Abstract
Oxygen affects the activity of multiple skeletogenic cells and is involved in many processes that are important for fracture healing. However, the role of oxygen in fracture healing has not been fully studied. Here we systematically examine the effects of oxygen tension on fracture healing and test the ability of hyperoxia to rescue healing defects in a mouse model of ischemic fracture healing. Mice with tibia fracture were housed in custom-built gas chambers and groups breathed a constant atmosphere of 13% oxygen (hypoxia), 21% oxygen (normoxia), or 50% oxygen (hyperoxia). The influx of inflammatory cells to the fracture site, stem cell differentiation, tissue vascularization, and fracture healing were analyzed. In addition, the efficacy of hyperoxia (50% breathing oxygen) as a treatment regimen for fracture nonunion was tested. Hypoxic animals had decreased tissue vascularity, decreased bone formation, and delayed callus remodeling. Hyperoxia increased tissue vascularization, altered fracture healing in un-complicated fractures, and improved bone repair in ischemia-induced delayed fracture union. However, neither hypoxia nor hyperoxia significantly altered chondrogenesis or osteogenesis during early stages of fracture healing, and infiltration of macrophages and neutrophils was not affected by environmental oxygen after bone injury. In conclusion, our results indicate that environmental oxygen levels affect tissue vascularization and fracture healing, and that providing oxygen to patients with fractures accompanied by ischemia may be beneficial.
Keywords: fracture, oxygen, hypoxia, hyperoxia, angiogenesis
Introduction
Inadequate blood supply is a significant contributing factor for delayed fracture healing or nonunion [1, 2]. In order to understand the mechanisms that impair fracture healing after disrupted blood flow and to explore new therapies to stimulate healing in an ischemic environment, we previously developed an animal model of ischemic fracture by ligating the femoral artery before creating a fracture in the tibia [3]. We found that ischemia created a hypoxic environment at the site of fracture [4], which led to cell death, delayed chondrocyte and osteoblast differentiation, and impaired fracture healing [3]. These findings inspired us to study the role of oxygen in fracture healing.
Oxygen is involved in multiple basic cellular processes that are important for normal fracture healing. First, oxygen is required for aerobic metabolism. Second, oxygen is required for activity of many enzymes, including hydroxylases, oxygenases, and cycloxygenases, which are involved in fracture healing. For example, lack of cycloxygenase-2 activity impairs bone repair by affecting osteoblast differentiation [5, 6]. Third, reduction in tissue oxygen interferes with the process of the collagen synthesis, because oxygen is required for hydroxylation of lysine and proline during collagen cross-linking and bundle formation [7]. Fourth, oxygen is an important signaling molecule, which regulates the expression of several angiogenic genes through the hypoxia inducible factor (Hif) pathway[8].
Additionally, tissue oxygen levels may modulate stem cell responses after fracture. Oxygen has been implicated in stem cell maintenance, mobilization, and recruitment to sites of injury [9–13]. In vitro experiments have demonstrated that oxygen tension has profound effects on skeletogenic cells, including osteoblasts, chondrocytes, and osteoclasts. Hyperbaric oxygen increases cell proliferation and mineralization of alveolar osteoblasts [14]. Under normobaric conditions, 2% oxygen applied to cells in the early phase of osteoblast differentiation decreases collagen production and mineralization compared to 20% oxygen [15]. Compared to 21% oxygen, 5% oxygen increases the differentiation of osteoblasts and their transformation to osteocytes [16]. Hypoxia also influences the expression of genes in cultured osteoblasts. Hypoxia decreases sclerostin expression [17], increases Wnt signaling [17], and increases BMP2 [18], IGF [19], and VEGF expression [20]. Similar to osteoblasts, chondrocytes in culture are also affected by oxygen levels. Hypoxia (2–5% oxygen) increases the expression of VEGF [21], collagen type II, glycosaminoglycan, and aggrecan [22–24]. Cultured chondrocytes tend to dedifferentiate and hypoxia can induce their redifferentiation [23]. Compared to osteoblasts, chondrocytes normally reside in avascular cartilage and have been speculated to be well-adapted to low oxygen tension [25], and these in vitro data have been used to support this idea. However, the growth plate is well perfused suggesting that oxygen may not be limiting for chondrocyte function in the growth plates [26]. Hypoxia also affects osteoclast activity. Changing culture conditions from 20% oxygen to 2% oxygen significantly stimulates osteoclast formation and bone resorption [27, 28].
While the effects of oxygen tension on skeletal cells have been extensively studied in vitro, little is known about the relevance of these studies to the situation in vivo. Most in vitro studies use 2–5% oxygen as the hypoxic conditions and results are compared to cultures in 20–21% oxygen, which is well-above the physiological state of tissues and cells in vivo. Further, the in vivo environment is much more complex. There are multiple cell types that have different metabolic demands. These cells are responding to a variety of growth factors and cytokines that interact to regulate the process of repair, and this complexity is not recapitulated in the in vitro experiments. Normally, Hif1α protein and VEGF increase when cells are hypoxic, but in the presence of inflammation and lactate, as in wounds, the effects is different, and oxygen promotes VEGF expression and angiogenesis [8, 29–31]. The goal of the current study was to determine the role of oxygen in bone repair in vivo and to explore the efficacy of non-hyperbaric hyperoxia on enhancement of fracture healing. We hypothesized that environmental oxygen alters fracture healing by regulating stem cell differentiation, angiogenesis, and inflammation during early fracture healing. We tested this hypothesis in a mouse model of tibia fracture healing.
Materials and Methods
Generation of tibia fractures
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California at San Francisco and at Dartmouth Medical School, Hanover, NH. Three-month-old male 129J/B6 mice (25–30g) were anesthetized with 0.03ml of a mixture of Ketamine (50mg/ml) and Medetomidine (0.5mg/ml). Closed transverse mid-diaphyseal fractures of the tibia were created with a three-point bending apparatus. Fractures were either stabilized with an external fixator or left unstabilized. In a second set of animals, the femoral artery was resected before creating tibia fractures, resulting in an ischemic environment that delays fracture healing [3]. After recovery, animals were allowed to ambulate freely and analgesics were provided for the first 72 hours (Buprenorphine, 0.03mg/mouse, ZT Sigma, St. Louis, MO). Animals that died during the post-operative period and those with comminuted fractures were excluded from further analyses.
