Abstract
The purpose of this experimental study was to evaluate the effect of chicken amniotic fluid (AF) on a cross section of rat sciatic nerves. Thirty adult male Sprague-Dawley rats weighing 275 to 300 g, were randomized into three groups treated with (1) amniotic fluid or AF (n=10), (2) normal saline or NS (n=10), and (3) sham surgery (n=10). The AF was aspirated from the amniotic cavity of incubating chick embryos at day 14. The sciatic nerve was exposed and sharply transected. Immediate epineurial repair was then performed. AF treated animals were given 2 ml/kg of the chick embryo AF subcutaneously, once daily, five times a week for up to 2 weeks. All animals were evaluated by sciatic functional index (SFI), electrophysiology, histology, and immunohistochemistry at days 28 and 56 after surgery. The SFI difference between AF and NS groups at days 21 and 28 after operation was statistically significant (P<0.05). The number of myelinated fibers in the AF group was significantly greater than that of the NS group at day 28 (P<0.05). At days 28 and 56 after operation, the nerve conduction velocity (NCV) mean of the AF group was faster than that of the NS group, but the difference was not statistically significant (P>0.05). The results of this study demonstrate that chick AF can enhance peripheral nerve regeneration.
Key Words: Amniotic fluid, Chick embryo, Nerve regeneration, Rat
Introduction
Peripheral nerves are often damaged by division, stretching, and crush. Poor outcomes may result from many factors, both intrinsic and extrinsic to the nervous system, including the type and level of injury, the presence of associated injury, the timing of surgery, and changes in the spinal cord neuron and end organ (Frostick, 1995 ▶).
As a supplement or natural medium, chick embryo amniotic fluid (AF) could support the development of two-cell mouse embryos (Esmaili and Rezazadeh Valojerdi, 2004 ▶).
AF is important for fetal health because it forms a protective sac around the fetus which prevents mechanical and thermal shocks, assists in the acid/base balance and contains nutritional growth factors and cytokines (Gitlin et al., 1972 ▶; Pitkin and Reynolds, 1975 ▶).
A number of investigators have worked on chick embryo AF. Ocampo et al. (1993) ▶ and Esmaili and Rezazadeh Valojerdi (2004) transferred preimplantation mammalian embryos into the amniotic cavity of developing chick embryos and observed better embryo growth. Conversely, Blakewood et al. (1989) ▶ did not find any significant differences between the use of aspirated AF and a control medium. Yet other investigators have reported culturing mouse embryos in human AF (Coetzee et al., 1989 ▶; Dorfmann et al., 1989 ▶) and chick embryo AF (Esmaili and Rezazadeh Valojerdi, 2004 ▶).
The amnion fluid possesses antimicrobial activity (Burdett et al., 1982 ▶). Several investigators have been interested in using natural fluids such as follicular and amniotic fluids as a source or supplement of culture medium (Coetzee et al., 1989 ▶; Dorfmann et al., 1989 ▶; Hemmings et al., 1994 ▶; Nakazawa et al., 1997 ▶; Wang et al., 1997 ▶).
Despite the availability of chicken AF, there is still controversy over the use of this fluid for mammalian embryo development (Esmaili and Rezazadeh Valojerdi, 2004 ▶) since its effects on peripheral nerves are yet to be determined. The purpose of this experimental study was thus to evaluate the effect of chicken AF on a cross section of rat sciatic nerves.
Materials and Methods
Animals
Thirty adult male Sprague-Dawley rats weighing 275 to 300 g, were randomized into three groups treated with (1) amniotic fluid or AF (n=10), (2) normal saline or NS (n=10), and (3) sham surgery (n=10). The left sciatic nerve was used as the experimental side and the right sciatic nerve as the control.
