Abstract
Green tea (Camellia sinensis) is one of the most popular beverages in the world and has been acknowledged for centuries as having significant health benefits. (−)-Epigallocatechin-3-gallate (EGCG) is the most abundant catechin in green tea, and it has been reported to have health benefit effects. Peroxisome proliferator-activated receptor γ coactivator (PGC)-1α is a crucial regulator of mitochondrial biogenesis and hepatic gluconeogenesis. The objective of this study was to investigate whether EGCG from green tea can affect the ability of transcriptional regulation on PGC-1α mRNA expression in HepG2 cells and 3T3-L1 adipocytes. To study the molecular mechanism that allows EGCG to control PGC-1α expression, the promoter activity levels of PGC-1α were examined. The PGC-1α mRNA level was measured using quantitative real-time PCR. The −970/+412 bp of PGC-1α promoter was subcloned into the pGL3-Basic vector that includes luciferase as a reporter gene. EGCG was found to up-regulate the PGC-1α mRNA levels significantly with 10 μmol/L of EGCG in HepG2 cells and differentiated 3T3-L1 adipocytes. PGC-1α promoter activity was also increased by treatment with 10 μmol/L of EGCG in both cells. These results suggest that EGCG may induce PGC-1α gene expression, potentially through promoter activation.
Keywords: EGCG, PGC-1α, promoter activity, HepG2 cells, 3T3-L1 adipocytes
INTRODUCTION
Green tea (Camellia sinensis) is the second most popular beverage in the world, and it has been acknowledged for centuries as having significant health benefits (1–4). The health benefits of green tea are attributed mainly to its high catechin content, including (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin (EC), (−)-epigallocate-chin (EGC), and (−)-epicatechin gallate (ECG). Among them, EGCG is the most abundant catechin in green tea, accounting for 30~50% of the catechin content (1). EGCG has been reported to have health beneficial effects against various chronic diseases such as cancer, heart disease, obesity, and others (1–4).
Peroxisome proliferator-activated receptor (PPAR) γ coactivator (PGC)-1 coactivators play an important role in the maintenance of lipid, glucose, and energy homeostasis, and thus are related with metabolic diseases such as obesity and diabetes (5). Among the PGC-1 family of coactivators, PGC-1α is highly responsive to different environmental stimuli including temperature, nutritional status, and physical activity, and coordinately regulates biological processes and metabolic pathways in a tissue-specific manner (6). PGC-1α was originally identified as a cold-inducible coactivator of PPARγ in brown adipose tissue (7), but recently, it has emerged as a potent energy regulator affecting on body energy expenditure (8). The transcriptional coactivator PGC-1α has been shown to be involved in pathways promoting fatty acids oxidation by increasing mitochondrial function and activity (7). PGC-1α plays the role of a crucial regulator of mitochondrial biogenesis, regulating mitochondrial DNA transcription via stimulating increased expression of mitochondrial transcription factor A (5), and nuclear respiratory factor (NRF)-1 and NRF-2 (5,9).
It has been proposed that EGCG affects physiological responses such as energy expenditure and fatty acid oxidation by regulation of gene expression in the liver and adipose tissue (4,10). We previously showed that EGCG effects on gene expression of hormone sensitive lipase (HSL) and uncoupling protein 2 (UCP2) in 3T3-L1 adipocytes (11,12), and regulates cholesterol 7 alpha-hydroxylase (CYP7A1) mRNA level in HepG2 cells (13). Nevertheless, it remains uncertain whether EGCG affects the PGC-1α gene expression in liver cells and adipocytes. Here, we hypothesized that EGCG may directly regulate PGC-1α gene expression in HepG2 cells and 3T3-L1 adipocytes. Therefore, we measured the mRNA levels and promoter activities of PGC-1α under EGCG treatment in both cells.
