Abstract
Resveratrol is widely promoted as a potential cancer chemopreventive agent, but a lack of information on the optimal dose prohibits rationally designed trials assessing efficacy. To challenge the assumption that ‘more is better’ we compared the pharmacokinetics and activity of a dietary dose with an intake 200-times higher. The dose response relationship and metabolite profile of [14C]-resveratrol in colorectal tissue of patients helped define clinically achievable concentrations. In ApcMin mice receiving a high-fat diet the low dose supressed intestinal adenoma development more potently than the higher dose. Efficacy correlated with increased AMP-activated protein kinase (AMPK) activation and the senescence marker p21. Non-linear dose responses were observed for AMPK and mTOR signalling in adenoma cells, culminating in autophagy and senescence. In human tissues low dietary exposures caused enhanced AMPK phosphorylation, autophagy and expression of the cytoprotective enzyme NQO1. These findings warrant revision of developmental strategies for diet-derived agents for cancer chemoprevention.
Chemoprevention offers enormous potential for reducing the burden of cancer in society. Trials of drugs such as tamoxifen and celecoxib provide proof of principle that the prevention of cancer through pharmaceutical intervention is feasible and cost-effective1–3; however, use of these agents in this context is severely hampered by an increased risk of serious side effects4,5. Diet-derived compounds are considered an attractive alternative to synthetic drugs for prevention of malignancies in healthy populations, with those that are consumed regularly by humans likely to have a good safety profile. However, despite extensive preclinical data indicating that phytochemicals and micronutrients can protect against cancer, these findings have failed to translate into successful outcomes in randomised controlled trials, and in some cases cancer incidence has actually increased in the intervention group6,7. These unexpected results have been partly attributed to a failure to identify the optimal preventive dose for clinical evaluation before embarking on large costly trials8,9. To date, little attention has been paid to this crucial issue, and instead the classic drug development philosophy has been adopted, that in terms of dosage, more is better. The situation is further confounded by a lack of appreciation of clinical pharmacokinetics, with the frequent use of concentrations in mechanistic in vitro studies that far exceed the levels attainable in human target tissues10.
A fundamental fact seems to have been overlooked in the development of cancer chemopreventive agents, in that diet-derived candidates are often identified on the basis of epidemiological observations indicating activity at low, chronic intake11,12. This would suggest that dietary achievable concentrations should be a focus of interest, but virtually nothing is known about the pharmacokinetics or activity of such low levels for any of the commonly investigated agents. This study aims to challenge the present developmental paradigm using a model phytochemical, resveratrol, which modulates multiple pathways pertinent to colorectal carcinogenesis13. Although resveratrol has been widely promoted as an agent worthy of clinical evaluation, current knowledge gaps, specifically identification of the optimal dose and key molecular targets in humans, prohibit the rational design of trials assessing chemopreventive efficacy. To address these deficiencies we compared the target tissue distribution and activity of a low dietary relevant dose, equivalent to the amount contained in a large glass of certain red wines14 with an intake 200-times higher that has previously been used in phase I clinical trials15,16. Our results show that low dietary exposures not only elicit biological changes in mouse and human tissues relevant to colorectal cancer chemoprevention, but they have superior efficacy compared to high doses, and should therefore be included in future preclinical testing strategies.
Results
Comparative plasma and tissue pharmacokinetics in humans
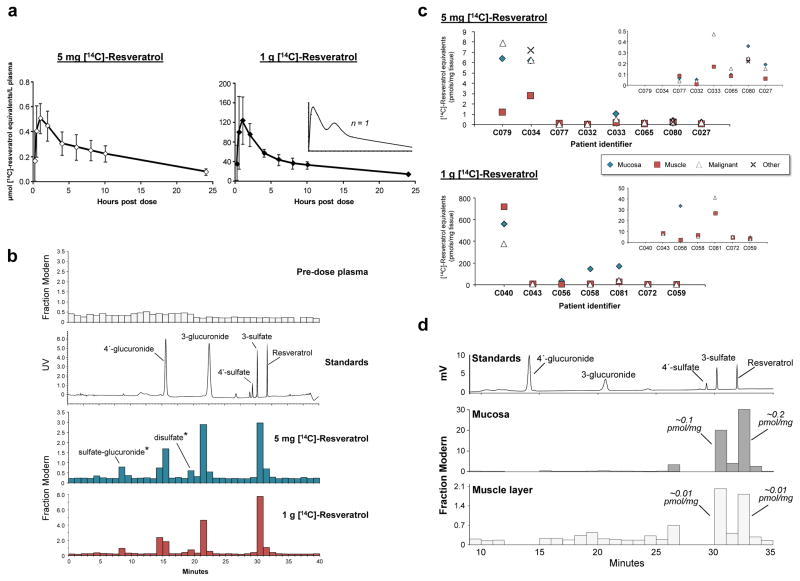
Resveratrol plasma pharmacokinetics are reasonably well characterised at high doses, but it is unlikely that quantities exceeding 1g can be taken chronically by healthy populations due to potential gastrointestinal symptoms17. The standard analytical techniques previously utilised are not sensitive enough to perform pharmacokinetic profiling of resveratrol or its metabolites generated by doses attainable through the diet. Therefore, we employed accelerator mass spectrometry18 in two trials to afford new insight into the distribution and metabolism of resveratrol over a clinically relevant range. Such studies necessitate administration of a trace amount (44 kBq) of [14C]-resveratrol, diluted with unlabelled compound to provide a dose of either 5mg or 1g. Following oral ingestion of a single dose by healthy volunteers plasma pharmacokinetic parameters for total [14C]-resveratrol equivalents increased in a linear manner, reaching average peak concentrations of 0.6 and 137 μmol/L for intakes of 5mg and 1g, respectively (Fig. 1a, Supplementary Table 1). Overall exposure as measured by the average area under the curve values (AUC) also differed by a factor of ~200 (5.2 and 940 μmol/L/h). At both doses, maximal plasma concentrations were typically observed around the 1h time point, with over half the volunteers (13/20) also exhibiting a second minor peak between ~4–10h. Importantly, circulating [14C]-labelled species were still detectable in all twenty subjects as late as 24h after resveratrol administration (Supplementary Table 1). Metabolite profiling of two randomly selected volunteers was achieved through coupling off-line HPLC separation with AMS analysis, which enables characterisation of the [14C]-labelled species based on chromatographic properties. Accordingly, both the dietary and pharmacological doses of resveratrol were found to be rapidly metabolised to sulfate and glucuronide conjugates with only a small fraction of parent compound remaining at tmax (Fig. 1b and Supplementary Table 1).
Figure 1. Comparison of the plasma pharmacokinetics and target tissue distribution of [14C]-resveratrol and its metabolites in humans following a low dietary achievable dose or high pharmacological dose.
(a–b) Healthy volunteers received a single [14C]-labelled oral dose of either 5 mg or 1 g resveratrol (44.5 kBq, 0.962 μSv) and plasma samples were taken over 24 h for determination of total [14C]-resveratrol equivalents by AMS analysis. (a) Graphs show average (± SD) concentrations for 10 volunteers per group, whilst the inset represents a single participant to illustrate the second peak maxima commonly observed with resveratrol due to enterohepatic recirculation. (b) Plasma metabolite profiles determined by HPLC-AMS analysis of selected samples from one patient on each resveratrol dose, taken 1 h after ingestion. Also included are a pre-dose plasma sample for determination of background levels of radiocarbon and a UV chromatogram from the analysis of authentic metabolite standards. Peaks designated by * were tentatively assigned on the basis of their chromatographic properties, since synthetic standards were not available. (c) Levels of [14C]-resveratrol equivalents in tissues of patients with colorectal cancer that received either 5 mg (n=8) or 1 g (n=7) resveratrol daily for 1 week prior to surgery, with the last dose being [14C]-radiolabelled. Where possible, malignant tissue and normal colorectal mucosa and muscle were obtained for each patient. For some participants, other tissue types (fat, ovarian tumour) were also available for analysis (Supplementary Table 2). One patient in the high dose group had surgery delayed by 6 days after taking [14C]-resveratrol and has been excluded. Enlargements are included as insets to enable comparisons at lower concentrations. (d) Metabolite profile in colorectal mucosa and muscle tissue of a patient that received 5 mg [14C]-resveratrol, determined by HPLC-AMS analysis. Peaks of radiocarbon in both tissue types correspond to resveratrol and its 3-sulfate, based on similarity of retention times to authentic standards, and the concentrations stated translate to μM, assuming 1g of tissue equates to 1mL.
