Abstract

UNESCO (the United Nations Educational, Scientific, and Cultural Organization) has declared 2015 the “International Year of Light and Light-Based Technologies”. In celebration of this proclamation, this Outlook provides a general history of light and its applications, from the earliest moments of the Big Bang through its present impact on all forms of life on the planet. Special emphasis is placed on fundamental advances in the generation and use of artificial light, as well as the harvesting and use of light from the Sun and other natural sources. During the past century, the role of light in the fields of physics, chemistry, and biology has expanded to include emerging fields such as environmental engineering, agriculture, materials science, and biomedicine. In this regard, future research challenges and new potential applications in these areas, in the context of “the central science”, are presented and discussed.
Short abstract
A scientific history of light is presented, from the Big Bang through its present impact on human existence. Applications and future research challenges in physics, chemistry, and biology involving light are presented and discussed.
Introduction
It is no exaggeration to say that light (defined broadly here as electromagnetic radiation) has played a crucial role in every aspect of human history, leading to amazing advances in science and technology, but also strongly influencing art, religion, and politics. Even before human existence, there was a period of time (from roughly 10 s after the Big Bang to 380,000 years later) when our universe consisted of nothing but photons.1 Think about this carefully for a moment: at one point (and a rather long point at that, compared to a human lifetime), everything we know about, and all that we have descended from, was light. As planet Earth formed and began to cool 4.5 billion years ago, life (replicative organized assemblies of matter) arose from myriad combinations of chemical reactions, many of which may have been light induced.2
The energy from our Sun is a faint remnant of these early events, without which we would not exist at all today. Modern physics, through the National Ignition Facility and Photon Science project at Lawrence Livermore National Laboratory, currently seeks to replicate the Sun’s fusion processes and to mimic the conditions of the early stages of the Big Bang using the largest array of high-powered lasers ever assembled for a single experiment.111 Controlled, sustainable fusion reactions are still a lofty goal, but if successful they may provide unlimited energy resources for the planet and, if human endeavor elects to expand beyond it, to the cosmos.
Many of the technological advances that society has enjoyed since the industrial revolution are the result of light-based phenomena. The development of smaller and smaller integrated circuits involves sophisticated surface photophysics and photochemistry,3 and the Herculean effort of synthetic chemists to produce brighter and more colorful molecules for electronic displays has made a significant impact on our ability to learn from and communicate with each other.4 Chemists have joined forces with biologists and physicists to bring modern medical imaging toward new frontiers in diagnoses and treatment.5 Optical detectors ensure product safety by finding defects in spray paint cans and juice bottle caps.6 The role of chemistry as the central science in the arrival of these technologies is obvious. The purpose of this Outlook is to examine light in the broadest possible sense through its history since the dawn of time, to summarize several scientific milestones that led to new light-based technologies, and finally to look ahead to the future at new research areas where chemists will play a role in bringing the next wave of benefits to society that use electromagnetic radiation. There is no intention here to be comprehensive; several books and Web sites are referenced, from which many detailed examples of light-induced chemical reactions can be extracted.
A Brief History of Light on Earth
About 3.5 billion years ago, the first photosynthetic organism arose from early chemistry.7 One can only wonder about this first assembly of molecules, which together could sense the Sun’s energy, create their own food, and replicate. The next few billion years of mutation, adaptation, and evolution led to different species that used light to observe their universe. Humans in particular applied their knowledge of light sources to solve practical problems such as the construction of safe and warm shelters, the development of organized agriculture, and the creation of visual arts and entertainment. Ancient Greeks and Romans had early models for theories of light and vision,8 and the ancient Egyptians experimented with lenses and sintered quartz (faience) to create dazzling ceramic colors.9 These iterative observations, applications, and theoretical developments are cogs in the wheel of what we now call the scientific method.
Humankind’s relationship with light probably began accidentally with lightning-induced fires, and light technology accelerated when humans began to control light and heat in the form of torches about 300,000 years ago.10 Three immediate consequences of controlled fire were (1) better protection from predators, (2) warmth, allowing migration to colder climates, and (3) cooked food, especially meat.11 All three of these benefits would lead to a better quality of life, as well as longer life expectancies. One can only imagine the anguish felt by early humans who watched their campfires extinguished by wind, rain, or snow. An additional feature of torches was their portability, important for a nomadic lifestyle. Controlling the intensity of a torch was one skill, and directing the light was another—initially this was accomplished using rocks carved from alabaster, or naturally curved objects such as seashells.12 This aspect of light technology grew eventually into the world of lenses, prisms, and fiber topics of which we take advantage today for safety, communications, and entertainment.
Chemistry played a key role in the history of light propagation and light directing devices: the quality of the glass in prisms and lenses and the abrasives that were used to polish them with precision were the result of a huge effort that started in ancient Greece13 and accelerated in The Netherlands in the 17th and 18th centuries.14 Janssen’s development of the microscope15 (ca. 1590) and Lippershey’s construction of the first telescope16 (ca. 1600) led to the invention of modern astronomy by Galileo, and enabled Robert Hook’s pioneering work on optical microscopy. In the biological arena, Robert Boyle studied bacterial bioluminescence in the mid-17th century, showing that it disappeared if oxygen was removed from the organisms. By the dawn of the age of enlightenment (both puns intended), scientists were poised to take light in many new directions for the betterment of society.
The influence of visible light on human behavior is exemplified by an early focus on color: The dyes and pigments used in textiles that traveled the Silk Road and the use of stained glass in churches are notable examples. These applications relied on a solid understanding of the chemistry of the dyes and their interactions with light. Color theory became of great interest to scientists such as Guyot (a color mixing expert)17 and the poet Goethe.18 The mixing of colors19 is currently a hot topic in the field of light-emitting diodes, where the quest for high quality white light continues. An early fascination of many early scientists was the relationship of light to sound, which led to several interesting but ultimately incorrect theories. Interestingly, the modern alternative medicine movement still shows an interest in propagating such hypotheses as truths.20
Color theory would not have captivated scientists without early investigations of the action of prisms on visible light. The Law of Refraction was first described in the year 984 by the Arab scientist Ibn-Sahl,21 and was well-understood at the time of Newton’s description of the colors from visible light that had passed through a prism. This was a groundbreaking discovery; up to that point, white light from the Sun was regarded as “pure”. But Newton’s genius did not stop there: he also noted that the light beam had been altered from a circular to an oblong shape. Furthermore, he noted that the original white light beam could be reconstructed with a lens and a second prism. With Newton’s prism work, light dispersion phenomena began to be quantified.22 Newton’s interest and important experiments on prisms are especially noteworthy. His election to the Royal Society at the age of 30 was based largely on these experiments, which are included in Robert P. Crease’s list of the ten most beautiful scientific experiments of all time.23
Major Milestones in the Science and Technology of Light: The 20th Century
The invention of the electric light bulb in the late 19th century was revolutionary and had a worldwide influence on human society. Chemists played a key role in this lighting technology by providing the most rugged materials for bulb filaments (initially carbon black, later various metal alloys).24 Modern technology for controlled lighting also takes advantage of new chemistries: energy efficient phosphors from compact fluorescent bulbs and white light emitting diodes (LEDs) both arose from strong efforts in synthetic organic and inorganic chemistry.25 As efficiencies of LEDs increase and their fabrication costs decrease, the Edison style bulb appears headed for extinction after almost a century of dominance.
The development of charge-coupled devices (CCDs), on which all digital photography (including that used on the Hubble telescope) is based, is another milestone.26 Modern CCD devices, along with high-quality quartz fiber optic cable, revolutionized the communications industry, and they continue to be improved upon for faster and more reliable data transmission. It is noteworthy that the photoelectric effect, as explained by Einstein, was one of the principles of quantum optics used to invent the CCD, and was one of many light-driven phenomena requiring the new quantum theory in the early 20th century. The list of Nobelists in Physics and Chemistry from this time shows intense activity in quantum physics, leading to a deeper fundamental understanding of the discrete nature of atomic and molecular energy levels, and of light having both particle and wavelike properties. Consequently, continuous wave (CW) spectroscopy over all regions of the electromagnetic spectrum flourished. Rarely was there a time (1905–1935) in scientific endeavor where theory and experiment pushed together for understanding complex ideas at such an intense pace.