Preparation of chick embryo amniotic fluid
Fertilized chick eggs from Lohman selected white Leghorn hens were incubated at 38 ± 1°C and a humidity of 50%. The embryos were broken into a Petri dish with all membranes intact, and the AF was collected using a 1 cc syringe with a 23 gauge needle. The AF was carefully aspirated using a pulled tip glass microcapillary pipette (20 µL) from the amniotic cavity of incubating chick embryos at day 14. AF was collected from 30 chick embryos according to development stage based on Hamburger and Hamilton (1951) ▶. An average amount of 0.5 ml of AF was collected from each embryo. The aliquots were centrifuged at 500 g for 15 min and their supernatants were filtered with a 0.22 µm sterile filter (Millipore, Sigma, USA). The samples were collected and stored at 4°C for a maximum of one week (Esmaili and Rezazadeh Valojerdi, 2004 ▶) to minimize protein degradation (Mirzajani et al., 2011 ▶). None of the samples showed visible signs of contaminating red blood cells when viewed under the microscope.
Surgical procedure
Following general anesthesia with intraperitoneal ketamine (90 mg/kg) and xylazine (10 mg/kg), the left hind limb was disinfected, shaved and prepped. The sciatic nerve was exposed at the sciatic notch via a gluteal muscle splitting incision and sharply transected. Immediate epineurial repair was then performed, using two 10-0 nylon sutures. In sham operations, the left sciatic nerves were briefly exposed. The muscles were reapproximated and the wound was closed and repaired with two 10-0 nylon epineurial sutures. After nerve injury, the AF treated animals were given 2 ml/kg of the chick embryo AF subcutaneously, once a day, five times a week for up to 2 weeks. The dosage and administration of AF were based on a previous study by Corpening et al. (2000) ▶. NS treated rats received the sterile NS injection in the same manner as AF treated animals. Surgery was performed under an operating microscope. After the surgery, animals were housed in individual cages with ad libitum food and water and a cycle of 12 h light/12 h dark.
Functional tests
To evaluate the sciatic functional index (SFI), on the day prior to and the 7th, 14th, 21st, 28th, 35th, 49th, and 56 days after the operation, Indian ink was applied to the plantar surface of the hind feet to cover all anatomical regions, and the animal was allowed to walk on a paper track and leave footprints. The footprints of both operated and unoperated limbs were used to calculate SFI using the formula developed by Bain et al., 1989 ▶. A value of 0 was considered normal, whereas an SFI of -100 meant total impairment, similar to results that could be obtained by a complete transaction of the sciatic nerve (Bain et al., 1989 ▶).
Electrophysiological study
The animals in each group were subjected to electrophysiological studies using Narco bio-system (USA) at days 28 and 56 post operation. During the test, their body temperature was kept constant between 36.5-37°C using a temperature control unit (Narco, USA). Under intraperitoneally urethane anesthesia (1 g/kg), the left sciatic nerve (operated side) was re-exposed by incision of the previous surgical site in the mid-thigh level. Stimulating electrodes were placed 20 mm apart on each side of the epineurial sutures, and a recording electrode was inserted into the gastrocnemius muscle. The difference in electromyography latency, amplitude and distance between proximal and distal stimulation sites was measured to calculate conduction velocity (Farjah et al., 2014 ▶).
Histological examination
At days 28 and 56 post operation, following electrophysiological assessment, the animals were sacrificed and 4 mm sections of the sciatic nerves, distal from the epineurial suture site, were removed and immediately fixed in 2.5% glutaraldehyde. The grafts were then embedded in paraplast paraffin, cut in 5 µm, and stained with toluidine blue. Morphometric analysis was performed using an image analyzing software (OLYSIA Biorefort, Olympus, Japan).
Immunohistochemistry
At days 28 and 56 post operation, anti S-100 (Dako, 1:200 dilution) was used as a myelin sheath marker for all groups. Briefly, specimens were post-fixed in 4% paraformaldehyde for 2 h. The tissue samples were embedded in paraffin and cut into 5 µm thick sections. According to the instructions of immunohistochemical staining kits, non-specific immunoreactions were blocked; sections were incubated in S-100 protein antibody solutions for 1 h at room temperature,washed three times with PBS and incubated in biotinylated anti-mouse, rabbit IgG solution for 1 h. Secondary antibody (horseradish peroxidase) solution was added to the sections using the diaminobenzidine method. Immuno-histochemical results were analyzed qualitatively using positive, more positive and clearly more positive terms (Choi et al., 2005 ▶).
Statistical analysis
Statistical analyses were carried out using a mixed-design (within and between groups comparisons). ANOVAs were computed with 95% confidence intervals using SPSS software (version 16.0). A post-hoc study was carried out to examine any significant differences between the groups. All data are presented as means ± SEM and P<0.05 was considered to be statistically significant.
