Abstract
Background
Diabetics remain at high risk of cardiovascular disease and mortality despite advancements in medical therapy. Noninvasive cardiac risk profiling is often more difficult in diabetics owing to the prevalence of silent ischemia with unrecognized myocardial infarction (MI), reduced exercise capacity, non-diagnostic electrocardiographic changes, and balanced ischemia from diffuse epicardial coronary atherosclerosis and microvascular dysfunction.
Methods and Results
A consecutive cohort of 173 diabetic patients (mean age 61.7±11.9, 37% female) with suspected myocardial ischemia underwent stress perfusion CMR. Patients were evaluated for adverse cardiac events following CMR with mean follow-up time of 2.9 ± 2.5 years. Mean HbA1C for the population was 7.9±1.8%. Primary endpoint was a composite of cardiac death and nonfatal MI. Diabetics with no inducible ischemia (n=94) experienced an annualized event rate of 1.4% compared to 8.2% (P=0.0003) in those with inducible ischemia (n=79). Diabetics without late gadolinium enhancement or inducible ischemia had a very low annual cardiac event rate (0.5%/year). Presence of inducible ischemia was the strongest unadjusted predictor (HR 4.86, P<0.01) for cardiac death and nonfatal MI. This association remained robust in adjusted stepwise multivariable Cox regression analysis (HR 4.28, P=0.02). In addition, categorical net reclassification index (NRI) using 5-year risk cutoffs of 5% and 10% resulted in reclassification of 43.4% of the diabetic cohort with NRI of 0.38 (95% CI 0.20–0.56, P<0.0001).
Conclusions
Stress perfusion CMR provided independent prognostic utility and effectively reclassified risk in diabetic patients referred for ischemic assessment. Further evaluation is required to determine if a noninvasive imaging strategy with CMR can favorably impact downstream outcomes and improve cost-effectiveness of care in diabetics.
Keywords: diabetes mellitus, magnetic resonance imaging, perfusion, ischemia, late gadolinium enhancement, cardiac death, myocardial infarction
Diabetes remains one of the leading causes of mortality and morbidity despite advancements in medical therapy, with prevalence continuing to increase worldwide.1 Diabetes is considered a coronary heart disease (CHD) risk equivalent, as diabetic patients without any history of myocardial infarction (MI) experience elevated risk of cardiac death and recurrent nonfatal MI similar to patients with established coronary artery disease (CAD).2, 3 Unfortunately, noninvasive risk assessment of diabetic patients remains challenging owing to multiple factors, including more advanced disease at time of presentation, limited exercise capacity due to peripheral arterial disease and microvascular complications, and increased prevalence of silent MI and multi-vessel coronary disease resulting in balanced ischemia.4, 5 Numerous studies have reported that diabetic populations with normal single-photon emission computed tomography (SPECT) or stress echocardiographic assessment may remain at high-risk of adverse cardiac events,6–9 particularly those who are unable to exercise and require pharmacological stress testing.10–12
Stress perfusion CMR has shown excellent diagnostic performance for the detection of CAD,13 and is able to assess both diffuse myocardial ischemia and microvascular dysfunction.14 This study was designed to evaluate the prognostic value of stress perfusion CMR in a consecutive diabetic cohort with suspected myocardial ischemia.
METHODS
Study Sample
We performed vasodilator stress perfusion CMR in 173 consecutive diabetic patients referred for evaluation of suspected myocardial ischemia. All patients were over the age of 21 (range 29 – 87 years of age) and the diagnosis of diabetes was established according to the current American Diabetics Association guidelines.15 Exclusion criteria included any typical contraindications to vasodilator stress testing, any metallic hazard for MRI, prior or known intolerance to gadolinium, severe renal impairment (glomerular filtration rate < 30 mL/min/1.73 m2), and pregnant state. Prior to CMR, trained study personnel collected detailed clinical data and medical history using standardized criteria.16 The institutional ethics review board approved the study, and informed consent was obtained from all patients. This cohort represents a combination of a patient subset from a larger study we have previously published (143 patients, 82.7%),16 as well as an additional 30 (17.3%) consecutive diabetic patients subsequently referred for stress CMR.
Stress CMR Protocol
Before 2006, stress CMR was performed with a 1.5 Tesla scanner (Signa CV/i, General Electric, Milwaukee, Wisconsin; 8-element coil), while studies after 2006 were conducted with a 3.0 Tesla magnet (Magnetom Trio, Siemens, Erlangen, Germany; 16-element coil). Our standardized stress CMR protocol included stress and rest myocardial perfusion, cine function, and late gadolinium enhancement (LGE) as previously published.16 All patients were instructed to refrain from caffeinated products 24 hours, and fast 6-hours, prior to stress CMR. A 12-lead electrocardiogram was obtained before and after stress CMR study, and both vital signs and cardiac rhythm were monitored throughout the entire examination. Gating was performed using vectorcardiography or pulse oximetry.
Both stress and rest myocardial perfusion images were acquired at 1.5 Tesla using a saturation-prepared, single-shot spoiled gradient echo sequence (repetition time 6 msec; echo time 2.3 msec; echo-train length 4; acceleration factor of 2 using SENSE; slice thickness: 8 mm) and at 3.0 Tesla using a saturation-recovery prepared turbo fast low-angle single shot (FLASH) sequence (TR 2.4 msec; TE 1 msec; acceleration factor of 2 using Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA; slice thickness of 10 mm). A bolus injection of 0.1 mmol/kg of gadolinium-based contrast agent (Magnevist, Bayer, Wayne, NJ) was administered at a rate of 4–5 mL/sec followed by 20 mL of saline flush at the same injection rate for both stress and rest perfusion imaging. Patients received vasodilator stress with either adenosine (Astellas Pharma US, Deerfield, Illinois) at a rate of 140 mcg/kg/min over 6 min, or regadenoson (Astellas Pharma US, Deerfield, Illinois) with a bolus injection of 0.4 mg. Both stress and rest myocardial perfusion images were acquired at 4 matching slice locations: basal, mid and distal short-axis, in addition to a 4-chamber long axis view. Medical personnel measured the blood pressure of all patient subjects before and after administration of the vasodilator stress agent. Cine imaging was performed in both contiguous short-axis (8 mm, 0 mm spacing) and long axis views (4-chamber, 2-chamber and 3-chamber) using steady-state free precession (SSFP) imaging (repetition time 3.4 msec; echo time 1.2 msec; matrix 256 × 256, slice thickness 8 mm). LGE imaging was performed 10–15 minutes after rest perfusion imaging using an inversion recovery gradient echo sequence with inversion time chosen to maximally null the septal myocardium.
CMR Image Analysis
Images were uploaded to an offline standard workstation (Mass Clinical 7.4®, Medis, Leiden, Netherlands) for post-processing and viewing, and the studies were interpreted by the consensus of two experienced reviewers (R.Y.K., B.H.) who were blinded to the clinical and follow-up data of the patients. Perfusion defect(s) consistent with inducible ischemia were defined by > 1 segment of subendocardial hypoperfusion, occurring during stress perfusion but not at rest or without matching LGE, persisted for at least 3 phases beyond peak myocardial enhancement, greater than 1 pixel wide, and followed a coronary distribution.17 Susceptibility artifacts and isolated papillary muscle or epicardial defects were not considered perfusion defects. Segmental analysis for perfusion, regional wall motion abnormalities, and LGE were performed using standard 16-segment AHA model.18 Inducible ischemia score was calculated by summing the number of segments with inducible ischemia. Infarct volume was quantified from subendocardial LGE imaging using standard criteria of ≥2 standard deviations of signal intensity beyond normal remote myocardium.