The connection between light and quantized states in matter led other research breakthroughs over the next few decades, the maser in the 1950s and the laser in the early 1960s being the most important. The visible light laser was revolutionary for both fundamental and applied science, even more so when Q-switching, mode-locking, and optical pumping technologies were introduced in the late 1960s.27 High-resolution optical spectroscopy dominated the landscape of chemical physics and physical chemistry for much of the next three decades, and applications as simple as hand-held laser pointers for lecturers, and as complicated as surgical devices, came quickly to the forefront of light-based technology just a short time later.
So far, I have discussed only visible light (although the warmth from our ancestor’s fire torches must have hinted at the invisible). An important nonvisible light source is X-rays, used in medical and industrial imaging, crystallography, and X-ray photoelectron spectroscopy, which is used mainly in surface science. This region of the electromagnetic spectrum is historically significant for its direct association with at least eight Nobel Prizes in Chemistry and Physics. The infrared (IR) region gives us many transmitters and detectors that are found in every day objects such as television remote controls and motion sensors in security systems.28 In scientific research, the near IR phosphorescence from singlet oxygen can be used to track its fate in cells as it is implemented in cancer therapies.29 Radio waves and microwaves are used extensively in astronomy,30 medical imaging,31 and satellite and cellular telephone and satellite communications systems.133 The global positioning system we currently take for granted is another major milestone for human society that is light-based (low frequency microwaves), and it has created a truly small world after all—do we ever not know where we are anymore?
The synchrotron and free electron laser technologies developed after WWII have also augmented the experimental arsenal of scientists interested in both fundamental and applied research using light. The impact of these large-scale, multiuser, broad wavelength range light sources has been profound. There are 48 synchrotron and 13 free electron laser facilities worldwide, serving tens of thousands of researchers per year.33 Applications of this research include medical and 3D biological imaging,34 analysis of geological materials,35 the study of chemical reaction dynamics,36 mechanisms of battery degradation,37,38 and determination of protein structure using X-ray crystallography.39 Many of these light sources have moved into “on-demand” mode with ultrafast pulsed X-rays, allowing for sophisticated time-resolved experiments on many different classes of material.40
There are several other notable 20th century scientific achievements involving light. One is the establishment of a nearly complete understanding of the mechanism of bacterial and plant photosynthesis.41 This process, which is the basis for all life on the planet, has been well mapped out in terms of its photophysics (the absorption of light by living organisms and the associated energy transfer pathways to photosynthetic reaction centers), its photochemistry (the electron transfer cascade that provides the energy gradient used to synthesize adenosine triphosphate (ATP) in cells), and its biochemistry (additional light-independent reactions such as those in the Calvin cycle).
A second major achievement is our deep understanding of vision (light-sensing, visual transduction) at the molecular level, especially in mammals.42 In a similar fashion to photosynthesis, the mechanism of visual transduction was elucidated using photophysical methods (ultrafast spectroscopy to establish lifetimes of excited states), photochemistry (measuring the time frame for 11-retinal’s cis–trans isomerization and the effect of this chemical change on the conformation of rhopodsin, an eye protein honored in Figure 1), and biochemistry (understanding the balance of Na+/K+ ion flow in the rods and cones and its regulation by signaling proteins). A third noteworthy accomplishment is the use of light in noninvasive photodynamic therapies for cancer. Understanding both the toxicity of singlet oxygen in cells and its controlled production using very efficient sensitizers, as well as light of the correct wavelength, has made modern skin cancer treatments fast and relatively inexpensive.43
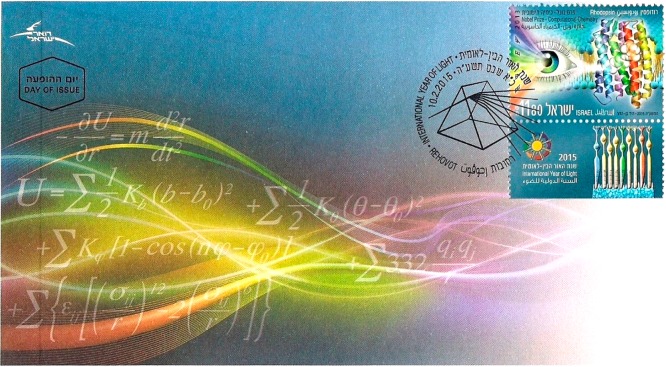
Figure 1.
A 2015 Israeli stamp honoring vision, the protein rhodopsin, and the International Year of Light. By courtesy of the Israel Philatelic Service, Israel Postal Company. Image credit David Ben-Hador.
Chemists desire to understand the mechanisms of bond breaking and bond forming processes, or about the movement of fundamental particles such as protons and electrons (and sometimes both). In terms of light-driven reactions, chemists turn to the well-known Jablonski diagram to understand the photophysical processes leading to reactive excited states.44 The terms absorption, excitation, intersystem crossing, vibrational relaxation, internal conversion, fluorescence, and phosphorescence are all familiar terms to photochemists in this regard. It is somewhat underappreciated that, in the mid-20th century, a great deal of controversy surrounded the latter phenomenon of phosphorescence from organic molecules. Jablonski himself45 and G. N. Lewis46 both argued for the existence of an excited triplet state as the emitting species in phosphoresence, but their ideas met with resistance from the brain trust of the time, mostly physicists such as Franck, Teller, and Livingston. With molecular orbital theory in its infancy, it was easy to see the reasoning for their skepticism: there was simply no place to put two unpaired electrons without violating the well-established parity rules for fundamental particles.
Lewis and his graduate student Michael Kasha persisted, and indeed insisted, in a landmark paper from 1947 covering more than 80 organic molecules, that phosphorescence was due to the relaxation of their excited triplet states.47 In spite of the successful paramagnetic susceptibility measurements of Lewis and Calvin on a phosphorescent molecule,48 the skeptics were unimpressed. Hard evidence that Lewis et al. were correct would come eventually from a laboratory just down the hall from Franck’s at the University of Chicago. Clyde Hutchison and his student Billy Mangum constructed an apparatus for the simultaneous detection of the optical emission and electron paramagnetic resonance (EPR) absorption of organic molecules such as naphthalene when they are subjected to ultraviolet excitation.49 By measuring the phosphorescence and the EPR spectrum simultaneously, they firmly established that the phosphorescent state was paramagnetic. The key to success in this experiment was to dope a small quantity of naphthalene molecules into a single organic crystal of durene. This created a crystal containing “dilute” naphthalene molecules, which would avoid triplet–triplet annihilation upon photoexcitation. Furthermore, mounting these crystals on a goniometer stage allowed an X-ray diffraction pattern to be measured, ensuring that the researchers knew the orientation of the naphthalene molecules relative to the applied magnetic field while carrying out the EPR experiment on their photoexcited triplet states. Rotation of either the goniometer stage axes or the external magnetic field led to predictable shifts in the positions and intensities of the EPR transitions.
This Hutchison–Mangum experiment was hardly simple: a hole had to be cut in the magnet pole piece to allow the UV excitation to reach the sample, and the entire EPR resonator, sample, and stage had to be cooled to 4.2 K. Their apparatus remains a marvel of modern engineering and is a great example of the use of two types of spectroscopy (and therefore two types of light—UV for the optical absorption and microwaves for the EPR spectrum) to solve a long-standing problem. Its beauty and complexity aside, this experiment settled, once and for all, a major scientific controversy regarding photo–excited triplet states that was almost two decades old.50
I close this section with a nod to several very recent advances in fundamental and applied science involving electromagnetic radiation. In 2015, a clever experiment using electrons to image light traveling in a nanowire was reported. This work demonstrated the dual nature of light, i.e., that light can simultaneously behave as a wave (through an interference pattern created by light traveling in opposite directions down the nanowire) and as a particle (by influencing the speed of a batch of electrons aimed near the wire).51 The beautiful picture that results from this experiment (Figure 2) is essentially a nanoscale confirmation of Einstein’s photoelectric effect.