Results
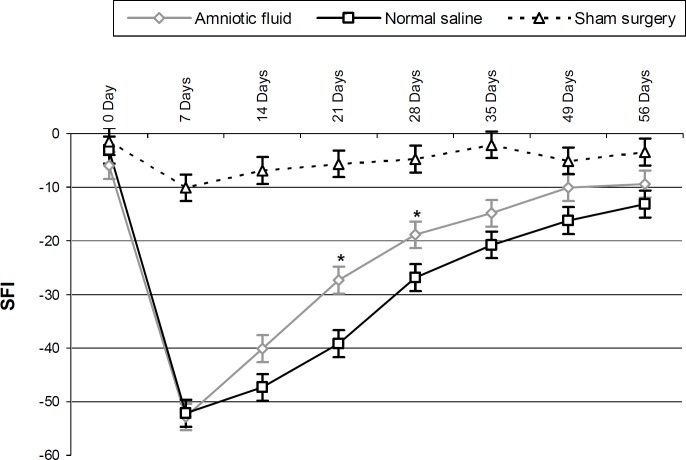
There were no post-operative deaths and no clinical evidence of wound infections was observed. SFI greatly decreased for AF and normal saline groups at day 7 after the operation. SFI improved from the first to the last evaluation in the experimental groups, at days 21 and 28 after operation, and the difference between AF and NS groups was statistically significant (P<0.05). In addition, the AF and normal saline groups were statistically different from the sham group (P<0.05). No statistically significant differences were found, however, between the AF and normal saline groups on day 56 post operation (P>0.05) (Fig. 1).
Fig. 1.
SFI before and after nerve injury in AF, normal saline, and sham surgery groups. * Difference between AF treated and normal saline groups (P<0.05, t-test). Results presented as means ± SEM
At days 28 and 56 post operation, the mean NCV of the AF group was faster than the NS group, but the difference was not statistically significant (P>0.05). The results of the electrophysiological study are presented in Table 1.
Table 1.
Comparison of NCV and AMP in each group at days 28 and 56 post operation
| Group | NCV (m/S) | AMP (mV) | ||
|---|---|---|---|---|
| 28cd | 56th | 28cd | 56th | |
| AF | 22.56±3.76 | 38.23±4.47 | 4.85±1.11 | 6.95±1.88 |
| NS | 16.01±2.98 | 34.01±3.82 | 3.69±1.88 | 6.21±1.28 |
| Sham surgery | 44.15±4.76* | 45.07±3.29* | 9.25±1.37* | 9.34±1.68* |
P<0.05, the difference between control/sham surgery and AF/NS groups were significant. The difference between AF and NS groups were not significant (P>0.05, One-Way-ANOVA). Results are means ± SEM
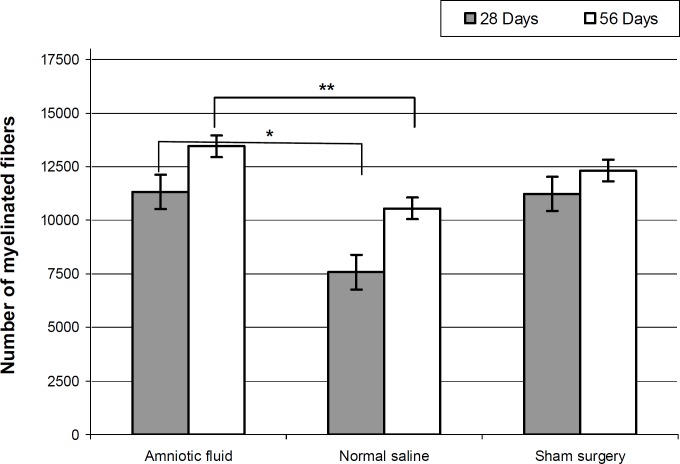
At post operation days 28 and 56, mean diameters (µm) of the regenerated myelinated fibers were 9.45 ±3.14, 13.81 ± 5.64 for the AF group, and 4.41 ± 2.12, 10.27 ± 3.45 for the normal saline group. Statistically significant differences were found between AF and NS at day 28 after the operation (P>0.05). The number of myelinated fibers in the AF group was significantly larger than that of the NS group (P<0.05) at day 28, but no significant difference was found at the 56th day (P<0.05) (Fig. 2).
