Abstract
Importance
It remains unclear whether telemonitoring approaches provide benefits for patients with heart failure (HF) after hospitalization.
Objective
To evaluate the effectiveness of a care transition intervention using remote patient monitoring in reducing 180-day all-cause readmissions among a broad population of older adults hospitalized with HF.
Design, Setting, and Participants
We randomized 1437 patients hospitalized for HF between October 12, 2011, and September 30, 2013, to the intervention arm (715 patients) or to the usual care arm (722 patients) of the Better Effectiveness After Transition–Heart Failure (BEAT-HF) study and observed them for 180 days. The dates of our study analysis were March 30, 2014, to October 1, 2015. The setting was 6 academic medical centers in California. Participants were hospitalized individuals 50 years or older who received active treatment for decompensated HF.
Interventions
The intervention combined health coaching telephone calls and telemonitoring. Telemonitoring used electronic equipment that collected daily information about blood pressure, heart rate, symptoms, and weight. Centralized registered nurses conducted telemonitoring reviews, protocolized actions, and telephone calls.
Main outcomes and measures
The primary outcome was readmission for any cause within 180 days after discharge. Secondary outcomes were all-cause readmission within 30 days, all-cause mortality at 30 and 180 days, and quality of life at 30 and 180 days.
Results
Among 1437 participants, the median age was 73 years. Overall, 46.2% (664 of 1437) were female, and 22.0% (316 of 1437) were African American. The intervention and usual care groups did not differ significantly in readmissions for any cause 180 days after discharge, which occurred in 50.8% (363 of 715) and 49.2% (355 of 722) of patients, respectively (adjusted hazard ratio, 1.03; 95% CI, 0.88-1.20; P = .74). In secondary analyses, there were no significant differences in 30-day readmission or 180-day mortality, but there was a significant difference in 180-day quality of life between the intervention and usual care groups. No adverse events were reported.
Conclusions and Relevance
Among patients hospitalized for HF, combined health coaching telephone calls and telemonitoring did not reduce 180-day readmissions.
Trial Registration
clinicaltrials.gov Identifier: NCT01360203
Heart failure (HF) is a prevalent condition in the United States, affecting 5.8 million patients,1 and is associated with high hospitalization and readmission rates, mortality, and cost of care.1-6 For patients with HF, discontinuities and lack of post-acute care monitoring can increase overall health care resource use through readmissions or worsened morbidity.7,8 Persistently high readmission rates for patients with HF suggest that further improvements to existing care transition approaches are needed,1,9 as evidenced by the readmission-related financial penalties of approximately $428 million affecting 2610 hospitals in the third year of the Centers for Medicare & Medicaid Services Hospital Readmission Reduction Program.10
Interventions to improve the care transition process have been shown to reduce readmissions while potentially improving morbidity and mortality in randomized clinical trials (RCTs),11-14 particularly for patients with HF.15 However, many of these interventions were tested in single centers with limited numbers of patients. Moreover, sustainability of research-derived care transition approaches is difficult, with many requiring intensive in-person interactions that are not always acceptable to patients16,17 and incurring costs to health professional organizations that may not be favorable under current health care financing arrangements.18 Telehealth technology, including mobile health and remote patient monitoring technologies, potentially provides more cost-effective solutions to the problems of financial viability and home visit acceptability by substituting for in-person interactions. However, its effectiveness to date (particularly in patients with HF) has been mixed. The largest RCT in the United States to date in this area, Telemonitoring to Improve Heart Failure Outcomes, did not show any significant benefit from its telehealth approach,19 perhaps because of the type of technology used, low adherence rates, lack of patient engagement before discharge, or handling of values that exceeded threshold variables.19,20 Another large RCT in Europe with high adherence rates and improved technology also showed no significant benefit.21 However, systematic reviews that include these studies continue to suggest significant reductions in mortality, morbidity, and HF-related hospitalizations.22-24
The objective of the Better Effectiveness After Transition–Heart Failure (BEAT-HF) study was to evaluate the effectiveness of a care transition intervention using remote patient monitoring in reducing 180-day all-cause readmissions among a broad population of older adults hospitalized with HF. It was designed to address issues identified with the Telemonitoring to Improve Heart Failure Outcomes RCT, including using newer remote monitoring approaches, engaging patients before discharge, and pairing remote monitoring with a telephone-based nurse care manager via scheduled contacts similar to in-person care transition programs.
Methods
Study Design
The BEAT-HF study was a prospective, 2-arm (with a 1:1 randomization) multicenter RCT conducted at 6 academic medical centers in California to compare usual care with a telehealth-based care transition intervention for older patients who are discharged home after inpatient treatment for decompensated HF.25 Five of the sites are part of the University of California system, including the University of California in Davis, Irvine, Los Angeles, San Diego, and San Francisco. The sixth location is Cedars-Sinai Medical Center in Los Angeles, which has a mixed-model medical staff that includes full-time faculty, a multispecialty group practice, and many independent private physicians. Three of the sites are major heart transplant centers, and an additional 3 serve as safety-net hospitals for their respective regions. Block randomization was conducted within each site using random blocks of 4 to 8 individuals via a web-based, computerized, random number generator. The study was approved by the University of California, Los Angeles (UCLA) institutional review board, and all other study institutions were subject to the UCLA institutional review board review. A data and safety monitoring board was convened for the study and reviewed data during the study enrollment period. The study was registered at clinicaltrials.gov (NCT01360203). The full study protocol can be found in the Supplement.