To ascertain whether a dietary-relevant dose of resveratrol can reach its purported target tissue we compared the distribution in normal colorectal mucosa and underlying muscle layer as well as malignant tissue samples obtained from patients that received either 5mg or 1g resveratrol daily for one week. The trial followed a window study design, taking advantage of the period between diagnosis and surgery for administering test agents, with the final dose, taken the evening before surgery, containing the [14C]-tracer. Resveratrol species reached the intestinal tissue of all patients, even at the dietary dose. As might be expected, levels decreased over time, with highest concentrations in those participants that experienced the shortest intervals between ingestion and surgery (Fig. 1C, Supplementary Table 2). Surprisingly, [14C]-resveratrol species were still detectable, albeit at low levels (0.11 pmol/mg), in the mucosa of a patient that suffered a six day delay of surgery, after taking the high dose [14C]-capsule. Greatest concentrations were achieved in tissue excised from the right-side of the colon, and there was a tendency (in 13/16 patients) for lower levels in the muscle compared to the surface mucosal layer. Although the large degree of inter-individual variability precludes direct comparisons, the difference between mucosa concentrations achieved in each patient group can be explained by the 200-fold dose discrepancy (range 0.05–6.38 and 4.46–560 pmol [14C]-resveratrol equivalents/mg tissue, for the 5mg and 1g dose, respectively). Concentrations attained in malignant tissue were similar to colonic mucosa, ranging from 0.04–7.9 pmol resveratrol equivalents/mg tissue in the 5mg group and 3–376 pmol/mg in the 1g patients. We also detected [14C]-resveratrol species in peritoneal fat, which was obtained from a proportion of patients, as well as a primary ovarian tumour taken from a participant with secondary colorectal deposits (Fig. 1c and Supplementary Table 2). Importantly, metabolite profiling revealed relatively high concentrations of parent resveratrol and its 3-sulfate, in both the mucosa and muscle tissue of a participant on 5mg daily. This finding parallels our previous observations in patients receiving 1g resveratrol19 and supports the gastrointestinal tract, over other internal tissues, as a potential target for resveratrol.
Superior cancer chemopreventive efficacy of low dose resveratrol
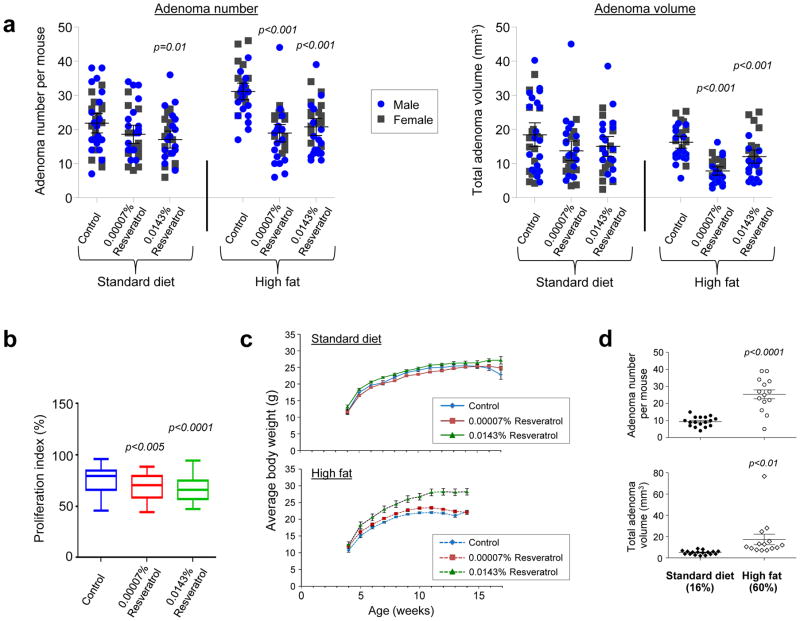
Having demonstrated detectable parent resveratrol in colorectal tissue of patients at both the pharmacological16,19 and dietary doses, we examined the ability of these exposures to prevent intestinal adenomas in the ApcMin mouse, a model of hereditary colorectal cancer characterised by a mutation in codon 850 of the adenomatous polyposis coli (Apc) gene. Since resveratrol is known to protect against age-related pathologies and early mortality associated with high fat in mice20–22, we compared the effects of resveratrol in animals maintained on a standard (SD) or high fat diet (HFD) from weaning. In two independent experiments involving male and female mice, only the higher resveratrol dose had a significant effect in animals receiving SD, where it caused a small (22%) reduction in adenoma number but failed to influence tumour volume. In contrast, when co-administered with a HFD the low dose of resveratrol (0.00007% w/w, equating to ~0.07 mg/kg body weight per day) significantly reduced adenoma number by ~40% and decreased the overall burden by ~52% relative to control animals of both gender (Fig. 2a). Surprisingly, although the high dose (0.0143% w/w; 14 mg/kg body weight) was also efficacious, it was consistently less potent, reducing adenoma number by one third, and burden by 25%. Inhibition of tumour development by resveratrol at both doses was associated with a small (6.5–9.3%) significant reduction in the proportion of proliferating cells in adenomas, but not histologically normal crypts of the small intestine or colon, in mice on the HFD, as measured by positive Ki-67 staining (Fig. 2b and Supplementary Fig. 1). In contrast, resveratrol had no effect on the extent of apoptosis, adjudged by cleaved caspase-3 immunostaining (Supplementary Fig. 1). Consistent with previous observations22, the higher dose was associated with significantly increased body weight in males (Fig. 2c and Supplementary Fig. 2), probably because of increased food consumption or less malabsorption due to lower tumour burden in these animals (Supplementary Fig. 3). Given that low dose resveratrol was only efficacious in mice on HFD, this raises the possibility that dietary feasible intakes may protect against the tumour promoting effects of fat, without reducing body weight (Fig. 2c and Supplementary Fig. 2). Indeed, comparison of tumour development in ApcMin mice on control SD and HFD, culled at the same time point (14 weeks of age) revealed a strong pro-carcinogenic effect of fat in this model (Fig. 2d).
Figure 2. Low dose resveratrol inhibits adenoma development in ApcMin mice on HFD more potently than a dose 200-fold higher.
Male and female mice were maintained on SD or HFD from weaning (4 weeks of age) supplemented with resveratrol (0.00007 or 0.0143%). Unless stated otherwise, mice on the SD (16% of calories from fat) were culled at 17 weeks whilst those on the HFD (60% of calories from fat) had to be killed at 14 weeks due to the tumour promoting effects of the latter. (a) Comparison of the number of adenomas per mouse and total adenoma volume in the small intestine of each animal. Data represent the mean ± SEM of 14–16 female plus 17–19 male mice per group. Significant treatment-related differences relative to the corresponding control diet group are shown. (b) Box plot showing the effect of resveratrol on the proliferative index in intestinal adenomas of ApcMin mice on HFD, as measured by immunohistochemical staining for nuclear Ki-67. Data represent the median percentage (plus 25th and 75th percentile) of Ki-67 positive cells per field, where 6 different visual fields were scored for each mouse (n = 6 males and 5 females per group). Whiskers indicate the maximum and minimum values. (c) Body weight of male ApcMin mice on SD or HFD alone or containing resveratrol. Data represent the mean ± SEM of 15–19 mice per group. High dose resveratrol significantly increased the body weight of mice on SD (p<0.05) and HFD (p<0.001) compared to corresponding controls; low dose resveratrol increased the body weight of animals on HFD only (p=0.05). (d) Effect of a control HFD on intestinal adenoma number and total volume compared to ApcMin mice on a SD. Animals in both groups (7–9 females plus 7–8 males) were culled at 14 weeks of age and data illustrate the mean ± SEM.