MATERIALS AND METHODS
Materials
Green tea EGCG (purity>95%) was purchased from Sigma (St. Louis, MO, USA). Human HepG2 cell line and 3T3-L1 cell line were obtained from the American Type Culture Collection (Manassas, VA, USA). Dulbecco’s modified Eagle’s medium (DMEM), pH 7.4 phosphate-buffered saline (PBS), fetal bovine serum (FBS), penicillin-streptomycine, TRIzol reagent, Moloney murine leukemia virus (M-MLV) reverse transcriptase, and Lipofectamin 2000 were purchased from Invitrogen (Grand Island, NY, USA). Universal SYBR Green PCR Master Mix was obtained from Qiagen (Chatsworth, CA, USA). A cell count kit (CCK)-8 was purchased from Dojindo Laboratories (Kumamoto, Japan). Luciferase reporter assay system, pGEM-T easy vector and pGL3 basic vector were purchased from Promega (Madison, WI, USA). pCMV-β galactosidase vector was obtained from Clontech (Palo Alto, CA, USA). Mlu I and Xho I restriction enzymes were purchased from Takara (Tokyo, Japan).
Cell culture
HepG2 cells were cultured in DMEM supplemented with 10% (v/v) FBS, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C under an atmosphere of 5% CO2. EGCG was dissolved with dimethylsulfoxide (DMSO), and the DMSO final concentration in the culture medium was 0.01%. Cells were treated with various concentrations of EGCG (0, 1, 5, or 10 μmol/L) in serum-free media for 24 h. In this experiment, cells were exposed to EGCG for 24 h or more based on the daily intake of EGCG at least 3~4 times. The control cells were treated with 0.01% DMSO without EGCG treatment. 3T3-L1 fibroblasts were initially cultured in DMEM supplemented with 10% (v/v) FBS, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C under an atmosphere of 5% CO2. To induce adipocyte differentiation, the 3T3-L1 cells were allowed to grow to confluence and were then cultured with a differentiation medium containing 0.5 mmol/L isobutyl methylxanthine, 1 μmol/L dexamethasone, and 5 μg/mL insulin. After exposure to the differentiation medium for 48 h, the 3T3-L1 cells were differentiated for an additional 7 days in DMEM supplemented with 10% FBS. Differentiated 3T3-L1 adipocytes were treated with various concentrations of EGCG (0, 1, 5, or 10 μmol/L) in 1% bovine serum albumin serum-free media for 24 h. The control cells were treated with 0.01% DMSO without EGCG. All measurements were performed in triplicate of three independent experiments.
Cell cytotoxicity assay
Cell viability was performed as described previously (14) using CCK-8 kit in accordance with the manufacturer’s instructions. Cells were treated with 0 (control), 1, 5, 10, 20, or 50 μM of EGCG for 24 or 48 h at 37°C. Absorbance was then measured using a Varioskan plate reader (Thermo Electron, Waltham, MA, USA) at 450 nm, and the results were presented as the percentage relative to the untreated control cells. Control cells were incubated with 0.01% DMSO without EGCG treatment.
Quantitative real-time PCR
Total RNA was extracted from HepG2 cells and differentiated 3T3-L1 adipocytes using TRIzol reagent. The corresponding cDNA was synthesized from 4 μg of RNA using M-MLV reverse transcriptase. After cDNA synthesis, quantitative real-time PCR was performed using Universal SYBR Green PCR Master Mix on a fluorometric thermal cycler (Corbett Research, Mortlake, Australia). Primers were designed using an online program (15). The sequences of the sense and antisense primers used for amplification were as follows: PGC-1α, 5′-GGA CAG AAC TGA GGG ACC GT-3′ and 5′-GCA GCA AAA GCA TCA CAG GT-3′; β-actin, 5′-GGA CCT GAC TGA CTA CCT CA-3′ and 5′-GCA CAG CTT CTC CTT AAT GT-3′. The ΔΔCt method was used for relative quantification. The ΔΔCt value for each sample was determined by calculating the difference between the Ct value of the target gene and the Ct value of β-actin as a reference gene. The normalized level of expression of the target gene in each sample was calculated using the formula 2−ΔΔCt. Values were expressed as a fold of the control.