Patient Population
Individuals admitted as hospital inpatients or on observation status were eligible if they were 50 years or older, were receiving active treatment for decompensated HF (defined as HF with the initiation of or an increase in diuretic treatment), were expected to be discharged to their home, and were capable of providing written informed consent in English, Spanish, Farsi, or Russian. Enrollment criteria were expanded in January 2012 to include all patients being actively treated for HF instead of just those having a principal diagnosis of HF. This change was made because patients deemed prospectively as not having a principal diagnosis of HF were being coded as patients with HF after their discharge because of patients with multiple active problems.
The study exclusions can be grouped into 3 main categories.25 First were patients who did not have the cognitive or physical ability (eg, dementia or weight >204 kg) or access to resources (eg, working telephone or usual source of care) required to participate fully in the BEAT-HF intervention. Second were patients already in a system of care providing more health professional contacts than the planned intervention (eg, living in a skilled nursing facility, receiving chronic hemodialysis, or awaiting or having received an organ transplant). Third were patients whose HF was due to acardiovascular condition that was expected to improve because of medical intervention (eg, percutaneous coronary intervention or interventional valve procedure during hospitalization).
Intervention
The intervention consisted of the following 3 components conducted by registered nurses: predischarge HF education, regularly scheduled telephone coaching, and home telemonitoring of weight, blood pressure, heart rate, and symptoms.25 The predischarge health education was conducted by a study nurse who was not part of the usual care team. The nurse guided patients through a booklet developed for patients with low health literacy that covered an explanation of HF, medication adherence, salt avoidance, fluid monitoring, exercising with HF, and daily checkup of weight and edema, as well as when to call the HF treatment team.26 The study nurse used the “teach-back” method to ensure patient understanding.27,28 The predischarge education also included a demonstration of how to use the remote home telemonitoring equipment and an explanation of why monitoring physiological variables is important for patients.
The electronic equipment (Bluetooth enabled; Bluetooth SIG, Inc) consisted of a wireless transmission pod, a weight scale, and a blood pressure and heart rate monitor integrated with a device that could display text questions and send simple text responses. Devices automatically transmitted data back to central servers for telemonitoring review by telephone call center study nurses based at the primary study site.
Intervention patients were scheduled to receive 9 telephone coaching calls over a 6-month period, generally from the same call center nurse, who had access to patients' medical histories and medication records. The nurse first contacted each enrolled patient 2 or 3 days after discharge from the hospital to reinforce the predischarge health coaching topics. Subsequent telephone nurse coaching then occurred on a weekly basis during the first month after discharge. After the first month, nurse coaching telephone calls were made monthly until the end of the 6-month study period. All telephone calls covered content reinforcing the predischarge education materials. Intervention patients were asked to use the telemonitoring equipment daily to transmit their weight, blood pressure, heart rate, and responses to 3 symptom questions, which were sent via cellular bandwidth to a secure server and were accessed daily by the telephone call center nurses. Readings that exceeded predetermined threshold variables generated a trigger for the telephone call center nurse to telephone the patient to investigate potential causes. When symptoms were concerning, patients were encouraged to contact their health professionals, although these individuals were also notified by the telephone call center nurses. If deemed necessary, the telephone call center nurses advised patients to call 911 or go to their nearest hospital emergency department. Telephone call center nurses also called patients who had stopped transmitting data to determine the reason and encourage the patient to resume daily monitoring.
Usual care at the sites included robust predischarge education and often a postdischarge follow-up telephone call.29 No additional surveillance was provided to control patients beyond whatever may have been requested as part of routine clinical practice, and the intervention did not substitute for usual care surveillance. Patients were not precluded from exposure to other readmission reduction or chronic disease management programs implemented by hospitals, physician groups, or health plans, such as education about HF, pharmacist consultation, and postdischarge telephone calls.
For all participants, enrollment nurses conducted baseline surveys via in-person interviews before randomization. On completion, patients were randomized via the web-based enrollment software, with randomization notification provided by the enrollment nurse. All participants were contacted for survey interviews at 7 days, 30 days, and 180 days after discharge by staff at the coordinating center who were unaware of the treatment randomizations. During these telephone interviews, information was collected about quality of life, satisfaction with care, and use of medications.
Outcome Measures
The primary outcome measure was 180-day all-cause readmission. Secondary outcomes reported herein include 30-day all-cause readmission, 30-day mortality, and 180-day mortality.25 Readmissions were identified from participating sites' hospitalization data, combined with California's inpatient discharge data for hospitalizations at nonstudy sites obtained from the California Department of Public Health Office of Statewide Health Planning and Development. Mortality was assessed using the Social Security and National Death Index, hospital data systems, contact with family members, and searches of obituaries.30 Quality of life was measured using the Minnesota Living With Heart Failure Questionnaire conducted via computer-assisted telephone interview.30
Statistical Analysis
Our sample size provided 80% power to detect a relative reduction of 28% in the primary outcome of 180-day readmission with a type I error of 0.05 after adjusting for within-hospital clustering. We conducted unadjusted intent-to-treat analyses. Individuals who had fully withdrawn consent were censored on the date of withdrawal in hazard models for the primary outcome and for secondary outcomes related to readmission and mortality. We conducted unadjusted analyses, followed by prespecified multivariable analyses to adjust for patient characteristics that may have been unequally distributed across treatment groups and may have influenced outcomes. These multivariable analyses include logistic regression models for readmission and mortality analyses. Models controlled for age, sex, race/ethnicity, insurance, comorbidities based on the Health Care Utilization Project methods,6 year and quarter of enrollment, social isolation as measured by the Lubben Social Network Scale score,31 and income level. Enrollment site was controlled for using random effects. Models also controlled for baseline quality-of-life scores in quality-of-life score analyses. Quality-of-life 30-day and 180-day analyses were only conducted for those individuals who reported quality-of-life data at the analyzed time point. After adjusting for days alive, adherence was measured in each measurement period separately for health coaching telephone calls (as the percentage of protocol-required calls that were completed) and for telemonitoring (as the percentage of days transmitting any type of data using telemonitoring).