Fig. 2.
Total number of regenerated myelinated nerve fibers after sciatic injury (n=5 on day 28 and n=5 on day 56 for each group). * The difference between amniotic fluid treated and normal saline groups at days 28 and ** 56 after operation, (P<0.05, One-Way-ANOVA). Results are presented as means ± SEM
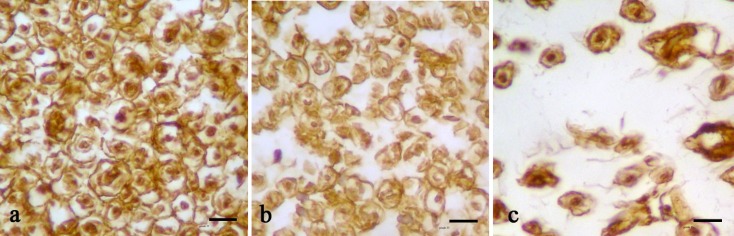
At day 56 after the operation, the AF group S-100 protein expression areas were extensively observed in the cross sections from the midpoint, indicating the existence of Schwann cells around the myelinated axons. In the AF group, the structure of regenerated axons was more similar to those of a normal nerve compared to the NS group (Fig. 3).
Fig. 3.
Immunohistochemical analysis of cross sections to the main axis of the regenerated nerve at day 56 after operation distal from the transected site of the sham surgery group (a), AF treated groups (b), and NM group (c). Positive staining of the myelin sheath-associated protein S-100 was observed. Regenerated nerve fibers containing Schwann cells, blood vessels, and myelinated axons throughout the tissue were present (scale bar 20 µm
Discussion
In the present study, we evaluated chick AF administration for sciatic nerve regeneration in adult rats. The results demonstrated that chick AF significantly enhanced peripheral nerve regeneration in vivo. To the best of our knowledge, this is the first study to examine the effect of chick AF on sciatic nerve regeneration. The enhancement of peripheral nerve regeneration in the AF group of this study is in agreement with the results reported earlier by Ozgenel and Filiz (2003) ▶ who showed that human AF improved peripheral nerve regeneration in rats (Ozgenel and Filiz, 2003 ▶). According to Esmaili and Rezazadeh Valojerdi (2004), AF acts as a supplement or a natural medium that supports the development of embryos.
In the present study, histological and functional results revealed that regeneration in the AF treatment group was superior to the NM group. Nevertheless, the NCV difference between the experimental groups was not statistically significant. NCV is dependent on axon diameter, myelination, and intermodal distance and determines the fastest conducting nerve fibers (Brown et al., 1991 ▶). Despite the damage caused to a large number of remaining fibers, a nerve may still have a few fibers that conduct very well. For this reason, nerve conduction velocity may evaluate the fastest and perhaps healthiest fibers rather than total nerve function (Kanaya et al., 1996 ▶).
The remarkable finding of this study was the accelerating effect of chick AF administration on axonal regeneration in rats. Chick AF can be obtained in large quantities and is inexpensive, sterilized, and easily stored. In addition, several growth factors have been previously discovered in the AF including nerve growth factor (NGF) (Chen et al., 2004 ▶), insulin like growth factors (IGFs) (Karcher et al., 2005 ▶), vascular endothelial growth factor (VEGF) (Burdett et al., 1982 ▶), and transforming growth factor-β1 (TGF-β1) (Corpening et al., 2000 ▶).
In this study, AF was collected from chick embryos at day 14 because of the peak in NGF concentration at days 15 and 16 (Mashayekhi et al., 2011b ▶), and IGF-I concentration at day 14 in the embryonic chicken (Scans et al., 1997 ▶). In addition, AF concentrations of TGF-β1 and VEGF increase from days 6 to 15 (Mirzajani et al., 2011 ▶) and days 6 to 11 (Mashayekhi et al., 2011a ▶), respectively.