Treatment with different levels of oxygen
After recovery from anesthesia, animals with tibia fractures were transferred into custom- built semi-sealed gas chambers. Oxygen levels in the chambers were maintained at 13% (hypoxia), 21% (normoxia), or 50% (hyperoxia) by infusing compressed nitrogen or oxygen for the duration of the entire experiment. Gas infusion was controlled by ProOx (BioSpherix Ltd, Redfield, NY). The carbon dioxide and humidity in the chambers were maintained at <0.5% and 65–75% respectively. Chambers were opened briefly every other day to change cages, food and water. All animals exhibited excellent tolerance to hypoxia and hyperoxia. No significant change of body weight was observed after surgery and oxygen treatment.
Examining oxygen tension at the fracture site
To determine whether breathing oxygen can alter the oxygen tension at fracture site, non-stabilized tibia fractures were created in 4 adult mice. Lithium phthalocyanine (LiPc), a paramagnetic material, was implanted using a 30G needle to the fracture site after bone injury. At 24 hours after injury, animals were anesthetized again. They were kept euthermic and then were allowed to breathe 13%, 21%, and 50% oxygen. Animals were allowed to breath each oxygen concentration for 20–30 minutes before oxygen tension at the fracture site was measured using electron paramagnetic resonance (EPR) before switching to the next oxygen concentration [4, 32].
To determine the correlation between local oxygen tension and tissue differentiation during fracture healing, LiPc crystals were implanted adjacent to the mid-diaphysis of the tibia immediately after creating non-stabilized tibia fractures. Tissue oxygenation was measured at 1, 3, 5, 7, and 10 days after injury with the animals breathing room air. Animals were sacrificed at 7 and 10 days post-fracture and fractured tissues were collected. Histological analysis was performed to determine the location of LiPc crystal and the types of tissues that the crystals were located in.
Histological, histomorphometric, and molecular analyses of fracture healing
Animals were sacrificed at 5–28 days after injury (n=4–6/group/time) and fractured tibiae were collected, fixed in 4% paraformaldehyde (PFA) at 4°C overnight, decalcified in 19% EDTA, dehydrated, and embedded in paraffin. Sagittal sections (10μm) through the entire block were prepared. To analyze fracture healing, every thirtieth slide was stained with HBQ staining, which stains bone red and cartilage blue. Histomorphometry was performed using an Olympus CAST system. The volume of callus (Vcallus) and the volumes of bone (Vbone), and cartilage (Vcartilage) within the callus were estimated using Cavalieri’s Principle.
To assess osteoblast and chondrocyte differentiation, in situ hybridization using probes that hybridize to markers of osteoblasts (Osteocalcin (Oc)) chondrocytes (Collagen type II (Col2)), and of chondrocyte maturation (Collagen type X (Col10), and vascular endothelial growth factor (VEGF)), was performed on sections adjacent to those used for histological staining [33].
Examination of fracture callus composition
Since non-stabilized fractures tend to angulate during repair, conventional mechanical testing such as three or four-point-bending is unreliable and insensitive. We turned to Fourier Transform Infrared (FTIR) spectroscopy to study the content and composition of mineral and matrix in the fracture calluses. Animals with non-stabilized tibia fracture were treated with hypoxia, normoxia, or hyperoxia for 21 days and fracture calluses were collected and fixed in 70% ethanol. Chemical composition of the fracture callous tissue was examined with FTIR spectroscopy. Samples were desiccated through an ethanol series followed by exposure in a desiccant chamber. For each sample, a homogenized powder mixture was created of 1% bone by weight in potassium bromide (KBr; Thermo Electron Corporation). The powder mixture was compressed using a manual die to create a solid, clear pellet for FTIR spectroscopy.
Spectroscopy was performed on a bench top interferometer system (Nexus 870, Thermo Electron Corporation). Spectra were acquired using 256 scans at a spectral resolution of 4cm−1. A background scan was recorded immediately following each sample scan to facilitate background correction. Following acquisition, the spectra were transferred to chemical imaging software (Isys, Spectral Dimensions, Inc.) for analysis. Spectra were baseline adjusted and the integrated areas of the amide I (1595–1720 cm−1), ν1 ν3 phosphate (PO4, 895–1215 cm−1), and carbonate (CO32-, 840–890 cm−1) bands were calculated. Mineral-to-matrix (PO4/amide I), carbonate-to-phosphate (CO32-/PO4), and carbonate-to-matrix (CO32-/amide I) ratios were calculated from integrated areas of the respective peaks. Additionally, peak heights were measured at specific wavenumbers: 1020 cm−1, 1030 cm−1, 1660 cm−1, and 1690 cm−1. From these, a series of absorbance ratios were calculated to determine additional spectroscopic parameters. The ratio of 1030 cm−1 to 1020 cm−1 represents the ratio of stoichiometric apatite to non-stoichiometric apatite, a measure of crystallinity. Finally, the ratio of 1660 cm−1 to 1690 cm−1 represents the proportion of non-reducible to reducible crosslinks in the collagen, indicative of collagen maturity.
Quantification of tissue vascularization
To analyze the effect of oxygen levels on vascular regeneration in non-ischemic fractures, mice were anesthetized and two pins were placed in the proximal and distal tibia 11mm apart. A transverse fracture was then created between these two pins. Mice were housed in the chambers of different oxygen levels (13%, 21%, and 50% of oxygen) for 3 days before sacrifice (n=4–6). This time point was chosen because vascular repair is usually abundant at the fracture site in mice by this time. Tissues between the two pins including fracture callus and surrounding muscles were collected for quantification of tissue vascularity. This procedure allows further analysis to be performed on comparable reference volumes between different samples without including too much normal tissues.
To analyze the effect of hyperoxia on vascular regeneration in ischemic fractures, mice with tibia fracture and femoral artery resection were housed in hyperoxic or normoxic chambers for 5 days before sacrifice (n=4–5). The whole fractured tibia with surrounding muscles was collected in order to assess collateral circulation, which is the major mechanism that restores blood supply to the distal limb after femoral artery resection. The reference volumes remain comparable. Five days was chosen because femoral artery delays fracture healing so vascular repair could be delayed as well.
Fractured tissues were fixed in 4% paraformaldehyde, decalcified in 19% EDTA, and embedded in Tissue-Tek OCT compound. Vertical uniform random (VUR) sections were generated through the whole tissue block. For each sample, 6–9 sections were selected using systemic random sampling, and immunohistochemistry was performed to visualize blood vessels using an anti-PECAM antibody (Platelet Endothelial Cell Adhesion Molecule). The length density and surface density of blood vessels were quantified using an Olympus CAST system as described before [34].