Importance of AMPK signalling in the chemopreventive effects of resveratrol
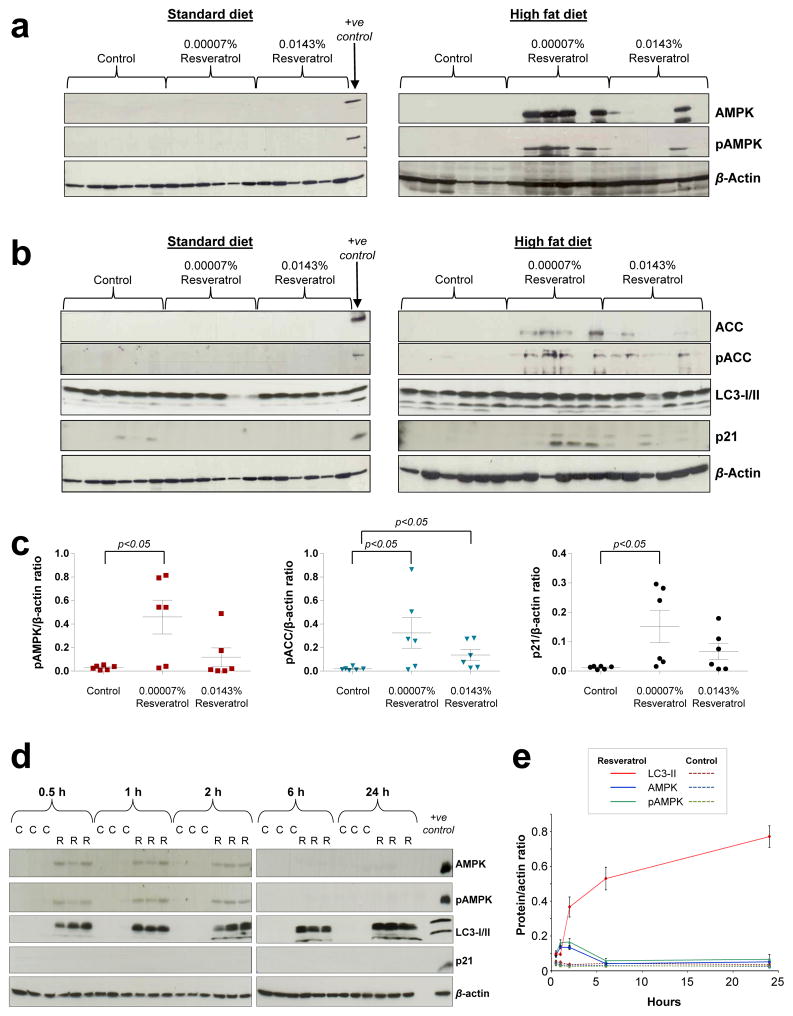
In female mice, efficacy was highly correlated with the expression and activation of the energy regulator AMPK in intestinal mucosa. Neither AMPKα protein nor its phosphorylated form were detectable in any mice on the SD or in the high fat control group, but both were evident in animals that ingested 0.00007% resveratrol, and to a lesser extent those on the high dose (Fig. 3a, c). This phenomenon was closely mirrored by an increased expression and phosphorylation of the AMPK target acetyl-CoA carboxylase (ACC) (Fig 3b, c). In contrast, a random pattern of AMPK and pAMPK expression was observed in mucosa of the male mice (Supplementary Fig. 4), probably due to overnight starvation prior to culling, which was not performed in the females; this was necessary to enable measurement of metabolic parameters in the fasting state, none of which were altered by intervention, apart from intestinal IGF1, which was decreased by the high dose resveratrol regardless of fat content (Supplementary Fig. 5). Furthermore, determination of the kinetics of AMPK activation in wild-type B57BL/6J male mice preconditioned on a HFD before receiving a single oral dose of resveratrol (2.1μg), revealed an extremely rapid response with increased expression and phosphorylation detected in normal intestinal mucosa just 30 min after administration (Fig. 3d, e). This low dose was equivalent to the total estimated amount ingested over the course of a day by animals receiving 0.00007% in their diet. AMPK activation persisted for only 2h before declining. This may explain the considerable variability of pAMPK levels in resveratrol-treated mice on HFD, as tissue concentrations may be dependent on when the mouse last ate. This finding also demonstrates that the ability of dietary-relevant doses of resveratrol to induce AMPK expression and phosphorylation in vivo is not gender specific. The mechanisms through which resveratrol specifically enhances the expression of AMPK in the mucosa of mice on a HFD are currently unclear.
Figure 3. Low dose dietary resveratrol activates AMPK and causes senescence in intestinal mucosa of mice on HFD.
(a–c) Expression and phosphorylation of AMPK and its downstream target ACC, together with levels of autophagy and senescence markers in tissue of female ApcMin mice maintained on SD or HFD, with or without resveratrol. The positive control sample is Apc10.1 cells exposed to 1 μM resveratrol. Mice were culled at 17 or 14 weeks of age for the standard and high fat groups, respectively. (c) Data represent the mean ± SEM of 6 mice per group. (d–e) Kinetics of AMPK activation and downstream effects in intestinal tissue of C57BL/6J wild-type male mice maintained on HFD which received a single gavage dose of resveratrol (2.1 μg per mouse; R) or vehicle control (C). Mice were culled post-dosing at the indicated time. (d) Representative immunoblots are shown for 3 mice per group. (e) Data represent the mean ± SEM of 4–6 mice per group.
One of the end products of AMPK activation is autophagy, a catabolic pathway required for the quality control of proteins/organelles and maintenance of energy homeostasis, which can also serve as a tumour suppressing mechanism23. We detected significantly enhanced levels of soluble microtubule-associated protein 1 light chain 3 (LC3-I) in mucosa tissue of resveratrol-treated mice, along with increased conversion to the lipid bound LC3-II, which is a constituent of autophagosomal membranes and marker of autophagy initiation (Fig. 3d, e). Upregulated autophagy appears to be a rapid but potentially short-term response to resveratrol since it was observed in animals that received the single dose but not in the chronically treated ApcMin mice (Fig. 3b, d, e). Conversely, p21 expression, a marker of senescence, was increased in ApcMin mice that ingested resveratrol with a HFD, whilst a single dose was insufficient to elevate p21 protein levels over the time frame monitored (Fig. 3b–e). Autophagy can facilitate establishment of the senescent phenotype24 and these data imply that autophagy precedes senescence in resveratrol treated mice.
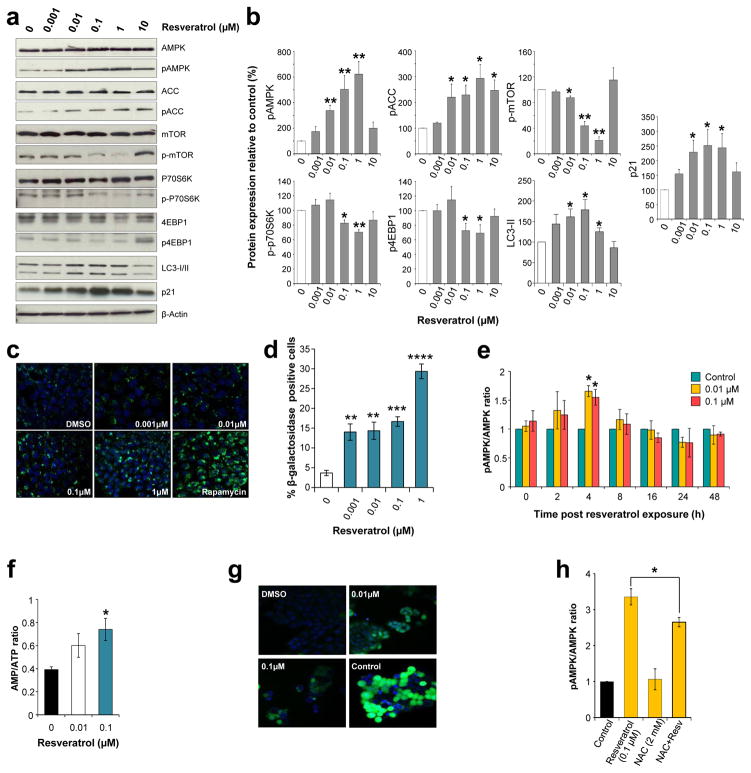
Apc10.1 cells derived from adenomas of ApcMin mice25 were used to further delineate the consequences of AMPK activation using a concentration range encompassing that detected in human colorectal tissue and plasma following both resveratrol doses. As the likely target in clinical chemoprevention, these cancer precursors provide a more relevant model than malignant cancer cells for assessing activity. The anti-tumour effects of resveratrol observed in vivo were recapitulated in Apc10.1 cells, which displayed increased autophagy measured as Cyto-ID Green-stained autophagic vacuoles, and elevated senescence, detected by β-galactosidase staining and p21 expression (Fig. 4a–d). After 6 days’ exposure to resveratrol the expression of AMPK remained stable but significant concentration-dependent activation was evident from 0.01μM and reached a maximum at 1μM (Fig. 4a, b). At 10μM, AMPK phosphorylation returned to basal levels. This bell-shaped dose response was also apparent for ACC phosphorylation, which was greatest at 1μM, reflecting the in vivo findings that lower exposures are more effective. Downstream targets of AMPK were altered in a similar manner (Fig. 4a, b); 1μM resveratrol caused the greatest reduction in phosphorylation of the mechanistic target of rapamycin (mTOR), and its downstream effectors 4EBP1 and S6K, which are involved in protein translation. These effects were independent of Akt, another mTOR regulator, since resveratrol had no effect on Akt expression and activation (Supplementary Fig. 6).
