a spectrum of facial outcomes. PAE at this premigratory period initiates a calcium transient that activates CaMKII and destabilizes transcriptionally active β-catenin, thereby initiating apoptosis within neural crest populations. Contributing to neural crest vulnerability are their low antioxidant responses. Ethanol-treated neural crest produce reactive oxygen species, and free radical scavengers attenuate their production and prevent apoptosis. Ethanol also significantly impairs neural crest migration, causing cytoskeletal rearrangements that destabilize focal adhesion formation; their directional migratory capacity is also lost. Genetic factors further modify vulnerability to ethanol-induced craniofacial dysmorphology, and include genes important for neural crest development including shh signaling, PDFGA, vangl2, and ribosomal biogenesis. Because facial and brain development are mechanistically and functionally linked, research into ethanol’s effects on neural crest also informs our understanding of ethanol’s CNS pathologies.
Keywords: neural crest, fetal alcohol spectrum disorders, craniofacial, apoptosis, calcium signaling, sonic hedgehog
Introduction to fetal alcohol spectrum disorders
Prenatal alcohol exposure (PAE) is the most common teratogen exposure in humans and is the greatest known cause of developmental disability. Conservatively, PAE adversely affects 9.1 to 50 per 1000 live births in the U.S. and 68.0 to 89.2 per 1000 in populations with high levels of alcohol abuse (May et al., 2007, 2009; Sampson et al., 1997). It causes lifelong behavioral and cognitive deficits that impair the ability to function in society, including executive function, learning, attention, and motor skills; IQ is generally less affected (Stratton et al., 1996; Mattson et al., 2010). Animal models reveal that PAE impairs multiple neurodevelopmental processes including neuronal induction, proliferation, survival, migration, synaptogenesis, and white matter formation; these deficits parallel the brain changes documented in affected individuals. The severity of this damage varies individually and is shaped by factors such as exposure pattern, dose, and timing, genetics, nutrition, and other environmental influences (May et al., 2008, 2009, 2011; Jacobson et al., 2004). A significant challenge for PAE is the paucity of clear diagnostic markers that permits identification of affected individuals. Individuals who have neurodevelopmental deficits and known PAE reside along the continuum of effects known as Fetal Alcohol Spectrum Disorders (FASD). Approximately 10% of these exhibit the diagnostic criteria of behavioral deficits, body growth reduction, and a distinctive craniofacial appearance and are defined clinically with Fetal Alcohol Syndrome (FAS) (Stratton et al., 1996). The reminder have equally significant neurodevelopmental impairments but apparently lack the facial and growth deficits (Mattson et al., 2010; Streissguth et al. 2004). Detailed anthropometric analyses recently revealed more subtle facial dysmorphologies in this latter population, suggesting that craniofacial dysmorphology is a useful diagnostic tool (Foroud et al., 2012). Because diagnosis facilitates patient access to support services, there is great interest in understanding the mechanisms underlying these craniofacial deficits and thereby their precise clinical context.
Unfortunately, a clear understanding of these underlying mechanisms has challenged investigators because, unlike many teratogens, ethanol lacks a single receptor. Instead, ethanol interacts, at levels as low as 0.02% (mg/dl) or 5 mM, with defined water-binding pockets within select proteins (Howard et al., 2011; Mihic et al., 1997). The best characterized of these mediate neurotransmission and thereby the anxiolytic, reward, and addictive properties of ethanol. The proteins that are targeted by ethanol during the embryo and fetal periods are less well characterized. Contrary to popular opinion, the embryo and fetus have greater ethanol vulnerability compared with adults. Fetal ethanol concentrations average two-thirds of maternal blood levels. However, fetal liver weakly metabolizes ethanol and thus the fetus depends on maternal catabolism for disposal; ethanol also persists in the amniotic fluid. Because behavioral and genetic variables preclude definition of a “safe” ethanol dose, public health recommendations advise abstention during pregnancy and lactation. Animal studies show that even social drinking exposures (0.02% to 0.08%) have lasting detrimental effects (Hamilton et al., 2014 and references therein). Some confusion arises because rodent studies generally utilize higher ethanol exposures (0.1% to 0.3%, or 22 mM to 66 mM) as compared with those experienced by humans, and this is because rodents catabolize ethanol more rapidly than do humans.
Craniofacial changes in FASD
Individuals with FASD often have a distinctive craniofacial appearance. The diagnostic criteria for FAS/partial FAS include the trio of small palpebral fissures, a flattened philtrum, and a thin upper lip. These changes are often accompanied by a flattened nasal bridge, micrognathia, epicanthal folds, and a reduced epicanthal and interpupillary distance (Hoyme et al., 2005; Klingenberg et al., 2010). These soft tissue changes are accompanied by underlying bone and cartilage changes with a flattened midface and mouth area. However, facial changes also occur in those who do not meet the U.S. Institute of Medicine (IOM) criteria for FAS/PFAS (partial FAS) (Stratton et al., 1996). A recent morphometric comparison of age-matched children having FAS/PFAS vs. heavy PAE vs. controls found that reductions in ear length, facial depth, and frontal width had the highest predictability for PAE (Foroud et al., 2012). The facial changes from PAE persist across multiple racial and ethnic backgrounds. While many of the bone and tissue changes suggest an adverse effect of PAE upon neural crest development, other craniofacial changes are likely secondary to the brain growth reductions that are also common in those with PAE (Lipinski et al., 2012; O’Leary-Moore et al., 2011). The facial changes observed in PAE strongly correlate with adverse neurobehavioral outcomes, and this strong association validates their diagnostic importance as useful markers for PAE (Foroud et al., 2012).
At its first discovery some suggested that FAS resulted from environmental or genetic factors unrelated to alcohol. However, elegant morphological work by Sulik and colleagues demonstrated that the facial changes observed in FAS could be reproduced in a mouse model of PAE, including the small palpebral fissures, midfacial flattening and reduction, and the elongated upper lip with deficient philtrum. This was succeeded in older embryos by substantial reductions of the prosencephalon, as well as the facial primordia and other brain regions (Sulik et al., 1981; Sulik and Johnston, 1983; Sulik 1984). Because brain size influences face size, the smaller prosencephalon likely contributed to the facial changes (see below). Additional mechanistic insight emerged from their identification of substantial cell degeneration that was largely restricted to the neuroepithelium and anterior neural folds, including regions enriched in neural crest progenitors (Sulik et al., 1981; Kotch and Sulik, 1992a). Similar facial changes occur following ethanol exposure in other species including non-human primate, chick, zebrafish, and Xenopus, suggesting that this represents an evolutionarily conserved action of ethanol and thus likely also operates in human PAE (Carvan et al., 2004; Cartwright and Smith, 1995; Sheller et al., 1988; Nakatsuji, 1983). Enumeration of neural crest populations following ethanol exposure confirms that ethanol significantly reduces cranial neural crest numbers (Flentke et al., 2011, 2014b; Garic et al., 2011). Thus, compelling morphological and cellular data affirm that PAE can produce a neurocristopathy.