Lactate analysis and VEGF ELISA
We analyzed the effect of oxygen levels on lactate accumulation and VEGF expression in the fractured limb at 3 days after fracture. Fractures were generated as described above. Immediately after collection, tissues were snap-frozen on dry ice and kept at −80°C until further processing. Tissues were homogenized in ice-cold distilled water with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and centrifuged. The supernatant was collected for analysis. Lactate levels were determined using an YSI 1500 Sport Lactate Analyzer (YSI Life Science, Yellow Springs, Ohio). VEGF levels were determined using an ELISA kit following the manufacture’s instruction (Roche, Indianapolis, IN). Measurements were normalized with sample weight.
Quantification of macrophages and neutrophils
To analyze the effect of oxygen on the infiltration of macrophages and neutrophils, sections adjacent to those used for quantification of tissue vascularization were selected and subjected to immunohistochemistry with antibodies F4/80 or Ly6g (eBioscience, San Diego, CA) to visualize macrophages and neutrophils respectively [35]. For each animal, 5–10 slides were selected using systemic random sampling. The number of macrophages and neutrophils within the fractured limbs was estimated using stereology. Data are expressed as number of cells/mm2 of tissue.
Statistics
Single factor ANOVA and t-test were used to determine the effects of oxygen levels on tissue vascularization and fracture healing. Nonparametric analyses (Kruskal-Wallis tests) were performed on FTIR data to evaluate differences in parameters among the three experimental groups. Data are presented as mean ± one standard deviation.
Results
Environmental oxygen levels affect local oxygen tension at the fracture site
We first determined the effect that ambient oxygen levels have on tissue oxygen levels at the fracture site. To achieve this we used Electron Paramagnetic Resonance spectroscopy. In this method a paramagnetic crystal is implanted into the fracture site, and the absolute amount of O2 can be determined. This technique is described in detail before [36]. Here, we determined that when animals were breathing room air, oxygen tension at the fracture site was 32.7±11.9mmHg. However, lowering the oxygen concentration in the air to 13% decreased the oxygen tension at the fracture site to 14.9±8.9mmHg (p<0.01 compared to room air). As expected, 50% oxygen led to an increase in oxygen tension to 68.4±10.3mmHg (p<0.01 compared to room air).
Environmental hypoxia inhibits fracture healing
We then determined the effects of different oxygen concentrations on the process of fracture healing. Mice with tibia fractures were housed in a gas chamber that maintained the oxygen concentration at 13% during the healing period (5–28 days). Compared to normoxic fractures, hypoxia treated fractures did not show a significant difference in bone and cartilage formation at 5 days after injury (Fig. 1) suggesting that overall tissue oxygen levels do not influence stem cell fate decisions during fracture healing. However, low oxygen was associated with decreased healing. At 10 days after injury, hypoxic fractures exhibited significantly less new bone and callus tissues compared to normoxic animals (Fig. 1A). By 28 days this trend was reversed to larger callus and more bone in these animals (Fig. 1A) suggesting that hypoxia delayed callus and bone remodeling at late stages of fracture healing. We then used FTIR to assess the composition of the tissues in the fracture callus, and we did not detect significant differences in fracture callus tissue composition between hypoxic and normoxic animals at 21 days.
Fig. 1.
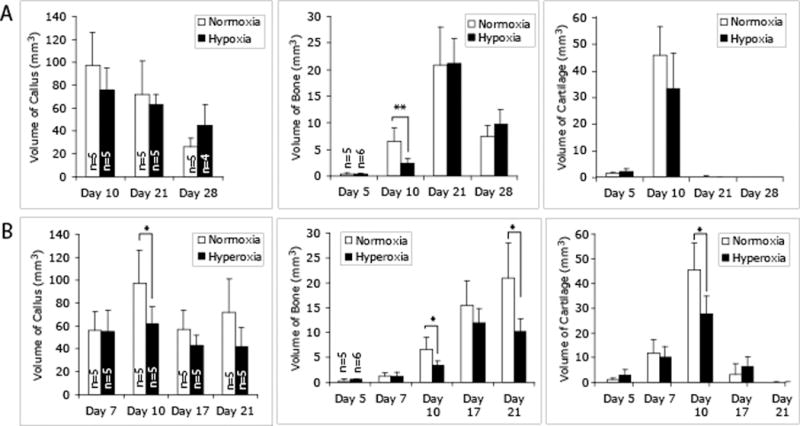
Effects of environmental oxygen on fracture healing in a mouse tibia fracture model. Histomorphometric analyses of the volume of callus, new bone, and cartilage in the callus were performed on HBQ stained tissue sections under a stereology microscope (n=4–6/group/time point). (A) Hypoxia-treated fractures. (B) Hyperoxia-treated fractures. * p<0.05, ** p<0.01.
Environmental hyperoxia alters fracture healing
In addition to reducing inspired oxygen levels, we wanted to test the effects of increasing atmospheric oxygen on fracture healing. To achieve this, mice with tibia fractures were housed in a gas chamber that contained 50% oxygen, and fracture healing was analyzed at 5–21 days after injury. Compared to normoxia, no significant effects of hyperoxia were detected at 5 and 7 days after fracture (Fig. 1B). At 10 days, hyperoxia significantly decreased the volumes of callus, bone, and cartilage compared to normoxia (Fig. 1B). At 17 days, no difference was observed, and at 21 days, hyperoxic fractures had significantly less bone compared to normoxic animals (Fig. 1B). No differences were detected in the composition of fracture callus by FTIR between hyperoxic and normoxic fractures at 21 days.
The effect of oxygen levels on inflammatory response
To determine whether oxygen levels affected the recruitment of inflammatory cells to the fracture site, the number of macrophages and neutrophils were quantified at 3 days after injury in animals breathing 13% (n=4), 21% (n=5), of 50% (n=5) oxygen. The number of macrophages is 186+/− 110/mm2 in hypoxic animals, 206+/− 106/mm2 in normoxic ones, and 242+/− 114/mm2 in hyperoxic fractures. The number of neutrophils is 207+/−62/mm2 in hypoxic fractures, 235+/−72/mm2 in normoxic ones, and 224+/−53/mm2 in hyperoxic ones. No significant difference in macrophage and neutrophil infiltration was detected among the three oxygen concentrations.
The effect of oxygen levels on angiogenesis
Since vascularization is required for bone healing, we examined the effect of oxygen on vascularity of the fracture site. To achieve this we quantified the length density and the surface density of blood vessels within the fractured limbs using Stereology (Olympus CAST system). Compared to non-fractured tibiae, fractured legs treated by normoxia had higher length density and surface density of blood vessels, suggesting that bone injury itself induces new blood vessel formation. Compared to normoxic fractures, hypoxia decreased length density and surface density of blood vessels while hyperoxia increased surface density of the vasculature, indicating that hypoxia has inhibitory effects on injury-induced new blood vessel formation and hyperoxia further stimulates vascular repair (Fig. 2).