Figure 2.
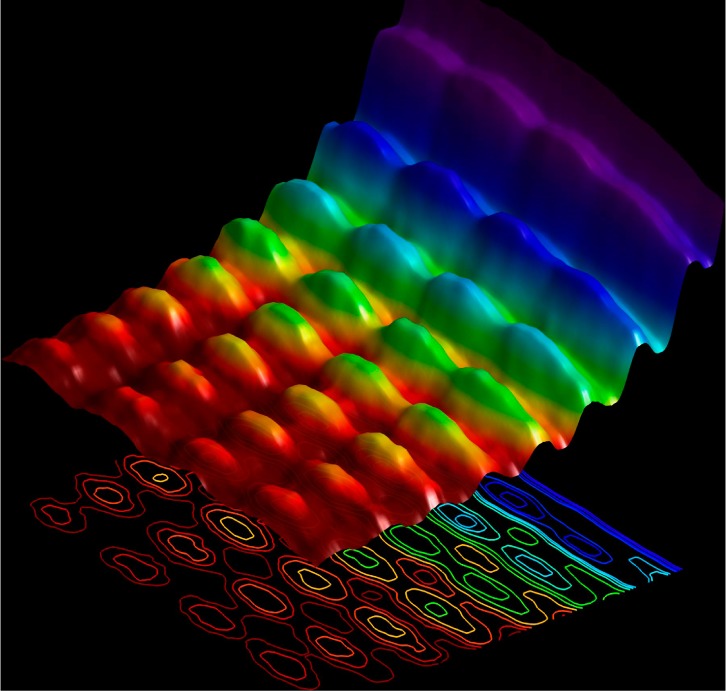
Picture of a standing wave interference pattern of light in a nanowire, with the different colors representing slow vs fast electrons used to detect the wave. Reprinted with permission from ref (51). Copyright 2014 Nature Publishing Group.
Two-dimensional spectroscopy was first developed in the NMR field and quickly became one of the most useful techniques for structural determination of organic and inorganic molecules, and it is now taught at the undergraduate level.52 The beauty of pulsed nuclear spins is their near-perfect manifestation of the essential features of quantum mechanics: coherences, relaxation, state mixing, and polarization are all easily observed and explained for simple two-spin systems. Recently, several laboratories have extended 2D methodologies to the IR, visible, and UV regions, including experiments with ultrafast pulses.53 Such techniques have allowed for exploration of vibrational and electronic coherences in highly complex systems such as light-harvesting antenna proteins. The existence of long-lived electronic quantum coherences in photosynthetic light-harvesting pigments has not been without controversy, and an excellent summary of these issues has recently been presented.54
In the realm of biological imaging, a great leap forward in our ability to investigate conformational motions of proteins was recently achieved by incorporating a minimally perturbative fluorescence quencher (thioamide) near a fluorescent amino acid elsewhere in the peptide sequence.55 This simple methodology promises to give ever more accurate experimental descriptions of important conformational changes in enzymatic reactions. It can also be used to investigate the protein misfolding phenomena associated with diseases such as Alzheimer’s.
It is worth noting that, in 2014, both the Physics and the Chemistry Nobel Prizes were awarded for research in light science: Betzig, Moerner, and Hell shared the Chemistry prize for their efforts to improve the resolution of optical microscopy,56 while the Physics prize was shared by Nakamura, Akasaki, and Amano for their development of the blue LEDs that revolutionized electronic displays and other technologies.57 For the foreseeable future, artificial lighting in all forms, from stadium instant replay screens to automobiles to theater lighting, will be dominated by LED technology. Solid-state lighting will also see increased use in the health care, communications, and information technology fields. And thanks to the Chemistry Prize winners, the so-called Abbe limit,58 which had dogged the resolution of biological imaging experiments for more than a century, is now relegated to scientific history.
The Future of Light and Light Technologies
The milestones listed above provoke an obvious question: where do we go from here? Future generations of scientists and engineers, and especially chemists, have many interesting challenges in front of them. A look back at the National Academy of Sciences 1992 book entitled “Science at the Frontier” lists artificial photosynthesis and computational neuroscience as topics where light and light technologies play major roles.59
Using the Sun
Harnessing the energy of our Sun in an efficient and cost-effective manner is a general problem for solar fuel production, the construction of photovoltaic devices, and generalized photocatalysis. To date, the most efficient solar energy conversion devices are cost prohibitive, while the less expensive materials such as silicon are poor visible light absorbers. A related problem in the area of solar fuels is the chemical reduction of carbon dioxide from the atmosphere, which has vexed scientists now for centuries. A logical goal for this century’s chemists would be to deal with three solar research related topics simultaneously: (1) the development of inexpensive, robust light-harvesting molecules that can be chemically coupled, in a scalable fashion, to a charge-separation device,60 (2) the construction of robust catalysts that operate in a practical pH range for both water oxidation61,62 and carbon dioxide reduction63,64 to provide a regenerative, nonpolluting fuel supply, and (3) the generation of a similarly structured device with new catalysts that can take these reactions one step further: mimicking the biosynthesis of glucose, i.e., not just providing energy output, but carrying out real photosynthesis for improvements to the food supply. Imagine a chip that synthesizes your body’s nutrients directly from the Sun, reducing the amount of land needed to grow crops. This could also have a ripple effect on the issue of fertilizer runoff and algae blooms, which are discussed in more detail below.