Clinical Outcomes and Follow-up
Patient follow-up was first performed by a thorough retrospective review of the available electronic medical record, followed by contacting patients using approved standardized study questionnaires. Mortality was also confirmed by the Social Security Death Index. The primary endpoint of the study was a composite measure of cardiac death or nonfatal MI. Secondary endpoints included the primary endpoint in addition to late coronary revascularization (at least 90 days of CMR), and a composite measure, which also included stroke and hospitalization for unstable angina, termed major adverse cardiac events (MACE).
Non-fatal myocardial infarction was defined from standardized criteria as the presence of a typical rise (> 99th percentile over upper limits of normal) and fall of serum cardiac biomarkers (troponin or CK-MB), along with at least one of the followings: symptoms of ischemia, development of pathologic Q-waves or ST-segment elevation on 12-lead ECG, or angiographic evidence of CAD.19 Mortality was confirmed from the medical record and/or from Social Security Death Index. All cases of mortality were adjudicated by the consensus of 2 study investigators, blinded to all CMR data, as either cardiac (due to acute MI, arrhythmia or pump failure) or non-cardiac. Unstable angina was defined by anginal symptoms requiring hospitalization in the presence of angiographically significant coronary stenosis. Stroke was defined as any event with the development of focal neurological deficits of central origin lasting at least 72 hours and resulting in a physical impairment or radiological evidence of damage consistent with stroke.
Statistical Analysis
Categorical variables were presented as counts with percentages, while continuous variables were expressed as means ± standard deviation or as median values with interquartile range depending on normality of distributions. Categorical variables were compared using the Fisher’s exact test, while comparisons for continuous data was performed using 2-sample Student t test or Wilcoxon rank-sum test, where appropriate. Kaplan-Meier analysis was performed for evaluation of MACE-free survival and stratified comparisons were evaluated by the log-rank test. Relationships between HbA1c levels and duration of diabetes with LGE and inducible ischemia score were evaluated with linear regression analysis. Univariable associations with the primary and secondary endpoints were determined by Cox proportional hazards regression. Multivariable Cox regression analysis was performed using stepwise selection of variables significant on univariable screen and forcing both age and gender into the model a priori. For the evaluation of annualized event rates, the entire cohort was classified into those with inducible ischemia, patients with subendocardial LGE but no inducible ischemia, and those without either inducible ischemia or LGE. A propensity score was generated using a logistic regression model using inducible ischemia as the dependent variable and known risk markers, patient age, gender, history of MI, history of PCI, LVEF, left ventricular end-diastolic volume index, and LV myocardial mass, as independent variables. Multivariable Cox regression analysis was then performed to associate inducible ischemia with the primary endpoints, adjusting for the propensity score. Net reclassification index was used to evaluate the incremental benefit of inducible ischemia by stress perfusion CMR using five-year risk categories of 5% (low risk) and 10% (high risk) based on prior studies in diabetics evaluating cardiac risk stratification and guidelines for management.20–22 Risk estimates were derived from multivariable Cox regression analysis for the addition of inducible ischemia to a multivariable model containing age, gender, and LVEF.
All statistical analysis was performed using commercially available software (SAS version 9.4, SAS Institute, Cary, North Carolina). A two-sided p-value of less than 0.05 was considered statistically significant.
RESULTS
Study Sample
A total of 173 consecutive diabetic patients were referred for stress perfusion CMR. All patients successfully underwent diagnostic stress CMR without any major complications, and all studies were of diagnostic quality. Baseline demographics stratified by the presence of inducible ischemia are summarized in Table 1. Both the mean body mass index (BMI) and HbA1C for the entire cohort were elevated (31.2± 6.9 and 7.9±1.8%, respectively). As expected, the stratum with inducible ischemia was significantly older, had a higher proportion of anginal chest pain, prior history of myocardial infarction, previous PCI and CABG, aspirin and beta-blocker use, and both ST-abnormalities and T-wave inversions on resting 12-lead electrocardiography.
Table 1.
Baseline Demographics of Total Cohort and Stratified by Presence or Absence of Inducible Ischemia by CMR
| Total (n = 173) |
Inducible Ischemia (n= 79) |
No Inducible Ischemia (n = 94) |
P-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 61.7±11.9 | 63.9±10.9 | 59.9±12.3 | <0.05* |
| Female | 64 (37%) | 23 (29%) | 41 (44%) | 0.06 |
| Body mass index (kg/m2) | 31.2±6.9 | 30.2±5.5 | 32.1±7.8 | 0.07 |
| Resting heart rate (bpm) | 72.0±14.1 | 72.1±13.2 | 71.9±14.8 | 0.92 |
| Hemoglobin A1c (%) | 7.9 ±1.8 | 7.9±1.7 | 7.9±1.9 | 0.90 |
| Coronary Risk Factors | ||||
| History of smoking (>10 pack-years) | 42 (24%) | 21 (27%) | 21 (22%) | 0.59 |
| History of hypertension | 141 (82%) | 67 (85%) | 74 (79%) | 0.24 |
| History of hypercholesterolemia | 132 (76%) | 64 (81%) | 68 (72%) | 0.15 |
| Anginal chest pain | 50 (29%) | 30 (38%) | 20 (21%) | <0.05* |
| History of MI | 41 (24%) | 32 (41%) | 9 (10%) | <0.0001* |
| History of PCI | 42 (24%) | 29 (37%) | 13 (14%) | <0.001* |
| History of CABG | 18 (10%) | 12 (15%) | 6 (7%) | 0.08 |
| History of CHF | 57 (33%) | 31 (39%) | 26 (28%) | 0.11 |
| Family history of CAD | 42 (24%) | 21 (27%) | 21 (22%) | 0.59 |
| Medications | ||||
| Oral hypoglycemic agent(s) | 85 (49%) | 33 (42%) | 52 (55%) | 0.12 |
| Insulin | 53 (31%) | 27 (34%) | 26 (28%) | 0.31 |
| ACEI or ARB | 108 (62%) | 49 (62%) | 59 (63%) | 1 |
| Statin | 140 (81%) | 67 (85%) | 73 (78%) | 0.21 |
| Aspirin | 123 (71%) | 65 (82%) | 58 (62%) | <0.01* |
| Beta-blocker | 117 (68%) | 61 (77%) | 56 (60%) | < 0.05* |
| Calcium channel blocker | 36 (21%) | 13 (16%) | 23 (24%) | 0.26 |
| Diuretics | 65 (38%) | 35 (44%) | 30 (32%) | 0.06 |
| Nitroglycerin | 33 (19%) | 22 (28%) | 11 (12%) | < 0.05* |
| Electrocardiogram | ||||
| Pathologic Q-waves | 32 (18%) | 19 (24%) | 13 (14%) | 0.12 |
| Left bundle branch block | 14 (8%) | 7 (9%) | 7 (7%) | 0.79 |
| Left ventricular hypertrophy | 15 (9%) | 7 (9%) | 8 (9%) | 1 |
| Resting ST-segment deviation | 25 (14%) | 16 (20%) | 9 (10%) | < 0.05* |
| T-wave inversion | 43 (25%) | 27 (34%) | 16 (17%) | < 0.01* |
| QRS prolongation (≥120 msec) | 23 (13%) | 11 (14%) | 12 (13%) | 0.83 |
| CMR | ||||
| Inducible ischemia score (# of segments) |
2.2±3.2 | 4.8±3.2 | 0±0 | < 0.0001* |
| Resting wall motion abnormality | 60 (35%) | 44 (56%) | 17 (18%) | < 0.0001* |
| LGE (subendocardial, present) | 88 (51%) | 56 (71%) | 32 (34%) | < 0.0001* |
| Global LGE (% of total myocardium) | 5.7±10.5 | 9.3±12.6 | 3.0±7.6 | < 0.001* |
| LVEF (%) | 51.8±17.6 | 48.7±18.9 | 54.3±16.1 | < 0.05* |
| LV end-diastolic volume index (ml/m2) |
94.9±39.9 | 101.3±46.9 | 90.0±32.8 | 0.07 |
| LV end-systolic volume index (ml/m2) |
51.6±41.5 | 60.0±49.8 | 45.1±32.6 | < 0.05* |
| LV mass index (g/m2) | 66.6±20.5 | 67.8±20.0 | 65.6±21.0 | 0.49 |
| RVEF (%) | 51.5±10.6 | 50.9±10.9 | 52.0±10.4 | 0.51 |
P-value of less than 0.05.