Fig. 2.
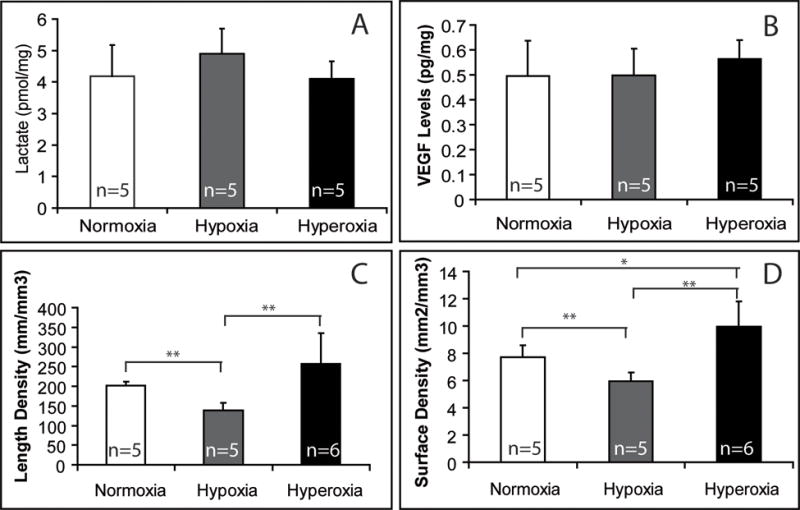
Effects of environmental oxygen on tissue vascularization at 3 days after fracture (n=5–6/group). Lactate and VEGF levels were measured in tissue lysates of fracture calluses. Length density and surface density of blood vessels were determined using stereology. * p<0.05, ** p<0.01.
To further examine the molecular mechanisms underlying the altered angiogenesis, the effect of environment oxygen on lactate accumulation and VEGF expression in the fractured limbs were examined at 3 days after injury. However, no significant difference was detected in the levels of lactate and VEGF in fractured tissues among animals treated with different oxygen concentrations (Fig. 2).
Effect of environmental oxygen levels on stem cell differentiation
To assess the effect of oxygen levels on early chondrocyte and osteoblast differentiation, in situ hybridization was performed on tissue sections of day 5 non-stabilized fractures (Fig. 3A–L). Chondrocytes expressing Col 2 were observed in fractures from all three oxygen groups. Both col 10 and VEGF transcripts were also detected in these cartilage islands. These data suggest that oxygen levels did not significantly affect the differentiation and maturation of chondrocytes. A marker of osteoblasts, osteocalcin, was also expressed in the area of periosteal reaction near fracture ends. No obvious difference in osteocalcin expression was observed among the three oxygen concentrations. Histomorphometry on the day 5 fractures demonstrated that hypoxia or hyperoxia did not alter the ratio of cartilage to bone formed at the fracture site (Fig. 3M), supporting the idea that oxygen levels do not favor cartilage over bone formation during early bone repair.
Fig. 3.
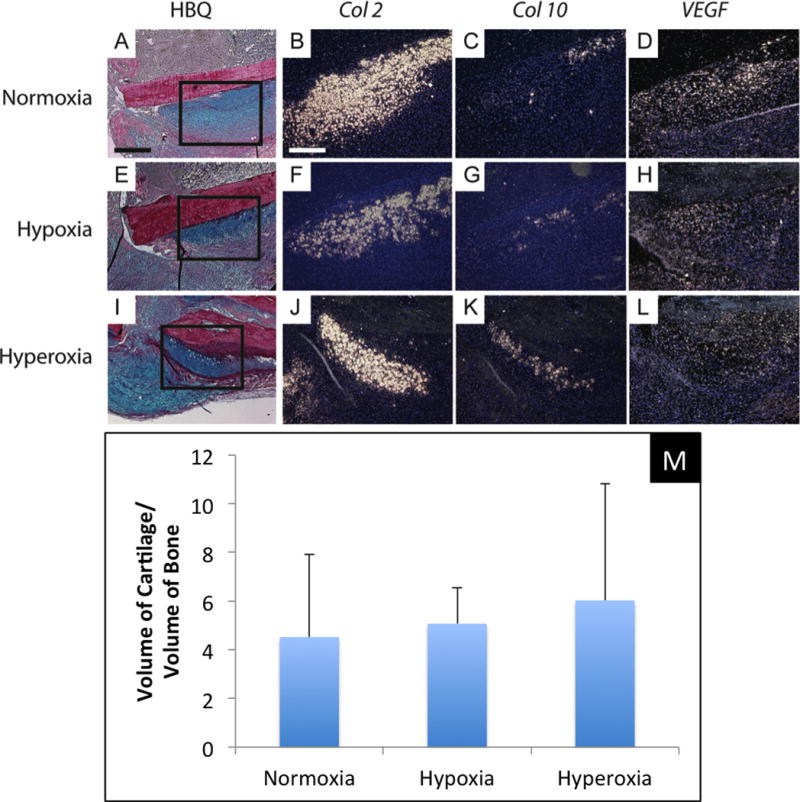
Environmental oxygen does not affect chondrocyte differentiation at 5 days after fracture. (A–D) Non-stabilized tibia fracture treated with normoxia. (E–H) Non-stabilized tibia fracture treated with hypoxia. (I–L) Non-stabilized tibia fracture treated with hyperoxia. (A,E,I) HBQ staining shows fracture callus and cartilage was stained blue. (B,F,J) Transcripts of collagen type II (Col 2), (C,G,K) collagen type X (Col 10), and (D,H,L) vascular endothelial growth factor (VEGF) were detected by in situ hybridization. Col 2 is a marker for chondrocytes while Col 10 and VEGF are markers for hypertrophic chondrocytes. No significant difference in the expression of these markers was detected between three groups. (M) Histomorphometric analysis shows similar ratio of the volume of cartilage and the volume of new bone in fracture calluses of three groups at 5 days after injury. Scale bar: A,E,I = 400μm, B–D,F–G,J–L = 200μm.
To further study the effect of hypoxia on stem cell differentiation, mice with stabilized tibia fractures, which normally heal through direct bone formation, were treated with 13% oxygen. Compared to normoxia, which showed a small amount of bone and cartilage formation, hypoxia significantly impaired fracture healing of the stabilized fractures at 10 days after injury (Fig. 4). There was a minimal amount of callus with no cartilage formation (Fig. 4) suggesting that ischemia-induced hypoxia did not evoke a switch in stem cell fate from osteoblast to chondrocyte differentiation.
