Abstract
A 55 year old HIV positive male had a skin lesion biopsy which showed atypical vascular proliferation within the superficial and deep dermis with mild atypia of lining endothelial cells. A sparse lymphoplasmacytic infiltrate surrounding the irregular vascular channels was noted. Immunohistochemistry highlighted the atypical blood vessels with the vascular markers CD31, CD34 and Factor VIII. The differential diagnosis included unusual vascular or lymphatic proliferations, stasis dermatitis, kaposiform hemangioendothelioma, progressive lymphangioma and angiosarcoma with focal Kaposi’s sarcoma features. Characteristic human herpes virus-8 positive staining helped support the diagnosis of patch stage of Kaposi’s sarcoma. Herein, we discuss the case findings, differential diagnosis and characteristic histological findings associated with the patch stage of Kaposi’s sarcoma which can be an elusive diagnosis.
Key words: Kaposi’s sarcoma, human herpes virus-8, patch stage
Introduction
Kaposi’s sarcoma is a low grade malignant neoplasm of vascular origin that can affect skin, mucocutaneous surfaces and solid organs. Cutaneous and mucosal Kaposi’s sarcoma has a typical progression from early patch to intermediate plaque to formation of tumor nodules.1 Clinically, this entity can be seen in four different settings: classical [non-human immunodeficiency virus (HIV) associated], endemic (African), transplant associated and HIV associated. The pathogenesis is causally linked to human herpes virus-8 (HHV-8) with virtually all Kaposi’s sarcoma lesions showing varying degrees of infection with the virus. Although Kaposi’s sarcoma can arise in different epidemiological contexts, it is most commonly seen with HIV infection with its presence being a criterion for diagnosis.2
In the early patch to plaque stage, Kaposi’s sarcoma can be restricted to the skin surface and surgical resection suffices as treatment with an excellent prognosis. The role of adjuvant therapy includes radiation therapy for multiple lesions in a restricted area and chemotherapy for disseminated disease. In transplant-associated Kaposi’s sarcoma, withdrawal of immunosuppression is the main mode of treatment. Acquired immunodeficiency syndrome (AIDS) associated Kaposi’s sarcoma patients often succumb to opportunistic infections rather than to progression of their Kaposi’s sarcoma.2
The patches in early stage are multiple pink, red, or purple macules, typically confined to the distal lower extremities.2 The histopathology of the early patch stage can be subtle often resembling granulation tissue and/or superficial and deep perivascular dermatitis. However, on closer inspection, these lesions have dilated, thin walled blood vessels with irregular outlines dissecting through primarily the collagen of the upper half of the dermis. The lining endothelial cells are flat, sparse and generally bland appearing with absent or mild atypia and no significant increase in mitosis. Characteristically, these inconspicuous vascular spaces are surrounded by a mononuclear infiltrate composed predominantly of plasma cells and lymphocytes.3 Conversely, patch stage can have more distinctive features with capillary and small vessel proliferations. Glomerulus-like vascular structures may sometimes be seen with oval shaped vessels lined by cuboidal endothelial cells in an angioendotheliomatous pattern. Hemosiderin laden macrophages and extravasated red blood cells seen more in the plaque stage, may also be rarely associated with the patch stage lesion.4 Despite distinct histological features, early patch stage of Kaposi’s sarcoma is commonly misdiagnosed, misinterpreted or missed on routine examination.
Case Report
A 55-year-old HIV positive male presented with an indistinct reddish flat lesion on the extensor surface of his right lower leg. Sections from the skin lesions showed vascular proliferation with primitive blood vessels dissecting between the collagen bundles within the superficial and deep dermis (Figure 1). Slight focal atypia of the lining endothelial cells was noted (Figure 2). There was a spindle cell proliferation within the surrounding dermis which appeared poorly circumscribed, associated with focal perivascular infiltrates of lymphocytes as well as many plasma cells (Figure 1). The overlying epidermis showed nothing of note. The Steiner silver stain on both biopsies showed no evidence of spirochetes or other organisms. Immunohistochemical stains showed HHV-8 nuclear staining of the spindle cells as well as focal staining of some of the endothelial cells lining the abnormal vascular spaces (Figure 3). CD34, CD31 and factor VIII positive staining outlined the endothelial cells of the abnormal vascular spaces. The overall clinical picture and characteristic histology was consistent with early patch stage of Kaposi’s sarcoma.
Figure 1.
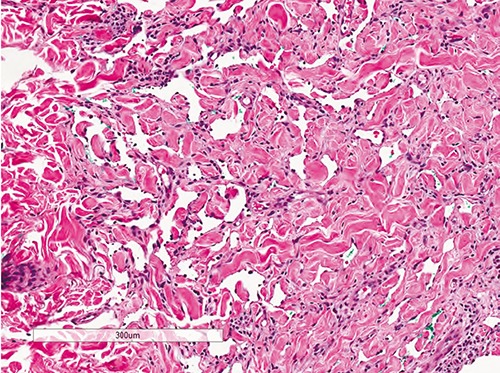
Dilated, angulated vessels in upper part of dermis dissecting through collagen bundles with minimal surrounding mononuclear infiltrate characteristic of patch stage of Kaposi’s sarcoma (Hematoxylin & Eosin, 100× magnification).