Construction of PGC-1α reporter gene
The human PGC-1α gene promoter from −970 bp to +412 bp was generated by PCR using human genomic DNA. The 5′-primer, bearing a Mlu I site, was 5′-TGT ACG CGT CCC TCA GTT CAC AGA CAT TCT-3′, and the 3′-primer, bearing a Xho I site, was 5′-TCT CTC GAG ACA GTG CCA AAG TCA CAT GGA-3′. Amplification of the PGC-1α promoter consisted of 95°C for 15 min, followed by 30 cycles at 95°C for 1 min, 64°C for 1 min, and 70°C for 2 min. The PGC-1α promoter fragment (−970/+412) was subcloned into the pGEM-T easy vector. It was then inserted into the pGL3 basic vector, which includes luciferase as a reporter gene.
Transfection and luciferase assay
Transfection experiments were carried out with Lipofectamin ® 2000 according to the manufacturer’s instructions. For transfection, 2 μg of the plasmid containing the PGC-1α reporter gene was employed, along with 1μg of pCMV-β galactosidase as an internal standard for the adjustment of transfection efficiency. Three hours after transfection, cells were treated with 0 (control), 1, 5, or 10 μmol/L of EGCG in serum-free medium for 40 h. For the luciferase assay, cells were washed with phosphate buffered saline and harvested with luciferase cell culture lysis reagent. PGC-1α promoter activity in the cells was measured with the Luciferase Reporter Assay System using a TD 20/20 luminometer (Turner Designs, Sunnyvale, CA, USA). β-galactosidase activity was assayed enzymatically using o-nitrophenyl-β-D-galactopyranoside as a substrate. Luciferase activity was calculated in relative light units and normalized to the β-galactosidase activity.
Statistical analysis
Data were expressed as mean±standard error of the mean (SEM). Statistical analyses were performed using SPSS software version 19 (IBM Corporation, Armonk, NY, USA). Significant differences among the treatment groups were assessed by one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests. The level P<0.05 and P<0.01 were considered to indicate statistically significant differences.
RESULTS
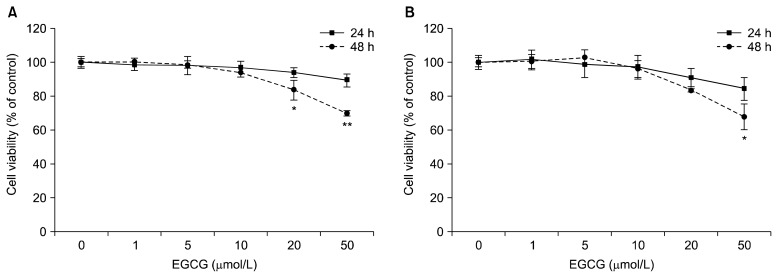
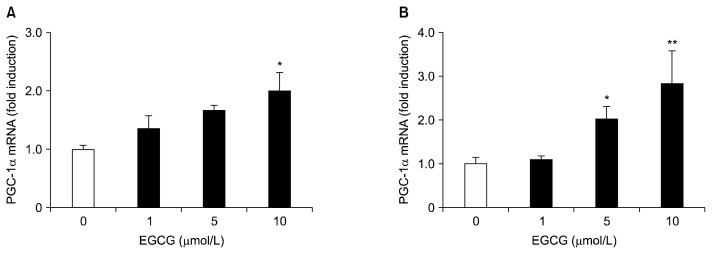
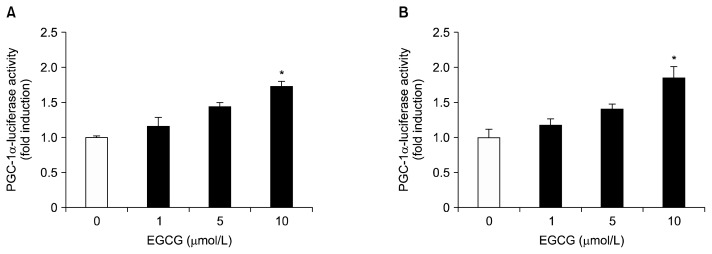
To investigate the potential cytotoxic effects of EGCG on HepG2 cells and differentiated 3T3-L1 adipocytes, the cells were treated with various concentrations (1~50 μmol/L) of EGCG for 24 and 48 h at 37°C. Cytotoxicity was found to be unaffected under 10 μmol/L of EGCG in both cells (Fig. 1A and 1B). Effects of EGCG on hepatic and adipogenic PGC-1α expression in HepG2 cells and differentiated 3T3-L1 adipocytes were measured by quantitative real-time PCR. Both cells were treated with different concentrations (0, 1, 5, or 10 μmol/L) of EGCG for 24 h. The mRNA levels of PGC-1α with 10 μmol/L of EGCG were significantly up-regulated by 2.0-fold in HepG2 cells, and by 2.84-fold in differentiated 3T3-L1 adipocytes, respectively, compared to the control (Fig. 2A and 2B). To further verify the up-regulation of the PGC-1α gene by EGCG, we assayed the promoter activity in HepG2 cells and differentiated 