Fig. 4.
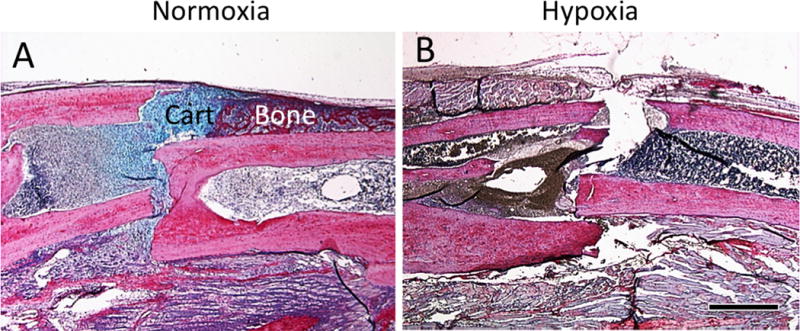
Hypoxia does not induce more cartilage formation in stabilized fractures. (A) Tibia fractures were rigidly stabilized with an external fixator. Under normoxic conditions, a small amount of bone and cartilage (Cart) is present at the fracture site. (B) Under hypoxic conditions, fracture healing is inhibited and no obvious bone and cartilage formation was observed in the callus. Scale bar = 1mm.
Next, we wanted to measure oxygen levels at sites of stem cell differentiation in order to determine if local oxygen levels corresponded to the type of tissue that formed in each region. To achieve this we implanted LiPC crystals into random areas adjacent to the bone fracture, and we used EPR oximetry to determine the oxygen concentration near the LiPC crystal. We observed a large degree of variation in oxygen measurements over the time course of this experiment (Fig. 5A), which is expected if there are regional differences in oxygen levels that correspond to differentiation of different tissue types. Indeed, at 7 and 10 days after injury, LiPc crystals were found in one of the following four locations (Fig. 5B–E): muscles (n=12), periphery of callus (n=5), cartilage in the callus (n=9), and endochondral bone (n=4). No statistical difference was detected in the oxygen levels among different locations at each time point analyzed (Fig. 5F). However, sites that were destined to form cartilage and undergo endochondral bone formation appeared to have low oxygen tension at one day after fracture compared to sites of muscle and periphery of callus, but this was transient and did not appear to be different at later time points (Fig. 5F).
Fig. 5.
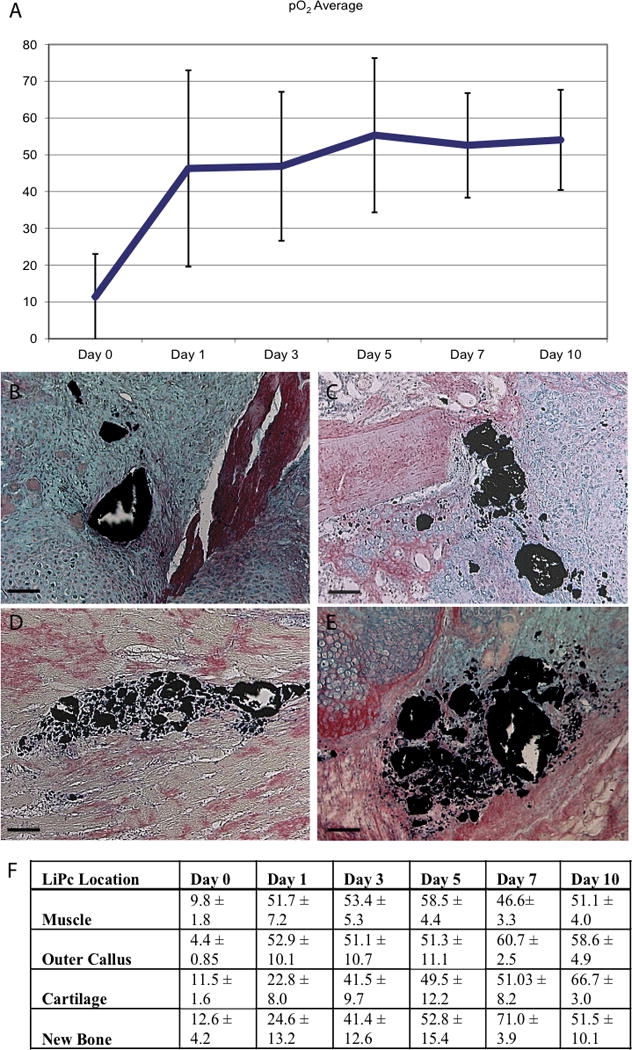
Longitudinal measurements of tissue O2 levels during fracture healing. (A) Longitudinal oxygen measurements made by EPR during the first 10 days of healing. (B–E) Histologic sections at day 10 illustrate that LiPc crystals are located in cartilage (B, C), in muscle (D), and in the outer region of the callus (E). (F) O2 levels differ depending on crystal location. In general areas of cartilage and bone formation have lower O2 levels than the outer area of the callus and the muscle during early fracture healing, especially at day 1 (p < 0.05). Scale bar=100μm.
Environmental hyperoxia improves fracture healing in an ischemic environment
Finally, hyperoxia appears to facilitate repair of cutaneous injuries, and we wanted to test whether hyperoxia may improve healing in situations where healing is severely compromised. Previously, we developed a model of delayed fracture union that resulted from resection of a segment of the femoral artery immediately prior to bone fracture. At 5 days after injury, we did not observe significant effects of hyperoxia on tissue vascularization (Fig. 6), but at 10 days after fracture, animals breathing 50% oxygen had significantly more callus tissue and formed more cartilage within the callus compared to ischemic mice that were housed in a normoxic atmospheric environment (Fig. 6, p<0.01). At this time point, hyperoxia also increased the amount of new bone (p=0.053) (Fig. 6). At day 28, histological analysis showed that 4 out of 5 fractures in animals housed in a normoxic environment did not exhibit bony bridging of the fractured bone ends. Instead, fibrous tissue and cartilage were present in the fracture gaps. In contrast, all fractures treated with hyperoxia (n=5) exhibited bony bridging at this time (Fig. 6).
Fig. 6.