ACEI=angiotensin-converting enzyme inhibitor, ARB=angiotensin II receptor blocker, CABG=coronary artery bypass grafting surgery, CAD=coronary artery disease, CHF=congestive heart failure, CMR=cardiac magnetic resonance imaging, LGE=late gadolinium enhancement, LV=left ventricular, LVEF = left ventricular ejection fraction, MI=myocardial infarction, PCI=percutaneous coronary intervention, RVEF=right ventricular ejection fraction.
CMR Characteristics
CMR findings for the entire cohort and stratified by the presence of inducible ischemia are shown in Table 1. Overall, the diabetic cohort had a relatively preserved LVEF (51.8±18%), 35% of patients had regional wall motion abnormalities, and 51% had the presence of subendocardial LGE. As expected, the stratum with inducible ischemia had a greater proportion of regional wall motion abnormalities, and subendocardial LGE, in addition to a greater burden of global LGE, measured as percentage of the total myocardium. LVEF was significantly lower, and indexed left ventricular end-systolic volume was significantly higher in diabetics with inducible ischemia by CMR. The mean inducible ischemia score in this stratum was 4.8±3.2 segments (16-segment AHA model).
Primary and Secondary Outcomes
Follow-up for clinical events was available for all patients in the cohort over a mean follow-up time of 2.9 ± 2.5 years. In total, there were 13 cases (7.8%) of adjudicated cardiac death, 12 patients (7.2%) suffered nonfatal MI, with a total of 21 events (12.1%) for the primary endpoint (4 patients suffered both nonfatal MI and cardiac death). A total of 38 diabetics (22%) underwent early revascularization (≤90 days from CMR), 16 (9.3%) underwent late revascularization (>90 days from CMR), 8 (4.8%) suffered a stroke, and 33 patients (19.9%) were hospitalized for unstable angina.
Kaplan Meier analysis for survival free from cardiac death or MI, stratified by the presence or absence of inducible ischemia, is shown in Figures 1A. Over 5-year follow-up, diabetics with no inducible ischemia by CMR had significantly higher event-free survival (P=0.004). Kaplan Meier analysis stratified by the presence of LGE (green line) or inducible ischemia (red line) for the primary composite endpoint demonstrated significantly lower event-free survival for both those patients with LGE and diabetics with inducible ischemia (P=0.006) (Figure 1B).
Figure 1. Kaplan-Meier survival curves for cardiac death + MI stratified by presence of (A) inducible ischemia and (B) both inducible ischemia and late gadolinium enhancement (LGE) in diabetics (N=173).
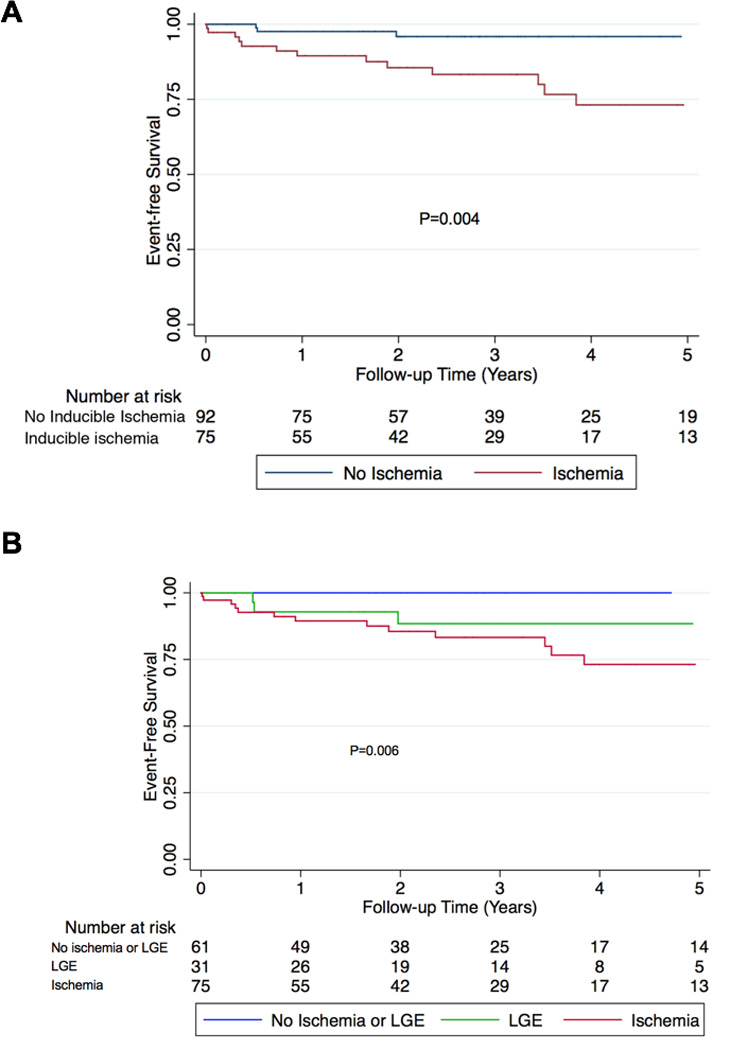
A) Kaplan-Meier curves stratified by the presence or absence of inducible ischemia demonstrates significantly lower survival from cardiac death and nonfatal MI in diabetics with inducible ischemia (P=0.004) over 5 years. B) Kaplan-Meier curves stratified by the presence of late gadolinium enhancement (green line), or presence of inducible ischemia (red line) shows significantly lower survival from cardiac death and nonfatal MI for patients with LGE and inducible ischemia (P=0.006). LGE = subendocardial late gadolinium enhancement.
Annual Event Rates
Estimated annualized event rates for the primary and secondary endpoints, stratified by the presence of inducible ischemia by CMR, are shown in Figure 2A. The entire cohort suffered an annual rate of cardiac death or nonfatal MI of 4.2%, 6.5% with the addition of late revascularization, and 12.2% for the secondary composite endpoint of MACE. Diabetics with no inducible ischemia had significantly lower annual event rates for all of the outcome measures, the primary composite endpoint of cardiac death or MI was 1.4% versus 8.2%, respectively (P=0.0003). Figure 2B displays annualized event rates for the primary and secondary endpoints of those patients with neither inducible ischemia or LGE (blue bars), LGE but no inducible ischemia (green bars), and all diabetics with inducible ischemia (red bars). For the primary composite endpoint, those diabetics without inducible ischemia or LGE had very low annual event rates for cardiac or nonfatal MI of 0.5%/year. For all secondary outcome measures, diabetics with LGE had higher annual event rates, whilst those patients with inducible ischemia had the highest annual event rates for both primary and secondary outcomes. There were no differences in annual event rates for either the primary or secondary outcome measures when diabetics with inducible ischemia were stratified by the presence of LGE (Figure 3). Event rates stratified by both presence of inducible ischemia and pre-test history of MI are shown in Figure 4. Although there was no significant difference between annual event rates amongst patients with no inducible ischemia irrespective of pre-test history of MI, diabetics with inducible ischemia and history of MI had the highest event rates amongst the entire cohort (11.8% versus 6.3%).