Figure 4. Low, dietary achievable concentrations of resveratrol activate AMPK signalling and cause autophagy and senescence in Apc10.1 mouse adenoma cells.
(a–b) Six days of repeated exposure to resveratrol enhances AMPK phosphorylation, inhibits mTOR signalling and increases markers of autophagy and senescence. Representative immunoblots are shown (a). (c) Detection of Cyto-ID Green-stained autophagic vacuoles visualised by fluorescence microscopy of live cells treated repeatedly with resveratrol for 6 days. Hoechst 33342-stained nuclei are in blue and rapamycin (500 nM) was used as a positive control. (d) Proportion of Senescence-Associated β-galactosidase positive stained cells after 6 days of repeated resveratrol treatment. (e) Kinetics of AMPK activation following exposure to resveratrol for 2 h, replacement of the media and further incubation without resveratrol for the times indicated. (f) AMP/ATP ratio determined by HPLC analysis. Cells were treated with resveratrol for 2 h, media was replaced and incubation continued for 4 h. (g) Levels of intracellular reactive oxygen species visualised using an Image-iT LIVE green ROS detection kit, 1 h after addition of resveratrol. Nuclei were counterstained blue with Hoechst 33342 and tert-butyl hydroperoxide (100 μM) was used as a positive control. (h) Effect of NAC on resveratrol-induced AMPK activation measured after 6 h co-incubation. All graphs illustrate the mean ± SEM of three independent experiments. Significant differences relative to control incubations are indicated by *(p<0.05), **(p<0.01), ***(p<0.001) and ****(p<0.0005).
Several different routes to AMPK activation have been demonstrated for resveratrol. However, the key studies have been performed with concentrations that exceed levels achievable in human plasma15,26–28. Given the reported dose-dependency of the mechanisms engaged26, we sought to identify the processes involved at lower clinically relevant concentrations. Short-term (2h) exposure of Apc10.1 cells to resveratrol followed by removal, to mimic the process of metabolic clearance in the mouse intestine, caused a transient increase in pAMPK after 4h, which correlated with a significant increase in the AMP/ATP ratio, consistent with ATP synthase inhibition (Fig. 4e–f). Co-incubation with the calcium chelator BAPTA or the CamKKβ inhibitor STO-609 had no effect (Supplementary Fig. 6), suggesting that inhibition of phosphodiesterase26 does not play a role in this system. Intriguingly, low concentrations of resveratrol induced a detectable increase in reactive oxygen species (ROS) within 1h (Fig. 4g). Therefore, we investigated whether increased oxidative stress may contribute to AMPK activation, as suggested for other activators29, including 2-deoxy-D-glucose30. Co-incubation with the antioxidant N-acetylcysteine (NAC)31 significantly blunted resveratrol-induced phosphorylation of AMPK, identifying a role for ROS at clinically achievable concentrations (Fig. 4h).
Low dose resveratrol exerts activity consistent with cancer chemoprevention in human colorectal tissue
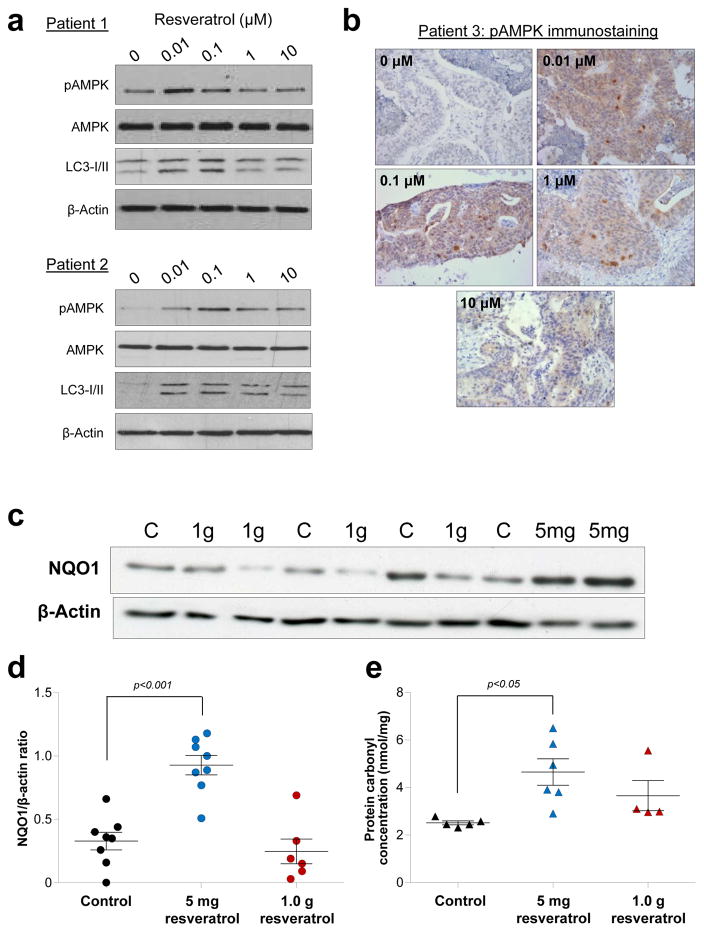
Analysis of AMPK signalling in colorectal tissue of the patients that received [14C]-resveratrol revealed random expression patterns, with no apparent difference between treated and control patients (Supplementary Fig. 7). As with the male ApcMin mice, this lack of effect may be due to the fact all patients were fasted overnight prior to surgery and would have been without food for differing lengths of time. To overcome this issue, we performed explant cultures of human colorectal tumours isolated from three individual patients and passaged in immuno-compromised NOD-SCID mice. Notably, the response to resveratrol exposure mimicked that observed in Apc10.1 cells, with rapid AMPK activation and increased autophagy at low concentrations (0.01–0.1μM) and a less pronounced or no effect with higher exposures (Fig. 5a–b).
Figure 5. Low concentrations of resveratrol activate AMPK and increase markers of oxidative stress in human colorectal tissues.
Exposure to resveratrol (2h) increases pAMPK levels and up-regulates autophagy in primary colorectal cancer explants, as assessed by Western blotting (a) and/or immunohistochemical staining (b) in samples from three different patients. Expression of NQO1 (c–d) and levels of protein carbonylation (e) in colorectal mucosa tissue of patients participating in the [14C]-resveratrol trial who received a dose of either 5 mg or 1 g daily for one week prior to surgery, or untreated control patients. Samples were analysed blind, and significant differences between control and treated groups are indicated. (c) A typical Western blot for NQO1. (d) Data represent the mean ± SEM of 6–8 patients per group. (e) Data show the mean ± SEM of 4–6 patients per group.
Further support for the existence of a non-conventional dose response in humans is provided by the observation that both NQO1 expression and protein carbonyl concentration were significantly increased in the colorectal mucosa of patients that received 5mg [14C]-resveratrol compared to those taking the 1g dose and the control group (Fig. 5c–e). NQO1 is a cytoprotective enzyme regulated by the transcription factor Nrf-2, which is activated by oxidative stress32, whilst quantitation of carbonyl groups provides a stable measure of amino acid oxidation33. Taken together, this study suggests that lower doses of resveratrol may be more active than higher supra-dietary intakes in humans and that the beneficial effects of resveratrol at such doses may be mediated by its pro-oxidant activity and upregulation of AMPK signalling.