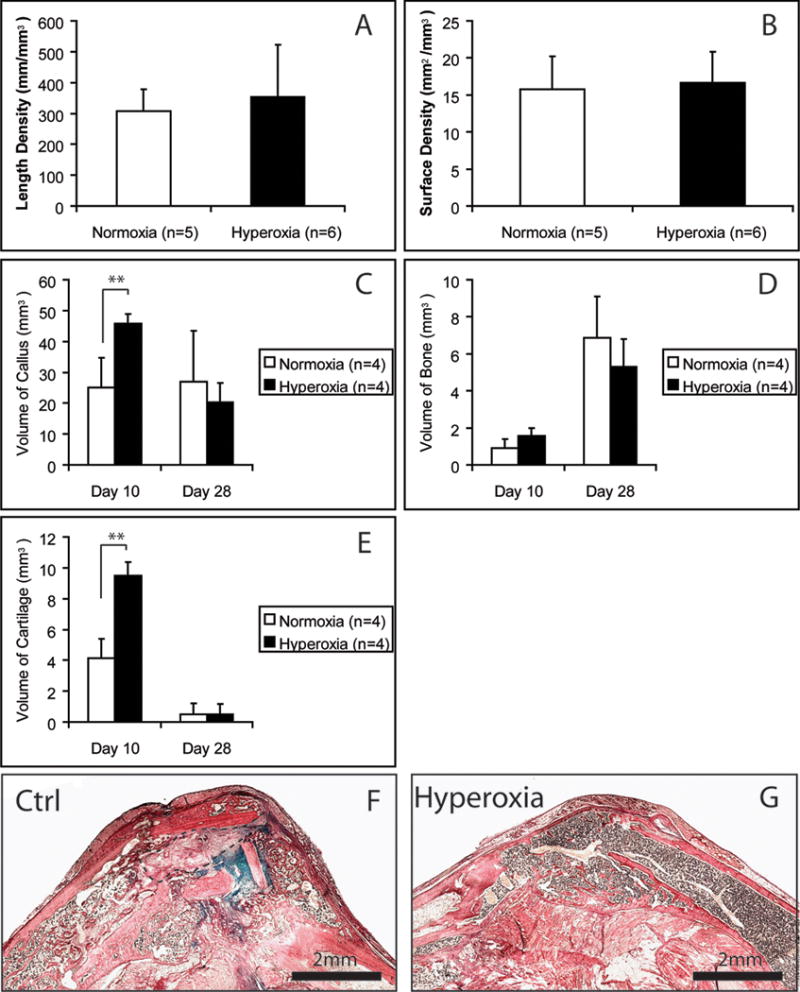
Environmental hyperoxia improves the repair of ischemic fractures. (A–E) Histomorphometry. ** p<0.01. Length density and surface density of blood vessels were analyzed at 5 days after fracture. (F) Ischemic fracture treated with normoxia did not heal completely at 28 days after fracture. HBQ staining and the blue color shows cartilage remnant in the callus. Outlined area is fibrous tissue. (G) Ischemic fracture treated with hyperoxia healed at 28 days after fracture and no cartilage remnant was detected. Scale bars = 2mm.
Discussion
Results from the current study confirmed that oxygen plays important roles during fracture healing. We found that reduced oxygen impaired bone repair, and this was associated with impaired angiogenesis. Hyperoxia also appeared to negatively impact fracture healing. Hyperoxia may lead to excessive production or accumulation of superoxide radicals that were detrimental to healing. Alternatively, the reductions in cartilage and bone that we observed could be related to an accelerated rate of healing in these animals. In other systems increased oxygen levels stimulates healing. Hyperbaric oxygen improves skin wound healing and fracture healing in animal models [37–39]. However, there is lack of strong evidence to support the clinical use of hyperbaric oxygen on fracture healing [40]. Our current data indicate that supplying oxygen to ischemic fractures improves fracture healing in our mouse model. Due to the wide availability and convenience of normobaric oxygen, further research in this direction is warranted.
Hypoxia is widely considered to be a driving force for angiogenesis. However, hyperoxia is also angiogenic in the context of inflammation. In particular, the Hif1/VEGF signaling pathway is a key regulator of angiogenesis during fracture healing [41]. While hypoxia is thought to stabilize Hif-1α, up-regulates the expression of VEGF, and induce angiogenesis [8], reactive oxygen species and lactate, both products of NADPH oxidases stimulate Hif1α stabilization directly and in combination [30]. Indeed, previous work has shown that environmental hypoxia inhibits while hyperoxia strongly stimulates angiogenesis [38]. Inhibitory effects of hypoxia on angiogenesis during fracture healing are likely due to multiple reasons. Oxygen is required for the activity of many enzymes including those that participate in collagen synthesis [38]. Alternatively or additionally, local hypoxia may have biological effects that are different from systemic hypoxia generated via modulating atmospheric oxygen levels. For example, atmospheric hypoxia creates systemic hypoxia whereby uninjured tissues adjacent and distant to the fracture callus are hypoxic. Therefore, the gradient of hypoxia at the injury site may be shallower in animals breathing low levels of oxygen. Within an individual cell, the decreased oxygen stabilizes Hif1α and initiates VEGF expression. In turn, VEGF is secreted and acts on adjacent cells possibly located in oxygenated environments that allow angiogenesis to proceed. Indeed, in an animal model of angiogenesis, Knighton et al. created a hole in the ear of a rabbit and found that a gradient of hypoxia is required for vascular repair [42]. In fracture models, measuring a gradient of oxygen is technically very challenging, but an O2 gradient is well described in soft tissue injuries [42].
Our data also demonstrate that hyperoxia stimulates angiogenesis in fractures. A multitude of data demonstrates that increased oxygen levels lead to increased angiogenesis, and the key metabolic component that stimulates this process appears to be lactate [29]. Lactate induces angiogenesis in an oxygen dependent manner, and this can be inhibited by inhibition of lactate dehydrogenase [30]. Lactate stabilizes Hif1α and leads to upregulation of VEGF in wounds [38]. Since hypoxia produces lactate via anaerobic glycolysis, this most likely led to the concept that hypoxia stabilizes Hif1α. Including hypoxia in the name of this molecule has been unfortunate, because this implied that hypoxia is the single mechanism by which Hif1α is normally stabilized. The vast majority of lactate produced in the body is through aerobic glycolysis [43, 44], and in wounds this stabilizes Hif1α possibly by oxidant signaling pathways in cells [31]. Thus, changes in our understanding of aerobic lactate production require a shift in thinking about Hif1α as well.
Indeed, in our fracture model we did not observe a relationship between inspired oxygen and lactate concentration at the fracture site. This result is consistent with other data showing that lactate levels are not correlated with pO2 [44]. During cutaneous wound healing lactate levels increase nearly five-fold as a result of the leukocyte oxidative burst and increased cell proliferation that occur after injury [30]. We observed a similar increase in lactate production after fracture. This is an important observation, because this increase in lactate may be sufficient to stabilize Hif1α induce VEGF expression and angiogenesis during fracture healing. This idea is strengthened by observations that hyperbaric oxygen up-regulates VEGF expression and promotes angiogenesis [38].