Figure 2. Estimated annualized event rates in diabetics stratified by the presence or absence of inducible ischemia and late gadolinium enhancement by stress perfusion CMR (N=173).
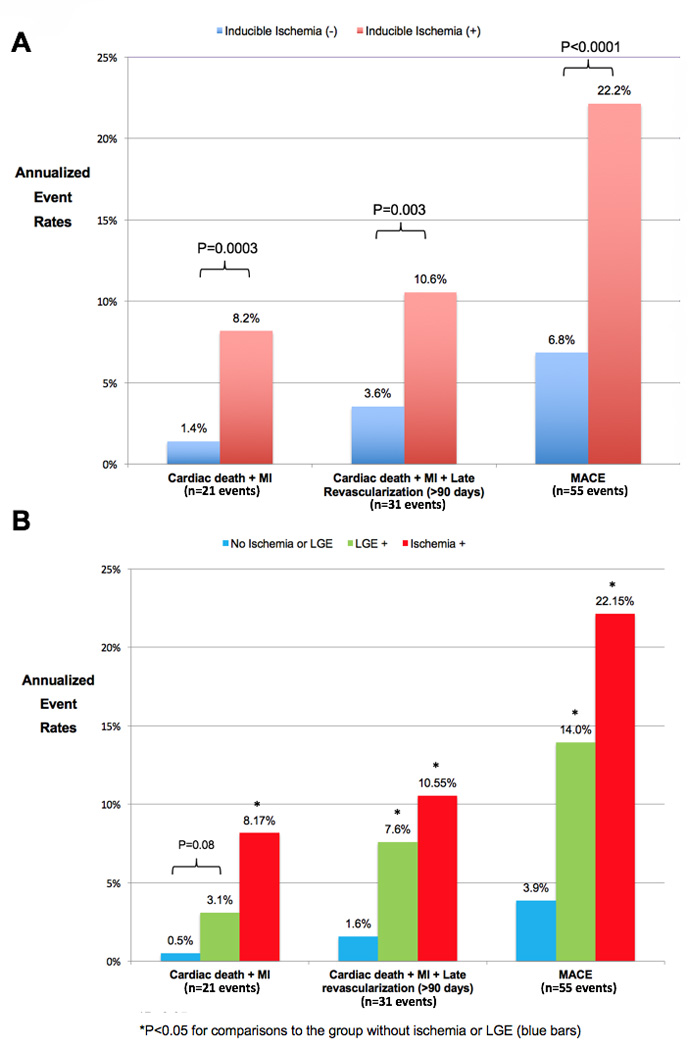
A) Annualized event rates for the primary and secondary outcome measures stratified by the presence (red bars) or absence (blue bars) of inducible ischemia by stress perfusion CMR demonstrate significantly lower annual event rates for both primary and secondary composite outcome measures. B) Annualized event rates for the primary and secondary outcome measures stratified by the presence of LGE (green bars), presence of inducible ischemia (red bars), or absence of both LGE and inducible ischemia (blue bars). *Indicate P values <0.05 for stratified comparisons between event rates. LGE = subendocardial late gadolinium enhancement, MACE = cardiac death, non-fatal MI, late revascularization (>90 days), stroke, and hospitalization for unstable angina, MI = myocardial infarction.
Figure 3. Estimated annualized event rates in diabetics with inducible ischemia by stress perfusion CMR stratified by the presence or absence of late gadolinium enhancement (N=79).
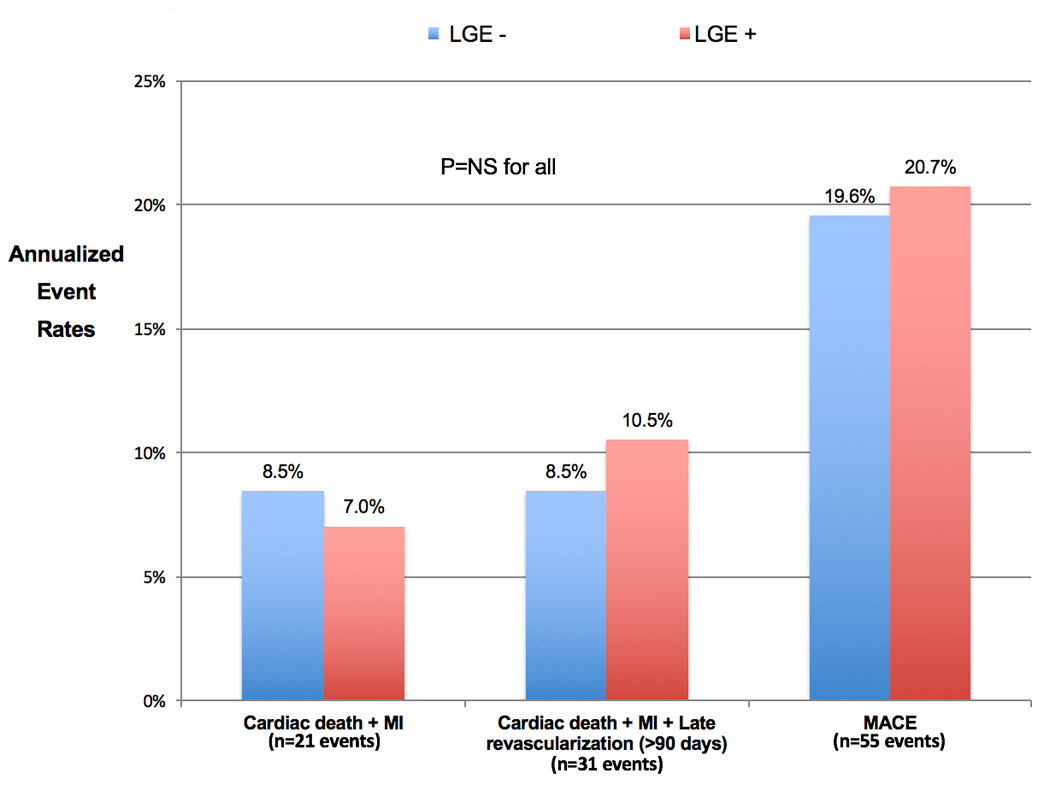
Annual event rates for the primary and secondary outcome measures for diabetics with inducible ischemia stratified by the presence or absence of LGE. LGE = subendocardial late gadolinium enhancement, MACE = cardiac death, non-fatal MI, late revascularization (>90 days), stroke, and hospitalization for unstable angina, MI = myocardial infarction.
Figure 4. Estimated annualized event rates of cardiac death and nonfatal MI in diabetics stratified by presence of inducible ischemia and pre-existing history of myocardial infarction.