Discussion
The ability of low, dietary feasible resveratrol doses to selectively prevent intestinal tumour development in ApcMin mice fed a HFD highlights several pertinent points important for advancing the field of chemoprevention. With recent results from trials such as SELECT, involving selenium and the antioxidant vitamin E, it is gradually being recognised that complex dose-response relationships exist for dietary-derived agents34,35. Furthermore, the lack of effect or even harm seen in trials with high-dose antioxidant supplements7 is consistent with the idea that low levels of ROS can trigger cellular defence mechanisms and are actually protective, which is suggestive of a non-linear dose-response, or hormesis36. In line with these emerging concepts, our observations in multiple models of murine and human colorectal cancer provide the first direct evidence that low intakes of resveratrol have greater anticancer efficacy than high doses.
The requirement for a HFD to reveal the efficacy of resveratrol explains why a dose ~14-fold higher than the maximal used here was necessary to produce a significant, albeit moderate (30%), reduction in adenomas in analogous studies involving ApcMin mice on a SD, whilst lower exposures were ineffectual37,38. This interaction with fat is in agreement with results emerging from clinical trials, where resveratrol at doses as low as 10mg daily, appears to have selective activity in obese humans39 or those with metabolic disorders, such as type 2 diabetes mellitus40,41. Of particular note is the study by Timmers et al. in which resveratrol (150mg per day) was found to mimic the effects of calorie restriction in obese men by lowering energy expenditure, and improving the metabolic profile and general health of participants39; in contrast, a comparable dose (75mg) had no effect in non-obese women with normal glucose tolerance42. Interestingly, a large daily dose 10-fold higher than that used by Timmers et al. failed to alter any metabolic parameters, even though the study followed a similar design and also involved obese men39,43. These reports, together with our observation that only the low 5mg daily dose of resveratrol increased biomarkers of oxidative stress in colorectal tissue of treated patients, and the fact that maximal AMPK activation and autophagy were achieved in human explants at submicromolar concentrations, lends further credence to the reality of a non-linear dose response in humans. Our in vivo results also emphasize a role for lifestyle and physiological factors in influencing an individual‘s response to intervention, which indicates the potential importance of personalising chemopreventive therapy.
Long-term maintenance of C57BL/6J mice, the background strain of ApcMin mice, on a HFD is a commonly used model of impaired glucose intolerance and early type 2 diabetes44, which is a well-established risk factor for colorectal cancer in humans45,46. Consumption of a HFD has previously been demonstrated to cause metabolic changes in ApcMin mice, with the degree of dysregulation increasing over time47. However, in the present study there was no evidence of the diabetic phenotype in mice on HFD, although it is difficult to draw direct comparisons because those on HFD were culled 3 weeks earlier than animals on SD. Resveratrol appears to counteract the tumour-promoting effects of a HFD without modulating the fasting levels of plasma biomarkers previously associated with health and survival benefits in middle aged mice20. Therefore, at the low doses used, it is likely that localised rather than systemic effects in colorectal cells are responsible for the chemopreventive efficacy of resveratrol, particularly as the phenotype observed in ApcMin mice was replicated in vitro.
Although widely perceived as an antioxidant our findings suggest that the transient pro-oxidant activity of resveratrol is responsible, at least in part, for activation of AMPK at very low concentrations. Whether the increased ROS involved are generated through inhibition of ATP synthase48, which also contributes to AMPK activation in adenoma cells via the classical route, remains to be determined. Countless in vitro studies have described pro-apoptotic effects of resveratrol in cancer cell lines13,49, however these necessitated the use of concentrations beyond those systemically achievable in humans15. We found no evidence of increased apoptosis, which is likely to be a consequence of toxicity at high concentrations, within the intestines of treated ApcMin mice; instead the anticancer effects of resveratrol seem to be mediated through the induction of autophagy and senescence. Autophagy is a short-term response and senescence the result of sustained exposure to low concentrations. Both processes have paradoxical roles in carcinogenesis23,50,51, but appear to serve as tumour supressing mechanisms in ApcMin mice on a HFD. It is therefore encouraging that the increased ROS stimulus and elevated autophagy also translate to human colorectal tissue exposed to concentrations of resveratrol generated in muscle and mucosa after a 5mg dose (~0.01–0.2 μM, Fig. 1d).
In summary, we provide compelling evidence of a bell-shaped dose response for resveratrol, with low doses having greater efficacy than high doses. We demonstrated that dietary achievable doses of resveratrol halt tumour progression in mice through induction of AMPK and senescence and that these effects translate to human tissue. Moreover, we unveiled how the tumour preventive efficacy of resveratrol is dependent on animal diet and, probably, human lifestyle, particularly behaviours that cause obesity and metabolic syndrome. This work warrants a deep rethinking of strategies aimed at implementing resveratrol, and potentially other diet-derived agents, for cancer chemoprevention and other therapeutic indications.
Materials and Methods
Resveratrol for preclinical experiments was purchased from Novanat Bioresources (Shanghai, China). Purity (99.9%) was confirmed by HPLC-UV and LC-MS/MS analysis. Resveratrol sulfate and glucuronide metabolite standards were synthesized in house according to published methods19. Apc10.1 cells derived from an ApcMin mouse adenoma were originally provided by Dr Carla De Giovanni (University of Bologna, Italy)25. Unless stated otherwise, all reagents were purchased from Sigma (UK).
Clinical trials
The trials were approved by the Liverpool UK Research Ethics Committee, the UK Medicines and Healthcare products Regulatory Authority, the Administration of Radioactive Substances Advisory Committee (ARSAC) and the Institutional Review Board at Lawrence Livermore National Laboratory. Both trials were conducted at The University Hospitals of Leicester NHS Trust.
Resveratrol (GMP grade) was manufactured by Orchid Chemicals and Pharmaceuticals Ltd. (Maharashtra, India) and supplied by Royalmount Pharma (Montreal, Canada). Resveratrol was formulated by Nova Laboratories (Leicester, UK) in hard gelatin capsules containing either 5 mg or 250 mg resveratrol. [14C]-Resveratrol was custom synthesized by BioDynamics Research Ltd. (Northamptonshire, UK) and radiodiluted with resveratrol (Royalmount Pharma). This material was then used for the manufacturer of [14C]-resveratrol capsules by Pharmaceutical Profiles (Nottingham, UK), which contained 5 mg total resveratrol and provided a labelled dose of 44.5 kBq (0.962μSv equivalent dose). The [14C]-radiolabeled capsules were only manufactured in the 5 mg dose size due to financial and logistical constraints. This meant that volunteers and patients in the 1 g dose groups actually received 4 × 250 mg resveratrol capsules plus a single 5 mg [14C]-resveratrol capsule, to provide a total dose of 1.005 g as their final dose prior to surgery, or as their only dose in the volunteer plasma pharmacokinetic study. The additional 5 mg of resveratrol was considered negligible for those taking 1 g daily.
Volunteer study
Twenty healthy volunteers over 18 years of age gave written informed consent and were recruited to the study. Volunteers agreed to abstain from ingesting large quantities of resveratrol-containing foods/drinks, vitamins and certain drugs for 5 days before the study. Bloods were screened for standard haematological and biochemistry parameters and only those with results within the normal limits were eligible to participate.
Healthy volunteers were fasted overnight and a baseline control blood sample was taken before they received a single dose of either 5 mg [14C]-resveratrol or 1.005 mg [14C]-resveratrol (4 × 250 mg, plus 5 mg [14C]-resveratrol). A further nine blood samples were taken at the following times post resveratrol ingestion: 0.25, 0.5, 1, 2, 4, 6, 8, 10, 24 h. There were no apparent differences in the demographics of the two dose groups relating to age, gender, ethnicity and body surface area. All subjects completed the study.
Colorectal cancer patient trial
Patients with resectable colorectal cancer were recruited into the study at the University Hospitals of Leicester, UK. Patients met the following eligibility criteria: histological diagnosis of colorectal malignancy, surgical resection scheduled as part of their management; willingness to abstain from ingesting large quantities of resveratrol-containing foods/drinks and vitamin supplements. Exclusion criteria included: neoadjuvant chemotherapy prior to resection; excessive alcohol intake; chemotherapy treatment or participation in another investigational drug study within 28 days of surgery; any malabsorption syndrome; chronic use of NSAIDs, steroids, warfarin or antiepileptic drugs that may interfere with resveratrol metabolism or biomarker analysis; evidence of abnormal liver function, defined by ALT >2.5x the upper limit of normal and serum bilirubin >1.5x the upper limit of normal; evidence of abnormal renal function, defined as creatinine clearance < 30mL/min; diabetes.