NGF is an important growth factor in cerebral cortical development due to the fact that it stimulates neuronal precursor cell proliferation (Mashayekhi and Salehi, 2007 ▶). NGF, originally identified as a neurite promoting factor in peripheral sensory and sympathetic neurons, and has been shown to function in the central nervous system (Chiaretti et al., 2008 ▶). The biological function of NGF is the maintenance and survival of the nervous system (Mashayekhi et al., 2011b ▶).
While IGF-I plays an important role in the regulation of mammalian growth (Ballard et al., 1990 ▶), TGF-β1 regulates the differentiation of neuronal, immune, mesenchymal and epithelial cell types (Massague et al., 2000 ▶). The importance of TGF-β1 signaling has been demonstrated in vascular morphogenesis (Pardali et al., 2010 ▶). During peripheral nerve regeneration, TGF-β1 up-regulates the beta fibroblast growth factor expression in the anterior horn motoneurons of the spinal cord (Pei et al., 2005 ▶). VEGF administration has also been shown to support and enhance the growth of regenerating nerve fibers, probably through a combination of endogenous, neurotrophic and neuroprotective effects (Pereira et al., 2011 ▶).
The mechanism of rat peripheral nerve regeneration caused by chick AF is unclear. Human, rat and chicken NGFs have been demonstrated to possess very similar biological activities (Ibanez et al., 1991 ▶). Chicken IGF-IR is 85% identical to that of humans (Holzenberger et al., 1996 ▶) and IGF-II is 60% identical to humans and bovine (Zhou et al., 1995 ▶). In addition, the AF IGF-I concentration is approximately 6 times more than that of plasma (Schmidek et al., 2001 ▶).
In conclusion, the present study shows that compared to normal saline, treatment with chick AF can better increase rat peripheral nerve regeneration.
Acknowledgements
This study was supported by the Urmia University of Medical Sciences (contract No. 1091). The authors thank all laboratory staff in cellular and molecular research center, School of Medicine, Urmia University of Medical Sciences.
References
- Bain, JR, Mackinnon, SE, Hunter, DA. Func-tional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr. Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- Ballard, FJ, Johnson, RJ, Owens, PC, Francis, GL, Upton, FM, McMurtry, JP, Wallace, JC. Chicken insulin-like growth factor-I: amino acid sequence, radio-immunoassay, and plasma levels between strains and during growth. Gen. Comp. Endocrinol. 1990;79:459–468. doi: 10.1016/0016-6480(90)90076-x. [DOI] [PubMed] [Google Scholar]
- Blakewood, EG, Jaynes, JM, Johnson, WA, Godke, RA. Using the amniotic cavity of the developing chick embryo for the in vivo culture of early-stage mammalian embryos. Poult. Sci. 1989;68:1695–1702. doi: 10.3382/ps.0681695. [DOI] [PubMed] [Google Scholar]
- Brown, CJ, Evans, PJ, Mackinnon, SE, Bain, JR, Makino, AP, Hare, G. Inter- and intraobserver reliability of walking-track analysis used to assess sciatic nerve function in rats. Microsurgery. 1991;12:76–79. doi: 10.1002/micr.1920120204. [DOI] [PubMed] [Google Scholar]
- Burdett, P, Lizana, J, Eneroth, P, Bremme, K. Proteins of human amniotic fluid. II. Mapping by two-dimensional electrophoresis. Clin. Chem. 1982;28:935–940. [PubMed] [Google Scholar]
- Chen, LH, Li, XB, Xiong, YL. Effects of a nerve growth factor isolated and purified from the venom of Naja naja atra on injured sciatic nerve in the adult cat. J. Sichuan Univ. 2004;35:194–197 . [PubMed] [Google Scholar]
- Chiaretti, A, Antonelli, A, Mastrangelo, A, Pezzotti, P, Tortorolo, L, Tosi, F, Genovese, O. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J. Neurotrauma. 2008;25:225–234. doi: 10.1089/neu.2007.0405. [DOI] [PubMed] [Google Scholar]