Analyses of human populations as well as animal models of PAE suggest that ethanol does not affect neural crest populations uniformly. The development of cranial neural crest populations is most commonly affected by ethanol, with reductions in their derived facial bone and cartilage, cranial nerves, tooth structure, and cardiac outflow tract. Trunk neural crest populations can undergo apoptosis in response to ethanol (Czarnobaj et al., 2014; Rovasio and Battiato, 2002). However, melanocyte changes are not observed in FASD, and changes to the parasympathetic and sympathetic nervous systems, if any, are not well defined. Ethanol’s apparent specificity for cranial neural crest is not understood and may reflect the greater plasticity and renewal capacity of non-cartilage neural crest, as well as inherent differences between these two populations (Czarnobaj et al., 2014; Wentzel and Eriksson, 2009).
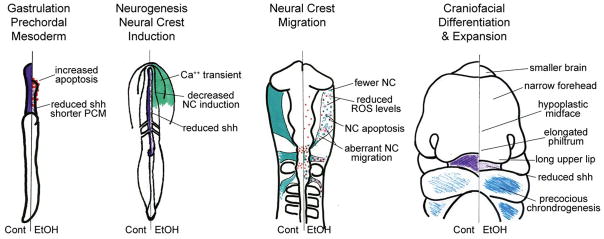
Research shows that ethanol adversely affects multiple events of neural crest development including induction, expansion, apoptosis, migration, and differentiation. These effects are summarized in Figure 1 and are detailed below. The complexity of ethanol’s action is consistent with its capacity to interact with multiple protein targets. For example, ethanol exposure during gastrulation generates a craniofacial appearance that differs from that generated by exposure during neural crest migration (i.e. Lipinski et al., 2012; see below). Thus the clinical criteria for the PAE face will continue to be refined as the ethanol-neural crest-brain interaction is better understood. In practice, however, PAE is seldom restricted to a single binge exposure. Women who abuse alcohol typically do so throughout the pregnancy, for example, with binge drinking every weekend. Thus what becomes labeled as the PAE face is a composite of ethanol’s multiple mechanisms.
Figure 1.
Effects of ethanol upon neural crest development. Although chick embryos are depicted, these changes have been documented in diverse vertebrate species including mouse, zebrafish, and Xenopus. The left-half of each embryo depicts normal development, and each right-half contrasts the consequences of ethanol exposure. Details of these changes are presented in the text. Purple, shh; teal, neural crest; blue, chondrogenesis; red dots, apoptosis; pink stars, ROS; teal squiggles, migrating neural crest. Cont, control; EtOH, ethanol; NC, neural crest; PCM, prechordal plate; ROS, reactive oxygen species.
Effects on midline, brain, and sonic hedgehog
Contributions to the craniofacial dysmorphology arise, in part, from ethanol’s disruption of midline expansion and sonic hedgehog (shh) signaling. Sulik was the first to suggest that the classic facial changes in FAS fall within the holoprosencephaly (HPE) spectrum (Sulik, 1984). Both mammalian and nonmammalian FASD models recreate these features and include midfacial hypoplasia, shortened and fused branchial arches, smooth philtrum, and hypocellularity and narrowing of the reduced telencephalon, prosencephalon and olfactory bulbs; exceptional high ethanol doses produce cyclopia (Aoto et al., 2008; Su et al., 2001; Carvan et al., 2004; Hong and Krause 2012; Li et al., 2007; Lipinski et al., 2012; Sulik, 1984). Mouse models show that this HPE phenotype is produced only when the ethanol exposure occurs during a narrow developmental window at gastrulation (e7.0), during the formation of the prechordal plate (Aoto et al., 2008; Kotch and Sulik, 1992; Sulik, 1984). At this stage, PAE may affect facial outcomes not through direct effects on neural crest, but indirectly through the impairment of neuroectoderm development. In both zebrafish and mouse, ethanol has been shown to significantly reduce the migration of the prechordal plate (Aoto et al., 2008; Blader and Strahl, 1998; Hong and Krauss, 2012). Accompanying this is substantial apoptosis within cells of the anterior prechordal mesendoderm (PME), and significantly reduced expression of several PME signals, including shh, fibroblast growth factor-8, and Foxa2 and goosecoid in the Notch pathway (Aoto et al., 2008; Hong and Krauss, 2012; Li et al., 2007). Endorsing a mechanistic role for SHH signaling is the demonstration that heterozygous loss-of-function in either SHH or Gli2 substantially magnify craniofacial dysmorphology and midline brain losses when ethanol exposure occurs at e7.0 (Kietzman et al., 2014).
Two distinct mechanisms have been identified to account for the loss of Shh. Ethanol has been shown to decrease shh at the protein level, through its suppression of cholesterol ester pools. As shown for the gastrulation-stage zebrafish embryo, this decreased availability of cholesterol esters pools reduces the substrate availability for the covalent esterification of the nascent N-terminal shh protein, which is necessary for the protein’s membrane association and shh signaling (Li et al., 2007). Increasing cholesterol-ester pools through exogenous means enhances shh activity in ethanol’s presence and rescues the zebrafish embryo’s morphology. Ethanol exposure has also been shown to activate protein kinase A (PKA), which is an endogenous repressor of Shh signaling (Aota et al. 2008). In mouse, ethanol treatment elevates PKA activity, as shown by the increased nuclear content of the PKA target pCREB within the anterior PME. Pretreatment with the PKA inhibitor KT5720 restores Shh expression to the anterior PME of ethanol-exposed mouse embryos. It also prevents apoptosis within the anterior PME. Both mechanisms are likely feasible, given ethanol’s pleiotrophic action, and the subsequent losses of shh would reduce the migration of the anterior PME and thereby reduce neuroectoderm induction especially along the anterior neural midline, contributing to HPE. Neural crest induction begins during gastrulation at the border between the neuroectoderm and ectoderm. Consistent with the proposed loss of neural crest induction, ethanol exposure at gastrulation (chick stage 4) causes a rapid decrease in several early neural crest markers and signals including Bmp4, Wnt6, FoxD3, and Snai2 Flentke et al. (2011). Additional research is needed to explore how ethanol affects the interactions between prechordal plate, neural plate, and induction of neural crest progenitors. In summary, ethanol’s suppression of midline induction, including formation of the neural plate and neural crest, contributes to the “typical” face (thin and elongated upper lip, absent philtrum, reduced midface) of individuals with FAS.