Results
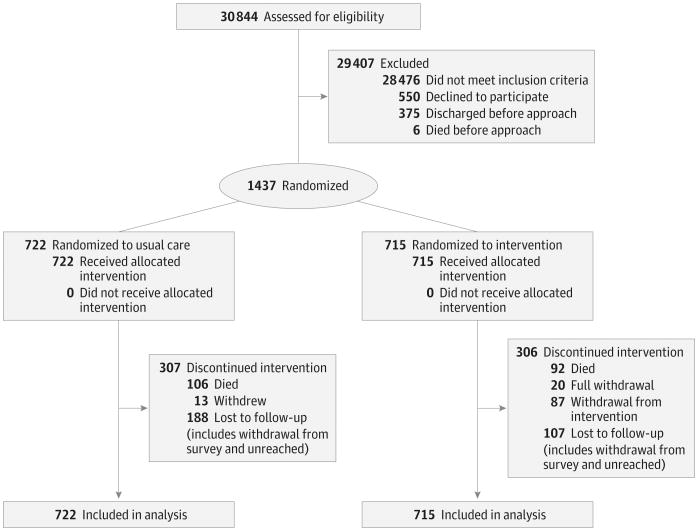
We assessed 30 844 individuals between October 12, 2011, and September 30, 2013, for study eligibility (Figure 1). Of these individuals, 28 476 did not meet inclusion criteria, including 18 005 patients without decompensated HF, 1383 transplant patients or candidates, and 1122 hemodialysis patients. Another 550 individuals declined to participate, and 381 individuals were discharged or died before they could be approached regarding the study. In total, 1437 patients were enrolled and randomized in the study, with 715 randomized to the intervention and 722 randomized to usual care. Thirty-three participants (20 intervention and 13 usual care) completely withdrew from the study. No individuals withdrew because of adverse events. There were no significant differences in participant characteristics (Table 1 and Table 2) between intervention and control participants. The median age of participants was 73 years. In total, 46.2% (664 of 1437) were female, 22.0% (316 of 1437) were African American, and 61.2% (880 of 1437) had a New York Heart Association classification of III or IV during their enrollment hospitalization.
Figure 1. BEAT-HF CONSORT Flow Diagram.
BEAT-HF indicates Better Effectiveness After Transition–Heart Failure; CONSORT, Consolidated Standards of Reporting Trials.
Table 1. Baseline Demographic Characteristics.
| Variable | Intervention | Usual Care |
|---|---|---|
| Age, median (interquartile range), y | 73 (62-84) | 74 (63-82) |
| Sociodemographics, Mean % (95% CI) | ||
| Female sex | 46.6 (42.9-50.2) | 47.1 (42.8-51.4) |
| Race/ethnicity | ||
| African American | 21.5 (18.5-24.5) | 22.7 (19.6-25.8) |
| Hispanic/Latino | 12.0 (9.6-14.3) | 10.9 (8.6-13.1) |
| White | 54.7 (51.0-58.4) | 54.3 (50.7-58.0) |
| Asian/Pacific Islander or other | 11.8 (9.4-14.2) | 12.1 (9.7-14.5) |
| Insurance | ||
| Private and other | 18.4 (15.5-21.3) | 17.6 (14.8-20.4) |
| Medicaid | 10.0 (7.7-12.2) | 10.4 (8.1-12.7) |
| Medicare | 44.9 (41.1-48.7) | 45.3 (41.6-49.0) |
| Medicare and Medicaid | 26.7 (23.3-30.0) | 26.7 (23.4-30.0) |
| Income, $ | ||
| <25 000 | 31.3 (27.8-34.7) | 31.7 (28.3-35.2) |
| 25 000 to 50 000 | 19.6 (16.7-22.5) | 20.9 (17.9-23.9) |
| >50 000 to 75 000 | 11.1 (8.8-13.4) | 12.1 (9.7-14.5) |
| >75 000 | 17.9 (15.1-20.7) | 13.1 (10.6-15.6) |
| Refused to answer or did not know | 20.2 (17.2-23.1) | 22.2 (19.1-25.3) |
| Social isolation | 21.4 (18.4-24.5) | 21.1 (18.0-24.1) |
Table 2. Baseline Comorbidities, Heart Failure Severity, and Discharge Medications.