As scientists endeavor to solve issues such as cheap solar power, they often strategize to build molecules or supramolecular systems that completely replicate natural systems. Alternatively, they can focus on mimicking one aspect of a natural system. For example, it does not make sense to build a whole plant for artificial photosynthesis, because much of the Sun’s energy transduced by the plant is directed toward activities other than the generation of food (the plant must grow, and it must reproduce). Artificial photosynthetic systems are (hopefully) free of these constraints, and in this regard considerable effort has been put forth to maximize the efficiencies of solar conversion devices. Fabrication costs for solar energy conversion devices remain high; therefore fossil fuels still dominate the energy landscape. Still, photovoltaics are now 1.8% of the energy industry and grew 43% last year. For the future it is worth questioning the effort made to synthesize the “best” catalyst or “best” light harvester without consideration of their costs. One can argue that fundamental principles must still be established to understand how and why solar energy devices work, but at some point the cheapest alternative fuel source will be the winner. Jean and co-workers have recently put forward an excellent summary of the current state of costs, molecular complexities, and efficiencies of photovoltaic materials.65
Another natural system of interest is the light-harvesting mechanism in butterfly wings, which has been optimized for maximum light absorption with cleverly constructed chromophores in layered arrays. Efforts to duplicate this system using nanopatterned layers of polymers are yielding very interesting results, but the researchers caution that many factors leading to improvement of photovoltaic efficiencies still need to be investigated, and they correctly identify cost as a possible limiting factor in this technology.66
Photobiology: Beyond the Human Body
Biological imaging and light-based medical treatments will continue to improve in terms of sensitivity and in the complexity of systems that can be studied. Optical tomography in the near IR region is now a reality,67 and it has proven possible to use fluorescence lifetime measurements to distinguish between healthy and unhealthy cells.68 An interesting challenge for chemists and biochemists is to be able to tag fluorophores or other radiative molecules to enable them to access specific cells or even specific regions once inside a cell.69 The higher their specificity, the more useful these molecules will be for diagnostics and therapy. Taking this a step further is the combination of optical and magnetic properties, in particular to make switchable properties (either optically switching a magnet’s polarity or magnetically switching light on and off).70 This is now being taken to an extreme with the development of wireless electricity using magnetic induction, but this has interesting implications for biological imaging as well.71
Bioluminescence remains a fascinating research topic for chemists and biochemists alike. A recent review of ocean-based bioluminescent phenomena by Haddock et al.72 shows an astounding “tree of life” diagram that shows luminescent organisms in almost every branch. These organisms typically use the well-understood luciferase protein to emit light, but other mechanisms are possible. Even more astounding is the recent publication of research showing ultraweak (undectable to the naked human eye) spontaneous photoemission from human bodies that can be correlated with diurnal rhythms.73 While it is well-established that human beings operate at power levels approximating a 100 W light bulb, the detection of human power dissipation in the form of light, however weak, is of interest in terms of our susceptibility to detection by other species (forgive me, but this includes extraterrestrials—sleep well). Back on plant Earth, a recent report on luciferase reactions suggests that deep tissue imaging may be possible via control of electron transfer quenching processes, a chemical reaction that shows great promise for diagnostic medical imaging.74
Neuroscience might seem out of place here, but I mention it in the context of a deeper general understanding of the senses (particularly vision) and memory in humans. For example, studies of the visual transduction process in the retina, the coupling of this information to the optic nerve, and the subsequent cognitive processes that create “sight”, and visual memories, is a fascinating topic that requires new knowledge of the chemistry, biology, and physics of each step. In just the past decade we have seen the construction of several artificial vision systems that make direct connections between the retina and the visual cortex of the human brain (Figure 3).75 Great progress is being made, and it begs the question of whether this technology can, in addition to helping blind people see, also enhance vision for those with the capability of sight? Can we take visual entertainment beyond the 3D IMAX experience, to the point where the viewer is “inside” the movie? Also, recent advances in the field of optogenetics has a direct connection to neuroscience and the visual transduction mechanism. For example, it has recently been reported that false memories can be optically “implanted” in certain mice using photoexcitation.76
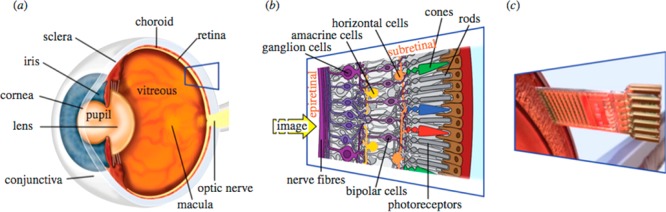
Figure 3.
Human eye. (a) The structures of the eye and (b) the retinal layers in detail. (c) The function of photoreceptors lost because of hereditary degeneration can be partially replaced by a subretinal chip. The chip carries a microphotodiode array with amplifiers and electrodes on a 3 mm × 3 mm area and is surgically placed subretinally in the location corresponding to the layer of degenerated photoreceptors. Reprinted with permission from ref (75). Copyright 2013 The Royal Society.
Much of the basic biochemistry of visual transduction is now understood, but research on the psychology and neurology of vision, and its connection to memory, is still at an early stage.77 This research area is connected to the bigger problem we face as aging planet: it is predicted by the Danish Aging Research Center that a child born in 2007 in Europe or the U.S. has a 50% probability of living to the age of 104.78 To take these statistics further, most of us will see, in our lifetime, a human living to the age of 150. However, most of our senses, including vision, begin to fail at the average age of 85. It is then worth questioning the value of those extra years if we are unable to see, smell, hear, taste, or touch. Vision, light, neurology, and psychology will all need to be blended together to make it an enjoyable longer ride.
This Outlook would not be complete without an acknowledgment of yet another Nobel Prize involving the interaction of light and matter: The 2015 Chemistry Nobel, announced just before this manuscript went to press, recognizes in part the outstanding work of Sancar and co-workers on DNA repair mechanisms as related to skin cancer. Sancar’s group in particular were pioneers in the use of molecular and cellular biology to map the process of nucleotide excision repair. This is a critical step correcting the damage to DNA caused by ultraviolet (UV) light from the atmosphere.79 When this normally very efficient DNA repair system is defective, the chances of a human being developing skin cancer are greatly increased after exposure to natural sunlight.
Light in Materials Science
Optical microscopy is used in biomedicine, but also finds great utility in materials and colloidal science.80 While observation of static structures will remain important, dynamic microscopy measurements are now possible and allow the tracking of moving colloidal and biological particles on both fast and slow time scales.81 This will be an especially important tool for the field of microfluidics and for the study of structured (non-Newtonian) fluids,82 where length scale correlations of flow with applied force are critical parameters for understanding this unusual state of matter. At the nanoscale, scanning probe microscopies (STM, AFM, and photonic force) will continue to have an impact in polymer and materials chemistry as well as molecular biology.83 A very exciting new development in nanoscale microscopy is the use of scanning tips as optical probes, where structural features of a surface can be probed simultaneously with optical excitation.84
Global appetite for computer memory continues to increase, and optically based information storage is clearly a growth area for improving memory density in electronic devices, and for increasing their read/write speeds. Light-switchable magnetic molecules also have potential in this regard.85 Quantum computers operate by the creation of a coherent superposition of states or entanglements, through which digital information can be transmitted and/or stored. It has been shown that such computers would be significantly faster than classical computers.86 Pulsed lasers are an obvious choice for the creation of the necessary coherences. With much of the mathematics of such computing systems already worked out and algorithms being developed, now is the time to begin looking for systems with the correct absorption and state-mixing properties for such a device. This field is emerging from theoretical predictions87 to verifiable experiments with photons.88 A possible photoinduced teleportation experiment using correlated radical pairs and a reporter electron spin is depicted in Figure 4. Here a reporter spin (a stable nitroxide radical) can be probed using pulsed electron paramagnetic resonance (EPR) to give information about a preceding photochemically induced electron transfer event, despite being uninvolved in the reaction. Since only quantum mechanical coupling rather than electron transfer is responsible for the EPR signal, this represents true teleportation of spin information rather than electron (spin) transfer.112
Figure 4.
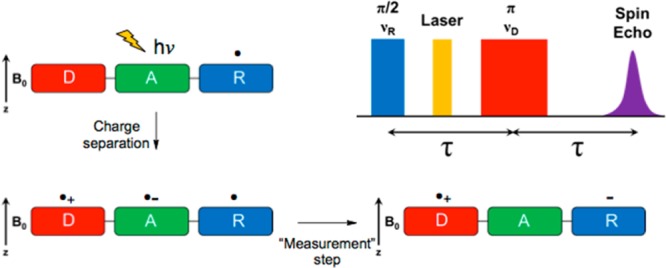
A spin teleportation experiment. The acceptor A (green molecule) is selectively photoexcited, and the triad undergoes sequential electron transfer reactions from a donor D (red) in the presence of a stable reporter spin R (blue). A pulsed electron paramagnetic resonance experiment that probes the reporter spin during the second electron transfer reaction can tell if this spin has been “teleported”. Image credit: Prof. M. R. Wasielewski.
Quantum dots are nanoparticles of semiconductor materials with tunable optical and electronic properties that lie between those of discrete molecules and bulk semiconductors.89 These particles have recently been used for television displays,90 and they show promise as tracers in biomedical and engineering applications, for example in the study of the corrosion of airplane wings.91 Quantum dots have been promoted as potential photovoltaic materials for solar power,92,93 but the lower cost of polymer-based materials has precluded their mass production at the present time. An attractive feature of quantum dots is the tunability of their emission spectra over a wide range in the visible region.94 Growing asymmetric (Janus particles) or heterogeneous quantum dots is attractive for instilling desirable properties such as solubility with one part of the particle and photocatalytic activity or photoactivated drug release on another part, or modulating electronic properties in novel ways (Figure 5).95
Figure 5.