3T3-L1 adipocytes. Cells were incubated with different concentrations (0, 1, 5, or 10 μmol/L) of EGCG for 40 h. The promoter activity of PGC-1α with 10 μmol/L of EGCG was significantly increased by 1.73-fold in HepG2 cells, and by 1.85-fold in differentiated 3T3-L1 adipocytes, respectively, compared to the control (Fig. 3A and 3B). Cotransfection with the control vector (pGL3-basic) showed a negligible effect on luciferase activity (data not shown).
Fig. 1.
Effects of EGCG on cell viability in HepG2 cells (A) and 3T3-L1 adipocytes (B). Cells were treated with 0 (control), 1, 5, 10, 20, or 50 μmol/L of EGCG and incubated for 24 or 48 h. Cell viability was determined using the WST-8 assay. Values are means±SEM (n=3) of three independent experiments. *P<0.05, **P<0.01 vs. control.
Fig. 2.
Effects of EGCG on the level of PGC-1α mRNA in HepG2 cells (A) and 3T3-L1 adipocytes (B). HepG2 cells and differentiated 3T3-L1 adipocytes were treated with 0 (control), 1, 5, or 10 μmol/L of EGCG for 24 h. The PGC-1α mRNA levels were determined by quantitative real-time PCR, and are expressed as fold-change over the control. Values are expressed as mean±SEM (n=3) of three independent experiments. *P<0.05, **P<0.01 vs. control.
Fig. 3.
Effects of EGCG on the promoter activity of PGC-1α in HepG2 cells (A) and 3T3-L1 adipocytes (B). HepG2 cells and differentiated 3T3-L1 adipocytes were transfected with PGC-1α (−970/+412 bp)/luciferase reporter gene and pCMV-β galactosidase, and were then incubated in serum-free media with the indicated concentrations of EGCG, from 0 to 10 μmol/L, for 40 h. Luciferase activity was calculated in relative light units and normalized to the β-galactosidase activity. Values are expressed as mean±SEM (n=3) of three independent experiments. *P<0.05 vs. control.
DISCUSSION
Green tea is one of the most popular beverages in the world, and it contains a number of bioactive components to improve health. As the major bioactive ingredients of green tea, polyphenols consists primarily of catechins including EGCG that is the most abundant and strongest bioactive chemical. When green tea is orally administered, various catechin compounds are metabolized and moved mainly via the digestive system. A recent study reported that administered 3H-EGCG was found in the digestive system, including the small intestine, colon and stomach of mice (16). In addition, 3H-EGCG was shown to be easily absorbed from the digestive tract and widely distributed into various organs such as liver, lung, heart, and kidney of mice (17), suggesting that EGCG may reach the liver and adipose tissue as intact in vivo.
On the other hand, EGCG has been shown to regulate the adipogenic and lipolytic pathways, resulting in the down regulation of CCAAT/enhancer binding protein-α and PPARγ, and the up-regulation of HSL and carnitine palmitoltransferase-1 in the 3T3-L1 adipocytes in obese mice (18). Moreover, EGCG inhibited lipogenesis through the phosphorylation of AMP-activated protein kinase and acetyl-CoA carboxylase in HepG2 cells (19).