| Intervention | Usual Care | |
|---|---|---|
| Comorbidities, Mean % (95% CI) | ||
| Valvular disease | 36.2 (32.6-39.9) | 34.3 (30.8-37.9) |
| Pulmonary circulation disease | 23.9 (20.6-27.0) | 22.9 (19.8-26.1) |
| Peripheral vascular disease | 13.3 (10.7-15.8) | 11.5 (9.2-13.9) |
| Other neurological disorder | 5.7 (4.0-7.5) | 5.9 (4.2-7.7) |
| Chronic pulmonary disease | 32.4 (28.9-35.9) | 32.5 (29.0-36.0) |
| Diabetes mellitus | ||
| Without chronic complications | 34.0 (30.4-37.6) | 35.8 (32.2-39.4) |
| With chronic complications | 10.8 (8.4-13.1) | 11.8 (9.4-14.2) |
| Hypothyroidism | 20.8 (17.7-23.8) | 20.3 (17.3-23.4) |
| Renal failure | 39.0 (35.3-42.7) | 42.7 (39.0-46.4) |
| Liver disease | 7.1 (5.1-9.0) | 5.6 (3.9-7.3) |
| Rheumatoid arthritis or collagen vascular disease | 4.6 (3.0-6.1) | 3.6 (2.2-5.0) |
| Obesity | 17.1 (14.2-19.9) | 16.5 (13.7-19.2) |
| Deficiency anemia | 34.0 (30.4-37.6) | 32.0 (28.6-35.5) |
| Depression | 10.6 (8.3-12.9) | 11.1 (8.8-13.5) |
| Hypertension | 81.7 (78.8-84.7) | 80.1 (77.1-83.1) |
| Heart Failure Severity, Mean % (95% CI) | ||
| Ejection fraction | 42.7 (41.3-44.3) | 43.0 (41.6-44.3) |
| New York Heart Association classification | ||
| I | 0.2 (0.0-0.5) | 0.7 (0.0-1.4) |
| II | 23.4 (20.0-26.9) | 25.8 (22.2-29.4) |
| III | 65.6 (61.8-69.4) | 63.9 (59.9-67.8) |
| IV | 10.8 (8.3-13.3) | 9.6 (7.2-12.0) |
| Discharge Medications, Mean % (95% CI) | ||
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 56.6 (52.8-60.3) | 54.6 (50.9-58.4) |
| β-Blocker | 73.2 (69.8-76.5) | 76.1 (72.9-79.4) |
| Digoxin | 16.7 (13.9-19.6) | 17.3 (14.5-20.2) |
| Loop diuretic | 80.3 (77.2-83.3) | 77.7 (74.6-80.9) |
| Aldosterone antagonist | 18.9 (15.9-21.8) | 19.7 (16.7-22.7) |
Overall, 82.7% (591 of 715) of intervention participants used the telemonitoring equipment. Among intervention patients, telemonitoring adherence was documented in 55.4% (396 of 715) of total days at 30 days and in 51.7% (370 of 715) of total days at 180 days, while telephone coaching adherence was 61.4% (439 of 715) of total telephone calls at 30 days and 68.0% (486 of 715) of total telephone calls at 180 days. There were 221 211 remote patient observations, including 18 531 observations that exceeded threshold variables, with a median of 22 (interquartile range [IQR], 8-48) per intervention patient. There were 3700 scheduled health coaching telephone calls completed, with a median of 6 (IQR, 3-8) per intervention patient.
Primary Outcome
The overall proportion of study participants who experienced our primary outcome (unadjusted, 180-day all-cause readmission) was 50.0% (718 of 1437) (Table 3). There was no significant difference detected in unadjusted (P = .42) or adjusted (P = .39) analyses between the proportion of intervention participants (50.8% [363 of 715]) or usual care participants (49.2% [355 of 722]) with 180-day all-cause readmission. The unadjusted hazard ratio for 180-day all-cause readmission with receipt of the intervention was 1.03 (95% CI, 0.89-1.19; P = .73). The adjusted hazard ratio for 180-day all-cause readmission with receipt of the intervention was 1.03 (95% CI, 0.88-1.20; P = .74) (Figure 2). Subgroup analyses for our primary outcome showed no significant differences for those 65 years or older, male or female sex, race/ethnicity categories, or New York Heart Association classification. None of the subgroups showed evidence of meaningful effect modification (Table 4).
Table 3. Primary and Secondary Outcomes.
| Variable | Total (N = 1437) | Intervention (n = 715) | Usual Care (n = 722) | P Value | Adjusted P Valuea |
|---|---|---|---|---|---|
| Readmission, No. (%) | |||||
| 30 d | 318 (22.1) | 162 (22.7) | 156 (21.6) | .63 | .63 |
| 180 d | 718 (50.0) | 363 (50.8) | 355 (49.2) | .54 | .39 |
| Mortality, No. (%) | |||||
| 30 d | 63 (4.4) | 24 (3.4) | 39 (5.4) | .06 | .04 |
| 180 d | 214 (14.9) | 100 (14.0) | 114 (15.8) | .34 | .30 |
| Readmission or Mortality, No. (%) | |||||
| 30 d | 359 (25.0) | 173 (24.2) | 186 (25.8) | .49 | .44 |
| 180 d | 792 (55.1) | 393 (55.0) | 399 (55.3) | .91 | .93 |
| Quality-of-Life Score, Mean (SD)b | |||||
| 30 d | 988 (31.23) | 485 (32.21) | 503 (30.28) | .25 | .34 |
| 180 d | 796 (30.49) | 383 (32.63) | 413 (28.50) | .02 | .02 |
Adjusted P values are from multivariable logistic regression models for readmission and mortality analyses. Models controlled for age, sex, race/ethnicity, insurance, income, social isolation, comorbidities, year, and quarter of enrollment, with enrollment site controlled for as random effects.
The Minnesota Living With Heart Failure Questionnaire30 has a total score that can range from 0 to 105. A lower score indicates less effect of heart failure on a patient's quality of life.