Patients were asked to abstain from consumption of foods and drinks containing resveratrol during the study period and gave written informed consent. Patients completed a dosing calendar and food diary during the study to assess compliance.
Patients took daily unlabelled resveratrol capsules for 6 days and then received the final [14C]-resveratrol dose either the evening before or morning of surgery, depending on the scheduled time of surgery. A control group without any intervention was also included. At surgical resection both normal and malignant tissue was sampled. The normal tissue was separated into mucosa and muscle. Any polyps that were present and any adherent peritoneal fat were also dissected for analysis. Benign tissue was obtained for all participants but it was not possible to obtain malignant samples from some patients with small tumours due to insufficient tissue after diagnostic samples were taken. One patient was recruited with a diagnosis of metastatic colorectal cancer with an ovarian Krunkenberg tumour deposit, however, on histological examination the malignant colorectal tissue was found to be a metastatic ovarian carcinoma. All other patients were confirmed histologically to have a primary colorectal adenocarcinoma.
Colorectal cancer patient demographics
| Control (no resveratrol) | 5 mg Resveratrol | 1 g Resveratrol | |
|---|---|---|---|
| Number of patients | 11 recruited 9 completed 2 incompletea |
10 recruited 8 completed 2 incompleteb |
10 recruited 7 completed 2 incompleteb,c 1 collected as controld |
| Age (years) mean ± SD (range) | 66.3 ± 10.9 (44–80) | 72.7 ± 12.3 (45–88) | 69.1 ± 11.5 (43–85) |
| Gender | 5 male, 6 female | 8 male, 2 female | 8 male, 2 female |
| Previous chemoradiotherapy | 4 | 0 | 1 |
Two patients in the control group did not complete the study as their operations were cancelled due to brain metastases and viscus perforation.
Three patients, two from the 5 mg dose group and one from the 1 g dose group did not complete the study as their tumours were found to be inoperable at laparotomy.
One patient received their completed the resveratrol course, including the [14C]-labelled dose but their surgery was then postponed for 6 days. Tissue was taken during the rescheduled surgery and is included, as appropriate in the analysis (Supplementary Table 2B).
One patient in the 1 g group was operated on before commencing resveratrol capsules, due to a cancellation slot becoming available. Tissue was collected as a control sample.
AMS analysis
Processing of samples for AMS analysis was performed in a designated laboratory, free from extraneous 14C-contamination. Human plasma (25 μL) and colorectal tissue (10 mg) samples were submitted for AMS analysis at Lawrence Livermore National Laboratory and were analysed neat to quantify the concentration of total [14C]-resveratrol species. All samples were converted to elemental carbon using standard protocols, by combustion to CO2, followed by reduction to filamentous graphite52. The resulting graphite was analysed by AMS, with each graphite sample analysed up to seven times for radiocarbon content or until the measurement variation was within ± 5%. The amount of 14C due to the presence of resveratrol species was calculated by subtracting background levels of radiocarbon detected in control colorectal tissue from untreated patients and plasma samples from volunteers obtained prior to ingestion of the [14C]-labelled dose. The concentration of [14C]-resveratrol equivalents was then determined by taking into account the specific activity, amount of tissue/plasma analysed and percentage of total carbon in each tissue type as determined by elemental analysis (3.4 % and 9.87 % for plasma and colon tissue, respectively). Pharmacokinetic parameters were modelled using WinNonlin Version 5.3 software (Pharsight Corporation, Mountain View, California, US).
For HPLC-AMS analysis of metabolite profiles, tissue was homogenised and extracted with methanol according to our standard protocol16. The remaining pellet was then subject to a second extraction with acetone and the supernatant was combined with the methanol fraction and concentrated to dryness. Plasma samples were extracted with acidified methanol as described previously53 and the supernatant concentrated under vacuum. Plasma and tissue extracts were then reconstituted in 50:50 methanol:water and subject to HPLC separation on a Waters Atlantis C18 column (4.6 mm × 150 mm, 3 μm) combined with a Waters Atlantis guard C18 column (4.6 mm × 20 mm, 5 μm), maintained at 35°C. A gradient mobile phase was used at a flow rate of 1 mL/min to maximise separation between resveratrol and all the potential metabolite peaks; this consisted of 2% isopropanol in 5 mM ammonium acetate (A) and 2% isopropanol in methanol (B) with a linear gradient as described below.
| Minutes | % Mobile phase A |
|---|---|
| 0 | 100 |
| 4 | 90 |
| 7 | 87 |
| 11 | 87 |
| 14 | 85 |
| 23 | 84 |
| 27 | 60 |
| 30 | 30 |
| 36 | 5 |
| 38 | 100 |
| 40 | 100 |
One minute fractions were collected throughout each HPLC run and these were evaporated to dryness then reconstituted in 200 μL 50:50 water:methanol prior to AMS analysis. Since HPLC fractions have negligible mass they were supplemented with a precise amount of carrier (1μL tributyrin, equivalent to 615 μg carbon), to provide sufficient carbon mass for optimal sample preparation and AMS analysis. Samples were analysed as outlined above and data are presented as Fraction Modern.
Analysis of protein carbonyls and NQO1 in human colorectal tissue
Colorectal mucosa samples from cancer patients participating in the [14C]-resveratrol trial, along with tissue from control untreated patients were analysed for markers of oxidative stress. All samples were coded and randomised and the analyses were performed blind. Sufficient tissue for NQO1 analysis was available from 8 patients in the 5 mg dose group and 7 patients that received 1 g resveratrol. Control tissue was obtained from 8 untreated patients. NQO1 expression was measured by Western blotting and normalised to actin. The effect of resveratrol on the NQO1/actin ratio was analysed using a T-test and Mann Whitney test. In both cases a significant difference was observed between the control and the low dose (5 mg) resveratrol group (p<0.001 for the T-test and p=0.001 for the Mann Whitney test). There was no significant difference between the control group and those patients that received the high (1 g) dose of resveratrol (p=0.37 for the T-test and p=0.64 for the Mann Whitney test). One extreme value for the NQO1/actin ratio (4.8) corresponding to a patient in the 1 g group was removed from the analysis as it was considered an outlier. A selection of mucosa samples (4–6 per patient group) were also subject to analysis using an OxiSelect™ protein carbonyl spectrophotometric assay (Cell Biolabs Inc.), according to the manufacturer’s instructions.
Animal studies
The animal experiments were performed under project licenses PPL40/2496 and 60/4370, granted to Leicester University by the UK Home Office. The experimental design was vetted by the Leicester University Local Ethical Committee for Animal Experimentation and met the standards required by the UKCCCR guidelines54. C57BL/6J ApcMin/+ (ApcMin) mice were bred in Leicester University Biomedical Services using animals originally obtained from Jackson Laboratory (Bar Harbor, ME, USA), and the ApcMin genotype was confirmed by PCR55. Wild-type C57BL/6J and NOD/SCID mice were purchased from Charles River and Harlan Laboratories (UK), respectively.
Efficacy studies in ApcMin mice
To assess resveratrol efficacy, an initial study was performed in male ApcMin mice and this was then repeated using female animals; the only difference between the experiments was that the male mice were starved overnight prior to culling whereas the females had free access to food throughout. After weaning at 4 weeks of age, animals were randomised to one of 6 different diets, with each group consisting of up to 25 mice. The following diets were prepared by IPS Product Supplies Ltd. (London, UK).