Effects on neural crest migration
In addition to its effects on midline formation, ethanol strongly suppresses neural crest migration. In response to ethanol challenge, fewer neural crest cells emigrate from the prosencephalon, mesencephalon, and rhomboencephalon (Cartwright and Smith, 1995; Rovasio and Battiato, 1995). Those that do emigrate largely follow the appropriate migratory routes (Cartwright and Smith 1995). However, at higher ethanol concentrations, a small number of neural crest cells (by merit of labeling for markers such as NC-1, snai2, or Sox9) instead appear within the lumen of the hindbrain and neural tube (Cartwright et al., 1998; Rovasio and Battiato, 2002), suggesting that migration and/or its cues are abnormal. Many of the migrating neural crest cells display apoptotic figures and can be co-labeled using TUNEL techniques (Cartwright et al. 1998; Garic et al. 2011; Rovasio and Battiato, 2002). Their abnormal migration was elegantly mapped in real-time using Sox10: EGFP zebrafish embryos (Boric et al. 2013). Under continuous ethanol exposure (100–200 mM), cranial neural crest migration loses its left-right symmetry with respect to the embryo’s midline and becomes profoundly asymmetric. The Sox10:EGFP-labeled cells travel a shorter distance over time and exhibit substantial retrograde motion along both the anteroposterior and mediolateral axes. These results suggest that ethanol impaired the cells’ migratory capacity, the substratum upon which they migrate, or both. Cell culture studies affirmed that the abnormal migration is inherent to the neural crest cells, at least in part. When cultured in ethanol’s presence (10.9 mM or 150 mM), fewer neural crest cells emerge from cranial-derived explants (Czarnobaj et al., 2014). The CNCs travel significantly shorter distances (50% of control values) and their migration is less directional, again with more retrograde movements (Czarnobaj et al., 2014; Rovasio and Battiato, 2002). At low ethanol (10.9mM) only the migration of cranial populations was impaired (Czarnobaj et al., 2014) and it took substantially higher ethanol levels (150mM) to impair trunk neural crest migration (Rovasio and Battiato, 2002). Importantly, this abnormal migration persisted for at least 24hr following ethanol removal, suggesting that ethanol caused lasting changes in neural crest migratory capacity (Rovasio and Battiato, 2002).
Insights into the basis for this impaired migration emerged from analyses of cellular morphology. Migrating neural crest normally have a stellate appearance with multiple filopodia. Ethanol exposure causes rapid (<30min) cytoskeletal remodeling within neural crest, with filopodia retractions and fewer focal adhesions (Hassler and Moran, 1986a, 1986b; Oyedele and Kramer, 2013; Rovasio and Battiato, 2002). Actin bundles are reorganized and thickened to define a linear, bipolar axis with significantly reduced arborization (Hassler and Moran, 1986a, 1986b; Oyedele and Kramer, 2013; Rovasio and Battiato, 2002). There are commensurate reductions in surface area and cell perimeter (Hassler and Moran, 1986a, 1986b; Oyedele and Kramer, 2013; Rovasio and Battiato, 2002), which may be dose- or substrate-dependent (Czarnobaj et al., 2014). The cytoskeletal remodeling and filopodia reductions persist for at least 24hrs after the ethanol removal, indicating that ethanol may have respecified the intracellular signals that govern cell migration (Rovasio and Battiato, 2002). Intracellular calcium is a potent regulator of focal adhesion assembly and cytoskeletal architecture, and one possibility is that the intracellular calcium transient elicited by ethanol (Debelak et al., 2003; see below) might also underlie this intracellular remodeling. Whether ethanol also alters the extracellular fibronectin/collagen stratum that directs neural crest migration is a question that needs investigation. Overall, data are compelling that ethanol exposure, even at pharmacologically “low” concentrations, impairs neural crest migration and this likely contributes to the facial deficits in FASD.
Effects on neural crest survival & apoptosis
Ethanol causes substantial cell death within the cranial neural crest (Figure 3; Kotch and Sulik, 1902; Cartwright and Smith, 1995; Rovasio and Battiato 2002) and eliminates as many as 50% of migrating neural crest cells, enumerated using markers such as Snai2, Sox9, HNK1, CRABP-I, and Crestin (Cartwright and Smith, 1995; Flentke et al., 2011, 2014b; Yamada et al., 2005). This cell death is apoptotic, as shown by its pyknotic appearance, by its labeling using classic apoptotic markers including Terminal Deoxynucleotidyl Transferase (TUNEL) and Annexin V-GFP reporters, and because the death is prevented using caspase-directed inhibitors (Cartwright et al., 1998; Dunty et al., 2001; Flentke et al., 2014b; Reimers et al., 2006). Prevention of their apoptosis using caspase inhibition normalizes the facial appearance, confirming that apoptosis contributes to the facial dysmorphology. Sensitivity to apoptosis is greatest when ethanol exposure occurs prior to the cells’ delamination and migration (Cartwright et al. 1998), and higher ethanol concentrations are necessary to initiate apoptosis within migrating cells.
Sulik and colleagues observed that cell populations that normally undergo programmed cell death appear to have the greatest sensitivity to ethanol-induced apoptosis (Sulik et al., 1988; Kotch and Sulik, 1992). This suggests the existence of factors that “prime” neural crest to apoptose. In the early chick embryo, ethanol causes two rounds of apoptosis. A first, modest peak occurs throughout the embryo within a few hours of ethanol addition (Debelak and Smith, 2000). However, a second and substantially greater neural crest apoptosis occurs at stages 12–13 and this death coincides with the endogenous cell death that occurs in neural crest progenitors in rhombomeres 3 and 5. However, ethanol did not up-regulate msx2 and bmp4 in the hindbrain, suggesting those cell death signals do not contribute to the apoptosis at this stage (Cartwright et al., 1998).
Extensive work in our laboratory has isolated the mechanism by which ethanol causes this apoptosis, and the basis for these cells’ heightened vulnerability. Ethanol is known to mobilize calcium through IP3-mediated mechanisms, for example, to activate oocytes (Winson and Maro, 1995). We found that, in the 3-somite chick embryo, ethanol concentrations as low as 9mM cause a rapid rise in intracellular calcium (Cai2+) levels within the early neural folds including neural crest (Figure 2; Debelak-Kragtorp et al., 2003). This represents a 6.03-fold increase in Cai2+ to 830 ± 59 nM, as quantified using ratiometric calcium imaging with Fura2 (Garic-Stankovic et al. 2006). The Cai2+ transient is elicited by aliphatic alcohols from C2 to C5 but not C6 or C7, suggesting the alcohol binding site accommodates n-alkanols through pentanol (Garic-Stankovic et al. 2006). Two-thirds of the Cai2+ originates from inositidyl-phosphate-triggered stores and the remainder from extracellular sources (Debelak-Kragtorp et al., 2003). The Cai2+ transient is mobilized through ethanol’s interaction with a G-protein-coupled receptor of unknown identity that is sensitive to inhibition by pertussis toxin. Ethanol’s activation of Gβγ and Gαi/o elevates intracellular IP3 levels through phospholipase Cβ, possibly PLCβ4 (Garic-Stankovic et al. 2005).
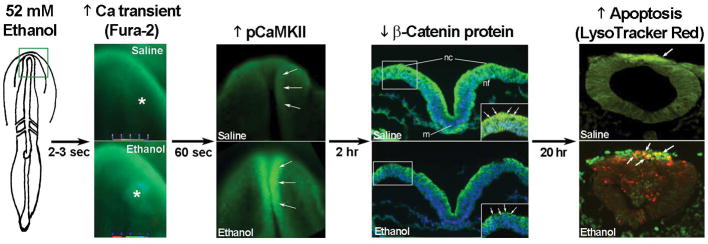
Figure 2.