Figure 2.
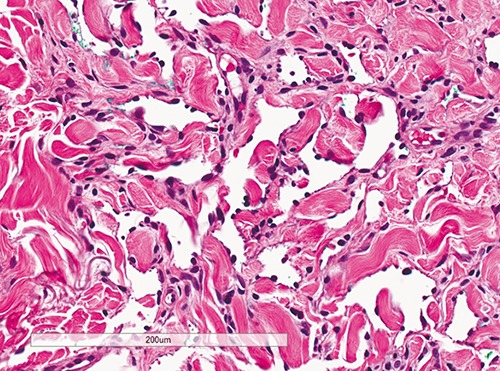
Flat to plump sparse endothelial cells lining angulated vessels in patch stage of Kaposi’s sarcoma with no mitosis or atypia (Hematoxylin & Eosin, 200× magnification).
Figure 3.
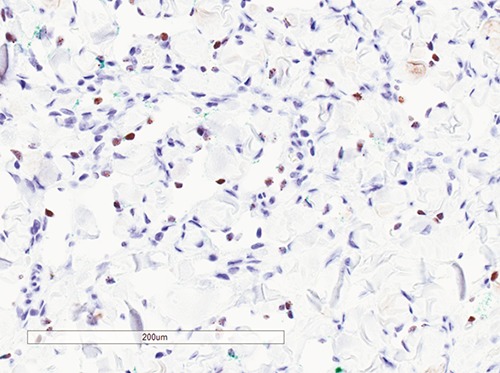
Immunohistochemistry for HHV-8 showing characteristic nuclear positivity in endothelial cells in patch stage of Kaposi’s sarcoma (HHV-8 immunohistochemistry, 200× magnification).
Discussion
The differential diagnosis for early patch stage Kaposi’s sarcoma includes capillary hemangioma and other unusual vascular/lymphatic proliferations, stasis dermatitis, acroangiodermatitis, kaposiform hemangioendothelioma, classic Kaposi’s sarcoma (plaque and nodular stage) and well differentiated angiosarcoma. Patch stage of Kaposi’s sarcoma can be differentiated from these entities by the characteristic histology and supported by immunohistochemical stains, as is demonstrated in our case.
Capillary hemangiomas are the most common type of hemangiomas and are characterized by thin walled capillaries of different shapes and sizes, with or without red blood cells in the lumen.2 In earlier lesions, the endothelial cells can be plump with compressed vascular lumens which progress to flattened endothelial cells arranged in a lobular configuration. Well-established lesions can demonstrate increased fibrosis, hyalinization of endothelial lined walls and subsequent occlusion of lumens.5
Immunohistochemistry highlights the endothelial cells with vascular markers (CD31, CD34, Factor VIII, and D2-40) but is characteristically negative for HHV-8 which helps differentiate this from patch stage of Kaposi’s sarcoma.
Lymphatic proliferations arising post radiation or in a setting of chronic lymphedema can have a similar histological picture with irregular, focally infiltrating vascular channels in a branching pattern dissecting through the superficial and deep dermis. These thin-walled dilated vessels are lined by endothelial cells with minimal to no atypia and can mimic Kaposi’s sarcoma.6
Acquired progressive lymphangioma or benign lymphangioendothelioma is a rare cutaneous condition affecting young individuals that microscopically, consist of anastomosing vascular structures dissecting through the superficial and deeper dermis. The lining endothelium is a single layer of flat, inconspicuous appearing cells with no evident mitoses. This lesion can sometimes be indistinguishable from well-differentiated angiosarcoma and patch stage of Kaposi’s sarcoma alike.7
Stasis dermatitis is a condition affecting middle aged to older individuals as a consequence of venous stasis. Clinically, it can vary on presentation from shiny and erythematous appearance to dry scaly lesions with secondary ulceration. Histologically, it can be identical to patch stage of Kaposi’s sarcoma with neoblood vessels arising in a lobular pattern in the superficial and deep dermis along with fibrosis. Inflammatory infiltrate ranging from histiocytes, lymphocytes and variable number of plasma cells can be present adjacent to these vascular spaces. Extravasated red blood cells, hyperplastic endothelial cells, and hemosiderin laden macrophages are also features that can be seen in this condition.8
Acroangiodermatitis or pseudo-Kaposi’s sarcoma is a rare cutaneous condition arising due to venous hypertension from severe chronic venous stasis in arteriovenous malformations (AVM), patients with paralyzed legs, hemodialysis patients with arteriovenous shunts, amputees and liver disease. Clinical presentation is often itchy, painful, confluent, purplishblue to brown papules and plaques on distal lower limbs. The histological features include capillary proliferations and perivascular inflammation characterized by eosinophils in the dermis with minimal epidermal pathology.9 The patch stage of Kaposi’s sarcoma is characterized by perivascular infiltrate of plasma cells and lymphocytes.