Oxygen and stem cell differentiation during fracture healing
In vitro data suggests that oxygen tension regulates stem cell differentiation. Hirao et al. demonstrated that pluripotent mesenchymal cells tend to differentiate into chondrocytes rather than osteoblasts in hypoxic culture conditions [45]. Hypoxia also has been shown to increase production of matrix by chondrocytes that differentiate in hypoxic conditions [46]. Pluripotent mesenchymal cells cultured at 5% oxygen increased the effectiveness of bone morphogenetic protein 2 induction of glycosaminoglycan production, suppressed alkaline phosphatase activity, and reduced mineralization of C3H10T1/2 cells [45]. However, results from this study and our previous in vivo work [3] are not consistent with these in vitro findings. Here, fractures were treated with environmental hypoxia and we did not observe increased cartilage formation, a change in the ratio to cartilage bone, or a change in the mode of healing from intramembranous to endochondral ossification in stabilized and hypoxic fractures. Maturation of cartilage and endochondral ossification were not significantly affected by hypoxia. Previously, we created ischemic fractures which led to severe hypoxia at the fracture site, but we did not observe alterations in chondrogenesis [3, 4]. These data strongly suggest that mechanical instability at the fracture site has a much greater role in regulating stem cell fate decisions during fracture healing.
Various in vitro and in vivo data suggest that oxygen regulates recruitment of stem cells. Hypoxia increases stem cell mobilization from bone marrow through the activation of SDF-1 pathway [13], and SDF-1 has been shown to be beneficial for fracture healing [47]. Interestingly, hyperbaric oxygen treatment also increases the number of circulating stem cells [10–12]. However, the importance of bone marrow derived stem cells to fracture healing is unknown. Current research has demonstrated that the majority of cells that directly participate in endochondral ossification by forming cartilage and bone during fracture healing are derived locally from the adjacent periosteum [48, 49]. Recruitment of bone marrow-derived mesenchymal stem cells to sites of bone injury are likely to help orchestrate the process of bone healing by contributing humoral factors that stimulate repair or modulate inflammation [50]. However, even if hypoxia induced mobilization and recruitment of more bone marrow derived stem cells to the fracture site in our studies, these cells did not appear to have a stimulatory effect on fracture healing since hypoxia inhibited bone repair.
Conclusions
Our data indicate that hypoxia does not play a primary role in regulating stem cell fate decisions during fracture healing. Changing the levels of oxygen that an animal breathed affected fracture repair but did not alter lactate levels, chondrogenesis, or osteogenesis but did affect angiogenesis. Hyperoxia stimulated and hypoxia suppressed angiogenesis during bone repair, and hyperoxia stimulated healing in ischemic environments. More work is required to determine if hyperoxic treatment could be used to stimulate bone healing in humans. Based on our observations and those of others we would like to adopt a model for regulation of cutaneous wound healing that has been championed by Hunt et al.[51]. In this model slight elevations in lactate levels due to aerobic glycolysis at the fracture site, and the associated oxidant signaling that results, leads to increased vascularity of the fracture site via the Hif1/VEGF signaling axis. We are currently testing this model to determine whether lactate is the primary regulator of angiogenesis during fracture healing as well as examining other potential roles of lactate during fracture repair.
Acknowledgments
We would like to thank Drs. Diane Hu, Thomas Hunt, and Mark Rollins for technical and intellectual support. This work was aided by the Orthopaedic Trauma Association (Basic Research Grant to C.L.), NIH-NIAMS (KO8-AR002164 and RO1-AR053645-01 to T.M.), and Zimmer Inc.
References
- 1.Dickson KF, Katzman S, Paiement G. The importance of the blood supply in the healing of tibial fractures. Contemporary orthopaedics. 1995;30:489–93. [PubMed] [Google Scholar]
- 2.Brinker MR, Bailey DE., Jr Fracture healing in tibia fractures with an associated vascular injury. The Journal of trauma. 1997;42:11–9. doi: 10.1097/00005373-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lu C, Miclau T, Hu D, et al. Ischemia leads to delayed union during fracture healing: a mouse model. J Orthop Res. 2007;25:51–61. doi: 10.1002/jor.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu C, Rollins M, Hou H, et al. Tibial fracture decreases oxygen levels at the site of injury. Iowa Orthop J. 2008;28:14–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Schwarz EM, Young DA, et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. The Journal of clinical investigation. 2002;109:1405–15. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie C, Liang B, Xue M, et al. Rescue of impaired fracture healing in COX-2−/− mice via activation of prostaglandin E2 receptor subtype 4. The American journal of pathology. 2009;175:772–85. doi: 10.2353/ajpath.2009.081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivirikko KI, Prockop DJ. Enzymatic hydroxylation of proline and lysine in protocollagen. Proceedings of the National Academy of Sciences of the United States of America. 1967;57:782–9. doi: 10.1073/pnas.57.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong GH. Regulation of angiogenesis by oxygen sensing mechanisms. Journal of molecular medicine. 2009;87:549–60. doi: 10.1007/s00109-009-0458-z. [DOI] [PubMed] [Google Scholar]
- 9.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–83. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 10.Thom SR, Bhopale VM, Velazquez OC, et al. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol. 2006;290:H1378–86. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–59. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10:1869–82. doi: 10.1089/ars.2008.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 14.Wu D, Malda J, Crawford R, et al. Effects of hyperbaric oxygen on proliferation and differentiation of osteoblasts from human alveolar bone. Connective tissue research. 2007;48:206–13. doi: 10.1080/03008200701458749. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaije C, Koedam M, van Leeuwen JP. Decreased oxygen tension lowers reactive oxygen species and apoptosis and inhibits osteoblast matrix mineralization through changes in early osteoblast differentiation. Journal of cellular physiology. 2012;227:1309–18. doi: 10.1002/jcp.22841. [DOI] [PubMed] [Google Scholar]
- 16.Hirao M, Hashimoto J, Yamasaki N, et al. Oxygen tension is an important mediator of the transformation of osteoblasts to osteocytes. Journal of bone and mineral metabolism. 2007;25:266–76. doi: 10.1007/s00774-007-0765-9. [DOI] [PubMed] [Google Scholar]