The aim of this study was to investigate the direct effects of EGCG on the hepatic and adipocyte PGC-1α gene expression, particularly whether EGCG affects the PGC-1α mRNA expression through the promoter activation of PGC-1α in HepG2 cells and differentiated 3T3-L1 adipocytes. Here, we examined the gene expression and promoter activity of PGC-1α in low concentrations under 10 μmol/L of EGCG, in the non-toxic range, in HepG2 cells and differentiated 3T3-L1 adipocytes.
To understand the underlying regulatory mechanisms of PGC-1α by EGCG, we evaluated the mRNA expression of PGC-1α in liver cells and adipocytes. In the present study, EGCG enhanced the mRNA level of PGC-1α with 10 μmol/L of EGCG in HepG2 cells and differentiated 3T3-L1 adipocytes. PGC-1α is an important regulator of tissue-specific metabolic functions, involved in the regulation of thermogenesis, hepatic gluconeogenesis, mitochondrial biogenesis, and energy expenditure (5). In brown adipose tissue, the level of PGC-1α is increased in cold-induced adaptive thermogenesis (7). Hepatic PGC-1α expression is induced by increasing gluconeogenesis in the liver of fasting mice (20). In skeletal muscle, it increases exercise-induced mitochondrial biogenesis and respiration (21). Adipocyte PGC-1α expression activates uncoupling protein activity and key features of mitochondrial biogenesis (5,7). PGC-1α activation has also been shown to attenuate high-fat diet-induced obesity by enhancing brown fat thermogenesis and adipose tissue oxidative metabolism (22). A recent study reported that EGCG strongly promoted mitochondrial biogenesis with an increase in SIRT1-dependent PGC-1α deacetylation in human cells from subjects with Down’s syndrome (23). In addition, EGCG was shown to suppress 1-methyl-4-phenyl-pyridine (MPP)-induced oxidative stress via the SIRT1/PGC-1α signaling pathway and to increase the PGC-1α mRNA level after treating PC12 cells with MPP+ and EGCG (24). Our results indicated that EGCG up-regulated PGC-1α mRNA expression in HepG2 cells and differentiated 3T3-L1 adipocytes. Hence, it is possible that EGCG may partially affect the physiological activity of PGC-1α in liver cells and adipocytes.
Enhancement of the PGC-1α mRNA level may stem from elevation of transcription and/or promotion of mRNA stability. To distinguish between these possibilities, the effects of EGCG on PGC-1α promoter activity in HepG2 cells and differentiated 3T3-L1 adipocytes were examined. PGC-1α promoter activity was increased by EGCG treatment in parallel with alteration of mRNA expression in both cells. Previously, we reported that EGCG enhanced the mRNA expression and promoter activity of UCP2 in 3T3-L1 adipocytes (12). In addition, EGCG showed an increase of CYP7A1 promoter activity and a similar increase of mRNA level in HepG2 cells (13). A recent study reported that EGCG inhibited transcriptional activity of the nuclear factor kappa-light-chain-enhancer of activated B cells-driven promoter in prostate cancer cells (25). In this study, EGCG was shown to stimulate the promoter activity of PGC-1α in HepG2 cells and differentiated 3T3-L1 adipocytes. These results support the hypothesis that EGCG may increase PGC-1α expression through its promoter activation. In conclusion, our results suggest that EGCG may directly regulate the gene expression of PGC-1α, which was associated with PGC-1α promoter activation in HepG2 cells and differentiated 3T3-L1 adipocytes. It is likely that EGCG plays a positive regulatory role in the transcriptional process of PGC-1α in the liver cells and adipocytes.