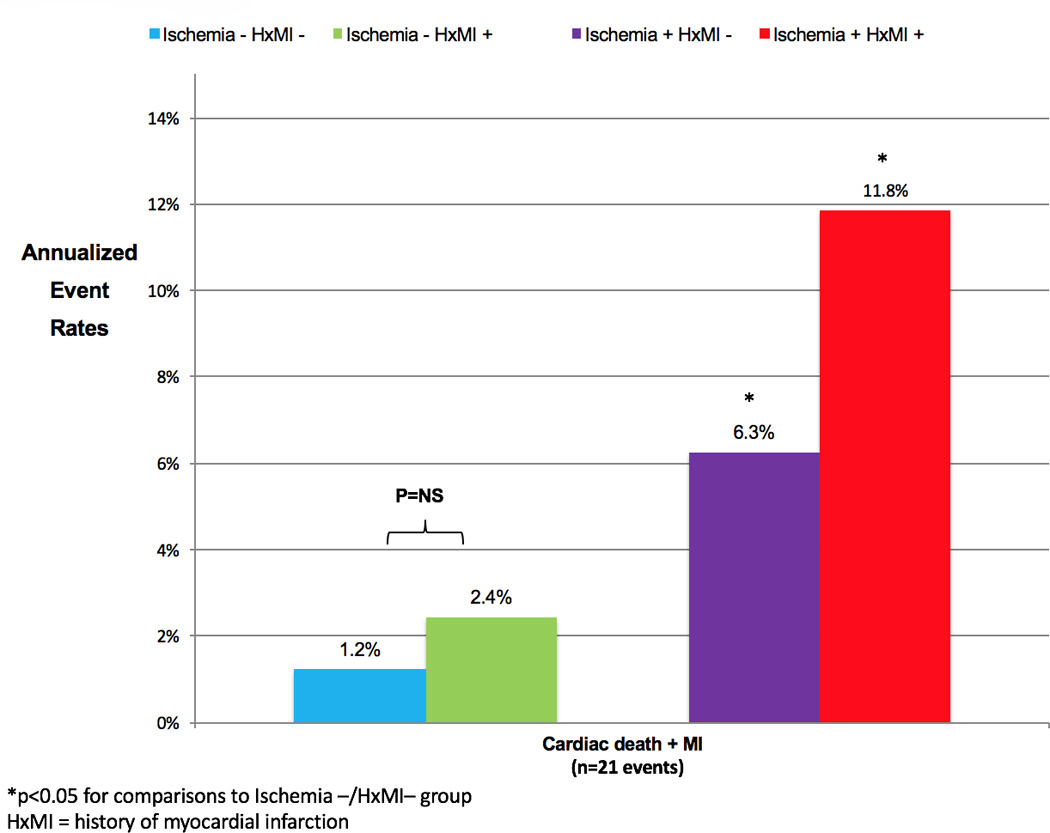
Annual event rates of cardiac death and nonfatal myocardial infarction for diabetics stratified by presence or absence of inducible ischemia and pre-existing history of myocardial infarction. HxMI = history of myocardial infarction, MI = myocardial infarction.
Incremental Prognostic Value of Inducible Ischemia
Univariable Cox regression analysis for the primary endpoint of cardiac death or nonfatal MI is shown in Table 2. Aspirin use (HR 8.35, 95% confidence interval 1.12–62.58, P=0.04), presence of LGE (HR 4.84, 95% confidence interval 1.06–22.09, P=0.04), and inducible ischemia (HR 4.86, 95% confidence interval 1.61–14.67, P<0.01) were all significant predictors of cardiac death or MI. For every additional segment of the 16-segment AHA model there was a HR 1.21 (95% confidence interval 1.10–1.33, P<0.0001) for the primary composite endpoint.
Table 2.
Cardiac Death and Non-fatal MI (n=21 events) Univariable and Multivariable Analyses
| Variable | Univariable Analysis | Multivariable Analysis§ | ||
|---|---|---|---|---|
| Unadjusted Hazard Ratio (95% CI) |
P-Value | Adjusted Hazard Ratio (95% CI) |
P-Value | |
| Demographics | ||||
| Age | 1.03 (0.99–1.07) | 0.14 | 1.03 (0.98–1.08) | 0.24 |
| Female | 1.01 (0.38–2.69) | 0.99 | 1.12 (0.33–3.73) | 0.86 |
| Body mass index (kg/m2) | 0.95 (0.88–1.03) | 0.19 | ||
| Hemoglobin A1c (%) | 0.86 (0.66–1.12) | 0.27 | ||
| Coronary Risk Factors | ||||
| History of smoking (>10 pack year) | 1.43 (0.61–3.32) | 0.41 | ||
| History of hypertension | 2.02 (0.47–8.77) | 0.35 | ||
| History of hypercholesterolemia | 0.81 (0.29–2.25) | 0.68 | ||
| Angina | 0.65 (0.21–1.96) | 0.44 | ||
| History of myocardial infarction | 2.21 (0.88–5.52) | 0.09 | ||
| History of PCI | 1.93 (0.76–4.94) | 0.17 | ||
| History of CABG | 0.38 (0.05–2.84) | 0.35 | ||
| Family history of CAD | 1.46 (0.55–3.84) | 0.45 | ||
| Medications | ||||
| ACEI | 0.93 (0.37–2.36) | 0.88 | ||
| Statin | 0.77 (0.28–2.15) | 0.62 | ||
| Aspirin | 8.35 (1.12–62.58) | 0.04* | ||
| Beta-blocker | 2.04 (0.67–6.19) | 0.21 | ||
| Calcium channel blocker | 0.74 (0.22–2.54) | 0.63 | ||
| Oral hypoglycemic agents | 0.84 (0.32–2.24) | 0.73 | ||
| Insulin | 0.49 (0.14–1.70) | 0.26 | ||
| ELECTROCARDIOGRAM | ||||
| Resting ST-segment deviation | 2.53 (0.90–7.14) | 0.08 | ||
| Pathologic Q waves | 1.24 (0.41–3.73) | 0.71 | ||
| Left bundle branch block | 1.97 (0.57–6.82) | 0.28 | ||
| Left ventricular hypertrophy | 0.60 (0.08–4.55) | 0.62 | ||
| T-wave inversion | 1.10 (0.40–3.05) | 0.86 | ||
| CMR | ||||
| Inducible ischemia | 4.86 (1.61–14.67) | < 0.01* | 4.21 (1.28–13.83) | 0.02* |
| Inducible ischemia score (number of segments) | 1.21 (1.10–1.33) | < 0.001* | ||
| Resting wall motion abnormalities | 1.91 (0.76–4.82) | 0.17 | ||
| LGE (subendocardial, present) | 4.84 (1.06–22.09) | 0.04* | ||
| Global LGE (% of total myocardium) | 1.00 (0.96–1.04) | 0.89 | ||
| LVEF (%) | 0.98 (0.96–1.01) | 0.19 | ||
| LV end-diastolic volume index (per 10 ml/m2) | 1.06 (0.95–1.17) | 0.30 | ||
| LV end-systolic volume index (per 10 ml/m2) | 1.06 (0.96–1.16) | 0.28 | ||
| LV mass index (g/m2) | 1.02 (0.98–1.03) | 0.09 | ||
| RVEF (%) | 0.96 (0.92–1.01) | 0.12 | ||
P-value of less than 0.05.
LVEF and the presence of LGE were not significant and removed from the final model in stepwise selection for P value of less than 0.05.
CABG=coronary artery bypass grafting surgery, CAD=coronary artery disease, CMR=cardiac magnetic resonance imaging, ECG=electrocardiography, LGE=late gadolinium enhancement, LV=left ventricular; LVEF=left ventricular ejection fraction, PCI=percutaneous coronary intervention, RVEF=right ventricular ejection fraction.
Multivariable Cox regression analysis was performed with stepwise selection of significant univariable predictors after forcing age and gender into the model a priori due to their established prognostic significance in the literature (Table 2).22 Inducible ischemia emerged as the only significant independent predictor for cardiac death or nonfatal MI (HR 4.21, 95% confidence interval, 1.28–13.83, P=0.02). When early revascularization was forced a priori into the model, inducible ischemia remained a significant adjusted predictor (HR 3.82, 95% confidence interval, 1.17–12.44, P=0.03). Inducible ischemia score was also a significant adjusted predictor (HR 1.20, 95% confidence interval 1.07–1.34, P=0.001) of the primary composite outcome measure. Multivariable Cox regression analysis was also used to evaluate for the secondary outcome measure of MACE using stepwise selection of significant univariable predictors and forcing age and gender into the model a priori. Once again, inducible ischemia emerged as the only significant independent predictor of MACE (HR 2.47, 95% confidence interval 1.34–4.56, P=0.004). Linear regression modelling was performed for prediction of inducible ischemia the following covariates: patient age, gender, history of MI, history of PCI, LVEF, left ventricular end-diastolic volume index, and LV myocardial mass (C-statistic 0.76, Chi-square 34.6, P<0.0001). An additional multivariable Cox regression analysis was performed using the derived propensity scores as a linear measure of covariate adjustment. Both the presence of inducible ischemia (HR 3.72, 95% confidence interval 1.15–12.02, P=0.03), and inducible ischemia score (HR 1.18, 95% confidence interval 1.06–1.32, P=0.003) remained significant predictors of cardiac death and nonfatal MI.