- Choi, BH, Ahu, SJ, Kim, BY, Huh, JH, Lee, SH, Jung, JH. Transplantation of cultured bone marrow stromal cells to improve peripheral nerve regeneration. Int. J. Oral Maxillofac Surg. 2005;34:537–542. doi: 10.1016/j.ijom.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Coetzee, JC, Sevenster, CB, Fourie, FL, van der Merwe, JV, Helberg, LA. In vitro culture of mouse embryos in human amniotic fluid. S. Afr. Med. J. 1989;76:62–63. [PubMed] [Google Scholar]
- Corpening, JW, Doerr, JC, Kristal, MB. Ingested bovine amniotic fluid enhances morphine antinociception in rats. Physiol. Behav. 2000;70:15–18. doi: 10.1016/s0031-9384(00)00244-4. [DOI] [PubMed] [Google Scholar]
- Dorfmann, AD, Bender, SD, Robinson, P, Fugger, EF, Bustillo, M, Reed, G, Schulman, JD. Cell-free human amniotic fluid as culture medium for mouse and human embryos. Fertil. Steril. 1989;51:671–674. doi: 10.1016/s0015-0282(16)60619-2. [DOI] [PubMed] [Google Scholar]
- Esmaili, F, Rezazadeh Valojerdi, M. Effect of six- and ten-day-old chick embryo amniotic fluid on develop-ment of two-cell mouse embryos. Exp. Anim. 2004;53:453–456. doi: 10.1538/expanim.53.453. [DOI] [PubMed] [Google Scholar]
- Farjah, GhH, Peirouvi, T, Heshmatian, B, Yasami, M, Dolatkhah, MA. The effect of short-term treatment of a gonadotropin-releasing hormone analog (buserelin) on sciatic nerve regeneration. Iranian J. Vet. Res. 2014;15:104–109. [Google Scholar]
- Frostick, SP. The physiology and metabolic con-sequence of muscle denervation. Int. Angiol. 1995;14:278–287. [PubMed] [Google Scholar]
- Gitlin, D, Kumate, J, Morales, C, Noriega, L, Arevalo, N. The turnover of amniotic fluid protein in the human conceptus. Am. J. Obstet. Gynecol. 1972;113:632–645. doi: 10.1016/0002-9378(72)90632-1. [DOI] [PubMed] [Google Scholar]
- Hamburger, V, Hamilton, HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hemmings, R, Lachapelle, MH, Falcone, T, Miron, P, Ward, L, Guyda, H. Effects of follicular fluid supplementation on the in vitro development of human pre-embryos. Fertil. Steril. 1994;62:1018–1021. doi: 10.1016/s0015-0282(16)57067-8. [DOI] [PubMed] [Google Scholar]
- Holzenberger, M, Lapointe, F, Leibovici, M, Lievre, CA. The avian IGF type 1 receptor: cDNA analysis and in situ hybridization reveal conserved sequence elements and expression patterns relevant for the development of the nervous system. Brain Res. Dev. Brain Res. 1996;97:76–87. doi: 10.1016/s0165-3806(96)00133-2. [DOI] [PubMed] [Google Scholar]
- Ibanez, C, Hallbook, F, Soderstrom, S, Ebendal, T, Persson, H. Biological and immunological pro-perties of recombinant human, rat, and chicken nerve growth factors: a comparative study. J. Neurochem. 1991;57:1033–1041. doi: 10.1111/j.1471-4159.1991.tb08254.x. [DOI] [PubMed] [Google Scholar]
- Kanaya, F, Firrell, JC, Breidenbach, WC. Sciatic functional index, nerve conduction tests, muscle con-traction, and axon morphometry as indicators of regeneration. Plast. Reconstr. Surg. 1996;98:1264–1271. doi: 10.1097/00006534-199612000-00023. [DOI] [PubMed] [Google Scholar]