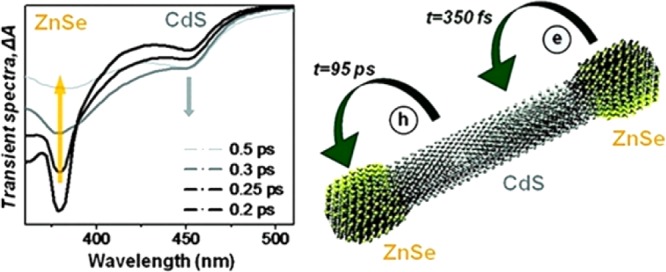
“Barbell”-type heterogeneous semiconductor nanostructures show unprecedented ultrafast charge carrier dynamics. Reprinted with permission from ref (95). Copyright 2010 The American Chemical Society.
Polymer photodegradation96 will continue to be an important topic for photochemists and photophysicists. In some cases, photodegradation is a desirable property, for example in recycling of plastics or in the activation of shape-memory polymers. For architectural coatings or in the aerospace industry, photodegradation is generally a detrimental property and is actively minimized, either with additives or with creative synthetic methodologies. In either case, spectroscopic investigations of the kinetics and mechanisms of these processes will be necessary, especially as new materials are synthesized or as new environmental regulations appear. Light-activated self-healing polymers are known, and have many analogies with shape-memory materials. The interplay of photothermal vs photochemical changes in macromolecules has recently become a frontier topic in this field.97
Agriculture is another area where light-based research will have a large impact. From water purification to phosphorus remediation to the control of toxic algae blooms, photochemistry and photophysics can play a large role. Phosphate runoff from farms and algae blooms are intimately connected,98 and photoactivated phosphorus remediation in the field would be an attractive solution. The situation is compounded by recent calls to increase production of crops such as corn for ethanol-based biofuels. The photochemical control of fertilizer release might also be a way to exert some control over phosphorus levels in watersheds. And while light-induced Fenton-type chemistry is a well-established method for water purification in some countries,99 it is time- and energy-consuming. Improvements to the efficiencies of these catalysts are definitely worth pursuing. As agriculture becomes more and more automated, one might imagine using a drone to deliver herbicides, pesticides, or fungicides to a specific crop during day or night using spectroscopic detection (both plants100 and animals101 can give drastically different images when irradiated with ultraviolet light, for example).
We will require new developments in computational chemistry for predicting the structures of excited states and open shell species such as free radicals that are often the products of light-induced reactions. Modern density functional theory methods generally do not fare well in this arena,102 and new approaches for dealing with electron correlation, spin–orbit coupling, and relativistic effects will be needed to support experimental findings and to provide predictive power for absorption and emission profiles, energies of paramagnetic species, and lifetime measurements in photophysics and photochemistry. This will be especially powerful for the development of photocatalysts for solar fuel devices and for new photovoltaic materials. Good calculations for surface-bound structures are also needed to corroborate interfacial characterization data, vide infra.
A General Comment about Interfacial Science, Spectroscopy, and Light
A key feature of many of the future challenges for chemists I have described here is the presence of an interface, for example a protein with water, a quantum dot with an organic solvent, or a catalyst with a semiconductor surface. In most of the applications that may result from such assemblies, a solid understanding of the nature of that interface will be critical to optimization of device performance. However, the chemical structure and reactivity of most interfaces are poorly understood. There are numerous techniques for the study of interfaces using many different types of spectroscopy, but a systematic characterization methodology is not presently available to the chemistry community. Some techniques work well for surfaces in a vacuum; others work better for surfactants in liquid solution. In other cases, molecules are physisorbed or chemisorbed on a surface with many different bonding motifs and orientations. With so many different measurements on different systems, the clear patterns that lead to predictive power have not emerged.
A physical organic chemistry approach is sorely needed in interfacial science today. This will require collaborative efforts for preparation of the cleanest and most uniform surfaces as well as the synthesis of molecules that can be chemically bonded to them. Spectroscopists of every ilk, using light of every wavelength, will confirm the structures of these systems. Others will need to examine chemical reactivity at these interfaces with time-resolved kinetic measurements, whether it is electron transfer, proton transfer, or a chemical transformation such as CO2 reduction.63 This will not be a simple undertaking, and it will take time to build a relevant structure/reactivity database for the most important interfaces. There is substantial room here for a talented group of chemists to have an impact on interfacial science similar, for example, to the impact that Marcus, Hush, Closs, and Miller had on electron transfer in the 1980s.103−107 Many of the light-based technologies listed here will benefit from this knowledge.
Closing Remarks
In the course of writing this manuscript,108−110 I was repeatedly drawn to one of my favorite literary passages, from Thomas Wolfe’s Look Homeward, Angel:
Each of us is all the sums he has not counted: subtract us into nakedness and night again, and you shall see begin in Crete four thousand years ago the love that ended yesterday in Texas.
The seed of our destruction will blossom in the desert, the alexin of our cure grows by a mountain rock, and our lives are haunted by a Georgia slattern, because a London cutpurse went unhung. Each moment is the fruit of 40 thousand years. The minute-winning days, like flies, buzz home to death, and every moment is a window on all time.
Between the lines of the above prose, I see the Big Bang, Newton’s prism, photosynthesis, DNA repair, spin teleportation, butterfly wings, microscopes, and countless other concepts and experiments that give me pause and wonder as I think about light in the broadest possible sense. I hope that you also find here, in some fashion, what you talk about when you talk about light, and that my musings might help you think about a new experiment or two. Enjoy what remains of this International Year of Light, and best wishes in your search for truth.
Acknowledgments
I thank Dr. N. V. Lebedeva for numerous suggestions that help improve the quality and focus of this manuscript during its evolution, and the National Science Foundation for their continued support of my light-based research program during the past three decades (currently through grants CHE-1111873 and CHE-1464817).
The authors declare no competing financial interest.
Footnotes
My apologies to the estate of Raymond Carver for abusing his wonderful title.
References
- Allday J.Quarks, Leptons and the Big Bang, 2nd ed.; IOP Publishing: 2002; p 98. [Google Scholar]
- Patel B. H.; Percivalle C.; Ritson D. J.; Duffy C. D.; Sutherland J. D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. 10.1038/nchem.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millot M.; Dubrovinskaia N.; Černok A.; Blaha S.; Dubrovinsky L.; Braun D. G.; Celliers P. M.; Collins G. W.; Eggert J. H.; Jeanloz R. Shock compression of stishovite and melting of silica at planetary interior conditions. Science 2015, 347, 418–420. [DOI] [PubMed] [Google Scholar]
- Somorjai G. A.; Li Y. Impact of surface chemistry. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 917–924. 10.1073/pnas.1006669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. S.; Klement P.; Jones A. M.; Ghimire N. J.; Yan J.; Mandrus D. G.; Taniguchi T.; Watanabe K.; Kitamura K.; Yao W.; Cobden D. H.; Xu X. Electrically tunable excitonic light-emitting diodes based on monolayer WSe2 p–n junctions. Nat. Nanotechnol. 2014, 9, 268–272. 10.1038/nnano.2014.26. [DOI] [PubMed] [Google Scholar]
- Fass L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. 10.1016/j.molonc.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raum K.; Ozguler A.; Morris S. A.; O’Brien W. D. Channel Defect Detection in Food Packages. IEEE transactions on ultrasonics, ferroelectrics, and frequency control 1998, 45, 30–40. [DOI] [PubMed] [Google Scholar]
- Blankenship R. E. Early Evolution of Photosynthesis. Plant Physiol. 2010, 154, 434–438. 10.1104/pp.110.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley P.Ancient philosophy, mystery, and magic: Empedocles and Pythagorean tradition; Oxford University Press: 1995. [Google Scholar]
- Lindberg D. C.Theories of Vision from Al-Kindi to Kepler; University of Chicago Press: 1976. [Google Scholar]
- Roebroeks W.; Villa P. On the earliest evidence for habitual use of fire in Europe. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 5209–5214. 10.1073/pnas.1018116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I will hold back from an outright proclamation that the instinct to barbecue is lodged somewhere in the human genome (about half of us are fairly well convinced it is firmly entrenched in the Y chromosome). Hard evidence in the field of evolutionary anthropology is skimpy in this regard. See, however, the following article, which I will call “food for thought” (pun intended):Fonseca-Azevedo K.; Herculano-Houzel S. Metabolic constraint imposes tradeoff between body size and number of brain neurons in human evolution. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 18571–18576. 10.1073/pnas.1206390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I am greatly indebted to Bill Williams and Associates, theater lighting specialists from Winnipeg, Canada, for maintaining a very interesting and thorough history of light at the following URL: http://www.mts.net/~william5/history/hol.htm. I borrowed significantly from this timeline to build my introduction.