Categorical net reclassification index was performed using 5-year risk cutoffs of 5% (low risk) and 10% (high risk) for the primary endpoint of cardiac death or nonfatal MI. The addition of inducible ischemia resulted in reclassification of 43.4% of the diabetic cohort with a categorical NRI of 0.38, (95% confidence interval of 0.20–0.56, P<0.0001). Continuous NRI was 0.60 (95% confidence interval of 0.15–1.05, P=0.009).
Association of Inducible Ischemia and LGE with Duration of Diabetes and HbA1c
The proportion of patients with inducible ischemia (blue bars) and LGE (red bars) stratified by increasing duration of diabetes (1–5 years, 6–10 years, and >10 years) amongst our cohort are shown in Figure 5A. There were no statistically significant differences in the prevalence of either inducible ischemia or LGE based on diabetic duration, however, patients with >10 year duration of diabetes had significantly greater burden of inducible ischemia than those with only 1–5 years of diabetes (Figure 5B). There was no association between HbA1c levels and either burden of inducible ischemia (inducible ischemia score) or global LGE (percentage of total myocardium) as shown in Figure 6A and 6B, respectively.
Figure 5. Linear regression analysis between inducible ischemia score and global late gadolinium enhancement percentage with HbA1c levels (N=173).
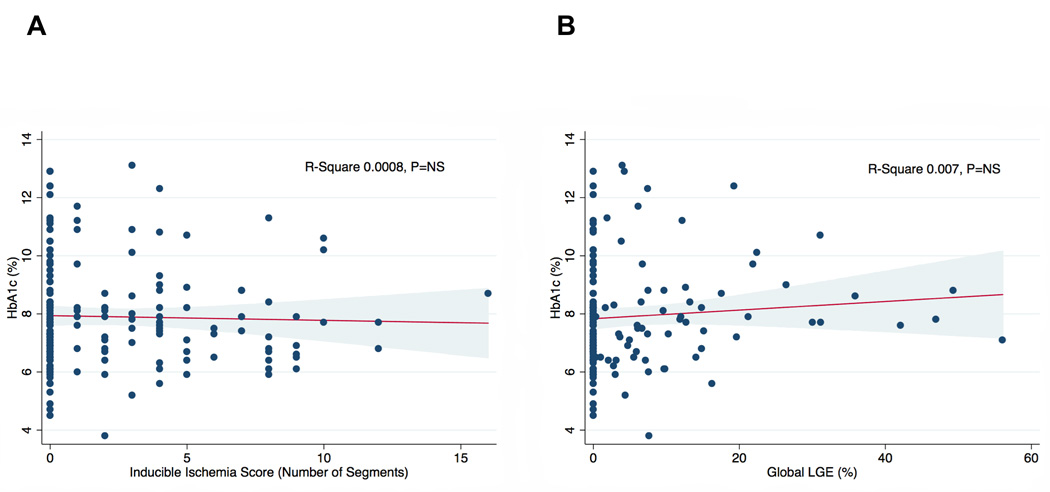
A) Linear regression analysis between inducible ischemia score (number of segments of 16-segment AHA model with inducible ischemia) and HbA1c levels. B) Linear regression analysis between global subendocardial LGE (percentage of total myocardium) and HbA1c levels. R-square and P values displayed in image. LGE = late gadolinium enhancement.
Figure 6. Proportion and burden of inducible ischemia and late gadolinium enhancement stratified by duration of diabetes (N=173).
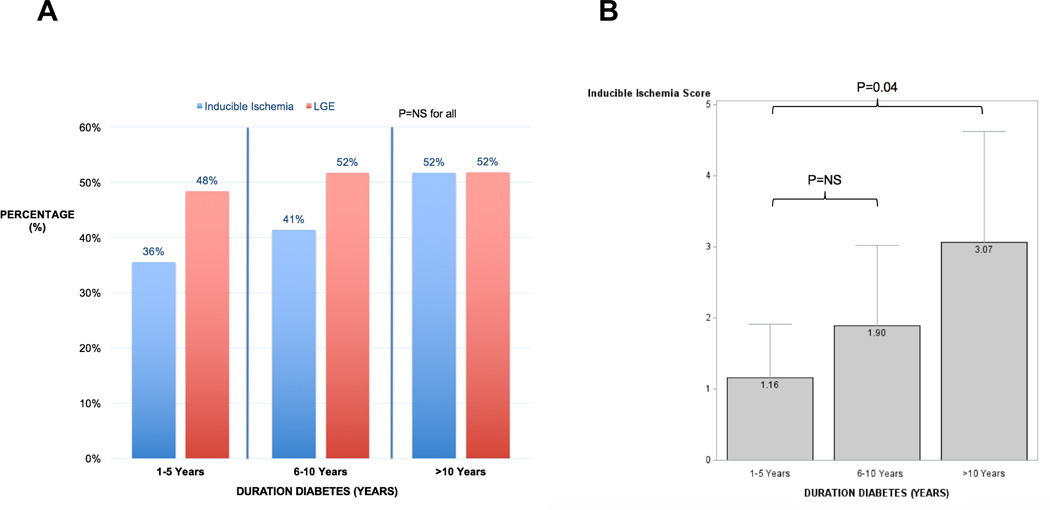
A) Proportion of diabetics with inducible ischemia (blue bars) and late gadolinium enhancement (red bars) stratified by duration of diabetes (1–5 years, 6–10 years, >10 years). B) Mean inducible ischemia score (number of segments of 16-segment AHA model with inducible ischemia) for different categories of duration of diabetes (1–5 years, 6–10 years, >10 years).
DISCUSSION
The results of this study demonstrate that the presence of inducible ischemia by stress perfusion CMR was associated with an almost five-fold increased likelihood of cardiac death and nonfatal MI amongst diabetics. On the other hand, the annual rate of cardiac death and nonfatal MI was very low at 0.5%/year amongst diabetics without inducible ischemia or late gadolinium enhancement on CMR. This robust prognostic association between CMR inducible ischemia and hard clinical events was independent of patients’ age, gender, and LVEF. Presence of inducible ischemia by stress perfusion CMR was effective in reclassifying over 40% of the current diabetic cohort into clinically relevant risk categories.