Key events in ethanol-induced apoptosis of chick cranial neural crest. The green box on the left panel indicates the headfold region where calcium is imaged in the 3-somite chick embryo. Exposure to 52mM ethanol instigates the mobilization of intracellular calcium stores (*) within the early head fold (boxed region) as quantified using Fura2. This selectively activates CaMKII within the anterior neural folds including neural crest (arrows) as detected using antibody directed against phospho-CaMKII (green signal, arrows). A dorsal view of the headfolds is depicted. Among other targets, CaMKII phosphorylates and destabilizes β-catenin protein (green signal at green arrows in boxed region) within the dorsal neural folds enriched in neural crest (D). Shown is a transverse section, dorsal at top, through the headfold of embryos having 3 somites; blue indicates DAPI-stained nuclei. Subsequently, there is significant apoptosis (red signal) within ethanol-exposed dorsal neuroprogenitors of the hindbrain including neural crest (strong green signal), detected using antibody against the neural crest marker snail2. The saline-treated control hindbrain displays little cell death. Shown is a transverse section through rhombomere 4, which normally lacks appreciable cell death, of embryos having 16–18 somites. Chick embryos normally have a low-level green autofluorescent background.
Although ethanol’s Cai2+ transient occurs throughout the neural folds and adjacent ectoderm, CaMKII is subsequently activated only within the dorsal roof of the neural folds, where neural crest progenitors reside (Figure 2). Ethanol’s action is selective as CaMKII activity does not increase in caudal regions, and calcium-dependent kinases such as PKC are not activated (Garic et al. 2011). Inhibition of CaMKII, using either small molecule inhibitors or over-expression of the dominant-negative construct K42M-CaMKII fully prevents the ethanol-induced apoptosis, whereas CaMKII forced-expression (T286D-CaMKII) is sufficient to induce neural crest death. This Cai2+ transient and CaMKII mediate this apoptosis in both chick and zebrafish (Flentke et al., 2011, 2014b), suggesting this mechanism is evolutionarily conserved and may also occur in humans.
Why is CaMKII pro-apoptotic to cranial neural crest? We discovered that CaMKII is capable of phosphorylating and destabilizing the transcriptional effector β-catenin, which provides essential trophic support during early neural crest development (Flentke et al., 2011, 2014a). Normally, β-catenin interacts with the transcription factors TCF and LEF to initiate gene expression (MacDonald et al., 2009). Canonical Wnt signaling enhances its transcriptional activity through its inhibition of GSK3β, which otherwise phosphorylates β-catenin and targets it for proteosomal degradation. However, other kinases and enzymes can also affect β-catenin stability including PKC, calpains, and non-canonical Wnt signaling via either Jnk/PCP or Ca/Wnt (Kohn and Moon, 2005). We found that, within 2hr of ethanol exposure, there is a significant loss of β-catenin protein, but not transcripts, within the dorsal neural folds including neural crest (Figure 2; Flentke et al., 2011). There is an accompanying loss of β-catenin/TCF/LEF transcriptional activity, as assessed using both TopFlash reporter constructs and qPCR for downstream targets including Snai2, FoxD3, and Wnt6, but not Bmp4 or TGFβ2 (Flentke et al. 2011). β-catenin over-expression rescues neural crest from ethanol-induced apoptosis, whereas dominant-negative TCF7 (ΔTCF) induces neural crest death in ethanol’s absence. Surprisingly, it is CaMKII and not GSK3β, PKC, or Jnk, that induces β-catenin instability. It does so by direct phosphorylation of β-catenin at three highly conserved CaMKII target sites at T332, T472, and S552 (Flentke et al. 2014a). Endorsing the critical importance of calcium signaling and β-catenin stability for ethanol’s action, a recent transcriptome analysis found that headfolds from a chick layer line having high sensitivity to ethanol-induced neural crest apoptosis had significantly lower expression of β-catenin and calmodulin as compared with a closely-related ethanol-resistant line, suggesting that changes in the endogenous level of canonical Wnt signaling could predispose an embryo to ethanol’s neurotoxic effects (Garic et al., 2014). Because noncanonical Wnt signaling normally contributes to the endogenous death within rhombomere 3/5 (Ellies et al., 2000), our data suggest that cranial neural crest has heightened sensitivity to apoptosis, in part, because ethanol either mimics or converges upon the calcium/Wnt signals that mediate this endogenous death. A major question is the identity of the downstream targets of β-catenin/canonical Wnt signaling that provide trophic support to neural crest, lost upon ethanol challenge.
Oxidative stress and neural crest survival
Ethanol is well known to induce cellular oxidative stress through mechanisms that include the suppression of oxidative phosphorylation and NADH accumulation from ethanol oxidation (Cunningham and Van Horn, 2003). Neural crest cells have lower levels of endogenous superoxide dismutase (SOD) activity (Davis et al. 1990) and this may increase their sensitivity to the stresses from reactive oxygen intermediates (ROS) (Chen and Sulik, 1996). Free radicals and oxidative stress contribute to the apoptosis of ethanol-exposed neural crest. Neural crest populations, either in culture or within the neural folds and early facial primordial, generate significant ROS levels when cultured with ethanol (Chen et al. 2013a; Davis et al. 1990; Kotch et al. 1995). Co-culture of ethanol-treated neural crest with exogenous free radical scavengers such as SOD, catalase, or α-tocopherol enhance cell survival and attenuate the craniofacial dysmorphology (Davis et al. 1990; Kotch et al. 1995). Importantly, forced expression of Nrf2, which interacts with the antioxidant response promoter element to induce cellular antioxidant responses, enhances neural crest survival and reduces their apoptosis in response to ethanol challenge (Chen et al. 2013a). Similar protection is conferred by small molecules that stimulate Nfr2 activity (Chen et al. 2013b; Yan et al. 2010).
The mechanism that might account for neural crest vulnerability to ROS production and free radical damage is unclear. It is unknown if ethanol acts through mechanisms similar to that in liver, such as the suppression of oxidative phosphorylation; it is also unknown if neural crest can oxidize ethanol, or if other mechanisms for ROS production are involved. One possible mechanism is an ethanol-mediated increase in free iron mobilization; iron chelators such as desferoxamine and phenanthroline enhance neural crest viability in ethanol’s presence, whereas mobilization of ferritin-bound iron enhances their vulnerability (Chen and Sulik, 2000). Short-term (6hr) ethanol exposure substantially suppresses SOD1 (0.688-fold, p=9.76×10−5) and glutathione peroxidase (GPX1, 0.28-fold, p=2.8×10−6) expression in chick neural headfolds (A Garic, ME Berres, SM Smith, unpublished data), which could enable cellular ROS levels to rise. By 24hr Nrf2, superoxide dismutase, catalase, and glutathione peroxidase levels are actually increased in ethanol-treated neural crest as compared with controls. Similar inductions of MnSOD and glutathione peroxidase-1 were observed in post-migratory neural crest after 48hr in continuous ethanol culture (Wentzel and Eriksson, 2008). These increases may be compensatory responses to earlier ROS elevations. Regardless, these elevations are apparently insufficient to confer cell protection, because, in the majority of these studies, the free radical scavengers mitigate the apoptosis but typically provide only partial protection against cell death (Chen et al. 2013a; Yan et al., 2013). It is unclear whether this partial protection reflects a technical issue, or if it instead suggests that ROS production is one of several elements that act in concert to induce neural crest death. Reflecting this complexity is the demonstration that trunk but not cranial neural crest induces CuZnSOD and EC-SOD in response to ethanol challenge (Wentzel and Eriksson, 2009). Differences in free radical scavenging capacity may contribute to the differential vulnerability of neural crest populations. Another unanswered question is whether there exist mechanistic relationships between the increased oxidative stress and ethanol’s other actions, such as the calcium transient or the losses of trophic support. These answers will substantially advance our understanding of why neural crest is so sensitive to teratogen insult.