Kaposiform hemangioendothelioma is a rare, locally aggressive tumor of infants and children which can initially present as a cutaneous lesion (75%) or retroperitoneal mass (18%). Histology is characterized by infiltrating sheets of compact spindle cells with slit-like spaces and can closely resemble Kaposi’s sarcoma.10 HHV-8 negativity is an important factor to differentiate between these two entities.
Classic Kaposi’s sarcoma is categorized as an intermediate vascular tumor according to World Health Organization (WHO) classification and clinically typified by larger, violaceous, raised plaques to ill-defined nodules. In the plaque stage, the vascular spaces appear more jagged with increased mononuclear cell infiltrate, siderophages and plump endothelial cells. This progresses to proliferating spindle cells located in the dermis or subcutaneous tissues with more obvious atypia and increased mitosis. The spindle cells may contain pink cytoplasmic globules representing degenerating red blood cells with intervening slit like spaces.2 Since nearly all Kaposi’s sarcoma lesions are associated with HHV-8, immunohistochemistry cannot differentiate between patch stage and later stages of Kaposi’s sarcoma and histology in conjunction with clinical picture remains the only key to distinguish the different stages.
Angiosarcomas are malignant vascular tumors that can histologically vary from well differentiated tumors difficult to distinguish from hemangiomas to poorly differentiated tumors that can mimic carcinomas or melanomas. Although primary angiosarcomas arise de novo, secondary angiosarcomas are known to arise post radiotherapy for malignant tumors (e.g. breast cancer) and in chronic lymphedema patients.11 Clinically, these neoplasms are aggressive with a dismal five year survival rate of 30% due to their ability to locally invade and metastasize widely. Cutaneous angiosarcomas often begin as well demarcated, asymptomatic, red nodules and can progress to fleshy, red-tan to gray-white masses with indistinct margins. The histological hallmark of angiosarcomas vary from mild to moderately atypical endothelial cells that line vascular structures in well differentiated neoplasms to severe atypia seen as undifferentiated spindle cell tumors without recognizable blood vessels. These lesions can focally show Kaposi’s sarcoma like features with vascular slit-like spaces and mild endothelial atypia. High mitotic count, necrosis and hemorrhage are commonly seen, especially with more poorly differentiated types.2 Patch stage of Kaposi’s sarcoma can be differentiated from well-differentiated angiosarcoma not only by the clinical history and progression but also by positivity for HHV-8 and showing nil to minimal endothelial cell atypia.
Conclusions
Patch stage of Kaposi’s sarcoma is a difficult histological diagnosis that can be readily missed if not thought of in the correct clinical setting. Although histology and immunomarking for HHV-8 can be quite distinctive, this entity needs to be kept in the differential for a HIV positive patient with a clinically and histologically vascular appearing mucocutaneous lesion.
References
- 1.Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med 2000;342:1027-38. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V, Abbas A, Aster J. Robbins basic pathology. 9th ed. Philadelphia: Saunder Elsevier; 2013. [Google Scholar]
- 3.Ackerman AB. The patch stage of Kaposi’s sarcoma. Am J Dermatopathol 1979;1:165-72. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RA, Burgess GH, Hoshaw RA. Patch stage Kaposi’s sarcoma. J Am Acad Dermatol 1980;2:509-12. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Ophthalmology. Basic and clinical science course (BCSC) 2010-2011. San Francisco: American Academy of Ophthalmology; 2011. [Google Scholar]
- 6.Requena L, Kutzner H, Mentzel T, et al. Benign vascular proliferations in irradiated skin. Am J Surg Pathol 2002;26:328-37. [DOI] [PubMed] [Google Scholar]
- 7.Guillou L, Fletcher CD. Benign lymphangioendothelioma (acquired progressive lymphangioma): a lesion not to be confused with well-differentiated angiosarcoma and patch stage Kaposi’s sarcoma: clinicopathologic analysis of a series. Am J Surg Pathol 2000;24:1047-57. [DOI] [PubMed] [Google Scholar]
- 8.Weedon D, ed. Weedon’s skin pathology. 3rd ed. London: Churchill Livingstone Elsevier; 2010. [Google Scholar]
- 9.Scholz S, Schuller-Petrovic S, Mali KH. Acroangiodermatitis in homozygous activated protein C resistance. Arch Dermatol 2005;141:396-7. [DOI] [PubMed] [Google Scholar]
- 10.Mac-Moune Lai F, To KF, Choi PC, et al. Kaposiform hemangioendothelioma: five patients with cutaneous lesion and long follow-up. Mod Pathol 2001;14:1087-92. [DOI] [PubMed] [Google Scholar]
- 11.Mendenhall WM, Mendenhall CM, Werning JW, et al. Cutaneous angiosarcoma. Am J Clin Oncol 2006;29:524-8. [DOI] [PubMed] [Google Scholar]