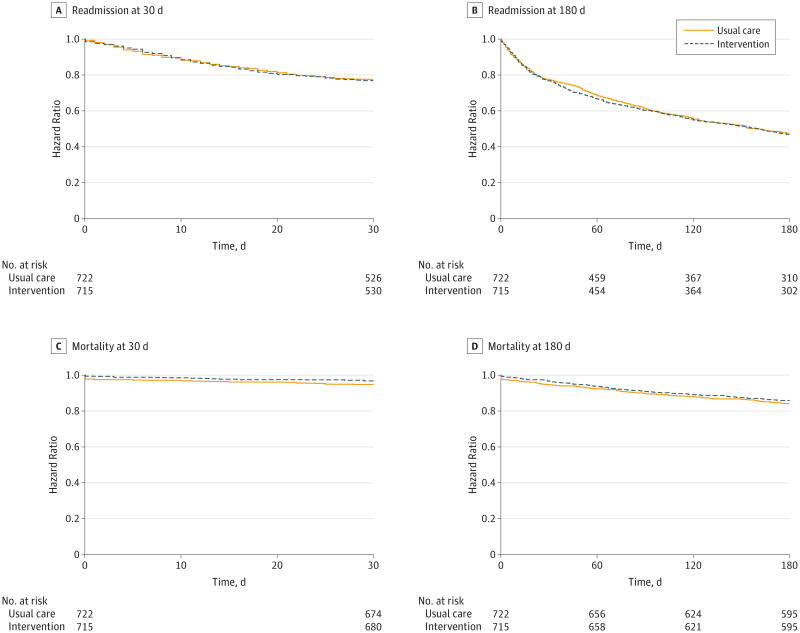
Figure 2. Hazard Ratios for Readmission and Mortality at 30 Days and 180 Days.
Dashed lines are for the intervention, and solid lines are for usual care. Adjusted hazard ratios, 95% CIs, and P values are from multivariable Cox proportional hazards regression models for readmission and mortality analyses. Models controlled for age, sex, race/ethnicity, insurance, income, social isolation, comorbidities, year, and quarter of enrollment, with enrollment site controlled for as random effects. A and B, The hazard ratio for 30-day readmission with the intervention is 1.03 (95% CI, 0.83-1.29; P = .77). The adjusted hazard ratio for 30-day readmission with the intervention is 1.01 (95% CI, 0.80-1.28; P = .91). The hazard ratio for 180-day readmission with the intervention is 1.03 (95% CI, 0.89-1.19; P = .73). The adjusted hazard ratio for 180-day readmission with the intervention is 1.03 (95% CI, 0.88-1.20; P = .74). C and D, The hazard ratio for 30-day mortality with the intervention is 0.61 (95% CI, 0.37-1.02; P = .06). The adjusted hazard ratio for 30-day mortality with the intervention is 0.53 (95% CI, 0.31-0.93; P = .03). The hazard ratio for 180-day mortality with the intervention is 0.88 (95% CI, 0.67-1.15; P = .32). The adjusted hazard ratio for 180-day mortality with the intervention is 0.85 (95% CI, 0.64-1.13; P = .26).
Table 4. Subgroup Analyses for the Primary Outcome.
| Variable | No. (%) | P Value | P Value for Interactiona | ||
|---|---|---|---|---|---|
| Total | Intervention | Usual Care | |||
| Age, y | |||||
| ≤65 (n = 452) | 212 (46.9) | 119 (51.1) | 93 (42.5) | .07 | .10 |
| >65 (n = 985) | 506 (51.4) | 244 (50.6) | 262 (52.1) | .65 | |
| Sex | |||||
| Male (n = 772) | 384 (49.7) | 196 (51.3) | 188 (48.2) | .39 | .38 |
| Female (n = 664) | 334 (50.3) | 167 (50.2) | 167 (50.5) | .94 | |
| Race/ethnicity | |||||
| African American (n = 316) | 168 (53.2) | 88 (57.5) | 80 (49.1) | .13 | .41 |
| Hispanic/Latino (n = 163) | 78 (47.9) | 44 (51.8) | 34 (43.6) | .30 | .85 |
| White (n = 779) | 374 (48.0) | 185 (47.6) | 189 (48.5) | .80 | NA |
| Asian/Pacific Islander or other (n = 171) | 98 (57.3) | 46 (54.8) | 52 (54.8) | .51 | .26 |
| New York Heart Association classification | |||||
| Not III or IV (n = 557) | 247 (44.3) | 115 (43.9) | 132 (44.8) | .84 | .49 |
| III or IV (n = 880) | 471 (53.5) | 248 (54.8) | 223 (52.2) | .45 | |
Abbreviation: NA, not applicable.
P values for interaction are from multivariable logistic regression models for 180-d readmission analyses. Models controlled for age, sex, race/ethnicity, insurance, income, social isolation, comorbidities, year, and quarter of enrollment, with enrollment site controlled for as random effects.
Secondary Outcomes
Findings for 30-day readmission mirrored those for 180-day readmission. The overall proportion of study participants with 30-day all-cause readmission was 22.7% (162 of 715) (Table 2). There was no significant difference detected in unadjusted (P = .56) or adjusted (P = .63) analyses between the proportion of intervention participants (22.7% [162 of 715]) or usual care participants (21.6% [156 of 722]) with 30-day all-cause readmission. The unadjusted hazard ratio for 30-day all-cause readmission with receipt of the intervention was 1.03 (95% CI, 0.83-1.29; P = .77). The adjusted hazard ratio for 30-day all-cause readmission with receipt of the intervention was 1.01 (95% CI, 0.80-1.28; P = .91) (Figure 2).