Other trophic support in neural crest
Although ethanol exposure at gastrulation clearly suppresses shh signaling (Aoto et al., 2008; Hong and Krauss, 2012), its effects upon this pathway in later neural crest development is unclear. Very high ethanol exposure (1% v/v, 171 mM) at the onset of neural crest migration (chick stage 9–10) suppressed the expression of shh, ptc and all three gli genes within the head primordia (Ahlgren et al., 2002). In these embryos, exogenous shh given as either protein or from retroviral expression reduced neural crest apoptosis, implicating a role for shh loss in this cell death. However, in whole embryo culture of similarly-staged mouse embryos (e7.8, 3–6 somites), the identical ethanol concentration did not alter the craniofacial expression of shh, ptc1, or gli1, indicating that signaling through the shh pathway was normal. Despite this, cranial neural crest populations were reduced and craniofacial morphology was abnormal (Yamada et al., 2005). The basis for these disparate results is unknown and requires additional investigation.
In addition to β-catenin/Wnts and shh signaling, ethanol exposure may impair other trophic factors that support neural crest survival. The neurotrophic growth factor Neurotrophin-3, but not ciliary neurotrophic factor, selectively reduced the severity of ethanol-induced abnormalities and enhanced both neural crest survival and proliferation when supplied exogenously commensurate with 100mM ethanol (Jaurena et al. 2011). Both migratory and premigratory neural crest express the Trk-C receptor, which strongly binds Neurotrophin-3, thus this may represent a direct effect on neural crest. Whether ethanol also affects neurotrophin-3 expression was not determined. In addition to its aforementioned effects on cholesterol-ester pools (Li et al., 2007), ethanol also depletes polyamines from the early mouse head primordia (e8.75 – e9.5). These dose-dependent reductions include putrescine, spermine, and spermidine, which are essential for DNA replication (Haghighi Poodeh et al., 2014). Premigratory neural crest cells are highly proliferative and emigrate synchronously in S-phase in the cell cycle (Burstyn-Cohen and Kalcheim, 2002). Such populations would have greater susceptibility to rate-limiting reductions in cellular metabolites such as cholesterol esters and polyamines, which could then produce cell-cycle arrest and initiate apoptosis. Indeed, ethanol does depress the proliferation of neural crest and neuroprogenitors (Jaurena et al., 2011; Giles et al., 2008). Again, given that ethanol does not have a single receptor, it is likely that multiple mechanisms shape neural crest sensitivity to apoptosis.
Genetic influences upon neural crest responses
Studies of monozygotic and dizygotic twins provided the earliest evidence that genetic factors can modulate fetal sensitivity to ethanol’s teratogenicity (Streissguth and Dehaene, 1993; Warren and Li, 2005). This genetic vulnerability extends to neural crest populations. For example, neural crest populations isolated from the mouse strain C57Bl/6J experience significantly greater apoptosis as compared to populations isolated from ICR mice and receiving equivalent ethanol exposures (50–200 mM; Chen et al., 2000). Differences in membrane GM1 ganglioside content may contribute to their differential vulnerability as levels are significantly lower in C57Bl/6J cells, are further reduced by ethanol, and GM1 supplementation attenuates ethanol’s fetal damage (Chen et al. 2000). Embryos from chick layer lines also significantly differ in the severity of craniofacial dysmorphology and neural crest apoptosis following equivalent ethanol exposure (Debelak and Smith, 2000; Su et al. 2001), as do zebrafish of the EK, AB, and TU backgrounds (Loucks and Carvan, 2004). That genetics influence craniofacial outcomes in diverse species suggests that similar genetic factors influence craniofacial outcomes in human exposures. This may partially explain why craniofacial dysmorphology occurs in a subset of individuals diagnosed with FASD. A genetic screen in zebrafish has identified multiple genes that modify craniofacial outcome in ethanol-exposed embryos including foxi1, hinfp, mars, pdgfa, plk1, and vangl2 (McCarthy et al. 2013; Swartz et al., 2014). Equally important is their finding that many genes important for neural crest development are not contributory, and this provides important evidence that ethanol selectively targets just a subset of signals and events to disrupt neural crest survival and development.
Additional insights into candidate genetic modifiers emerge from transcriptome comparisons of ethanol-sensitive and -resistant embryos. Three independent analyses of early headfolds, two using mouse and one using chicken, have identified common gene clusters that distinguish ethanol vulnerability in naive and/or ethanol-treated headfolds (Green et al. 2007; Downing et al. 2012; Garic et al., 2014). Gene clusters that significantly distinguished the ethanol and/or strain responses in at least two studies include ribosome biogenesis, RNA splicing, glycolysis/gluconeogenesis, tight junctions, and proteasomes. Ribosome biogenesis was the only KEGG cluster common to all three studies. Its consistent identification is especially interesting because, in addition to its translational role, it is a sensor of cellular stress. Under conditions wherein ribosome assembly is disrupted, for example when energy is limiting, some ribosomal proteins directly interact with MDM2, an ubiquitinase which normally silences p53. Ribosome protein-MDM2 interactions thus enhance cellular p53 activities including cell cycle arrest and apoptosis (Chakraborty et al., 2011; Fumagalli and Thomas 2011). Early neural crest is sensitive to disruptions in ribosome biogenesis, perhaps due to their rapid proliferation. The human ribosomopathies Diamond Blackfan anemia and Treacher-Collins Syndrome display craniofacial dysmorphologies similar to FASD, and, for Treacher-Collins syndrome, cause cell cycle arrest and extensive apoptosis within the cranial neural crest (Narla and Ebert, 2010; Trainor, 2010). In early mouse and chick headfolds that are enriched in neural crest, ethanol dramatically suppresses ribosome protein expression as early as 4hr to 6hr following ethanol challenge (Green et al. 2007; Downing et al. 2012; A Garic, ME Berres, SM Smith, unpublished). Investigation of these changes relative to ethanol-induced cell death is now underway. Thus unbiased transcriptome analyses provide novel insights into the mechanisms underlying ethanol’s neurocristopathy, as well as into allelisms affecting individual vulnerability to ethanol’s damage.