Standard AIN-93 diet in which 16 % of the calories are provided by fat
Standard AIN-93 containing 0.00007 % resveratrol (0.7 mg/kg diet)
Standard AIN-93 containing 0.0143 % resveratrol (14.3 mg/kg diet)
High-fat AIN-93 diet, in which 60 % of the calories are provided by fat
High-fat AIN-93 containing 0.00007 % resveratrol
High-fat AIN-93 containing 0.0143 % resveratrol
The identity of each diet with respect to resveratrol content was confirmed by HPLC analysis using an established assay developed in-house53. Diets were changed on a weekly basis and the mass remaining weighed to estimate the amount of food consumed per cage and then by each mouse. Individual cages housed 2–4 mice. Orientation studies indicated that the high-fat diet accelerated adenoma development; therefore, mice on the standard diet were sacrificed at 17 weeks of age and those on the high-fat diet at 14 weeks. Occasionally, mice had to be culled early due to welfare issues that were unrelated to adenoma development; therefore these mice were excluded from the analysis. Animals were killed by cardiac exsanguination under terminal anaesthesia, and the intestinal tract was removed and flushed with PBS. Blood was collected in lithium heparin tubes and plasma was obtained by centrifugation. The multiplicity, location and size of intestinal adenomas were recorded as described previously56. Intestinal tissue was either fixed in formalin and paraffin embedded or the mucosa was collected by scraping with a spatula and flash frozen in liquid nitrogen, and then stored at −80°C.
An identical smaller study was also performed on a separate occasion to directly compare adenoma development in mice on the standard versus high-fat control diet (n=7–9 females plus 7–8 males per group). All animals were culled at 14 weeks of age and tissues were collected and processed as described above.
Kinetics of AMPK activation in Wild-type C57BL/6J mice
Forty male C57BL/6J mice aged 8–10 weeks were placed on AIN-93 high-fat diet and randomised to two groups. After three weeks, mice (n=20) received either a single dose of resveratrol (2.1 μg in 100 μL 2% ethanol in water) by gavage or an equivalent volume of vehicle (control) only This dose of resveratrol approximates to the total daily intake of a mouse maintained on a diet containing 0.00007 % resveratrol, assuming it consumes 3 g of food per day. Groups of four mice were culled at the following time points after administration of resveratrol or vehicle: 0.5, 1, 2, 6, 24 h. Intestinal mucosa was collected and immediately flash frozen, as described above.
Tumour passaging
Colorectal tumours obtained from patients were passaged in mice to provide cancer tissue for explant cultures ex vivo. Adult male NOD/SCID (NOD/SCID NOD.CB17/JHliHsd-Prkdcscid) mice were fed on normal irradiated diet (5LF-5) for maintenance. Sections of tumours from surgical resections (~2mm thick) were placed in media 199, and washed twice in media 199 containing 2% Antibiotic-Antimycotic (Invitrogen) prior to implantation. The tumour tissue was inserted into the right and/or left flank of a mouse. Body weight and tumour size were measured twice a week. Mice were sacrificed and the tumour excised before they reached the size limit designated in the animal project licence. Tissue was used immediately for explant culture.
Measurement of metabolic parameters in plasma and tissue of male ApcMin mice
Plasma glucose, triglyceride and cholesterol levels were measured using individual commercially available colorimetric or fluorometric assay kits supplied by Cayman Chemical (Tallinn, Estonia). Samples were analysed according to the manufacturer’s protocols and signal intensity quantified using a FLUOstar OPTIMA plate scanner (BMG Labtech, Germany). For glucose and triglyceride concentrations, the absorption was quantified at 514nm and 540nm, respectively. In the cholesterol assay, fluorescence was measured using excitation wavelengths of 565–580nm and emission wavelengths of 585–595 nm. Plasma insulin levels were quantified using an Ultrasensitive Mouse Insulin ELISA assay from Mercodia (Uppsala, Sweden). Plasma and intestinal mucosa levels of IGF1 were determined using a Mouse IGF-I Quantikine ELISA Kit from R&D Systems (Minnesota, U.S.). All analysis was conducted in accordance with the manufacturer’s instructions.
Immunohistochemistry
Immunohistochemistry was performed using the Novolink Polymer Detection system (Leica Biosystems, Newcastle, UK) according to the manufacturer’s instructions. For formalin fixed mouse tissue the anti-Ki-67(ab15580, Abcam) and Caspase-3 (9661, Cell Signalling) were used at 1:1000 and 1:200 dilutions, respectively. For analysis of human explant tissues the phosphorylated AMPK antibody (2535, Cell Signalling) was used at a 1:100 dilution.
Cell culture
Apc10.1 cells were cultured in low glucose Dulbecco’s Modified Eagle’s Medium, supplemented with 20% foetal calf serum (Invitrogen) at 37 °C, 5% CO2. The primary antibodies used to assess protein levels in Apc10.1 cell lysates were all supplied by Cell Signalling Tech., apart from those for p21 and β-actin, which were purchased from Santa Cruz Biotechnologies. Dose response studies (Fig. 4a–b) for the expression of AMPKα, pAMPKα (Thr172), ACC, pACC (Ser79), mTOR, p-mTOR (Ser2448), P70S6K, p-P70S6K (Thr389), 4EBP1, p-4EBP1 (Thr70), p21 and LC3-I/II were conducted by treating Apc10.1 cells daily with resveratrol (0.001–1 μM) for six days. Cells were harvested 4 h after the last treatment, lysates produced and protein expression determined by Western blotting.
For the senescence-associated β-galactosidase assay, Apc10.1 cells were treated daily with resveratrol (0.001–1 μM) for six days. Four hours after the last treatment, cells were stained using a senescence β-galactosidase staining kit (Cell Signalling Tech.) according to the manufacturer’s instructions and viewed under a Nikon Eclipse TE2000 inverted microscope (200x total magnification). The percentage of SA-β-galactosidase positive stained cells was determined by counting the number of blue cells of the total cell number in several fields of view in three independent experiments. The same treatment protocol was followed for the analysis of autophagy using the Cyto-ID® Autophagy detection kit (Enzo Life Sciences, Switzerland). Rapamycin (500 nM) was included as a positive control.
For the analysis of resveratrol-induced reactive oxygen species (ROS), Apc10.1 cells were incubated with resveratrol (0.01 or 0.1 μM) or DMSO solvent control only, for 1 h. Levels of intracellular ROS were visualised using an Image-iT™ LIVE green ROS detection kit (Invitrogen) according to the manufacturer’s protocol. Nuclei were counterstained blue with Hoechst 33342 and tert-butyl hydroperoxide (100 μM) was used as a positive control. Stained cells were visualised using a confocal microscope at 408 nm for Hoechst and 488 nm for FITC.
To ascertain the role of reactive oxygen species in activating AMPK. Apc10.1 cells were pre-incubated with N-acetylcysteine (2mM) or DMSO vehicle for 30 min prior to addition of resveratrol (0.1 μM) or DMSO (control), then incubated for a further 6 h. Cells were harvested and the lysates subject to Western blotting to determine the ratio of AMPK to pAMPK.
A similar protocol was used to investigate whether resveratrol activated AMPK via effects on calcium signalling. In these experiments, cells were pre-exposed to BAPTA-AM (20 μM) or STO-609 (5 mg/mL) for 30 min. Resveratrol (0.01 or 0.1 μM) was then added and incubation continued for a further 6 h. Cells were then harvested, lysed and analysed by Western blotting.
HPLC assay for intracellular AMP, ADP and ATP
Analysis of AMP, ADP and ATP content in cells was performed using an adaptation of a published method57. Apc10.1 cells were treated with resveratrol or DMSO solvent control for 2 h, the media was then replaced and incubation continued for a further 4 h. Perchloric acid (0.15 mL of a 5% solution) was added to cell lysates and an aliquot of each supernatant (0.15 mL) was mixed with 0.1 mL of 1M K2CO3, centrifuged and the supernatant filtered prior to analysis. Extracts were subject to analysis on an Ultrasphere ODS column (250 × 4.6 mm, 5μm, HiChrom, UK) using a gradient mobile phase, consisting of (A) 0.1 M KH2PO4 buffer (pH 6) and (B) 10% acetonitrile in 0.1 M KH2PO4, at a flow rate of 1 mL/min, with UV detection at 256 nm. The gradient was 0% B for 9 min, and increased to 25% B over 6 min, then to 100% B by 17 min and remained at this for 5 min, before returning to the starting conditions. Identity was confirmed by comparison of retention time with authentic standards and the AMP/ATP ratio was calculated from the ratio of peak areas.