The overall proportion of study participants with 30-day all-cause mortality was 4.4% (63 of 1437) (Table 2). Nonsignificant differences were detected in unadjusted analysis (P = .06) and significant differences in adjusted analysis (P = .04) between the proportion of intervention participants (3.4% [24 of 715]) or usual care participants (5.4% [39 of 722]) with 30-day all-cause mortality. The unadjusted hazard ratio for 30-day all-cause mortality with receipt of the intervention was 0.61 (95% CI, 0.37-1.02; P = .06), and the adjusted hazard ratio for 30-day all-cause mortality with receipt of the intervention was 0.53 (95% CI, 0.31-0.93; P = .03) (Figure 2). Review of the timing of deaths indicates that this finding was because of in-hospital death differences after randomization, which would make it less likely to be owing to the intervention.
The overall proportion of study participants with 180-day all-cause mortality was 14.9% (214 of 1437) (Table 2). There was no significant difference detected in unadjusted (P = .34) or adjusted (P = .30) analyses between the proportion of intervention participants (14.0% [100 of 715]) or usual care participants (15.8% [114 of 722]) with 180-day all-cause mortality. The hazard ratio for 180-day all-cause mortality with receipt of the intervention was 0.88 (95% CI, 0.67-1.15; P = .32), and the adjusted hazard ratio for 180-day all-cause mortality with receipt of the intervention was 0.85 (95% CI, 0.64-1.13; P = .26) (Figure 2).
The overall mean, 30-day quality-of-life score was 31.23, and the overall mean, 180-day quality-of-life score was 30.49 (Table 3). There was a significant difference in 180-day quality-of-life scores between the intervention participants (mean, 28.50) and the control participants (mean, 32.63) in unadjusted (P = .02) and adjusted (P = .02) analyses.
Discussion
The BEAT-HF study is one of the largest RCTs of remote patient telemonitoring in an HF population. It was designed to determine the effectiveness of the intervention using a broad population of patients hospitalized with HF that would be consistent with actual practice. Similar to other large RCTs of telemonitoring, we did not find significant effects of the BEAT-HF intervention on all-cause readmission within the first 30 or 180 days. The physiological signals of changes in daily weights and increased symptoms may not provide adequate warning of impending decompensation in patients with HF.20,32 Trials of implanted hemodynamic monitoring systems in ambulatory patients with HF have shown that weight is a poor surrogate for filling pressures and is not a reliable signal for impendent decompensation.33 However, readmission is also increasingly recognized as a complex phenomenon, the cause of which is not solely limited to physiological variables.34 In addition, all participating sites were already focused on readmissions among patients with HF because of impending potential penalties from the Hospital Readmission Reduction Program and had implemented readmission reduction efforts.29 Similar types of interventions potentially could show effects among patients who have not previously been the focus of readmission reduction efforts.
Although the primary and secondary readmission outcome measures were not met, we found that the BEAT-HF intervention had significant effects on adjusted analyses of the prespecified secondary outcome measure of 30-day mortality. However, review of deaths suggests that this finding was a result of in-hospital death differences after randomization, which would make it less likely to be due to the intervention.
We also found that the BEAT-HF intervention had significant effects on quality of life among 180-day survey respondents. Findings for quality of life are limited by survey response rates. Although these rates were stable between 30 days and 180 days after accounting for mortality, survey nonrespondents differed in baseline characteristics from survey respondents and potentially could have different quality-of-life outcomes. Further study would be required to validate this finding because the BEAT-HF trial was not specifically designed or powered for this outcome.
The BEAT-HF study had several limitations. Because the major source of the BEAT-HF funding was derived from American Recovery and Reinvestment Act of 2009 funds, we could not extend enrollment beyond September 30, 2013. Doing so may have strengthened the 30-day mortality findings because the study was not powered for this specific outcome. The study sites are all academic medical centers in California, which could limit generalizability. However, half of the sites included safety-net hospitals, and the broad patient eligibility criteria increase generalizability. The use of other types of personnel instead of registered nurses potentially could have affected study outcomes. The intervention was not directly integrated with the physician practices caring for the patients, which is increasingly possible with advances in electronic health records. The effectiveness of transition of care, disease management, and telemonitoring interventions may be highly dependent on how they are integrated and adhered to in practice.32 Adherence to the BEAT-HF intervention appears to have been a critical factor. Despite deploying several strategies to promote patient engagement and foster adherence with the telemonitoring and telephone call center intervention, only 61.4% (439 of 715) and 55.4% (396 of 715) of patients randomized to the intervention were more than 50% adherent to telephone calls and telemonitoring, respectively, within the first 30 days. Remote patient monitoring has also experienced significant technological change, including increasing use of tablets and other remote sensors. Newer approaches, such as implantable devices, could increase adherence or provide better information to identify problems after discharge.
Conclusions
The BEAT-HF study found that a combination of remote patient monitoring with care transition management did not reduce 180-day all-cause readmission after hospitalization for HF. Hospitalizations in the first 30 days and 180-day mortality were also not reduced with the intervention. Individuals participating in this intervention may experience quality-of-life improvements at 180 days. However, further studies would be needed to confirm these findings.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grant R01HS019311 from the Agency for Healthcare Research and Quality; by grant RC2HL101811 from the National Heart, Lung, and Blood Institute (NHLBI); by grant UL1TR000124 from the National Center for Advancing Translational Science (NCATS) of the University of California, Los Angeles, Clinical and Translational Science Institute; by grant 66336 from the Robert Wood Johnson Foundation; by the Sierra Health Foundation; by the University of California Center for Health Quality and Innovation; and by the participating institutions. All grants were to Dr Ong except for the NHLBI (to Dr Mangione, the final principal investigator) and the NCATS (to Steven M. Dubinett, MD).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Ong and Ms Edgington had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ong, Romano, Aronow, Auerbach, Black, De Marco, Escarce, Evangelista, Ganiats, Greenberg, Greenfield, Kaplan, Mangione, Banafsheh Sadeghi, Sarrafzadeh, Fonarow.