Future Directions
We now understand much about how ethanol disrupts neural crest development and produces the craniofacial deficits of FAS that were first described over 40 years ago. Ethanol’s ability to interact with multiple signaling pathways permits it to simultaneously enhance some pathways (i.e. calcium mobilization) while repressing others (i.e. sonic hedgehog). Identification of these heterogeneous actions informed the realization that PAE produces a spectrum of facial outcomes. In addition to dose and timing of exposure, growing evidence documents that genetic background also modulates ethanol’s facial dysmorphology. Ethanol’s ability to adversely affect neural crest induction, proliferation, apoptosis, migration and perhaps differentiation has enhanced our understanding of what a facial dysmorphology “means” when diagnosing FASD. Because neural crest is also a stem cell lineage, this work also informs our understanding of ethanol’s neurodevelopmental damage, and, ultimately, the development of prenatal and postnatal interventions that will ameliorate ethanol’s damage.
Many questions remain unanswered. Some of these include: What is the mechanism by which ethanol impedes prechordal plate induction – might ethanol’s calcium transients play a role here? – and does ethanol alter targets in addition to shh to attenuate neural crest induction? Is there a role for ethanol in altering neural crest differentiation and lineage determination? It was shown some years ago that ethanol causes the precocious differentiation of chondrocytes in facial mesenchyme explants (Hoffman and Kulyk, 1999). This is similar to ethanol’s pro-differentiation effects on neurogenesis, and apart from this one report, the neural crest effect remains uninvestigated. Finally, why are neural crest cells so susceptible to apoptosis? Their loss of β-catenin is clearly contributory, but is there a role for other effectors in hastening their demise? Answers to these questions will further reveal how ethanol disrupts craniofacial morphogenesis, and, hopefully, support the development of diagnostics that improve patient access to interventions.
Acknowledgments
The authors acknowledge the many investigators who contributed to this area and apologize that not all could be recognized here due to space limitations. Supported by NIH Merit Award #AA11085 to S.M.S.
References
- Ahlgren SC, Bronner-Fraser M. Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Curr Biol. 1999;9:1304–1314. doi: 10.1016/s0960-9822(00)80052-4. [DOI] [PubMed] [Google Scholar]
- Ahlgren SC, Thakur V, Bronner-Fraser M. Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure. Proc Natl Acad Sci USA. 2002;99:10476–10481. doi: 10.1073/pnas.162356199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto K, Shikata Y, Higashiyama D, Shiota K, Motoyama J. Fetal ethanol exposure activates protein kinase A and impairs Shh expression in prechordal mesendoderm cells in the pathogenesis of holoprosencephaly. Birth Defects Res A Clin Mol Teratol. 2008;82:224–231. doi: 10.1002/bdra.20447. [DOI] [PubMed] [Google Scholar]
- Blader P, Strahle U. Ethanol impairs migration of the prechordal plate in the zebrafish embryo. Dev Biol. 1998;201:185–201. doi: 10.1006/dbio.1998.8995. [DOI] [PubMed] [Google Scholar]
- Boric K, Orio P, Viéville T, Whitlock K. Quantitative analysis of cell migration using optical flow. PLoS One. 2013;8(7):e69574. doi: 10.1371/journal.pone.0069574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Kalcheim C. Association between the cell cycle and neural crest delamination through specific regulation of G1/S transition. Dev Cell. 2002;3:383–395. doi: 10.1016/s1534-5807(02)00221-6. [DOI] [PubMed] [Google Scholar]
- Cartwright MM, Smith SM. Stage dependent effects of ethanol on cranial neural crest cell development: Partial basis for the phenotypic variations observed in Fetal Alcohol Syndrome. Alcohol Clin Exp Res. 1995;19:1454–1462. doi: 10.1111/j.1530-0277.1995.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Cartwright MM, Tessmer LA, Smith SM. Ethanol-induced neural crest apoptosis is coincident with their endogenous death but is mechanistically distinct. Alcohol Clin Exp Res. 1998;22:142–149. [PubMed] [Google Scholar]
- Carvan MJ, 3rd, Loucks E, Weber DN, Williams FE. Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis. Neurotoxicol Teratol. 2004;26:757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Cavieres MF, Smith SM. Genetic and developmental modulation of cardiac deficits in prenatal alcohol exposure. Alcohol Clin Exp Res. 2000;24:102–109. [PubMed] [Google Scholar]
- Chakraborty A, Uechi T, Kenmochi N. Guarding the ‘translation apparatus’: defective ribosome biogenesis and the p53 signaling pathway. WIREs RNA. 2011;2:507–522. doi: 10.1002/wrna.73. [DOI] [PubMed] [Google Scholar]
- Chen SY, Periasamy A, Yang B, Herman B, Jacobson K, Sulik KK. Differential sensitivity of mouse neural crest cells to ethanol-induced toxicity. Alcohol. 2000;20:75–81. doi: 10.1016/s0741-8329(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu J, Chen SY. Over-expression of Nrf2 diminishes ethanol-induced oxidative stress and apoptosis in neural crest cells by inducing an antioxidant response. Repro Tox. 2013a;42:102–109. doi: 10.1016/j.reprotox.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu J, Chen SY. Sulforaphane protects against ethanol-induced oxidative stress and apoptosis in neural crest cells by the induction of Nrf2-mediated antioxidant response. Br J Pharmacol. 2013b;169:437–448. doi: 10.1111/bph.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Sulik KK. Free radicals and ethanol-induced cytotoxicity in neural crest cells. Alcohol Clin Exp Res. 1996;20:1071–1076. doi: 10.1111/j.1530-0277.1996.tb01948.x. [DOI] [PubMed] [Google Scholar]
- Chen SY, Sulik KK. Iron-mediated free radical injury in ethanol-exposed mouse neural crest cells. J Pharmacol Exp Ther. 2000;294:134–140. [PubMed] [Google Scholar]
- Chen SY, Yang B, Jacobson K, Sulik KK. The membrane disordering effect of ethanol on neural crest cells in vitro and the protective role of GM1 ganglioside. Alcohol. 1996;13:589–595. doi: 10.1016/s0741-8329(96)00073-0. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Van Horn CG. Energy availability and alcohol-related liver pathology. Alcohol Res Health. 2003;27:291–299. [PMC free article] [PubMed] [Google Scholar]
- Czarnobaj J, Bagnall KM, Bamforth JS, Milos NC. The different effects on cranial and trunk neural crest cell behaviour following exposure to a low concentration of alcohol in vitro. Arch Oral Biol. 2014;59:500–512. doi: 10.1016/j.archoralbio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Davis WL, Crawford LA, Cooper OJ, Farmer GR, Thomas DL, Freeman BL. Ethanol induces the generation of reactive free radicals by neural crest cells in vitro. J Craniofac Genet Dev Biol. 1990;10:277–293. [PubMed] [Google Scholar]
- Debelak KA, Smith SM. Avian genetic background modulates the neural crest apoptosis induced by ethanol exposure. Alcohol Clin Exp Res. 2000;24:307–314. [PubMed] [Google Scholar]