- Karcher, DM, McMurtry, JP, Applegate, TJ. Developmental changes in amniotic and allantoic fluid insulin-like growth factor (IGF)-I and -II concentrations of avian embryos. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005;142:404–409. doi: 10.1016/j.cbpa.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Mashayekhi, F, Dianati, E, Masomi Moghadam, L. Quantitative analysis of nerve growth factor in the amniotic fluid during chick embryonic development. Saudi J. Biol. Res. 2011b;18:209–212. doi: 10.1016/j.sjbs.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi, F, Salehi, Z. Infusion of anti-nerve growth factor into the cisternum magnum of chick embryo leads to decrease cell production in the cerebral cortical germinal epithelium. Eur. J. Neurol. 2007;14:181–186. doi: 10.1111/j.1468-1331.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Mashayekhi, F, Zahiri, S, Mirzajani, E, Nickpay A. Developmental changes in amniotic fluid vascular endothelial growth factor levels of chick embryo. Ann. Biol. Res. 2011a;2:94–99. [Google Scholar]
- Massague, J, Blain, SW, Lo, RS. TGF beta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Mirzajani, E, Dejhagah, S, Mashayekhi, F, Nikpay, A, Siyam, S. Amniotic fluid TGF-β1 concentration during chick embryonic development. Ann. Biol. Res. 2011;2:185–190. [Google Scholar]
- Nakazawa, T, Ohashi, K, Yamada, M, Shinoda, S, Saji, F, Mura, Y, Araki, H. Effect of different con-centrations of amino acids in human serum and follicular fluid on the development of one-cell mouse embryos in vitro. J. Reprod. Fertil. 1997;111:327–332. doi: 10.1530/jrf.0.1110327. [DOI] [PubMed] [Google Scholar]
- Ocampo, MB, Ocampo, LC, Suzuki, K, Mori, T, Ueda, J, Shimizu, H, Kanagawa, H. Development to the blastocyst stage of pig embryos cultured in the amniotic fluid of developing chick embryos. J. Vet. Med. Sci. 1993;55:889–891. doi: 10.1292/jvms.55.889. [DOI] [PubMed] [Google Scholar]
- Ozgenel, GY, Filiz, G. Effects of human amniotic fluid on peripheral nerve scarring and regeneration in rats. J. Neurosurg. 2003;98:371–377. doi: 10.3171/jns.2003.98.2.0371. [DOI] [PubMed] [Google Scholar]
- Pardali, E, Goumans, MJ, ten Dijke, P. Signaling by members of the TGFbeta family in vascular mor-phogenesis. Trends Cell Biol. 2010;20:556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Pei, YY, Duan, SB, Cai, WJ, Yi, XN, Zeng, ZC, Zhang, JW, Xu, YZ, Zou, QY, Wen, XD. Effects of transforming growth factor-beta 1 on the peripheral nerve regeneration of rats. J. Cent. South. Univ. 2005;30:447–451. [PubMed] [Google Scholar]
- Pereira Lopes, FR, Lisboa, BC, Frattini, F, Almeida, FM, Tomaz, MA, Matsumoto, PK, Langone, F, Lora, S, Melo, PA, Borojective, R, Han, SW, Martinez, MB. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol. Appl. Neurobiol. 2011;37:600–612. doi: 10.1111/j.1365-2990.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- Pitkin, RM, Reynolds, WA. Fetal ingestion and metabolism of amniotic fluid protein. Am. J. Obstet. Gynecol. 1975;123:356–363. doi: 10.1016/s0002-9378(16)33436-6. [DOI] [PubMed] [Google Scholar]
- Scans, CG, Thommes, RC, Radecki, SV, Buonomo, FC, Woods, JE. Ontogenic changes in the circulating concentrations of insulin-like growth factor (IGF)-I, and -II, and IGF-binding proteins in the chicken embryo. Gen. Comp. Endocrinol. 1997;106:265–270. doi: 10.1006/gcen.1997.6876. [DOI] [PubMed] [Google Scholar]
- Schmidek, A, Hare, T, Milakofsky, L, Nibbio, B, Epple, A. Insulin-like growth factor-I affects amino compounds in the fluids of the chicken embryo. Gen. Comp. Endocrinol. 2001;123:235–243. doi: 10.1006/gcen.2001.7650. [DOI] [PubMed] [Google Scholar]
- Wang, WH, Abeydeera, LR, Cantley, TC, Day, BN. Effects of oocyte maturation media on development of pig embryos produced by in vitro fertilization. J. Reprod. Fertil. 1997;111:101–108. doi: 10.1530/jrf.0.1110101. [DOI] [PubMed] [Google Scholar]
- Zhou, M, Ma, Z, Sly, WS. Cloning and expression of the cDNA of chicken cation-independent mannose-6-phosphate receptor. Proc. Natl. Acad. Sci. USA. 1995;92:9762–9766. doi: 10.1073/pnas.92.21.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]