- Goes F. J., Optical Quality Lenses in Antiquity. In The Eye in History Jaypee Brothers Medical Publishers: 2013; p162. [Google Scholar]
- Ruestow E. G.The Microscope in the Dutch Republic: The Shaping of Discovery; Cambridge University Press: New York, 1996. [Google Scholar]
- van Heurck H.The Microscope: Its Construction and Management ; Including Technique, Photo-micrography, and the Past and Future of the Microscope; Crosby Lockwood and Son: New York, 1893; p 41. [Google Scholar]
- King H. C.The History of the Telescope; Dover: 2011. [Google Scholar]
- Crone R. A.A History of Color: The Evolution of Theories of Lights and Color; Kluwer Academic: 1999. [PubMed] [Google Scholar]
- von Goethe J. W.Theory of Colours; Dover: 2006. [Google Scholar]
- For an excellent interactive online demonstration of color mixing as related to human vision, see: https://phet.colorado.edu/en/simulation/color-vision.
- Reid J. S. The Special Relationship Between Sound And Light, With Implications For Sound And Light Therapy. Subtle Energ. Energy Med. 2006, 17, 215–231. [Google Scholar]
- Rashed R. A pioneer in anaclastics: Ibn Sahl on burning mirrors and lenses. Isis 1990, 81 (3), 464–491. 10.1086/355456. [DOI] [Google Scholar]
- Newton I.Opticks (English and Latin Edition), 1st ed.; Octavo: 1998. (originally published in 1704).
- Crease R. P.The Prism and the Pendulum; Random House: New York, 2003. [Google Scholar]
- Coolidge W. D. Metallic tungsten and some of its applications. Trans. Am. Inst. Electr. Eng. 1912, XXXI (Part 1), 1219–1228. 10.1109/T-AIEE.1912.4768477. [DOI] [Google Scholar]
- Akselrod G. M.; Argyropoulos C.; Hoang T. B.; Ciracì C.; Fang C.; Huang J.; Smith D. R.; Mikkelsen M. H. Probing the mechanisms of large Purcell enhancement in plasmonic nanoantennas. Nat. Photonics 2014, 8, 835–840. 10.1038/nphoton.2014.228. [DOI] [Google Scholar]
- Janesick J. R.Scientific charge-coupled devices; SPIE Press: 2001; p 4. [Google Scholar]
- Townes C. H.How the Laser Happened: Adventures of a Scientist; Oxford University Press: 1999. [Google Scholar]
- Rogalski A. History of infrared detectors. Opto-Electronics Review 2012, 20, 279–308. 10.2478/s11772-012-0037-7. [DOI] [Google Scholar]
- Ogilby P. R. Singlet oxygen: there is indeed something new under the sun. Chem. Soc. Rev. 2010, 39, 3181–3209. 10.1039/b926014p. [DOI] [PubMed] [Google Scholar]
- Wilson T.; Rohlfs K.; Hüttemeister S.. Tools of Radio Astronomy (Astronomy and Astrophysics Library), 6th ed.; Springer: 2014. [Google Scholar]
- Vlaardingerbroek M. T.; Boer J. A.. Magnetic Resonance Imaging, 3rd ed.; Springer: 2003. [Google Scholar]
- The Handbook of Global Media and Communication Policy, 1st Ed.; Mansell R.; Raboy M., Eds.; John Wiley & Sons, Ltd.: 2014. [Google Scholar]
- Alpern E.It All Comes to Light; U. S. Department of Energy Office of Science; 2015: http://science.energy.gov/news/featured-articles/2015/07-07-15/.
- Spizzirri J.3D potential through laser annihilation; 2015: http://www.alcf.anl.gov/articles/3d-potential-through-laser-annihilation.
- Arns C. H.; Knackstedt M. A.; Pinczewski M. V.; Lindquist W. B. Accurate estimation of transport properties from microtomographic images. Geophys. Res. Lett. 2001, 28, 3361–3364. 10.1029/2001GL012987. [DOI] [Google Scholar]
- Wernet Ph.; Kunnus K.; Josefsson I.; Rajkovic I.; Quevedo W.; Beye M.; Schreck S.; Grübel S.; Scholz M.; Nordlund D.; Zhang W.; Hartsock R. W.; Schlotter W. F.; Turner J. J.; Kennedy B.; Hennies F.; de Groot F. M. F.; Gaffney K. J.; Techert S.; Odelius M.; Föhlisch A. Orbital-specific mapping of the ligand exchange dynamics of Fe(CO)5 in solution. Nature 2015, 520, 78–81. 10.1038/nature14296. [DOI] [PubMed] [Google Scholar]
- Lin F.; Markus I. M.; Nordlund D.; Weng T.-C.; Asta M. D.; Xin H. L.; Doeff M. M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. 10.1038/ncomms4529. [DOI] [PubMed] [Google Scholar]
- Lin F.; Nordlund D.; Weng T.-C.; Zhu Y.; Ban C.; Richards R. M.; Xin H. L. Phase evolution for conversion reaction electrodes in lithium-ion batteries. Nat. Commun. 2014, 5, 3358. 10.1038/ncomms4358. [DOI] [PubMed] [Google Scholar]
- Atkinson S. C.; Armistead J. S.; Mathias D. K.; Sandeu M. M.; Tao D.; Borhani-Dizaji N.; Tarimo B. B.; Morlais I.; Dinglasan R. R.; Borg N. A. The Anopheles-midgut APN1 structure reveals a new malaria transmission–blocking vaccine epitope. Nat. Struct. Mol. Biol. 2015, 22, 532–539. 10.1038/nsmb.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarris L.On-demand X-rays at Synchrotron Light Sources; 2015: https://newscenter.lbl.gov/2015/05/26/on-demand-x-rays/.