Acquisition, analysis, or interpretation of data: Ong, Romano, Edgington, Aronow, Auerbach, Black, De Marco, Hanna, Ganiats, Greenberg, Greenfield, Kaplan, Kimchi, Liu, Lombardo, Bahman Sadeghi, Sarrafzadeh, Tong, Fonarow.
Drafting of the manuscript: Ong, Edgington, Aronow, Auerbach, Kaplan, Bahman Sadeghi.
Critical revision of the manuscript for important intellectual content: Ong, Romano, Aronow, Auerbach, Black, De Marco, Escarce, Evangelista, Hanna, Ganiats, Greenberg, Greenfield, Kaplan, Kimchi, Liu, Lombardo, Mangione, Banafsheh Sadeghi, Sarrafzadeh, Tong, Fonarow.
Statistical analysis: Ong, Edgington, Hanna, Kaplan, Liu.
Obtained funding: Ong, Evangelista, Ganiats, Kimchi, Sarrafzadeh, Fonarow.
Administrative, technical, or material support: Romano, Aronow, Auerbach, Black, Ganiats, Greenfield, Kaplan, Lombardo, Bahman Sadeghi, Banafsheh Sadeghi, Sarrafzadeh, Tong, Fonarow.
Study supervision: Ong, Auerbach, De Marco, Escarce, Ganiats, Greenberg, Greenfield.
Conflict of Interest Disclosures: Dr De Marco reported being a consultant to Boston Scientific, Cardiokinetics, and Gambro and reported serving on advisory boards for Otsuka and Bayer. Dr Fonarow reported being a consultant to Amgen, Bayer, Baxter, Medtronic, and Novartis. No other disclosures were reported.
Group Information: In addition to the authors of the present article, other members of the Better Effectiveness After Transition–Heart Failure (BEAT-HF) Research Group (in alphabetical order) are Bruce Davidson, PhD, MBA, Hassan Ghasemzadeh, PhD, Michael Gropper, MD, MPH, and Michelle Mourad, MD. The BEAT-HF project managers are Arjang Ahmadpour, BS, Wendy Davila, RN, Susanne Engel, MBA, Ronald Jacolbia, BS, Herman Lee, BS, Laura Linares, RN, Elizabeth Michel, RN, Meghan Soulsby Weyrich, MS, and Elizabeth Zellmer, BS. The BEAT-HF postdischarge intervention nurses are Lida Esbati-Mashayekhi, RN, Elisabeth Haddad, RN, Marian Haskins, RN, Tianne Larson, RN, and Kathryn Pratt, RN. The BEAT-HF enrollment nurses are Hendry Ansorie, RN, Kymberly Aoki, RN, Ruth Baron, RN, Eileen Brinker, RN, Maureen Carroll, RN, Annette Contasti, RN, Anne Fekete, RN, Vivian Guzman, RN, Linda Larsen, RN, Lisa Martinez, RN, Sharon Myers, RN, Melanee Schimmel, RN, Amanda Schnell-Heringer, RN, Arlene Taylor, RN, Tracy Tooley, RN, Geoffrey van den Brande, RN, and Evanthia Zaharias, RN. The BEAT-HF research staff are John Billimek, PhD, Rocio Castaneda, BS, Ma Elloi Delos Reyes, BS, Dana Fine, BS, Tammy Lo, BS, Xoan Luu, BA, Socorro Ochoa, BA, Mayra Perez, BA, David Rincon, BA, Fidelia Sillas, BA, Visith Uy, BS, Esther Wang, BS, Haiyong Xu, PhD, Stella Yala, MD, and Tingjian Yan, PhD.
Additional Information: The telemonitoring system vendor (IDEAL Life; IDEAL Life Inc) was paid for its services and had no role in the study design, the analysis of the data, or the writing of the manuscript. The study was designed by one of us (Dr Ong.), data were gathered by site coordinators and the UCLA coordinating center, and the data were analyzed by 2 of us (Dr Ong and Ms Edgington). One of us (Dr Ong) drafted the manuscript and made the decision to submit it for publication. All authors contributed to subsequent revisions and vouch for the accuracy and completeness of the data and analyses.
Additional Contributions: Meghan Soulsby Weyrich, MS, provided editorial assistance with the manuscript, for which she did not receive compensation outside of her usual salary. The California Department of Public Health Office of Statewide Health Planning and Development and the Social Security and National Death Index assisted with the data collection. We thank the BEAT-HF project managers, nurses, and research staff for their assistance with the project.
References
- 1.Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol. 2013;61(4):391–403. doi: 10.1016/j.jacc.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Thom T, Haase N, Rosamond W, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee [published corrections appear in Circulation. 2006;113(14):e696 and 2006;114(23):e630] Circulation. 2006;113(6):e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 3.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011;364(16):1582] N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Medicare & Medicaid Services. [Accessed September 28, 2015];Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html.