Cardiovascular disease remains the leading cause of death amongst diabetics, and the morbidity and mortality from CAD is two to four fold greater in comparison with nondiabetics.23, 24 Risk stratification amongst diabetics with established techniques, including stress echocardiography and SPECT, may have limited sensitivity, particularly for those diabetics unable to undergo exercise assessment. Numerous studies have found high rates of annual cardiac death and nonfatal MI, ranging from 1.9 to 6.0%, amongst diabetics with normal stress imaging, particularly beyond 2-years.6–11, 25, 26 Marwick and colleagues evaluated the prognostic value of stress echocardiography in 937 symptomatic diabetics and reported an annualized mortality rate of 4% in the first 5 years for those with a normal stress echocardiogram.11 Only 36% of this cohort underwent exercise stress, and referral for a pharmacological test portended a 4-fold higher risk of death.11 Kalmesh and colleagues observed a 6% annual rate of cardiac death and nonfatal MI in 236 diabetics with normal stress echocardiography,7 while Cortigliani and colleagues reported an annual cardiac event rate of 5.5% in diabetics over the age of 65.8 High event rates in diabetics with normal SPECT imaging has also been reported. Giri and colleagues observed a cardiac death rate of 3.9% for diabetics with normal SPECT imaging,6 while Ghatak and colleagues observed an annual cardiac event rate of 3.4% amongst diabetics with a normal pharmacological SPECT study.25 In over 1200 symptomatic diabetics, Kang and colleagues found an annual event rate of 1.9% for those with a normal SPECT, and 3-fold increased risk of adverse events for diabetics unable to undergo exercise stress.26 These results are particularly concerning due to the high proportion of diabetics who have exercise intolerance due to numerous factors, such a peripheral neuropathy, advanced age, skin ulceration, and peripheral arterial disease.4 On the basis of these results, some groups have advocated for serial risk stratification every 2–3 years in diabetics,9, 12, 27 which is not highly feasible due to rising costs and prevalence of diabetes, as well as the risk of cumulative ionizing radiation from SPECT imaging.28
Diabetics suffer more severe coronary atherosclerosis due to accelerated endothelial dysfunction,29, 30 are more likely to have silent ischemia with prior ischemic injury at time of presentation,31 microvascular dysfunction,32 and diffuse epicardial coronary disease,33 which not only portends a worsened prognosis but may also reduce the sensitivity and specificity of established imaging techniques for risk stratification.34 In this cohort of 173 diabetic patients, over 30% had resting regional wall motion abnormalities and over 50% had evidence of prior myocardial infarction. Despite these baseline LV abnormalities that may reduce the diagnostic and prognostic performance of both stress echocardiography and SPECT imaging,12, 34 all patients successfully underwent pharmacological stress perfusion CMR, and we observed excellent prognostic utility and risk reclassification. Ischemic assessment by stress perfusion CMR is not limited by the presence of balanced ischemia or microvascular dysfunction, and is the gold standard for assessment of myocardial fibrosis and identification of prior ischemic injury. Diabetics with a history of pre-existing MI and inducible ischemia had the highest annual rates of cardiac death and MI (11.8%), while diabetics without any history of MI or inducible ischemia had significantly lower event rates (1.2%/year, Figure 4). The combined assessment of inducible ischemia and subendocardial LGE was able to identify a sub-group of diabetics who enjoyed a very low annual rate of adverse cardiac events and very high event-free survival (cardiac death and nonfatal MI) by Kaplan-Meier analysis. This is in keeping with other studies evaluating the prognostic importance of LGE in patients with silent or clinically unrecognized infarction,35 as well as the prognostic utility of inducible ischemia in higher risk cohorts, such as those with established CAD.16 The combined diagnostic feasibility, prognostic utility, and safety (lack of ionizing radiation) of stress perfusion CMR may provide an effective clinical tool for risk stratification amongst diabetics with suspicion of cardiovascular disease.
Recent clinical trials evaluating more intensive glycemic control amongst diabetics have not demonstrated significant reductions in adverse cardiac events, and one study even reported an increased risk of cardiac death.36–38 Interestingly, we observed no significant association between HbA1c levels and the degree of either inducible ischemia or burden of LGE, expressed as a percentage of the total myocardium (Figure 5). However, patients with a longer duration of diabetes had a greater burden of inducible ischemia (Figure 6), suggesting a link between the degree of atherosclerosis, vascular dysfunction and duration of diabetes. Inducible ischemia score by CMR was also an independent predictor of cardiac death and MI in this cohort with an adjusted HR of 1.20 (95% confidence interval 1.07–1.34, P=0.001) for every 1 additional LV segment (of 16-segment AHA model) with inducible ischemia. The potential reduction in adverse cardiac events by more aggressive medical or invasive therapies in diabetics with greater ischemic burden requires prospective evaluation.
The design of this study was non-randomized, retrospective, single-center, and had a male preponderance, therefore the results must be taken in consideration of these inherent limitations. Furthermore, the relatively small sample size reduces the number of covariates that may be included in multivariable analysis to adjust for any confounding. Despite these limitations, the study cohort reflects real-world clinical evaluation of suspected myocardial ischemia in diabetics by clinical cardiologists in a large tertiary care center over a greater than 10-year period. In addition, the multivariable model was adjusted for powerful predictors of adverse cardiac events, such as LVEF. A second limitation includes bias introduced by the results of the stress perfusion CMR, which may have impacted downstream management decisions by referring clinicians. However, when we adjusted our multivariable model for early revascularization, inducible ischemia by stress perfusion CMR remained an independent predictor of our primary outcome measure. Furthermore, as the benefit of revascularization in diabetics with stable angina remains unclear,39, 40 a prospective imaging-guided trial would need to be conducted to determine the impact on cardiovascular outcomes. Finally, this cohort did not include any asymptomatic diabetics, in whom the potential prognostic utility of stress perfusion CMR is currently unknown, and the CMR protocol did not allow for quantitative myocardial blood flow analyses that may provide additional prognostic information. Both of these limitations should be topics of future investigation.
In conclusion, stress perfusion CMR was shown to be highly feasible with excellent prognostic utility in a high risk cohort of symptomatic diabetic patients who were referred for ischemic assessment. The absence of inducible ischemia and subendocardial LGE by CMR identified diabetic patients at very low risk for cardiac mortality and nonfatal MI. Further evaluation is required to determine whether early risk stratification may impact downstream cardiac therapies and ultimately lead to improved patient outcomes.
Clinical Perspective.
Diabetics represent a high risk cohort of patients who are both at increased risk of coronary artery disease and adverse cardiac events. Due to a high prevalence of silent ischemia amongst diabetics, many have already suffered a prior silent myocardial infarction upon first presentation to medical care. Standard techniques for risk stratification of coronary artery disease may lack sensitivity due to the high pre-test risk of diabetics, particularly for those unable to exercise who undergo pharmacological testing. We evaluated the prognostic utility of stress perfusion cardiac MRI amongst 173 consecutively referred diabetics with clinical suspicion of myocardial ischemia. The absence of inducible ischemia or late gadolinium enhancement on pharmacological stress perfusion CMR identified patients at very low risk of cardiac mortality and nonfatal myocardial infarction (0.5%/year). In contrast, the presence of late gadolinium enhancement consistent with a prior myocardial infarction identified diabetics at higher risk of adverse cardiac events (3.1%/year), and those with inducible ischemia suffered the highest annual event rates (8.2%/year). The presence of inducible ischemia by stress perfusion CMR effectively risk reclassified over 40% of diabetics beyond clinical risk factors and ejection fraction using risk cutoffs of 5% and 10% (NRI 0.38, 95% CI 0.20–0.56, P<0.0001). These findings suggest pharmacological stress perfusion CMR may offer a robust non-invasive risk stratification tool without ionizing radiation exposure for diabetics with suspected myocardial ischemia.
Acknowledgments
Dr. Raymond Kwong is supported by NIH grant R01-HL091157 and a research grant from Astellas Pharmaceuticals.
Footnotes
DISCLOSURES
All other authors have no financial disclosures relevant to the content of this manuscript.