- Debelak-Kragtorp KA, Armant DR, Smith SM. Ethanol-induced cephalic apoptosis requires phospholipase C-dependent intracellular calcium signaling. Alcohol Clin Exp Res. 2003;27:515–523. doi: 10.1097/01.ALC.0000056615.34253.A8. [DOI] [PubMed] [Google Scholar]
- Downing C, Balderrama-Durbin C, Kimball A, Biers J, Wright H, Gilliam D, Johnson TE. Quantitative trait locus mapping for ethanol teratogenesis in BXD recombinant inbred mice. Alcohol Clin Exp Res. 2012;36:1340–1354. doi: 10.1111/j.1530-0277.2012.01754.x. [DOI] [PubMed] [Google Scholar]
- Dunty WC, Jr, Chen SY, Zucker RM, Dehart DB, Sulik KK. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol Clin Exp Res. 2001;36:1340–1354. [PubMed] [Google Scholar]
- Ellies DL, Church V, Francis-West P, Lumsden A. The WNT antagonist cSFRP2 modulates programmed cell death in the developing hindbrain. Development. 2000;127:5285–5295. doi: 10.1242/dev.127.24.5285. [DOI] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Amberger E, Hernandez M, Smith SM. Calcium-mediated repression of β-catenin and its transcriptional signaling mediates neural crest cell death in an avian model of fetal alcohol syndrome. Birth Defects Res A Clin Mol Teratol. 2011;91:591–602. doi: 10.1002/bdra.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Hernandez M, Smith SM. CaMKII represses transcriptionally-active β-catenin to mediate acute ethanol neurodegeneration and can phosphorylate β-catenin. J Neurochem. 2014a;128:523–535. doi: 10.1111/jnc.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentke GR, Klingler RH, Tanguay RL, Carvan MJ, 3rd, Smith SM. An evolutionarily-conserved mechanism of calcium-dependent neurotoxicity in a zebrafish model of FASD. Alcohol Clin Exp Res 2014. 2014b Feb 11; doi: 10.1111/acer.12360. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Wetherill L, Vinci-Booher S, Moore ES, Ward RE, Hoyme HE, Robinson LK, Rogers J, Meintjes EM, Molteno CD, Jacobson JL, Jacobson SW. Relation over time between facial measurements and cognitive outcomes in fetal alcohol-exposed children. Alcohol Clin Exp Res. 2012;36:1634–1646. doi: 10.1111/j.1530-0277.2012.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli S, Thomas G. The role of p53 in ribosomopathies. Sem Hematol. 2011;48:97–105. doi: 10.1053/j.seminhematol.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Garic A, Berres ME, Smith SM. High-Throughput Transcriptome Sequencing Identifies Candidate Genetic Modifiers of Vulnerability to Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2014 doi: 10.1111/acer.12457. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garic A, Flentke GR, Amberger E, Hernandez M, Smith SM. CaMKII activation is a novel effector of alcohol’s neurotoxicity in neural crest stem/progenitor cells. J Neurochem. 2011;118:646–57. doi: 10.1111/j.1471-4159.2011.07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garic-Stankovic A, Hernandez MR, Chiang PJ, Debelak-Kragtorp KA, Flentke GR, Armant DR, Smith SM. Ethanol triggers neural crest apoptosis through the selective activation of a pertussis toxin-sensitive G protein and a phospholipase Cβ-dependent Ca2+ transient. Alcohol Clin Exp Res. 2005;29:1237–1246. doi: 10.1097/01.alc.0000172460.05756.d9. [DOI] [PubMed] [Google Scholar]
- Garic-Stankovic A, Hernandez M, Flentke GR, Smith SM. Structural constraints for alcohol-stimulated Ca2+ release in neural crest, and dual agonist/antagonist properties of n-octanol. Alcohol Clin Exp Res. 2006;30:552–559. doi: 10.1111/j.1530-0277.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- Giles S, Boehm P, Brogan C, Bannigan J. The effects of ethanol on CNS development in the chick embryo. Reprod Toxicol. 2008;25:224–230. doi: 10.1016/j.reprotox.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Green ML, Singh AV, Zhang Y, Nemeth KA, Sulik KK, Knudsen TB. Reprogramming of genetic networks during initiation of the Fetal Alcohol Syndrome. Dev Dyn. 2007;236:613–631. doi: 10.1002/dvdy.21048. [DOI] [PubMed] [Google Scholar]
- Haghighi Poodeh S, Alhonen L, Salonurmi T, Savolainen MJ. Ethanol-induced impairment of polyamine homeostasis - a potential cause of neural tube defect and intrauterine growth restriction in fetal alcohol syndrome. Biochem Biophys Res Comm. 2014;446:173–178. doi: 10.1016/j.bbrc.2014.02.079. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, Bird CW, Davies S, Savage DD. Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behav Brain Res. 2014;269:44–54. doi: 10.1016/j.bbr.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler JA, Moran DJ. Effects of ethanol on the cytoskeleton of migrating and differentiating neural crest cells: possible role in teratogenesis. J Craniofac Genet Dev Biol Suppl. 1986a;2:129–136. [PubMed] [Google Scholar]
- Hassler JA, Moran DJ. The effects of ethanol on embryonic actin: a possible role in teratogenesis. Experientia. 1986b;42:575–577. doi: 10.1007/BF01946710. [DOI] [PubMed] [Google Scholar]
- Hoffman LM, Kulyk WM. Alcohol promotes in vitro condrogenesis in embryonic facial mesenchyme. Int J Dev Biol. 1999;43:167–174. [PubMed] [Google Scholar]
- Hong M, Krauss RS. Cdon mutation and fetal ethanol exposure synergize to produce midline signaling defects and holoprosencephaly spectrum disorders in mice. PLoS Genet. 2012;8(10):e1002999. doi: 10.1371/journal.pgen.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, Slesinger PA, Davies DL, Das J, Trudell JR, Harris RA. Alcohol-binding sites in distinct brain proteins: the quest for atomic level resolution. Alcohol Clin Exp Res. 2011;35:561–1573. doi: 10.1111/j.1530-0277.2011.01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg Wo, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurena MB, Carri NG, Battiato NL, Rovasio RA. Trophic and proliferative perturbations of in vivo/in vitro cephalic neural crest cells after ethanol exposure are prevented by neurotrophin 3. Neurotoxicol Teratol. 2011;33:422–430. doi: 10.1016/j.ntt.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Kietzman HW, Everson JL, Sulik KK, Lipinski RJ. The teratogenic effects of prenatal ethanol exposure are exacerbated by Sonic Hedgehog or GLI2 haploinsufficiency in the mouse. PLoS One. 2014;9(2):e89448. doi: 10.1371/journal.pone.0089448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP, Wetherill L, Rogers J, Moore E, Ward R, Autti-Rämö I, Fagerlund A, Jacobson SW, Robinson LK, Hoyme HE, Mattson SN, Li TK, Riley EP, Foroud T CIFASD Consortium. Prenatal alcohol exposure alters the patterns of facial asymmetry. Alcohol. 2010;44:649–457. doi: 10.1016/j.alcohol.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Kotch LE, Chen SY, Sulik KK. Ethanol-induced teratogenesis: free radical damage as a possible mechanism. Teratology. 1995;52:128–136. doi: 10.1002/tera.1420520304. [DOI] [PubMed] [Google Scholar]
- Kotch LE, Sulik KK. Experimental fetal alcohol syndrome: proposed pathogenic basis for a variety of associated facial and brain anomalies. Am J Med Genet. 1992a;44:168–176. doi: 10.1002/ajmg.1320440210. [DOI] [PubMed] [Google Scholar]