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korea funded by the Korean Government (No. NRF-2013R1A1A2009522) and BK 21 plus (22A20130012143).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res. 2006;50:176–187. doi: 10.1002/mnfr.200500102. [DOI] [PubMed] [Google Scholar]
- 3.Grove KA, Lambert JD. Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. J Nutr. 2010;140:446–453. doi: 10.3945/jn.109.115972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MS, Kim CT, Kim Y. Green tea (−)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann Nutr Metab. 2009;54:151–157. doi: 10.1159/000214834. [DOI] [PubMed] [Google Scholar]
- 5.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-α. Int J Obes. 2005;29:S5–S9. doi: 10.1038/sj.ijo.0802905. [DOI] [PubMed] [Google Scholar]
- 7.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 8.Cantó C, Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 10.Klaus S, Pültz S, Thöne-Reineke C, Wolfram S. Epigal-locatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes. 2005;29:615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- 11.Lee MS, Kim CT, Kim IH, Kim Y. Inhibitory effects of green tea catechin on the lipid accumulation in 3T3-L1 adipocytes. Phytother Res. 2009;23:1088–1091. doi: 10.1002/ptr.2737. [DOI] [PubMed] [Google Scholar]
- 12.Lee MS, Kim Y. (−)-Epigallocatechin-3-gallate enhances uncoupling protein 2 gene expression in 3T3-L1 adipocytes. Biosci Biotechnol Biochem. 2009;73:434–436. doi: 10.1271/bbb.80563. [DOI] [PubMed] [Google Scholar]
- 13.Lee MS, Park JY, Freake H, Kwun IS, Kim Y. Green tea catechin enhances cholesterol 7α-hydroxylase gene expression in HepG2 cells. Br J Nutr. 2008;99:1182–1185. doi: 10.1017/S0007114507864816. [DOI] [PubMed] [Google Scholar]
- 14.Lee MS, Shin Y, Jung S, Kim CT, Kim IH, Kim Y. Effects of high hydrostatic pressure extract of Korean fresh ginseng on hepatic lipid accumulation and AMPK activation in HepG2 cells. J Food Nutr Res. 2015;3:40–45. doi: 10.12691/jfnr-3-1-7. [DOI] [Google Scholar]
- 15.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mole Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 16.Fujiki H, Imai K, Nakachi K, Shimizu M, Moriwaki H, Suganuma M. Challenging the effectiveness of green tea in primary and tertiary cancer prevention. J Cancer Res Clin Oncol. 2012;138:1259–1270. doi: 10.1007/s00432-012-1250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H. Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–1776. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 18.Chan CY, Wei L, Castro-Muñozledo F, Koo WL. (−)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion by inhibition of cell proliferation and suppression of adipose phenotype expression. Life Sci. 2011;89:779–785. doi: 10.1016/j.lfs.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Kim JJY, Tan Y, Xiao L, Sun YL, Qu X. Green tea polyphenol epigallocatechin-3-gallate enhance glycogen synthesis and inhibit lipogenesis in hepatocytes. BioMed Res Int. 2013;2013:920128. doi: 10.1155/2013/920128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 21.Matravadia S, Martino VB, Sinclair D, Mutch DM, Holloway GP. Exercise training increases the expression and nuclear localization of mRNA destabilizing proteins in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2013;305:R822–R831. doi: 10.1152/ajpregu.00590.2012. [DOI] [PubMed] [Google Scholar]
- 22.Jun HJ, Joshi Y, Patil Y, Noland RC, Chang JS. NTPGC-1α activation attenuates high-fat diet-induced obesity by enhancing brown fat thermogenesis and adipose tissue oxidative metabolism. Diabetes. 2014;63:3615–3625. doi: 10.2337/db13-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenti D, De Rasmo D, Signorile A, Rossi L, de Bari L, Scala I, Granese B, Papa S, Vacca RA. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down’s syndrome. Biochim Biophys Acta. 2013;1832:542–552. doi: 10.1016/j.bbadis.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Ye Q, Ye L, Xu X, Huang B, Zhang X, Zhu Y, Chen X. Epigallocatechin-3-gallate suppresses 1-methyl-4-phenylpyridine-induced oxidative stress in PC12 cells via the SIRT1 /PGC-1α signaling pathway. BMC Complement Altern Med. 2012;12:82. doi: 10.1186/1472-6882-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee S, Siddiqui MA, Dayal S, Ayoub YZ, Malathi K. Epigallocatechin-3-gallate suppresses proinflammatory cytokines and chemokines induced by Toll-like receptor 9 agonists in prostate cancer cells. J Inflamm Res. 2014;7:89–101. doi: 10.2147/JIR.S61365. [DOI] [PMC free article] [PubMed] [Google Scholar]