Explant cultures
Explant cultures were performed with primary colorectal cancer samples originating from three different patients after passage in mice. Tumour tissues were freshly excised from NOD/SCID mice and placed in DMEM (low glucose media) supplemented with 1% fetal calf serum and 2% antibiotic-antimycotic (explant culture media). Tissues were minced to an approximate size of 2mm3. For each concentration of resveratrol and the solvent control, nine pieces of tumour were placed in an insert, within a single well of a six well plate and explant culture media (1.5 mL) was added. The explants were incubated overnight at 37°C, 5% CO2. The next day the explant media was removed and fresh media supplemented with either resveratrol or vehicle control (DMSO) was added and incubated at 37° C for 2 h, before being harvested for either immunohistochemistry or Western blotting. For the analysis of protein expression by Western blotting, all 9 pieces of tissue in a single well were combined and lysed.
Statistical analysis
The effect of resveratrol dose on the expression of NQO1 protein in human colorectal tissue was analysed using a T-test and Mann Whitney test. The effect of resveratrol treatment on adenoma volume and number in ApcMin mice was modelled using regression analysis (of log-transformed data for volume). Mouse body weight data over time were analysed using mixed effects linear regression. The immunohistochemistry and in vitro data were analysed using a T-test.
Supplementary Material
Acknowledgments
We thank Carla De Giovanni for the Apc.10.1 cells. Work was supported by Cancer Research UK (C325/A6691 and C1362/A18081) with assistance from the Leicester Experimental Cancer Medicine Centre (C325/A15575 Cancer Research UK/UK Department of Health). AK was funded by a studentship from the Libyan Government. AMS analysis was performed at the Research Resource for Biomedical AMS Laboratory, operated at LLNL and supported by the NIH National Centre for Research Resources, Biomedical Technology Program grant #P41RR13461.
References
- 1.Cuzick J, et al. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 2.Hershman D, Sundararajan V, Jacobson JS, Heitjan DF, Neugut AI, Grann VR. Outcomes of tamoxifen chemoprevention for breast cancer in very high-risk women: a cost effectiveness analysis. J Clin Oncol. 2011;20:9–16. doi: 10.1200/JCO.2002.20.1.9. [DOI] [PubMed] [Google Scholar]
- 3.Steinbach G, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein L, et al. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91:1654–62. doi: 10.1093/jnci/91.19.1654. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 6.Klein EA, et al. Vitamin E and the risk of prostate cancer – The selenium and vitamin E cancer prevention trial (SELECT) J Am Med Assoc. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omenn GS, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 8.Goodman GE, Schaffer S, Omenn GS, Chen C, King I. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from β-carotene and retinol efficacy trial. Cancer Epidemiol Biomarkers Prev. 2003;12:518–526. [PubMed] [Google Scholar]
- 9.Scott EN, Steward WP, Gescher AJ, Brown K. Development of dietary phytochemical chemopreventive agents: biomarkers and choice of dose for early clinical trials. Cancer Prev Res. 2009;2:525–530. doi: 10.1158/1940-6207.CAPR-08-0223. [DOI] [PubMed] [Google Scholar]
- 10.Scott E, Steward WP, Gescher AJ, Brown K. Resveratrol in human cancer chemoprevention – choosing the “right” dose. Mol Nutr Food Res. 2011;56:7–13. doi: 10.1002/mnfr.201100400. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JR. β-Carotene: a miss for epidemiology. J Natl Cancer Inst. 1999;91:2068–2069. doi: 10.1093/jnci/91.24.2068. [DOI] [PubMed] [Google Scholar]
- 12.Patterson SL, Maresso KC, Hawk E. Cancer chemoprevention: successes and failures. Clin Chem. 2013;59:94–101. doi: 10.1373/clinchem.2012.185389. [DOI] [PubMed] [Google Scholar]
- 13.Pezzuto JM. Resveratrol as an inhibitor of carcinogenesis. Pharm Biol. 2008;46:443–573. [Google Scholar]
- 14.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 15.Brown VA, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel KR, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical Trials of resveratrol in Resveratrol and Health. Ann N Y Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown K, Tompkins EM, White INH. Applications of accelerator mass spectrometry for pharmacological and toxicological research. Mass Spectrom Rev. 2006;25:127–145. doi: 10.1002/mas.20059. [DOI] [PubMed] [Google Scholar]
- 19.Patel KR, et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci Transl Med. 2013;5:205ra133. doi: 10.1126/scitranslmed.3005870. [DOI] [PubMed] [Google Scholar]
- 20.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1–14. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Pearson KJ, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmelman AC. The dynamic nature of autophagy in cancer. Genes Dev. 2011;25:1999–2010. doi: 10.1101/gad.17558811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young AR, Narita M. Connecting autophagy to senescence in pathophysiology. Curr Opin Cell Biol. 2010;22:234–240. doi: 10.1016/j.ceb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 25.De Giovanni C, et al. Apc10.1: an ApcMin/+ intestinal cell line with retention of heterozygosity. Int J Cancer. 2004;109:200–206. doi: 10.1002/ijc.11690. [DOI] [PubMed] [Google Scholar]
- 26.Park SJ, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawley SA, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zmijewski JW, et al. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 2010;285:33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Liang B, Shirwany NA, Zou MH. 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoSOne. 2011;6:e17234. doi: 10.1371/journal.pone.0017234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ju TC, et al. AMPK-α1 functions downstream of oxidative stress to mediate neuronal atrophy in Huntington’s disease. Biochim Biophys Acta. 2014;1842:1668–1680. doi: 10.1016/j.bbadis.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1–Nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 33.Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol Med. 2003;9:169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 34.Kristal AR, et al. Baseline selenium status and effects of selenium and vitamin E supplementation on prostate cancer risk. J Natl Cancer Inst. 2014;106:djt456. doi: 10.1093/jnci/djt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christensen MJ. Selenium and prostate cancer prevention: what next – if anything? Cancer Prev Res. 7:781–785. doi: 10.1158/1940-6207.CAPR-14-0197. [DOI] [PubMed] [Google Scholar]
- 36.Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nature Med. 2014;20:709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]
- 37.Sale S, et al. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) on adenoma development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int J Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler CC, Rainwater L, Whelan J, McEntee MF. Dietary resveratrol does not affect intestinal tumorigenesis in Apc(Min/+) mice. J Nutr. 2004;134:5–10. doi: 10.1093/jn/134.1.5. [DOI] [PubMed] [Google Scholar]
- 39.Timmers S, et al. Calorie restriction-like effect of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutrition Res. 2012;32:537–541. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Brasnyo P, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 42.Yoshino J, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poulsen MM, et al. High-dose resveratrol supplementation in obese men - an invesigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winzell MS, Ahrén B. The high-fat diet-fed mouse. A model for studying mechanisms of glucose tolerance and type 2 diabetes. Diabetes. 2004;53:S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 45.Larsson AC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 46.Aleksandrova K, et al. Metabolic syndrome and risks of colon and rectal cancer: The European Prospective Investigation into Cancer and Nutrition study. Cancer Prev Res. 2011;4:1873–1883. doi: 10.1158/1940-6207.CAPR-11-0218. [DOI] [PubMed] [Google Scholar]
- 47.Day AD, et al. Linking inflammation to tumorigenesis in a mouse model of high-fat-diet-enhanced colon cancer. Cytokine. 2013;64:454–462. doi: 10.1016/j.cyto.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Mechanisms of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci USA. 2007;104:13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gescher AJ, Steward WP. Relationship between mechanisms, bioavailability and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev. 2003;12:953–957. [PubMed] [Google Scholar]
- 50.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Mancera PA, Young ARJ, Narita M. Inside and out: the activities of senescence in cancer. Nature Rev Cancer. 2014;14:547–558. doi: 10.1038/nrc3773. [DOI] [PubMed] [Google Scholar]
- 52.Brown K, Dingley KH, Turteltaub KW. Accelerator mass spectrometry for biomedical research. Methods in Enzymology, Elsevier Academic Press Inc. 2005;402:423–43. doi: 10.1016/S0076-6879(05)02014-8. [DOI] [PubMed] [Google Scholar]
- 53.Boocock DJ, et al. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J Chromatogr B. 2007;848:182–187. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Workman P, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luongo C, Moser AR, Gledhill S, Dove WF. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 1994;54:5947–5952. [PubMed] [Google Scholar]
- 56.Perkins S, et al. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11:535–540. [PubMed] [Google Scholar]
- 57.Stocchi V, et al. Simultaneous extraction and reverse-phase high-performance liquid chromatographic determination of adenine and pyridine nucleotides in human red blood cells. Anal Biochem. 1985;146:118–124. doi: 10.1016/0003-2697(85)90405-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.