- 17.Genetos DC, Toupadakis CA, Raheja LF, et al. Hypoxia decreases sclerostin expression and increases Wnt signaling in osteoblasts. Journal of cellular biochemistry. 2010;110:457–67. doi: 10.1002/jcb.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng WP, Yang SN, Lai CH, et al. Hypoxia induces BMP-2 expression via ILK, Akt, mTOR, and HIF-1 pathways in osteoblasts. Journal of cellular physiology. 2010;223:810–8. doi: 10.1002/jcp.22104. [DOI] [PubMed] [Google Scholar]
- 19.Steinbrech DS, Mehrara BJ, Saadeh PB, et al. Hypoxia increases insulinlike growth factor gene expression in rat osteoblasts. Annals of plastic surgery. 2000;44:529–34. doi: 10.1097/00000637-200044050-00012. discussion 534–5. [DOI] [PubMed] [Google Scholar]
- 20.Steinbrech DS, Mehrara BJ, Saadeh PB, et al. Hypoxia regulates VEGF expression and cellular proliferation by osteoblasts in vitro. Plastic and reconstructive surgery. 1999;104:738–47. doi: 10.1097/00006534-199909030-00019. [DOI] [PubMed] [Google Scholar]
- 21.Lin C, McGough R, Aswad B, et al. Hypoxia induces HIF-1alpha and VEGF expression in chondrosarcoma cells and chondrocytes. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2004;22:1175–81. doi: 10.1016/j.orthres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Coyle CH, Izzo NJ, Chu CR. Sustained hypoxia enhances chondrocyte matrix synthesis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2009;27:793–9. doi: 10.1002/jor.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duval E, Leclercq S, Elissalde JM, et al. Hypoxia-inducible factor 1alpha inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1alpha-dependent redifferentiation of chondrocytes. Arthritis and rheumatism. 2009;60:3038–48. doi: 10.1002/art.24851. [DOI] [PubMed] [Google Scholar]
- 24.Henderson JH, Ginley NM, Caplan AI, et al. Low oxygen tension during incubation periods of chondrocyte expansion is sufficient to enhance postexpansion chondrogenesis. Tissue engineering. Part A. 2010;16:1585–93. doi: 10.1089/ten.tea.2009.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajpurohit R, Koch CJ, Tao Z, et al. Adaptation of chondrocytes to low oxygen tension: relationship between hypoxia and cellular metabolism. Journal of cellular physiology. 1996;168:424–32. doi: 10.1002/(SICI)1097-4652(199608)168:2<424::AID-JCP21>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Farnum CE, Lenox M, Zipfel W, et al. In vivo delivery of fluoresceinated dextrans to the murine growth plate: imaging of three vascular routes by multiphoton microscopy. The anatomical record. Part A, Discoveries in molecular, cellular, and evolutionary biology. 2006;288:91–103. doi: 10.1002/ar.a.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnett TR, Gibbons DC, Utting JC, et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. Journal of cellular physiology. 2003;196:2–8. doi: 10.1002/jcp.10321. [DOI] [PubMed] [Google Scholar]
- 28.Muzylak M, Price JS, Horton MA. Hypoxia induces giant osteoclast formation and extensive bone resorption in the cat. Calcified tissue international. 2006;79:301–9. doi: 10.1007/s00223-006-0082-7. [DOI] [PubMed] [Google Scholar]
- 29.Hunt TK, Aslam R, Hussain Z, et al. Lactate, with oxygen, incites angiogenesis. Advances in experimental medicine and biology. 2008;614:73–80. doi: 10.1007/978-0-387-74911-2_9. [DOI] [PubMed] [Google Scholar]
- 30.Hunt TK, Aslam RS, Beckert S, et al. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxidants & redox signaling. 2007;9:1115–24. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milovanova TN, Bhopale VM, Sorokina EM, et al. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. Journal of applied physiology. 2009;106:711–28. doi: 10.1152/japplphysiol.91054.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu C, Saless N, Hu D, et al. Mechanical stability affects angiogenesis during early fracture healing. Journal of orthopaedic trauma. 2011;25:494–9. doi: 10.1097/BOT.0b013e31822511e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu C, Miclau T, Hu D, et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–7. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu C, Hansen E, Sapozhnikova A, et al. Effect of age on vascularization during fracture repair. J Orthop Res. 2008;26:1384–9. doi: 10.1002/jor.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing Z, Lu C, Hu D, et al. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3:451–8. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan N, Williams BB, Hou H, et al. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxidants & redox signaling. 2007;9:1169–82. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman RJ. Hyperbaric oxygen therapy for wound healing and limb salvage: a systematic review. PM R. 2009;1:471–89. doi: 10.1016/j.pmrj.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Hunt TK, Ellison EC, Sen CK. Oxygen: at the foundation of wound healing–introduction. World J Surg. 2004;28:291–3. doi: 10.1007/s00268-003-7405-x. [DOI] [PubMed] [Google Scholar]
- 39.Yablon IG, Cruess RL. The effect of hyperbaric oxygen on fracture healing in rats. J Trauma. 1968;8:186–202. doi: 10.1097/00005373-196803000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Bennett MH, Stanford R, Turner R. Hyperbaric oxygen therapy for promoting fracture healing and treating fracture non-union. Cochrane Database Syst Rev. 2005:CD004712. doi: 10.1002/14651858.CD004712.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Shen X, Wan C, Ramaswamy G, et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2009;27:1298–305. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–70. [PubMed] [Google Scholar]
- 43.James JH, Luchette FA, McCarter FD, et al. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. 1999;354:505–8. doi: 10.1016/S0140-6736(98)91132-1. [DOI] [PubMed] [Google Scholar]
- 44.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. The Journal of physiology. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirao M, Tamai N, Tsumaki N, et al. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. The Journal of biological chemistry. 2006;281:31079–92. doi: 10.1074/jbc.M602296200. [DOI] [PubMed] [Google Scholar]
- 46.Lafont JE, Talma S, Hopfgarten C, et al. Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J Biol Chem. 2008;283:4778–86. doi: 10.1074/jbc.M707729200. [DOI] [PubMed] [Google Scholar]
- 47.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis and rheumatism. 2009;60:813–23. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Naik A, Xie C, et al. Periosteal stem cells are essential for bone revitalization and repair. Journal of musculoskeletal & neuronal interactions. 2005;5:360–2. [PubMed] [Google Scholar]
- 49.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2009;24:274–82. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caplan AI, Correa D. The MSC: an injury drugstore. Cell stem cell. 2011;9:11–5. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trabold O, Wagner S, Wicke C, et al. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2003;11:504–9. doi: 10.1046/j.1524-475x.2003.11621.x. [DOI] [PubMed] [Google Scholar]