- 5.Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407–413. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 6.Ong MK, Mangione CM, Romano PS, et al. Looking forward, looking back: assessing variations in hospital resource use and outcomes for elderly patients with heart failure. Circ Cardiovasc Qual Outcomes. 2009;2(6):548–557. doi: 10.1161/CIRCOUTCOMES.108.825612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen GA, Bartely A, Coleman E, et al. Transforming Care at the Bedside How-to Guide: Creating an Ideal Transition Home for Patients With Heart Failure. Cambridge, MA: Institute for Healthcare Improvement; 2008. [Google Scholar]
- 8.Amoah B, Boutwell A, Schall M, Sevin C, Shapiro E, Taylor J. Getting Started Guide: Improving Care for Patients With Heart Failure: Focus on Ambulatory Care. Cambridge, MA: Institute for Healthcare Improvement; 2008. [Google Scholar]
- 9.Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation. 2013 Measures Updates and Specifications Report: Hospital-Level 30-Day Risk-Standardized Readmission Measures for Acute Myocardial Infarction, Heart Failure, and Pneumonia (Version 6.0) New Haven, CT: Mar, 2013. [Google Scholar]
- 10.Rau J. Medicare fines 2,610 hospitals in third round of readmission penalties. [Accessed November 26, 2015];Kaiser Health News. http://khn.org/news/medicarereadmissions-penalties-2015/. Published October 2, 2014.
- 11.Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 12.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675–684. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 13.Coleman EA, Parry C, Chalmers S, Min SJ. The Care Transitions Intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 14.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 16.Voss R, Gardner R, Baier R, Butterfield K, Lehrman S, Gravenstein S. The Care Transitions Intervention: translating from efficacy to effectiveness. Arch Intern Med. 2011;171(14):1232–1237. doi: 10.1001/archinternmed.2011.278. [DOI] [PubMed] [Google Scholar]
- 17.Katz MH. Interventions to decrease hospital readmission rates: who saves? who pays? Arch Intern Med. 2011;171(14):1230–1231. doi: 10.1001/archinternmed.2011.309. [DOI] [PubMed] [Google Scholar]
- 18.Stauffer BD, Fullerton C, Fleming N, et al. Effectiveness and cost of a transitional care program for heart failure: a prospective study with concurrent controls. Arch Intern Med. 2011;171(14):1238–1243. doi: 10.1001/archinternmed.2011.274. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai AS. Home monitoring heart failure care does not improve patient outcomes: looking beyond telephone-based disease management. Circulation. 2012;125(6):828–836. doi: 10.1161/CIRCULATIONAHA.111.031179. [DOI] [PubMed] [Google Scholar]
- 21.Koehler F, Winkler S, Schieber M, et al. Telemedical Interventional Monitoring in Heart Failure Investigators. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the Telemedical Interventional Monitoring in Heart Failure study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 22.Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JG. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015;(10):CD007228. doi: 10.1002/14651858.CD007228.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandor A, Gomersall T, Stevens JW, et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta-analysis. Heart. 2013;99(23):1717–1726. doi: 10.1136/heartjnl-2013-303811. [DOI] [PubMed] [Google Scholar]
- 24.Pandor A, Thokala P, Gomersall T, et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207. v–vi. doi: 10.3310/hta17320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Black JT, Romano PS, Sadeghi B, et al. BEAT-HF Research Group. A remote monitoring and telephone nurse coaching intervention to reduce readmissions among patients with heart failure: study protocol for the Better Effectiveness After Transition–Heart Failure (BEAT-HF) randomized controlled trial. Trials. 2014;15:124. doi: 10.1186/1745-6215-15-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeWalt DA, Malone RM, Bryant ME, et al. A heart failure self-management program for patients of all literacy levels: a randomized, controlled trial [ISRCTN11535170] BMC Health Serv Res. 2006;6:30. doi: 10.1186/1472-6963-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howie-Esquivel J, Carroll M, Brinker E, et al. A strategy to reduce heart failure readmissions and inpatient costs. Cardiol Res. 2015;6(1):201–208. doi: 10.14740/cr384w. doi: http://dx.doi.org/10.14740/cr384w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White M, Garbez R, Carroll M, Brinker E, Howie-Esquivel J. Is “teach-back” associated with knowledge retention and hospital readmission in hospitalized heart failure patients? J Cardiovasc Nurs. 2013;28(2):137–146. doi: 10.1097/JCN.0b013e31824987bd. [DOI] [PubMed] [Google Scholar]
- 29.Vasilevskis EE, Kripalani S, Ong MK, et al. Variability in implementation of interventions aimed at reducing readmissions among patients with heart failure: a survey of teaching hospitals [published online November 17, 2015] Acad Med. doi: 10.1097/ACM.0000000000000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rector TS, Kubo SH, Cohn JN. Patients self-assessment of their congestive heart failure, part 2: content, reliability and validity of a new measure, the Minnesota Living With Heart Failure Questionnaire. Heart Fail. 1987;3:198–209. [Google Scholar]
- 31.Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006;46(4):503–513. doi: 10.1093/geront/46.4.503. [DOI] [PubMed] [Google Scholar]
- 32.Desai AS, Stevenson LW. Connecting the circle from home to heart-failure disease management. N Engl J Med. 2010;363(24):2364–2367. doi: 10.1056/NEJMe1011769. [DOI] [PubMed] [Google Scholar]
- 33.Zile MR, Bennett TD, St John Sutton M, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118(14):1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 34.Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803–1812. doi: 10.1001/jamainternmed.2015.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.