- Blankenship R. E.Molecular Mechanisms of Photosynthesis, 2nd ed.; Wiley-Blackwell: 2014. [Google Scholar]
- Molecular Mechanisms in Visual Transduction; Stavenga D. G., de Grip W. J., Pugh E. N. Jr., Eds.; Handbook of Biological Physics Vol. 3; North-Holland: 2000. [Google Scholar]
- Photodynamic Therapy: Fundamentals, Applications and Health Outcomes; Hugo A. G., Ed.; Nova Science Pub. Inc.: 2015. [Google Scholar]
- Jablonski A. Efficiency of Anti-Stokes Fluorescence in Dyes. Nature 1933, 131, 839–840. 10.1038/131839b0. [DOI] [Google Scholar]
- Jablonski A. Uber den Mechanismus der Photolumineszenz von Farbstoffphosphoren. Z. Phys. 1935, 94, 38–46. 10.1007/BF01330795. [DOI] [Google Scholar]
- Lewis G. N.; Lipkin D.; Magel T. T. Reversible Photochemical Processes in Rigid Media. A Study of the Phosphorescent State. J. Am. Chem. Soc. 1941, 63, 3005. 10.1021/ja01856a043. [DOI] [Google Scholar]
- Lewis G. N.; Kasha M. Phosphorescence and the Triplet State. J. Am. Chem. Soc. 1944, 66, 2100–2116. 10.1021/ja01240a030. [DOI] [Google Scholar]
- Lewis G. N.; Calvin M. Paramagnetism Of The Phosphorescent State. J. Am. Chem. Soc. 1945, 67, 1232. 10.1021/ja01223a513. [DOI] [Google Scholar]
- Hutchison C. A. Jr.; Mangum B. W. Paramagnetic Resonance Absorption in Naphthalene in its Phosphorescent State. J. Chem. Phys. 1958, 29, 952. 10.1063/1.1744621. [DOI] [Google Scholar]
- Hutchison C. A. Jr.; Mangum B. W. Paramagnetic Resonance Absorption in Naphthalene in its Phosphorescent State. J. Chem. Phys. 1961, 34, 908. 10.1063/1.1731693. [DOI] [Google Scholar]
- Piazza L.; Lummen T. T. A.; Quiñonez E.; Murooka Y.; Reed B. W.; Barwick B.; Carbone F. Simultaneous observation of the quantization and the interference pattern of a plasmonic near-field. Nat. Commun. 2015, 6, 6407. 10.1038/ncomms7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebolin H.Basic One- and Two-Dimensional NMR Spectroscopy; Wiley: New York, 2010. [Google Scholar]
- West B. A.; Giokas P. G.; Molesky B. P.; Ross A. D.; Moran A. M. Toward Two-Dimensional Photon Echo Spectroscopy with 200 nm Laser Pulses. Opt. Express 2013, 21, 2118–2125. 10.1364/OE.21.002118. [DOI] [PubMed] [Google Scholar]
- Lambert N.; Chen Y.-N.; Cheng Y.-C.; Li C.-M.; Chen G.-Y.; Nori F. Quantum biology. Nat. Phys. 2013, 9, 10–18. 10.1038/nphys2474. [DOI] [Google Scholar]
- Goldberg J. M.; Speight L. C.; Fegley M. W.; Petersson E. J. Minimalist Chromophores to Monitor Protein Dynamics: Thioamide Quenching of Selectively-Excitable Fluorescent Amino Acids. J. Am. Chem. Soc. 2012, 134, 6088–6091. 10.1021/ja3005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2014/.
- http://www.nobelprize.org/nobel_prizes/physics/laureates/2014/.
- Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung [Contributions to the Theory of the Microscope and of Microscopic Perception]. Archiv für Mikroskopische Anatomie [Archive for Microscopic Anatomy] (in German); Verlag von Max Cohen & Sohn: Bonn, Germany, 1873; Vol. 9, pp 413–468. [Google Scholar]
- Greenwood A.Science at the Frontier; The National Academies Press: 1992. [Google Scholar]
- Creatore C.; Chin A. W.; Parker M. A.; Emmott S. Emergent models for artificial light-harvesting. Front. Mater. 2015, 10.3389/fmats.2015.00006. [DOI] [Google Scholar]
- Lewis N. S.; Nocera D. G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (43), 15729–15735. 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion J. J.; House R. L.; Papanikolas J. M.; Meyer T. J. Chemical approaches to artificial photosynthesis. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 15560–15564. 10.1073/pnas.1212254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. J.; Meyer G. J.; Fujita E. Molecular Approaches to the Photocatalytic Reduction of Carbon Dioxide for Solar Fuels. Acc. Chem. Res. 2009, 42, 1983–1994. 10.1021/ar9001679. [DOI] [PubMed] [Google Scholar]
- Kumar B.; Llorente M.; Froehlich J.; Dang T.; Sathrum A.; Kubiak C. P. Photochemical and Photoelectrochemical Reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. 10.1146/annurev-physchem-032511-143759. [DOI] [PubMed] [Google Scholar]
- Jean J.; Brown P. R.; Jaffe R. L.; Buonassisi T.; Bulovic V. Pathways for solar photovoltaics. Energy Environ. Sci. 2015, 8, 1200–1219. 10.1039/C4EE04073B. [DOI] [Google Scholar]
- Ko D.-H.; Tumbleston J. R.; Gadisa A.; Aryal M.; Liu Y.; Lopez R.; Samulski E. T. Light-trapping nano-structures in organic photovoltaic cells. J. Mater. Chem. 2011, 21, 16293–16303. 10.1039/c1jm12300a. [DOI] [Google Scholar]
- Guo B.; Wang Y.; Peng C.; Zhang H. L.; Luo G. P.; Le H. Q.; Gmachl C.; Sivco D. L.; Peabody M. L.; Cho A. Y. Laser-based mid-infrared reflectance imaging of biological tissues. Opt. Express 2004, 12, 208–219. 10.1364/OPEX.12.000208. [DOI] [PubMed] [Google Scholar]
- Abi Haidar D.; Leh B.; Zanello M.; Siebert R. Spectral and lifetime domain measurements of rat brain tumors. Biomed. Opt. Express 2015, 6, 1219–1233. 10.1364/BOE.6.001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloway N. T.; Choob M.; Sanz A.; Sheetz M. P.; Miller L. W.; Cornish V. W. Optimized Fluorescent Trimethoprim Derivatives for in vivo Protein Labeling. ChemBioChem 2007, 8, 767–774. 10.1002/cbic.200600414. [DOI] [PubMed] [Google Scholar]
- Nakatsuji S. Recent progress toward the exploitation of organic radical compounds with photo-responsive magnetic properties. Chem. Soc. Rev. 2004, 33, 348–353. 10.1039/b306449m. [DOI] [PubMed] [Google Scholar]
- Karalis A.; Joannopoulos J. D.; Soljačic M. Efficient wireless non-radiative mid-range energy transfer. Ann. Phys. 2008, 323, 34–48. 10.1016/j.aop.2007.04.017. [DOI] [Google Scholar]
- Haddock S. H. D.; Moline M. A.; Case J. F. Bioluminescence in the Sea. Annu. Rev. Mar. Sci. 2010, 2, 443–493. 10.1146/annurev-marine-120308-081028. [DOI] [PubMed] [Google Scholar]
- Kobayashi M.; Kikuchi D.; Okamura H. Imaging of Ultraweak Spontaneous Photon Emission from Human Body Displaying Diurnal Rhythm. PLoS One 2009, 4 (7), e6256. 10.1371/journal.pone.0006256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura H.; Kojima R.; Kamiya M.; Kobayashi E.; Komatsu T.; Ueno T.; Terai T.; Hanaoka K.; Nagano T.; Urano Y. New Class of Bioluminogenic Probe Based on Bioluminescent Enzyme-Induced Electron Transfer: BioLeT. J. Am. Chem. Soc. 2015, 137 (12), 4010–4013. 10.1021/ja511014w. [DOI] [PubMed] [Google Scholar]
- Stingl K.; Bartz-Schmidt K. U.; Besch D.; Braun A.; Bruckmann A.; Gekeler F.; Greppmaier U.; Hipp S.; Hörtdörfer G.; Kernstock C.; Koitschev A.; Kusnyerik A.; Sachs H.; Schatz A.; Stingl K. T.; Peters T.; Wilhelm B.; Zrenner E. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc. R. Soc. London, Ser. B 2013, 280, 20130077. 10.1098/rspb.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Ramirez S.; Pang P. T.; Puryear C. B.; Govindarajan A.; Deisseroth K.; Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 2012, 484, 381–385. 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson L. H.; Hottowy P.; Weiner G. A.; Dabrowski W.; Litke A. M.; Chichilnisky E. J. High-Fidelity Reproduction of Spatiotemporal Visual Signals for Retinal Prosthesis. Neuron 2014, 83 (1), 87–92. 10.1016/j.neuron.2014.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K.; Doblhammer G.; Rau R.; Vaupel J. W. Ageing populations: the challenges ahead. Lancet 2009, 374 (9696), 1196–1208. 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.; Adar S.; Selby C. P.; Lieb J. D.; Sancar A. Genome-wide analysis of human global and transcription-coupled excision repair of UV damage at single-nucleotide resolution. Genes Dev. 2015, 29, 948–960. 10.1101/gad.261271.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicuta P.; Donald A. M. Microrheology: a review of the method and applications. Soft Matter 2007, 3, 1449–1455. 10.1039/b706004c. [DOI] [PubMed] [Google Scholar]