- Kotch LE, Sulik KK. Patterns of ethanol-induced cell death in the developing nervous system of mice; neural fold states through the time of anterior neural tube closure. Int J Dev Neurosci. 1992b;10:273–279. doi: 10.1016/0736-5748(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Li YX, Yang HT, Zdanowicz M, Sicklick JK, Qi Y, Camp TJ, Diehl AM. Fetal alcohol exposure impairs Hedgehog cholesterol modification and signaling. Lab Invest. 2007;87:231–240. doi: 10.1038/labinvest.3700516. [DOI] [PubMed] [Google Scholar]
- Lipinski RJ, Hammond P, O’Leary-Moore SK, Ament JJ, Pecevich SJ, Jiang Y, Budin F, Parnell SE, Suttie M, Godin EA, Everson JL, Dehart DB, Oguz I, Holloway HT, Styner MA, Johnson GA, Sulik KK. Ethanol-induced face-brain dysmorphology patterns are correlative and exposure-stage dependent. PLoS One. 2012;7(8):e43067. doi: 10.1371/journal.pone.0043067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks E, Carvan MJ., III Strain-dependent effects of developmental ethanol exposure in zebrafish. Neurotox Teratol. 2004;26:745–755. doi: 10.1016/j.ntt.2004.06.017. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Adnams CM, Jones KL, Riley EP CIFASD. Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2013;37:517–528. doi: 10.1111/j.1530-0277.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–71. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais AS, Robinson LK, Manning M, Buckley D, Hoyme HE. Maternal risk factors predicting child physical characteristics and dysmorphology in fetal alcohol syndrome and partial fetal alcohol syndrome. Drug Alcohol Depend. 2011;119:18–27. doi: 10.1016/j.drugalcdep.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy N, Wetherill L, Lovely CB, Swartz ME, Foroud TM, Eberhart JK. Pdgfra protects against ethanol-induced craniofacial defects in a zebrafish model of FASD. Development. 2013;140:3254–3265. doi: 10.1242/dev.094938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji N. Craniofacial malformation in Xenopus laevis tadpoles caused by the exposure of early embryos to ethanol. Teratology. 1983;28:299–305. doi: 10.1002/tera.1420280220. [DOI] [PubMed] [Google Scholar]
- Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115:3196–3205. doi: 10.1182/blood-2009-10-178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary-Moore SK, Parnell SE, Lipinski RJ, Sulik KK. Magnetic resonance-based imaging in animal models of fetal alcohol spectrum disorders. Neuropsychol Rev. 2011;21:167–185. doi: 10.1007/s11065-011-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyedele OO, Kramer B. Nuanced but significant: how ethanol perturbs avian cranial neural crest cell actin cytoskeleton, migration and proliferation. Alcohol. 2013;47:417–426. doi: 10.1016/j.alcohol.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Reimers MJ, La Du JK, Periera CB, Giovanini J, Tanguay RL. Ethanol-dependent toxicity in zebrafish is partially attenuated by antioxidants. Neurotoxicol Teratol. 2006;28:497–508. doi: 10.1016/j.ntt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Rovasio RA, Battiato NL. Role of early migratory neural crest cells in developmental anomalies induced by ethanol. Int J Dev Biol. 1995;39:421–422. [PubMed] [Google Scholar]
- Rovasio RA, Battiato NL. Ethanol induces morphological and dynamic changes on in vivo and in vitro neural crest cells. Alcohol Clin Exp Res. 2002;26:1286–1298. doi: 10.1097/01.ALC.0000026102.73486.65. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Charren SK, et al. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sheller B, Clarren SK, Astley SJ, Sampson PD. Morphometric analysis of Macaca nemestrina exposed to ethanol during gestation. Teratology. 1988;38:411–417. doi: 10.1002/tera.1420380503. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Diagnosis, Epidemiology, Prevention, and Treatment. Washington, D.C: National Academy Press; 1996. Fetal Alcohol Syndrome; p. 213. [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Dehaene P. Fetal alcohol syndrome in twins of alcoholic mothers: concordance of diagnosis and IQ. Am J Med Genet. 1993;47:857–861. doi: 10.1002/ajmg.1320470612. [DOI] [PubMed] [Google Scholar]
- Su B, Debelak KA, Tessmer LL, Cartwright MM, Smith SM. Genetic influences on craniofacial outcome in an avian model of prenatal alcohol exposure. Alcohol Clin Exp Res. 2001;25:60–69. [PubMed] [Google Scholar]
- Sulik KK. Critical periods for alcohol teratogenesis in mice, with special reference to the gastrulation stage of embryogenesisMechanisms of Alcohol Damage in Utero. Ciba Foundation Symposium; Pitman, London. 1984. pp. 124–141. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Cook CS, Webster WS. Teratogens and craniofacial malformations: relationships to cell death. Development. 1988;103(suppl):213–232. doi: 10.1242/dev.103.Supplement.213. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC. Sequence of developmental alterations following acute ethanol exposure in mice: craniofacial features of the fetal alcohol syndrome. Am J Anat. 1983;166:257–269. doi: 10.1002/aja.1001660303. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214:936–938. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Swartz ME, Wells MB, Griffin M, McCarthy N, Lovely CB, McGurk P, Rozacky J, Eberhart JK. A screen of zebrafish mutants identifies ethanol-sensitive genetic loci. Alcohol Clin Exp Res. 2014;38:694–703. doi: 10.1111/acer.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA. Craniofacial birth defects: the role of neural crest cells in the etiology and pathogenesis of Treacher Collins syndrome and the potential for prevention. Am J Med Genet Part A. 2010;152A:2984–2994. doi: 10.1002/ajmg.a.33454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KR, Li TK. Genetic polymorphisms: impact on the risk of fetal alcohol spectrum disorders. Birth Defects Res Part A. 2005;73:195–203. doi: 10.1002/bdra.20125. [DOI] [PubMed] [Google Scholar]
- Wentzel P, Eriksson UJ. Altered gene expression in neural crest cells exposed to ethanol in vitro. Brain Res. 2009;1305:S50–S60. doi: 10.1016/j.brainres.2009.08.057. [DOI] [PubMed] [Google Scholar]
- Winston NJ, Maro B. Calmodulin-dependent protein kinase II is activated transiently in ethanol-stimulated mouse oocytes. Dev Biol. 1995;170:350–352. doi: 10.1006/dbio.1995.1220. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Nagase T, Nagase M, Koshima I. Gene expression changes of sonic hedgehog signaling cascade in a mouse embryonic model of fetal alcohol syndrome. J Craniofac Surg. 2005;16:1055–1061. doi: 10.1097/01.scs.0000183470.31202.c9. [DOI] [PubMed] [Google Scholar]
- Yan D, Dong J, Sulik KK, Chen SY. Induction of the Nrf2-driven antioxidant response by tert-butylhydroquinone prevents ethanol-induced apoptosis in cranial neural crest cells. Biochem Pharmacol. 2010;80:144–149. doi: 10.1016/j.bcp.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]