REFERENCES
- 1.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160:517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Sattar N. Revisiting the links between glycaemia, diabetes and cardiovascular disease. Diabetologia. 2013;56:686–695. doi: 10.1007/s00125-012-2817-5. [DOI] [PubMed] [Google Scholar]
- 4.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30:203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 5.Natali A, Vichi S, Landi P, Severi S, L'Abbate A, Ferrannini E. Coronary atherosclerosis in Type II diabetes: angiographic findings and clinical outcome. Diabetologia. 2000;43:632–641. doi: 10.1007/s001250051352. [DOI] [PubMed] [Google Scholar]
- 6.Giri S, Shaw LJ, Murthy DR, Travin MI, Miller DD, Hachamovitch R, Borges-Neto S, Berman DS, Waters DD, Heller GV. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation. 2002;105:32–40. doi: 10.1161/hc5001.100528. [DOI] [PubMed] [Google Scholar]
- 7.Kamalesh M, Matorin R, Sawada S. Prognostic value of a negative stress echocardiographic study in diabetic patients. Am Heart J. 2002;143:163–168. doi: 10.1067/mhj.2002.119377. [DOI] [PubMed] [Google Scholar]
- 8.Cortigiani L, Bigi R, Sicari R, Landi P, Bovenzi F, Picano E. Prognostic value of pharmacological stress echocardiography in diabetic and nondiabetic patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2006;47:605–610. doi: 10.1016/j.jacc.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Baroncini LA, Borsoi R, Vidal ME, Valente NJ, Veloso J, Pecoits Filho R. Assessment of dipyridamole stress echocardiography for risk stratification of diabetic patients. Cardiovasc Ultrasound. 2015;13:35. doi: 10.1186/s12947-015-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozanski A, Gransar H, Hayes SW, Friedman JD, Hachamovitch R, Berman DS. Comparison of long-term mortality risk following normal exercise vs adenosine myocardial perfusion SPECT. J Nucl Cardiol. 2010;17:999–1008. doi: 10.1007/s12350-010-9300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marwick TH, Case C, Sawada S, Vasey C, Short L, Lauer M. Use of stress echocardiography to predict mortality in patients with diabetes and known or suspected coronary artery disease. Diabetes Care. 2002;25:1042–1048. doi: 10.2337/diacare.25.6.1042. [DOI] [PubMed] [Google Scholar]
- 12.Kamalesh M, Feigenbaum H, Sawada S. Challenge of identifying patients with diabetes mellitus who are at low risk for coronary events by use of cardiac stress imaging. Am Heart J. 2004;147:561–563. doi: 10.1016/j.ahj.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121:2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes A. Standards of medical care in diabetes--2014. Diabetes Care. 2014;(37 Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 16.Shah R, Heydari B, Coelho-Filho O, Murthy VL, Abbasi S, Feng JH, Pencina M, Neilan TG, Meadows JL, Francis S, Blankstein R, Steigner M, di Carli M, Jerosch-Herold M, Kwong RY. Stress cardiac magnetic resonance imaging provides effective cardiac risk reclassification in patients with known or suspected stable coronary artery disease. Circulation. 2013;128:605–614. doi: 10.1161/CIRCULATIONAHA.113.001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner A, Bruder O, Schneider S, Nothnagel D, Buser P, Pons-Lado G, Dill T, Hombach V, Lombardi M, van Rossum AC, Schwitter J, Senges J, Sabin GV, Sechtem U, Mahrholdt H, Nagel E. Current variables, definitions and endpoints of the European cardiovascular magnetic resonance registry. J Cardiovasc Magn Reson. 2009;11:43. doi: 10.1186/1532-429X-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. American Heart Association Writing Group on Myocardial S and Registration for Cardiac I. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K, Alpert JS, White HD Joint ESCAAHAWHFTFftRoMI. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Jr, Smith SC, Jr, Spertus JA, Williams SV. American College of Cardiology F, American Heart Association Task Force on Practice G, American College of P, American Association for Thoracic S, Preventive Cardiovascular Nurses A, Society for Cardiovascular A, Interventions, Society of Thoracic S. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Rutter MK, Wahid ST, McComb JM, Marshall SM. Significance of silent ischemia and microalbuminuria in predicting coronary events in asymptomatic patients with type 2 diabetes. J Am Coll Cardiol. 2002;40:56–61. doi: 10.1016/s0735-1097(02)01910-1. [DOI] [PubMed] [Google Scholar]
- 22.Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, Deedwania P, Eckel RH, Ershow AG, Fradkin J, Inzucchi SE, Kosiborod M, Nelson RG, Patel MJ, Pignone M, Quinn L, Schauer PR, Selvin E, Vafiadis DK. American Heart Association Diabetes Committee of the Council on L, Cardiometabolic Health CoCCCoC, Stroke Nursing CoCS, Anesthesia CoQoC, Outcomes R and the American Diabetes A. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38:1777–1803. doi: 10.2337/dci15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2:120–126. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- 24.Jacoby RM, Nesto RW. Acute myocardial infarction in the diabetic patient: pathophysiology, clinical course and prognosis. J Am Coll Cardiol. 1992;20:736–744. doi: 10.1016/0735-1097(92)90033-j. [DOI] [PubMed] [Google Scholar]
- 25.Ghatak A, Padala S, Katten DM, Polk DM, Heller GV. Risk stratification among diabetic patients undergoing stress myocardial perfusion imaging. J Nucl Cardiol. 2013;20:529–538. doi: 10.1007/s12350-013-9731-1. [DOI] [PubMed] [Google Scholar]
- 26.Kang X, Berman DS, Lewin HC, Cohen I, Friedman JD, Germano G, Hachamovitch R, Shaw LJ. Incremental prognostic value of myocardial perfusion single photon emission computed tomography in patients with diabetes mellitus. Am Heart J. 1999;138:1025–1032. doi: 10.1016/s0002-8703(99)70066-9. [DOI] [PubMed] [Google Scholar]
- 27.Elhendy A, Arruda AM, Mahoney DW, Pellikka PA. Prognostic stratification of diabetic patients by exercise echocardiography. J Am Coll Cardiol. 2001;37:1551–1557. doi: 10.1016/s0735-1097(01)01199-8. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Einstein AJ, Fazel R, Krumholz HM, Wang Y, Ross JS, Ting HH, Shah ND, Nasir K, Nallamothu BK. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: a population-based analysis. J Am Coll Cardiol. 2010;56:702–711. doi: 10.1016/j.jacc.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ertek S, Cicero AF, Cesur M, Akcil M, Altuner Kayhan T, Avcioglu U, Korkmaz ME. The severity of coronary atherosclerosis in diabetic and non-diabetic metabolic syndrome patients diagnosed according to different criteria and undergoing elective angiography. Acta Diabetol. 2011;48:21–27. doi: 10.1007/s00592-010-0211-7. [DOI] [PubMed] [Google Scholar]
- 30.Hsueh WA, Lyon CJ, Quinones MJ. Insulin resistance and the endothelium. Am J Med. 2004;117:109–117. doi: 10.1016/j.amjmed.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 31.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 32.Quinones MJ, Hernandez-Pampaloni M, Schelbert H, Bulnes-Enriquez I, Jimenez X, Hernandez G, De La Rosa R, Chon Y, Yang H, Nicholas SB, Modilevsky T, Yu K, Van Herle K, Castellani LW, Elashoff R, Hsueh WA. Coronary vasomotor abnormalities in insulin-resistant individuals. Ann Intern Med. 2004;140:700–708. doi: 10.7326/0003-4819-140-9-200405040-00009. [DOI] [PubMed] [Google Scholar]
- 33.Morgan KP, Kapur A, Beatt KJ. Anatomy of coronary disease in diabetic patients: an explanation for poorer outcomes after percutaneous coronary intervention and potential target for intervention. Heart. 2004;90:732–738. doi: 10.1136/hrt.2003.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albers AR, Krichavsky MZ, Balady GJ. Stress testing in patients with diabetes mellitus: diagnostic and prognostic value. Circulation. 2006;113:583–592. doi: 10.1161/CIRCULATIONAHA.105.584524. [DOI] [PubMed] [Google Scholar]
- 35.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 36.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, Investigators V. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 37.Gerstein HC, Riddle MC, Kendall DM, Cohen RM, Goland R, Feinglos MN, Kirk JK, Hamilton BP, Ismail-Beigi F, Feeney P, Group AS. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:34i–43i. doi: 10.1016/j.amjcard.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 39.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS, Group CTR. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 40.Group BDS, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]