- Kaufman L. J.; Weitz D. A. Direct imaging of repulsive and attractive colloidal glasses. J. Chem. Phys. 2006, 125, 074716. 10.1063/1.2227386. [DOI] [PubMed] [Google Scholar]
- Psaltis D.; Quake S. R.; Yang C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 2006, 442, 381–386. 10.1038/nature05060. [DOI] [PubMed] [Google Scholar]
- Sasmal D. K.; Lu H. P. Single-Molecule Patch-Clamp FRET Microscopy Studies of NMDA Receptor Ion Channel Dynamics in Living Cells: Revealing the Multiple Conformational States Associated with a Channel at Its Electrical Off State. J. Am. Chem. Soc. 2014, 136, 12998–13005. 10.1021/ja506231j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin J. M.; Berweger S.; Jones A. C.; Raschke M. B. Nano-optical imaging and spectroscopy of order, phases, and domains in complex solids. Adv. Phys. 2012, 61 (6), 745–842. 10.1080/00018732.2012.737982. [DOI] [Google Scholar]
- Thies S.; Sell H.; Schütt C.; Bornholdt C.; Näther C.; Tuczek F.; Herges R. Light-Induced Spin Change by Photodissociable External Ligands: A New Principle for Magnetic Switching of Molecules. J. Am. Chem. Soc. 2011, 133 (40), 16243–16250. 10.1021/ja206812f. [DOI] [PubMed] [Google Scholar]
- Simon D. R. On the power of quantum computation. Proceedings, 35th Annual Symposium on Foundations of Computer Science 1994, 116–123. 10.1109/SFCS.1994.365701. [DOI] [Google Scholar]
- Salikhov K. M.; Golbeck J. H.; Stehlik D. Quantum teleportation across a biological membrane by means of correlated spin pair dynamics in photosynthetic reaction centers. Appl. Magn. Reson. 2007, 31 (1–2), 237–252. 10.1007/BF03166259. [DOI] [Google Scholar]
- Wang X.-L.; Cai X.-D.; Su Z.-E.; Chen M.-C.; Wu D.; Li L.; Liu N.-L.; Lu C.-Y.; Pan J.-W. Quantum teleportation of multiple degrees of freedom of a single photon. Nature 2015, 518, 516–519. 10.1038/nature14246. [DOI] [PubMed] [Google Scholar]
- Kobr L.; Gardner D. M.; Smeigh A. L.; Dyar S. M.; Karlen S. D.; Carmieli R.; Wasielewski M. R. Fast Photo-driven Electron Spin Coherence Transfer: A Quantum Gate Based on a Spin Exchange J-Jump. J. Am. Chem. Soc. 2012, 134, 12430–12433. [DOI] [PubMed] [Google Scholar]
- Kim J. Y.; Voznyy O.; Zhitomirsky D.; Sargent E. H. 25th Anniversary Article: Colloidal Quantum Dot Materials and Devices: A Quarter-Century of Advances. Adv. Mater. 2013, 25, 4986–5010. 10.1002/adma.201301947. [DOI] [PubMed] [Google Scholar]
- https://www.newscientist.com/article/dn23591-quantum-dot-displays-make-your-tv-brighter-than-ever/.
- Safai M., Georgeson G., Method and apparatus for nondestructive corrosion detection using quantum dots, US Patent, 7925452 B2.
- Semonin O. E.; Luther J. M.; Beard M. C. Quantum dots for next-generation photovoltaics. Mater. Today 2012, 15, 508–515. 10.1016/S1369-7021(12)70220-1. [DOI] [Google Scholar]
- Nozik A. J.; Beard M. C.; Luther J. M.; Law M.; Ellingson R. J.; Johnson J. C. Semiconductor Quantum Dots and Quantum Dot Arrays and Applications of, Multiple Exciton Generation to Third-Generation Photovoltaic Solar Cells. Chem. Rev. 2010, 110, 6873–6890. 10.1021/cr900289f. [DOI] [PubMed] [Google Scholar]
- Chen L.-J.; Lin J.-D.; Lee C.-R. An optically stable and tunable quantum dot nanocrystal-embedded cholesteric liquid crystal composite laser. J. Mater. Chem. C 2014, 2, 4388–4394. 10.1039/c4tc00128a. [DOI] [Google Scholar]
- Hewa-Kasakarage N. N.; El-Khoury P. Z.; Tarnovsky A. N.; Kirsanova M.; Nemitz I.; Nemchinov A.; Zamkov M. Ultrafast carrier dynamics in type II ZnSe/CdS/ZnSe nanobarbells. ACS Nano 2010, 4, 1837–1844. 10.1021/nn100229x. [DOI] [PubMed] [Google Scholar]
- Yousif E.; Haddad R. Photodegradation and photostabilization of polymers, especially polystyrene: review. SpringerPlus 2013, 2, 398. 10.1186/2193-1801-2-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habault D.; Zhang H.; Zhao Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244–7256. 10.1039/c3cs35489j. [DOI] [PubMed] [Google Scholar]
- Carpenter S. R. Eutrophication of aquatic ecosystems: Bistability and soil phosphorus. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 10002–10005. 10.1073/pnas.0503959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrini O.; Oliveros E.; Braun A. M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. 10.1021/cr00018a003. [DOI] [Google Scholar]
- Lang M.; Stober F.; Lichtenthaler H. K. Fluorescence emission spectra of plant leaves and plant constituents. Radiat. Environ. Biophys. 1991, 30 (4), 333–347. 10.1007/BF01210517. [DOI] [PubMed] [Google Scholar]
- Gaffin D. D.; Barker T. N. Comparison of scorpion behavioral responses to UV under sunset and nighttime irradiances. J. Arachnol. 2014, 42 (1), 111–118. 10.1636/Hi12-91.1. [DOI] [Google Scholar]
- Reiher M. A Theoretical Challenge: Transition-Metal Compounds. Chimia 2009, 63, 140–145. 10.2533/chimia.2009.140. [DOI] [Google Scholar]
- Marcus R. A. On the Theory of Oxidation-Reduction Reactions Involving Electron Transfer. I. J. Chem. Phys. 1956, 24, 966. 10.1063/1.1742723. [DOI] [Google Scholar]
- Hush N. S. Distance dependence of electron transfer rates. Coord. Chem. Rev. 1985, 64, 135–157. 10.1016/0010-8545(85)80047-3. [DOI] [Google Scholar]
- Huddleston R. K.; Miller J. R. Determination of Electron-Transfer Rate Constants from Data on Tunneling to Randomly Distributed Acceptors in a Rigid Medium. J. Phys. Chem. 1982, 86, 200–203. 10.1021/j100391a014. [DOI] [Google Scholar]
- Closs G. L.; Miller J. R. Intramolecular Long-Distance Electron-Transfer in Organic-Molecules. Science 1988, 240, 440–447. 10.1126/science.240.4851.440. [DOI] [PubMed] [Google Scholar]
- Miller J. R.; Calcaterra L. T.; Closs G. L. Intramolecular Long-Distance Electron-Transfer in Radical-Anions – the Effects of Free-Energy and Solvent on the Reaction Rates. J. Am. Chem. Soc. 1984, 106, 3047–3049. 10.1021/ja00322a058. [DOI] [Google Scholar]
- A short article similar in spirit to this one was recently published in Diamonds, the seasonal newsletter of the Diamond Advance Light Source in the UK. Unfortunately the article has no byline but it can be found at the following URL: http://www.diamond.ac.uk/Home/News/LatestFeatures/27_04_15.html. Also, an unbylined editorial highlighting similar themes related to the International Year of Light appeared here:
- Francl M. The enlightenment of chemistry. Nat. Chem. 2015, 7, 761–762. 10.1038/nchem.2354. [DOI] [PubMed] [Google Scholar]
- For an interesting historical look at other advances in photochemistry, see:Roth H. D. Twentieth century developments in photochemistry. Brief historical sketches. Pure Appl. Chem. 2001, 73 (3), 395–403. 10.1351/pac200173030395. [DOI] [Google Scholar]